Abstract
The study examines the morpho-physiological differences in leaf characteristics between two species of C4 plants: sorghum and maize. The research was conducted in field conditions where plants were rainfed. Both species different varieties were cultivated at two distinct sites that exhibited variations in soil texture and drought stress incidence according to the vegetation condition index (VCI). Samples were collected during various growth stages to analyze the relative water content (RWC), proline levels, and stomatal density. Sorghum plants displayed higher RWC, proline levels, and stomatal density than maize plants. In sorghum, the biochemical traits, such as the proline content, may play a more critical role in withstanding water-limited conditions than in maize in our experiment. Under the same water restriction period, sorghum showed higher RWC levels. Sorghum plants reduced stomatal density under more water-limited conditions, which proves its plasticity. Additionally, early maturation played a crucial role in both species. The early sorghum variety KWS Kallisto and maize variety Walterinio KWS had a more stable yield at both sites. Nevertheless, the highest yields were found in the later varieties, KWS Hannibal and KWS Inteligens. The higher proline levels and the relative water content are drought-tolerant mechanisms and may be used to indicate drought intensity in field conditions. Our findings spotlight the influence of genetic diversity and genotype-environment interactions in determining crop responses to drought stress, providing valuable information for future breeding programs to enhance drought tolerance in crops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drought, abiotic stress, holds significant importance due to its adverse impact on plant physiology, biochemical processes (Per et al., 2017), and substantial anatomical alterations. Additionally, drought influences growth (Daryanto, et al., 2016), enzyme functionality, stomatal morphology (He et al., 2020), stomatal closure (Marček et al., 2019), photosynthesis, carbon fixation (Liu et al., 2015), and relative water content (RWC) (Marček et al., 2019). As a result, drought poses a threat to agricultural production on a global scale, particularly in the face of current climate change conditions (Girvetz et al., 2009; Romm, 2015; Shrestha, et al., 2012; Xu, et al. 2016), which are projected to amplify the frequency and severity of drought occurrences (Středa et al., 2019).
In the Czech Republic, where the experiment was conducted (the South Moravian Region), soil moisture content during the growing season has been decreasing over the past six decades, and drought events have become significantly more frequent (Meitner, et al., 2023). More extended periods of drought alternate with one-off high rainfall totals. While it is impossible to precisely predict the timing of drought exposure for plants in field conditions, patterns in seasonal drought occurrence can be identified. Based on these patterns, farmers can make informed decisions regarding using early- or late-maturing varieties of their chosen crop species, taking advantage of drought escape. Therefore, to maintain the yield of maize and sorghum in this area, it is necessary to study drought tolerance under field conditions, and isolating the specific effects of various environmental factors and stresses on plant growth, yield quantity, and quality can be challenging. However, investigating drought tolerance adaptations in natural conditions can provide insights into natural selection processes and aid in identifying new stress tolerance mechanisms (Kooyers, 2015).
Understanding plant responses to drought involves a spectrum of morpho-physiological, biochemical, cellular, and molecular processes. These responses involve enhancements in root systems, leaf structures, osmotic adjustment, RWC, and stomatal regulation (Ilyas et al., 2021). Moreover, the differing reactions of distinct plant species to drought become apparent in their adaptive strategies. For instance, certain plant varieties employ drought escape as a mechanism to mitigate stress impact by completing their life cycles earlier, thereby avoiding the onset of drought (Fukai & Cooper, 1995; Taiz & Zeiger, 2010), which is particularly beneficial when late summer drought occurs (Bowers, 1995) in temperate climate zones.
Drought can lead to desiccation in susceptible cells, depleting compatible solutes like proline and sugars. The accumulation of proline, an osmolyte and amino acid, is one of the various metabolic responses of plants to salt and drought stress. Proline biosynthesis and subsequent signalling contribute to maintaining redox balance in both standard and stressful environments (Hayat et al., 2012; Per et al., 2017), and genes involved in proline metabolism are upregulated during water deficit (Badigannavar et al., 2018). The precise role of proline in stress tolerance remains a subject of debate. However, it is believed that, to stabilise subcellular structures, scavenge free radicals, buffering cellular redox potential, and initiate gene expression. Proline also exhibits cross-tolerance to different types of stress (Kaur & Asthir, 2015). Research indicates a correlation between heightened proline accumulation induced by stress conditions and intensified growth impediment, potentially serving as an indicative factor to eliminate the most vulnerable varieties (Arteaga et al., 2020; Fariaszewska et al., 2020).
Stomatal density may also play a crucial role in enhancing drought tolerance. Plants with fewer stomata tend to exhibit greater drought tolerance (Kusvuran et al., 2010) and are more efficient in water utilization (Caine et al., 2019). However, the relationship between stomatal density and drought response can vary among plant species. Contrary to certain findings, soybean plants have shown that higher stomatal density may enhance water supply by elevating stomatal conductance, particularly on the upper leaf surface, enhancing transpiration even during water stress (Buttery et al., 1993). Similarly, while wheat plants exhibit increased stomatal density under drought conditions, some studies have reported contrasting observations (Öztürk & Korkut, 2018). Some investigations (Liao et al., 2005) have demonstrated that wheat plants with lower stomatal density showed improved water use efficiency, highlighting the complexity of the relationship. The density and size of stomata, along with guard cells, have been linked to water use efficiency in plants (Dunn et al., 2019; Xu & Zhou, 2008). Consequently, the response of stomatal density to drought stress is not solely determined by the plant species but is also influenced by the specific genetic makeup or genotype (Mansouri et al., 2016).
In this context, the hypothesis arises that plant species with varying origins and evolutionary adaptations might exhibit diverse responses to drought stress. Specifically, research indicates that maize, generally considered less drought-tolerant than sorghum (Hasan et al., 2017; Queiroz et al., 2019), could display dissimilar reactions under water stress compared to sorghum due to their inherent differences in drought tolerance mechanisms. Previous studies have highlighted variations in growth inhibition, gas exchange, and water use efficiency between maize and sorghum under drought conditions (Hasan et al., 2017). Sorghum, originating from Africa and adept at surviving dry seasons and high temperatures (Beyene et al., 2015), often demonstrates diverse responses and sensitivity levels among its genotypes (Abraha et al., 2015). These distinctions in maize and sorghum responses to water stress underscore the need for a comparative analysis of their reactions under drought conditions.
The study aims to fill the crucial gaps in understanding of how maize and sorghum respond differentially to agricultural drought, and evaluate the suitability and effectiveness of their cultivation by analyzing morpho-physiological traits such as proline levels, RWC, and stomatal density. The study is concurrently assessing the aboveground biomass yield of selected varieties across various sites differing primarily in soil texture and moisture content. The presented article describes the experimental plant material and the methods used, evaluates the course of the weather at the experimental locations, and presents the results of individual morpho-physiological properties and yield of maize and sorghum varieties. Such an investigation furthers our understanding of plant stress responses and holds potential implications for agricultural practices and breeding programs targeting drought resilience.
Materials and Methods
Plant Material
For this experiment, ten temperate sorghum varieties and ten temperate maize varieties were chosen, as outlined in Table 1. The selection of varieties for both species was primarily based on their earliness, which served as the main criterion in the decision-making process. The categorization of earliness was determined based on information provided by the seed supplier. Among the sorghum varieties, the majority consisted of Sorghum bicolor (L. Moench) and Sorghum × drummondii (Nees ex. Steud.) hybrids, many of which are commonly cultivated in temperate climates for silage production due to their higher aboveground biomass. In the case of maize, earliness was characterized by the FAO number, which corresponds to the moisture content of the grain. The early maize varieties were identified by having the lowest FAO number.
Field Conditions
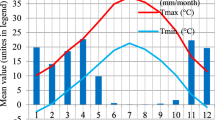
The plants were grown at four sites (1, 2, 3a and 3b), but each species was grown at only two different sites. These sites are located close to the South Moravian Region, Czech Republic (49.023N, 16.618E), and are classified as climate class Dfb. The average temperature for this location in which the sites are located is 10.3 °C with a precipitation sum of 491.1 mm per year (Orság et al., 2022). At the beginning of the experiment, sites 3a and 3b were considered more drought-stressed due to higher sand particle content (for more information, see Supplementary Information). A meteorological station located close to sites 1 and 2 recorded precipitation with a Met One 370 rain gauge (Met One Instruments, USA) and temperature at the height of 2 m above ground with a Vaisala HMP155A Merici sensor (Vaisala, Finland).
Because the month’s total precipitation creates a worse depiction of rainfall distribution, the conventional graphical representation of the quantity of monthly precipitation distribution (Fig. 1) leads to a poorer interpretation. As a result, we have developed a straightforward measure noted as the Rain Distribution Scale (RDS). The scale is based on monthly rainfall measurements expressed in millimetres, which are then converted into dimensionless values (Table 2). For instance, 1.2 mm of rain in one day is worth 2 on the scale.
This scale is based on the precipitation pattern in the given climatic conditions. The scale values for each day are then entered into the radar chart. The numbers on the outer line represent the day number. An average monthly scale value can be calculated from the sum of the monthly values of the scale, which also characterizes the given month.
The Vegetation Condition Index (VCI), derived from remote-sensing data (Jiao et al., 2016), effectively differentiates varying levels of water availability across diverse sites. Vegetation indices, such as the Normalized Difference Vegetation Index (NDVI), are extensively employed in agriculture due to their capacity to monitor changes in crop biomass and health (Becker-Reshef, et al., 2010; Dorigo, et al., 2007). These indices also serve in studying the impacts of drought (Liang et al., 2017; Liu & Kogan, 2007; Sholihah et al., 2016; Zambrano et al., 2016), exhibiting strong correlations with other drought indices such as the Palmer Drought Severity Index. This correlation underscores the NDVI’s utility in effectively monitoring drought conditions (Jiao et al., 2016).
The vegetation condition index (VCI) was calculated using the geographic information system software ArcGIS (Esri, USA) to determine the per cent drought area during the chosen dates at the sites in 2022. Images recorded by a drone during the growing season of 2022 were used to calculate the VCI. First, the normalized difference vegetation index (NDVI) was calculated, and the VCI was then calculated as the per cent area of drought occurrence, as follows (Faridatul & Ahmed, 2020):
NDVIi is the NDVI value for a specific pixel in the month, and NDVImax and NDVImin are the same pixel’s maximal and minimal NDVI values for similar periods.
Sites 3a and 3b are at the exact location but consist of two fields with different crop rotations. The dimensions of the plots were 14.0 × 2.7 m for sorghum and 7.0 × 3.0 m for maize. The plants of each variety were grown in randomised plots in two repetitions. The sowing depth was 0.08 m for maize with a row spacing of 0.75 m, and for sorghum, the sowing depth was 0.03 m with a row spacing of 0.45 m. The maize plants were fertilized with urea at a rate of 140 kg nitrogen/hectare at both sites in spring, two weeks before the sowing date; sorghum was not fertilized in the given field conditions, as it is not a practice and can grow well without fertilizers. All plants were solely rainfed. The preceding crop species were grain maize at sites 1 and 2, winter barley at site 3a, and spring poppy at site 3b. For details about soil chemical composition, see Supplementary Information.
Uneven Rain Distribution and The Vegetation Condition Index
The following charts (Fig. 2) graphically represent rain distribution in the growing season of 2022. According to the sum of precipitation for the given month, July seems to be the month with enough precipitation; however, the sum number of precipitation is affected by the storms and a few heavy showers of rain, and between these extremes of heavy rains, there were other extremes such as no rain and high temperatures. Therefore, the created scale (Rain Distribution Scale) gives us a sum of scale values of 1.4, which is lower than in June, in which the sum of month precipitation is lower, but the distribution is higher than in July.
The spring months, April and May, were the most drought-stressed. Meanwhile, the summer months of June, July and August had higher rain precipitation and scale values. However, it is mainly due to summer storms and heavy rains accompanying these storms. The spring months’ precipitation mainly affects vegetative growth; meanwhile, the summer and autumn months affect reproductive stages such as apex development, flower and seed set, and the quality of seeds (maturation).
Even though the selected sites were located relatively close to each other, the different soil structures and the different vegetation periods of the two investigated species affected the incidence of drought at these sites, which was calculated from the NDVI (Table 3). The NDVI results were graphically visualized (as shown in Fig. 3) and used to calculate the VCI (Table 4). The highest drought percentage incidence was recorded in May when the extreme values were recorded at site 1 (98.6% of extreme drought).
Aboveground Biomass Harvest and Other Observed Traits
The plants of sorghum and maize were harvested by hand in three replications at different times according to their dry matter content of aboveground biomass (in sorghum, about 28%; in maize, about 33%), which were monitored by continuous sampling. The plants were cut about 0.01 m above the ground. The size of the harvested area was for sorghum (5.0 × 0.45 m) and maize (5.0 × 0.75 m). The harvested biomass was weighed, subsequently crushed and homogenised. This material was dried in two stages in an oven (BMT Venticell ECO, Czech Republic). In the first stage, the drying period was 24 h under 65 °C; in the second stage, the period was 4 h under 105 °C. Finally, the harvest of dry aboveground biomass was calculated for tonnes per hectare.
The sorghum height was also measured as the differences between the sorghum varieties were significantly greater than in maize. The height was measured before the harvest in three replications.
Proline Content Determination
The samples used for proline determination were collected at the times indicated in Table 4. In all cases, two replicates of approximately 1 × 5 cm leaf parts were cut from the middle part of the second oldest (second-to-bottom leaf) and second youngest (second-to-top leaf) leaves of two plants. The samples were collected in the field and transported to the laboratory in a portable cool box where the temperature was below 10° C. They were then stored at -80 °C in the laboratory until analysis.
With slight modifications, the samples were prepared for spectrophotometric determination (Magné & Larher, 1992). First, the extraction medium was prepared with 1% ninhydrin solution (prepared from ≥ 99% pure solid ninhydrin, Roth, Germany) dissolved in a 60:40 (v/v) glacial acid: water solution (99% pure glacial acid was diluted to 60%, Roth, Germany; and deionized water was used for the solution). Standards for proline spectrophotometric determination were prepared to obtain a gradient from 0.04 mM to 1 mM proline (L-proline, 98,5% pure, Roth, Germany; was dissolved in the extraction medium); the calibration set underwent the same procedure as did the samples from the field (excluding the steps of freezing and weighing). The field samples were taken from the freezer, weighed (average weight 100 mg), and ground in liquid nitrogen using a mortar and pestle. After the nitrogen had evaporated, the samples were transferred with a spatula to individual tubes, to which 1.5 ml of ninhydrin solution was added. The tubes were shaken by hand for 10 s and placed into a boiling water bath for 1 h. Then, the tubes were cooled to room temperature by placing them on ice packs; after cooling, 1.5 ml of toluene (99%; Lach-Ner, Czech Republic) was added for chromophore extraction. The tubes were vigorously shaken by hand for 15 s, then the phases were separated, and the absorbance of the stable upper phase was measured at 520 nm after 24 h; toluene served as a blank sample.
Leaf RWC Determination
Leaf RWC is the percentage water content at the sampling time relative to the water content at full turgor (Alam et al., 2020). One prerequisite before each sampling time was the absence of rain for at least 3–4 days. As mentioned in Proline Content Determination, the samples of the same size were again collected at times indicated in Table 4 and placed into plastic tubes sealed with lids. The field samples were transported in the cool box to the field station as soon as possible, where all the samples were individually weighed (fresh weight). The leaves were subsequently cut into approximately 1 × 1 cm pieces and returned to the tubes, to which water was added until all parts of each sample were fully submerged. After 3 h, the water was poured from the tubes, and the samples were dried using filter paper, weighed (saturated weight), and then dried in an oven at 103 °C for 2 h (the drying time and temperatures were modified). After that, the samples were weighed again (dry weight), and the RWC was determined (González & González-Vilar, 2001).
Stomatal Density Determination
The samples for stomatal density determination were taken, as seen in Table 4. In the field, transparent nail polish was applied to the third youngest leaf, in the middle of the leaf on both the upper and the lower sides. After approximately 1 min, transparent adhesive tape was pressed onto the dry polish so that the polish adhered well to the tape. Then, the tape with the polish imprint on the leaf surface was peeled off and adhered to a glass slide. The stomatal density was then determined by light microscopy. The number of stomata was counted within an area of 1 × 1 mm at 200 × magnification. Two replicates for each sample were included.
Statistical Analyses
Statistical analyses were performed in XLSTAT (Lumivero, USA), an MS Excel add-on software program. Differences between the results obtained for the two species, the different varieties and the different sites were analysed with the nonparametric Kruskal‒Wallis test, and the Steel–Dwass–Critchlow–Fligner test was used for post hoc testing for multiple pairwise comparisons. Spearman correlation (rs) was used to analyse the relationships among the observed traits, including the number of stomata, proline contents, RWC and aboveground biomass yield. The PCA (Principal Component Analysis) graphically represented the Spearman correlation values. The graphical representations (Figs. 2, 4, 5) were made in Excel (version 2306, Microsoft, USA), and Figs. 6, 7, 8, 9, 10, 11, 14,15 were made in SigmaPlot (Systat Software Inc., USA). Figures 12, 13 were made by XLSTAT.
Results and Discussion
The results obtained from the field experiment investigating the responses of maize and sorghum to agricultural drought exhibit several key insights that support the initial hypothesis regarding their differential reactions to drought stress.
Proline and RWC Relationship in Maize and Sorghum Under Field Conditions
Under the analysed field conditions, a negative correlation between the RWCs and the proline content of the leaves was found. Although the correlation between RWC and proline content in sorghum species was not statistically significant at site 3a (rs = -0.212; p = 0.560), it was at site 1 (rs = -0.648; p = 0.049). The association was extremely weak in maize (site 2 rs = -0.018; site 3b rs = -0.200), and the link was not shown to be statistically significant. In contrast to maize, the values of RWC and proline content of sorghum values are relatively widely dispersed in the graphical depiction of the data in Figs. 4, 5. One explanation for this is the diverse genetic background of the selected sorghum cultivars, some of which are hybrids of Sorghum bicolor and Sorghum arundinaceum (also known as Sorghum × drummondii, Sudan grass).
Sorghum bicolor has a greater drought tolerance than the Sorghum-Sudan grass hybrid, according to an experiment comparing the drought tolerance of maize, sorghum, and sorghum-sudan grass species (Schittenhelm & Schroetter, 2014). From the values of RWC and proline content, it can be inferred that proline content in sorghum has a more significant impact on water deficit than maize. However, it is essential to acknowledge that plants produce a variety of other chemicals, such as fructans (Benkeblia, 2022; Hendry, 1993; Vijn & Smeekens, 1999) and the phytohormone abscisic acid (Waterland et al., 2010; Sah, et al., 2016), which also play a significant role in the response and survival of plants under water shortage. Determining their levels in field conditions may provide an interesting perspective on the mechanisms of drought tolerance of crops under field conditions.
The Selected Morpho-Physiological Parameters Differences in Both Species.
To investigate traits’ statistical differences, we performed the Kruskal–Wallis test for each species and factor at each site, as plants under sub-optimal conditions at different sites may react differently to maintain their yield parameters (Table 5).
Relative Water Content (RWC) Analysis
The average values for each variety within the species are represented in Fig. 6 and Fig. 7. A statistically significant difference was found between the values of RWC in sorghum and maize (Table 5). The higher RWC values were found for sorghum, which showed an average value of 90.7% for the entire observed period. Meanwhile, the average value in maize was 87.0% under the studied field conditions.
RWCs of 100 to 90% are related to stomatal closure, cell expansion, and growth reduction as cells reach the maximal water content. At RWCs of 90 to 80%, changes in the tissue composition and alterations in photosynthesis and respiration rates occur (González & González-Vilar, 2001); hence, we assumed that some changes in photosynthesis may have occurred in maize plants, as the average value of RWC fell under 90%. The RWC below 80% is associated with a water potential of -1.5 MPa or less, which leads to metabolic changes, decreased photosynthesis, increases in respiration and proline content and accumulation of ABA (González & González-Vilar, 2001). The higher RWC observed in sorghum, a more drought-tolerant species than maize (Schittenhelm & Schroetter, 2014; van Oosterom et al., 2021), is intriguing. This finding suggests that sorghum potentially sustains higher water levels in its leaves over an extended period, potentially due to improved water use efficiency (Danalatos, et al., 2009; Hasan et al., 2017) and enhanced water absorption through its more robust root system compared to maize (Schittenhelm & Schroetter, 2014).
Differences of statistical significance in RWC among varieties were found only in sorghum (Table 5). The highest average RWC values were found in varieties like Hannibal (93.4%), KWS Bulldozer (93.4%), and KWS Tarzan (93.1%). The observed varietal differences in RWC under drought conditions align with prior reports in various species such as maize, wheat, potato, soybean, and mungbean. (Hossain, et al., 2015; Nazran et al., 2019; Pokharel & Pandrey, 2012; Soltys-Kalina et al., 2016). The highest RWC values of the sorghum varieties were not affected by the time to maturity, as three different varieties presented the highest values and had different earliness. Nevertheless, the timing of drought in the early stage of development plays a vital role in this outcome, as the spring months of April and May seemed less precipitation-rich. Therefore, genotype and the interaction between genotype and environmental/field conditions played more prominent and essential roles in determining RWC than just the earliness of the sorghum varieties.
Environmental influence on RWC was evident between the sampled sites and different dates. Site-specific differences in sorghum RWC were profound, with site 3a recording the highest average values (92.9%) compared to site 1 (88.4%). These variations could be attributed to soil characteristics favouring plant growth at site 1 due to higher clay content, while the more favourable water regime at site 3a lessened drought severity. It is possible that during the drought period, the roots may have been coarser in clay loam, but in soils with a higher content of sand particles, fine roots may have formed easily, and it may have helped plants absorb water from the soil (Garg et al., 2020).
Temporal variations in RWC were observed for both sorghum and maize. The highest RWC values for both species were recorded in August (sorghum, 92.4%; maize, 93.2%), presumably influenced by abundant rainfall early that month. The lowest RWC values were recorded in June for sorghum (88. 7%) and in July for maize (82.3%). Additionally, variations in growth stages between the two species, with maize sown earlier than sorghum in the given climate, contributed to differences in RWC levels across sampling months.
Proline Content
The data revealed significant differences in proline content between sorghum and maize (Table 5). Sorghum exhibited higher proline levels, averaging 8.7 micromoles/100 mg of fresh leaf weight, compared to maize, which had an average of 5.3 micromoles/100 mg of fresh leaf weight. These findings align with the observed higher Relative Water Content (RWC) in sorghum under the specific field conditions (Figs. 8 and 9).
The variability in proline accumulation capacity among species has been found in previous studies (Yamada et al., 2005). Sorghum varieties exhibited distinct maximal proline accumulation abilities (Blum & Ebercon, 1976), potentially contributing to their ability to maintain higher RWC levels. Studies involving exogenous proline applications in plants, such as cauliflower, have shown enhanced RWC due to increased internal proline content (EL-Bauome et al., 2022). This exogenous proline application also increases chlorophyll content and crop yield through the increased activities of crucial enzymes like superoxide dismutase, peroxidase, and catalase (Hanif et al., 2021). Further evidence supports the link between proline accumulation and drought tolerance, with more drought-tolerant varieties showing higher proline levels (Quilambo, 2004). The opposite reaction was found in drought-resistant rice varieties that exhibited lower proline contents (Dien, et al., 2019). However, barley plants have also demonstrated proline accumulation under stressful conditions (Frimpong et al., 2021). From the results of our experiment, the plants of maize and sorghum can also achieve higher proline levels under drought conditions in the field. Nevertheless, sorghum plants exhibited higher levels of proline than maize, which may be one of many drought tolerance mechanisms of sorghum.
Significant variations in proline content were observed exclusively among sorghum varieties. Early sorghum varieties, specifically Nutri Honey (10.6 micromoles/100 mg of fresh leaf weight) and KWS Kallisto (9.8 micromoles/100 mg of fresh leaf weight), displayed the highest average proline content. This trend is characterized by relatively high proline levels in more drought-tolerant varieties, as found in other crops like wheat and sunflower (Ghosh et al., 2022; Keyvan, 2010; Manivannan et al., 2007). Compared to other sorghum varieties, KWS Kallisto stands out as potentially more drought-tolerant. In contrast, maize variety SY Ignis exhibited proline levels akin to certain sorghum varieties, yet it demonstrated a significant difference in aboveground dry biomass between the two sites.
Statistically significant differences in proline content between sites were found only for maize. Plants from site 2 displayed an average proline content of 4.1 micromoles/100 mg of fresh leaf weight, contrasting with the higher 7.9 micromoles/100 mg of fresh leaf weight in plants from site 3b. For sorghum, although average proline content differed slightly between sites as 8.9 micromoles/100 mg of fresh leaf weight in the samples from site 1 and 8.4 micromoles/100 mg of fresh leaf weight in those from site 3a, these variations were not statistically significant. The differences in maize proline content between sites suggest differential drought stress levels, particularly in June when maize at site 3b experienced higher stress than at site 2. The consistently higher proline content in sorghum, regardless of site differences, points out a continuous strategy of proline production, possibly as a "prepare to drought strategy".
Regarding sampling dates, statistically significant differences in proline content were evident for both sorghum and maize. In sorghum, peak proline content coincided with the lowest RWC in June, correlating with the sowing of sorghum varieties, indicating higher stress during this initial growth stage. Conversely, maize exhibited its highest proline content and lowest RWC in July, a month marked by prolonged drought.
Stomatal Density
Differences in stomatal density between sorghum and maize, both belonging to the C4 species, were evident in this study. Sorghum exhibited a higher stomatal density, averaging 152.4 stomata/1 mm2 leaf tissue, whereas maize showed a lower density with an average of 118.2/1 mm2 (Figs. 10 and 11). This distinction in stomatal density could be attributed to an evolutionary trait, potentially representing an anatomical adaptation in response to differing levels of drought tolerance between these species.
The stomatal density in sorghum varied significantly across different sites. Site 3a displayed the highest stomatal density (161.2 stomata/1 mm2), followed by site 1 (143.6 stomata/1 mm2). Site 1 was identified as experiencing higher drought stress based on the Vegetation Condition Index (VCI). Our observations suggest a potential adaptive response in sorghum, indicating a possible decrease in stomatal density under more arid conditions. Analogous findings in other studies have identified a lower stomatal density in more drought-tolerant plant species such as barley, wheat, rice, and tomato (Caine et al., 2019; Hughes et al., 2017; Kulkarni & Deshpande, 2006; Lestari, 2016; Li et al., 2017). This aligns with our findings, suggesting that a reduction in stomatal density may be a mechanism sorghum employs to adapt to more severe drought stress.
Additionally, our study revealed fluctuations in stomatal density across different sampling dates, with the highest density recorded in both sorghum and maize in August, the final month of sampling. There was a consistent increase in stomatal density with plant growth. Future research could explore the growth stages at which stomatal density stabilizes in sorghum and maize by extending the sampling period beyond August, especially since the harvest of aboveground biomass continued until October. This prolonged study could provide valuable insights into the cessation of stomatal density increase during the growth cycle of these plant species.
Influence of Environmental Factors on Inter-parameter Relationships and Species Responses
A detailed correlation matrix can be seen in Supplementary Information. The results of PCA analysis can be seen in Figs. 12 and 13. The varieties and parameters located in the opposite quadrants of the chart reacted differently in our study. The investigated parameters were fresh aboveground biomass yield, aboveground biomass yield at 28% in sorghum and 33% in maize, height (sorghum), RWC, proline content and stomatal density. The varieties closest to each other in the graph reacted similarly in the given parameters.
Under conditions of reduced water availability, both sorghum and maize plants exhibited decreased leaf RWC, accompanied by elevated proline content, indicating heightened water deficit stress. This elevation in proline content revealed a significant negative correlation with yield, a correlation specifically observed in sorghum (rs = − 0.806 at site 1; rs = − 0.733 at site 3a).
In sorghum, increased plant height correlated positively with RWC at both sites (rs = 0.830 and rs = 0.721), suggesting a possible relationship between plant stature and root system development, thereby influencing water absorption. It has been shown in maize that varieties with the leafy trait, also characterized by taller plants, have longer roots and a greater root system surface area (Costa et al., 2002), but this theory needs to be tested in sorghum.
At site 1, more drought-affected according to the Vegetation Condition Index (VCI), sorghum plants exhibited reduced stomatal density, although the variety’s response strongly impacted yield. A robust positive correlation emerged between earliness and yield, indicating that later maturing sorghum varieties demonstrated higher yields. In contrast, at site 3a, the plants’ reaction to parameters differed, displaying lower proline content but higher RWC values. This suggests the existence of lower water deficit stress (as indicated by VCI); nevertheless, RWC exhibited an increase alongside plant height.
Exploring the response of sorghum varieties to different sites (Fig. 12), KWS Kallisto, exhibiting early maturity, demonstrated consistent and stable yields similar to Latte at site 1, displaying similar RWC values, dry mass yield, and dry matter content. The similarity in maturity rate and height between KWS Kallisto and Latte may elucidate their proximity in the graph. KWS Hannibal, a high-yielding variety, differed substantially in its reaction to the sites. It exhibited lower proline content, elevated stomatal density, and increased dry matter and fresh biomass. Therefore, KWS Hannibal has the genetic potential to yield the highest from the selected sorghum varieties; however, its yield and yield characteristics are unstable and unpredictable in drought-stressed conditions.
Maize plants, likewise, showed diverse behaviours across the two sites. Increased proline content at site 2 correlated positively with the dry matter content of maize plants (rs = 0.794), indicating a response to water-limited conditions. Enhanced stomatal density correlated with increased leaf dry matter content at site 2 (rs = 0.685), likely resulting from expanded leaf dimensions. Conversely, at site 3b, maize plants exhibited increased stomatal density when proline content was decreased (rs = -0.676), suggesting a reduction of stomatal density during increased proline levels, which can be a potential mechanism for drought tolerance in maize.
Analyzing the reaction of maize varieties to different sites (Fig. 13), at site 2, Walterinio KWS shared a quadrant with KWS Koletis and KWS Inteligens, where SY Orpheus also resided. KWS Inteligens displayed similar proline and stomatal density content to Walterinio KWS, with comparable dry matter content observed in KWS Inteligens and SY Orpheus. However, at site 3b, Walterinio KWS diverged from KWS Inteligens by higher RWC values and lower proline content. These variations in response could suggest diverse drought response mechanisms among these varieties, including biochemical processes apart from proline content influencing RWC values.
The Yield Performance
The fresh aboveground biomass yield is a crucial trait for agronomists cultivating crops for fodder or silage purposes. Typically, sorghum plants exhibit superior yield under intensified drought-stress conditions compared to maize. While the quality of sorghum silage closely resembles that of maize silage (Fazaeli et al., 2006; Nusrathali et al., 2021; Podkówka & Podkówka, 2011), sorghum’s aboveground biomass comprises a higher lignin content relative to maize (Wahyuni et al., 2019; Zardin et al., 2017), potentially leading to decreased digestibility. Nevertheless, including the brown midrib (BMR) trait in sorghum varieties reduces lignin synthesis, enhancing digestibility. Consequently, a combination of sorghum and maize silage presents a promising alternative, especially when maize struggles to produce sufficient fresh biomass to feed animals (Cattani et al., 2017).
Therefore, it is desired that crop plants yield the highest yield possible. From this view (Figs. 14 and 15), the highest yield in sorghum was found in KWS Hannibal (intermediate; 21.7 t/ha at site 1 and 27.1 t/ha at site 3a) at both sites, followed by KWS Bulldozer (late; 20.4 t/ha) at site 1, and Big Kahuna (late; 25.9 t/ha) at site 3a. With the variety, Big Kahuna was a technical problem because its late earliness made it impossible to harvest the aboveground biomass at close to 28% in the given weather conditions in 2022. So, the plants were harvested at the lower dry matter before the plants could develop low-temperature damage in October. KWS Bulldozer, KWS Hannibal and Big Kahuna are quite tall plants, with KWS Hannibal being the tallest of the selected varieties (3.8 m at site 1; 3.1 m at site 3a) compared to the shortest Ruzrok plants (2.3 m at site 1; 2.0 m at site 3a). The height and good tillering ability were caused by genetic predisposition, which contributed to the highest yields in intermediate and late sorghum varieties. The highest yield in maize was found in KWS Inteligens (intermediate;24.1 t/ha) at site 1 and (19.0 t/ha) at site 3b. In this case, the later varieties performed better than the earlier ones, suggesting that rate maturity also plays a significant role in maize.
These results support our hypothesis, because it implies that plant with varying origins are prone to exhibit diverse responses to drought stress. Despite the first view, when scientists look for a more drought-tolerant crop, the most stable yield is sought above the highest yield. For plant breeders, the combination of stable and high yield is the most required trait. Nevertheless, the complexity of this trait, with the high number of genes involved, makes it difficult to achieve by classical plant breeding methods. The most stable yield was found in the sorghum variety KWS Kallisto (early; 16.2 t/ha at site 1 and 15.7 t/ha at 3a) and the maize variety Walterinio KWS (semi-early; 17.8 t/ha at sites 2 and 17.2 at site 3b). The early varieties probably used a drought avoidance strategy (Shavrukov et al., 2017). The maturity rate affects the yield. The early varieties usually yield less (Degener & Kappas, 2015) due to the reduced time for yielding components. For more information on average values for each parameter and variety, see Supplementary Information.
Conclusion
The study revealed species-specific responses to drought stress in maize and sorghum, underlining sorghum’s superior drought tolerance compared to maize under the studied field conditions. The sorghum plants presented higher RWCs, proline contents and stomatal density numbers than the maize plants. Moreover, there were differences in proline contents among the sorghum varieties, and some of the varieties yielded highly, perhaps because they modulated their RWCs and proline contents as drought tolerance mechanisms to maintain their yields. However, in maize, these parameters did not significantly affect yield. The sorghum variety KWS Hannibal (intermediate) yielded the highest yield. The most significant yield difference between the two sites (6.2 t/ha) was found in Big Kahuna (late). The maturity rate played a technical problem under the given weather conditions for the silage harvest of Big Kahuna. Therefore, the sorghum variety KWS Hannibal seems like a good candidate for our weather/climatic conditions, as its yield was among the highest, even with the second greatest difference in yields (5.5 t/ha), as the agronomists are interested more in the highest yield than the stable yield which was found in variety KWS Kallisto (early). In maize, the suitable variety was KWS Inteligens (intermediate), as the differences between yields were in the middle (5.1 t/ha) from the selected varieties. Nevertheless, the most adaptable variety seemed to be Walterinio KWS (semi-early). It may indicate that earliness still plays a decisive role in drought escape strategy in spring-sown crops grown in temperate climates. The findings also highlight the significance of genetic diversity and genotype-environment interactions in determining the morpho-physiological responses and yield performances of these crop species under agricultural drought, providing valuable insights for future breeding programs and strategies to enhance drought tolerance in crops.
Data Availability
All relevant data are in the paper and the Supplementary Information file, found at https://doi.org/10.5281/zenodo.8206566.
References
Abraha, T., Githiri, S. M., Kasili, R., Araia, W., & Nyende, A. B. (2015). Genetic variation among Sorghum (Sorghum bicolor L. Moench) Landraces from Eritrea under post-flowering drought stress conditions. American Journal of Plant Sciences, 06, 1410–1424. https://doi.org/10.4236/ajps.2015.69141
Alam, A., Emmanuel, I., Victor, O., Caroline, U., Rizvi, A. H., Kumar, T., et al. (2020). Screening of some selected Indian Maize cultivars to simulated drought condition. Indian Journal of Agricultural Research. https://doi.org/10.18805/IJARe.A-5247
Arteaga, S., Yabor, L., Díez, M. J., Prohens, J., Boscaiu, M., & Vicente, O. (2020). The use of proline in screening for tolerance to drought and salinity in Common Bean (Phaseolus vulgaris L.) genotypes. Agronomy. https://doi.org/10.3390/agronomy10060817
Badigannavar, A., Teme, N., de Oliveira, A. C., Li, G., Vaksmann, M., Viana, V. E., Ganapathi, T. R., & Sarsu, F. (2018). Physiological, genetic and molecular basis of drought resilience in sorghum [Sorghum bicolor (L.) Moench]. Indian Journal of Plant Physiology, 23, 670–688. https://doi.org/10.1007/s40502-018-0416-2
Becker-Reshef, I., Vermote, E., Lindeman, M., & Justice, C. (2010). A generalized regression-based model for forecasting winter wheat yields in Kansas and Ukraine using MODIS data. Remote Sensing of Environment, 114, 1312–1323.
Benkeblia, N. (2022). Insights on fructans and resistance of plants to drought stress. Frontiers in Sustainable Food Systems. https://doi.org/10.3389/fsufs.2022.827758
Beyene, A., Hussien, S., Pangirayi, T., & Mark, L. (2015). Physiological mechanisms of drought tolerance in sorghum, genetic basis and breeding methods: a review. African Journal of Agricultural Research, 10, 3029–3040. https://doi.org/10.5897/AJAR2015.9595
Blum, A., & Ebercon, A. (1976). Genotypic responses in sorghum to drought stress. III. Free proline accumulation and drought resistance 1. Crop Science, 16, 428–431. https://doi.org/10.2135/cropsci1976.0011183X001600030030x
Bowers, G. R. (1995). An early soybean production system for drought avoidance. Journal of Production Agriculture, 8, 112–118. https://doi.org/10.2134/jpa1995.fpage
Buttery, B. R., Tan, C. S., Buzzell, R. I., Gaynor, J. D., & MacTavish, D. C. (1993). Stomatal numbers of soybean and response to water stress. Plant and Soil, 149, 283–288. https://doi.org/10.1007/BF00016619
Caine, R. S., Yin, X., Sloan, J., Harrison, E. L., Mohammed, U., Fulton, T., Biswal, A. K., Dionora, J., Chater, C. C., Coe, R. A., Bandyopadhyay, A., Murchie, E. H., Swarup, R., Quick, W. P., & Gray, J. E. (2019). Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytologist, 221, 371–384. https://doi.org/10.1111/nph.15344
Cattani, M., Guzzo, N., Mantovani, R., & Bailoni, L. (2017). Effects of total replacement of corn silage with sorghum silage on milk yield, composition, and quality. Journal of Animal Science and Biotechnology. https://doi.org/10.1186/s40104-017-0146-8
Costa, C., Dwyer, L. M., Zhou, X., Dutilleul, P., Hamel, C., Reid, L. M., & Smith, D. L. (2002). Root morphology of contrasting maize genotypes. Agronomy Journal, 94, 96–101. https://doi.org/10.2134/agronj2002.9600
Danalatos, N. G., Archontoulis, S. V., and Tsiboukas, K. (2009). Comparative analysis of sorghum vs corn growing under optimum and under water/nitrogen limited conditions in central Greece: 17th European Biomass Conference and Exhibition. June 29–July 3 2009. Hamburk, Germany
Daryanto, S., Wang, L., Jacinthe, P.-A., & Hui, D. (2016). Global synthesis of drought effects on maize and wheat production. PLOS One. https://doi.org/10.1371/journal.pone.0156362
Degener, J. F., & Kappas, M. (2015). Differences in biomass yield development of early, medium, and late maize varieties during the 21st century in Northern Germany. Environmental Sciences Europe. https://doi.org/10.1186/s12302-015-0042-9
Dien, D. C., Mochizuki, T., & Yamakawa, T. (2019). Effect of various drought stresses and subsequent recovery on proline, total soluble sugar and starch metabolisms in Rice (Oryza sativa L.) varieties. Plant Production Science, 22, 530–545. https://doi.org/10.1080/1343943X.2019.1647787
Dorigo, W. A., Zurita-Milla, R., de Wit, A. J., Brazile, J., Singh, R., & Schaepman, M. E. (2007). A review on reflective remote sensing and data assimilation techniques for enhanced agroecosystem modeling. International Journal of Applied Earth Observation and Geoinformation, 9, 165–193.
Dunn, J., Hunt, L., Afsharinafar, M., Meselmani, M. A., Mitchell, A., Howells, R., et al. (2019). Reduced stomatal density in bread wheat leads to increased water-use efficiency. Journal of Experimental Botany, 70, 4737–4748. https://doi.org/10.1093/jxb/erz248
EL-Bauome, H. A., Abdeldaym, E. A., Abd El-Hady, M. A. M., Darwish, D. B. E., Alsubeie, M. S., El-Mogy, M. M., Basahi, M. A., Al-Qahtani, S. M., Al-Harbi, N. A., Alzuaibr, F. M., Alasmari, A., Ismail, I. A., Dessoky, E. S., and Doklega, S. M. A.(2022). Exogenous proline, methionine, and melatonin stimulate growth, quality, and drought tolerance in cauliflower plants. Agriculture https://doi.org/10.3390/agriculture12091301
Fariaszewska, A., Aper, J., Van Huylenbroeck, J., De Swaef, T., Baert, J., & Pecio, Ł. (2020). Physiological and biochemical responses of forage grass varieties to mild drought stress under field conditions. International Journal of Plant Production, 14, 335–353. https://doi.org/10.1007/s42106-020-00088-3
Faridatul, M. I., & Ahmed, B. (2020). Assessing agricultural vulnerability to drought in a heterogeneous environment: a remote sensing-based approach. Remote Sensing. https://doi.org/10.3390/rs12203363
Fazaeli, H., Golmohhammadi, H. A., Al-Moddarres, A., Mosharraf, S., & Shoaei, A. A. (2006). Comparing the performance of sorghum silage with Maize silage in feedlot calves. Pakistan Journal of Biological Sciences, 9, 2450–2455. https://doi.org/10.3923/pjbs.2006.2450.2455
Frimpong, F., Anokye, M., Windt, C. W., Naz, A. A., Frei, M., van Dusschoten, D., & Fiorani, F. (2021). Proline-mediated drought tolerance in the barley (Hordeum vulgare L.) isogenic line is associated with lateral root growth at the early seedling stage. Plants. https://doi.org/10.3390/plants10102177
Fukai, S., & Cooper, M. (1995). Development of drought-resistant cultivars using physiomorphological traits in rice. Field Crops Research, 40, 67–86. https://doi.org/10.1016/0378-4290(94)00096-U
Garg, A., Bordoloi, S., Ganesan, S. P., Sekharan, S., & Sahoo, L. (2020). A relook into plant wilting: observational evidence based on unsaturated soil–plant-photosynthesis interaction. Scientific Reports. https://doi.org/10.1038/s41598-020-78893-z
Ghosh, U. K., Islam, M. N., Siddiqui, M. N., Cao, X., Khan, M. A. R., & Wicke, S. (2022). Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biology, 24, 227–239. https://doi.org/10.1111/plb.13363
Girvetz, E. H., Zganjar, C., Raber, G. T., Maurer, E. P., Kareiva, P., & Lawler, J. J. (2009). Applied climate-change analysis: the climate wizard tool. PLoS One. https://doi.org/10.1371/journal.pone.0008320
González, L., and González-Vilar, M. (2001). "Determination of Relative Water Content," in Handbook of Plant Ecophysiology Techniques (Netherlands: Kluwer Academic Publishers Group), 207–212
Hanif, S., Saleem, M. F., Sarwar, M., Irshad, M., Shakoor, A., Wahid, M. A., & Khan, H. Z. (2021). Biochemically triggered heat and drought stress tolerance in rice by Proline application. Journal of Plant Growth Regulation, 40, 305–312. https://doi.org/10.1007/s00344-020-10095-3
Hasan, S. A., Rabei, S. H., Nada, R. M., & Abogadallah, G. M. (2017). Water use efficiency in the drought-stressed sorghum and maize in relation to expression of aquaporin genes. Biologia Plantarum, 61, 127–137. https://doi.org/10.1007/s10535-016-0656-9
Hayat, S., Hayat, Q., Alyemeni, M. N., Wani, A. S., Pichtel, J., & Ahmad, A. (2012). Role of proline under changing environments. Plant Signaling & Behavior, 7, 1456–1466. https://doi.org/10.4161/psb.21949
He, X., Xu, L., Pan, C., Gong, C., Wang, Y., Liu, X., & Yu, Y. (2020). Drought resistance of Camellia oleifera under drought stress: changes in physiology and growth characteristics. PLOS One. https://doi.org/10.1371/journal.pone.0235795
Hendry, G. A. F. (1993). Evolutionary origins and natural functions of fructans - a climatological, biogeographic and mechanistic appraisal. New Phytologist, 123, 3–14. https://doi.org/10.1111/j.1469-8137.1993.tb04525.x
Hossain, M. M., Lam, H.-M., & Zhang, J. (2015). Responses in gas exchange and water status between drought-tolerant and -susceptible soybean genotypes with ABA application. The Crop Journal, 3, 500–506. https://doi.org/10.1016/j.cj.2015.09.001
Hughes, J., Hepworth, C., Dutton, C., Dunn, J. A., Hunt, L., Stephens, J., Waugh, R., Cameron, D. D., & Gray, J. E. (2017). Reducing stomatal density in barley improves drought tolerance without impacting on yield. Plant Physiology, 174, 776–787. https://doi.org/10.1104/pp.16.01844
Ilyas, M., Nisar, M., Khan, N., Hazrat, A., Khan, A. H., Hayat, K., Fahad, S., Khan, A., & Ullah, A. (2021). Drought tolerance strategies in plants: a mechanistic approach. Journal of Plant Growth Regulation, 40, 926–944. https://doi.org/10.1007/s00344-020-10174-5
Jiao, W., Zhang, L., Chang, Q., Fu, D., Cen, Y., & Tong, Q. (2016). Evaluating an enhanced vegetation condition index (VCI) based on VIUPD for drought monitoring in the Continental United States. Remote Sensing. https://doi.org/10.3390/rs8030224
Kaur, G., & Asthir, B. (2015). Proline: a key player in plant abiotic stress tolerance. Biologia Plantarum, 59, 609–619. https://doi.org/10.1007/s10535-015-0549-3
Keyvan, S. (2010). The effects of drought stress on yield, relative water content, proline, soluble carbohydrates and chlorophyll of bread wheat cultivars. Journal of Animal and Plant Sciences, 8, 1051–1060.
Kooyers, N. J. (2015). The evolution of drought escape and avoidance in natural herbaceous populations. Plant Science, 234, 155–162. https://doi.org/10.1016/j.plantsci.2015.02.012
Kulkarni, M., & Deshpande, U. (2006). Anatomical breeding for altered leaf parameters in tomato genotypes imparting drought resistance using leaf strength index. Asian Journal of Plant Sciences, 5, 414–420. https://doi.org/10.3923/ajps.2006.414.420
Kusvuran, S., Dasgan, H. Y., Kuçukkomurcu, S., and Abak, K. (2010). Relationship between drought tolerance and stomata density in melon. Acta Horticulturae. https://doi.org/10.17660/ActaHortic.2010.871.39
Lestari, E. G. (2016). The relation between stomata index and drought resistant at rice somaclones of Gajahmungkur, Towuti, and IR 64. Biodiversitas Journal of Biological Diversity. https://doi.org/10.13057/biodiv/d070112
Li, Y., Li, H., Li, Y., & Zhang, S. (2017). Improving water-use efficiency by decreasing stomatal conductance and transpiration rate to maintain higher ear photosynthetic rate in drought-resistant wheat. The Crop Journal, 5, 231–239. https://doi.org/10.1016/j.cj.2017.01.001
Liang, L., Sun, Q., Luo, X., Wang, J., Zhang, L., Deng, M., Di, L., and Liu, Z. (2017). Long-term spatial and temporal variations of vegetative drought based on vegetation condition index in China. Ecosphere. https://doi.org/10.1002/ecs2.1919
Liao, J.-X., Chang, J., & Wang, G.-X. (2005). Stomatal density and gas exchange in six wheat cultivars. Cereal Research Communications, 33, 719–726. https://doi.org/10.1556/CRC.33.2005.2-3.140
Liu, W. T., & Kogan, F. N. (2007). Monitoring regional drought using the Vegetation Condition Index. International Journal of Remote Sensing, 17, 2761–2782. https://doi.org/10.1080/01431169608949106
Liu, Z., Guan, D., Moore, S., Lee, H., Su, J., & Zhang, Q. (2015). Climate policy: Steps to China’s carbon peak. Nature, 522, 279–281. https://doi.org/10.1038/522279a
Magné, C., & Larher, F. (1992). High sugar content of extracts interferes with colorimetric determination of amino acids and free proline. Analytical Biochemistry, 200, 115–118. https://doi.org/10.1016/0003-2697(92)90285-F
Manivannan, P., Jaleel, C. A., Sankar, B., Kishorekumar, A., Somasundaram, R., Lakshmanan, G. M. A., & Panneerselvam, R. (2007). Growth, biochemical modifications and proline metabolism in Helianthus annuus L. as induced by drought stress. Colloids and Surfaces b: Biointerfaces, 59, 141–149. https://doi.org/10.1016/j.colsurfb.2007.05.002
Mansouri, D., Rassaa, N., Chalh, A., Bnejdi, B., & Gazzah, E. L. (2016). Stomata development variability of ten wheat genotypes under early water stress. Journal of New Sciencies: Agriculture and Biotechnology, 35, 1969–1975.
Marček, T., Hamow, K. Á., Végh, B., Janda, T., Darko, E., & Lambreva, M. D. (2019). Metabolic response to drought in six winter wheat genotypes. PLOS ONE. https://doi.org/10.1371/journal.pone.0212411
Meitner, J., Balek, J., Bláhová, M., Semerádová, D., Hlavinka, P., Lukas, V., Jurečka, F., Žalud, Z., Klem, K., Anderson, M. C., Dorigo, W., Fischer, M., & Trnka, M. (2023). Estimating drought-induced crop yield losses at the cadastral area level in the Czech Republic. Agronomy, 13(7), 1669. https://doi.org/10.3390/agronomy13071669
Nazran, A., Ahmed, J. U., Karim, A. J. M. S., & Ghosh, T. K. (2019). Physiological responses of mungbean (Vigna radiata) varieties to drought stress. Bangladesh Journal of Agricultural Research, 44, 1–11. https://doi.org/10.3329/bjar.v44i1.40899
Nusrathali, N., Mufeeth, M., Ahamed, A., Majeed, U., and Musthafa, M. (2021). Comparison of chemical composition and quality of maize, sorghum and hybrid Napier grass CO-3 silages using bag or bucket silos. Journal of Bangladesh Agricultural University. https://doi.org/10.5455/JBAU.70271
van Oosterom, E. J., Kulathunga, M. R. D. L., Deifel, K. S., McLean, G. B., Barrasso, C., Wu, A., Messina, C., and Hammer, G. L. (2021). Dissecting and modelling the comparative adaptation to water limitation of sorghum and maize: role of transpiration efficiency, transpiration rate and height. In silico Plants. https://doi.org/10.1093/insilicoplants/diaa012
Orság, M., Fischer, M., Trnka, M., Brotan, J., Pozníková, G., & Žalud, Z. (2022). Trends in Air Temperature and Precipitation in Southeastern Czech Republic, 1961–2020. Acta Universitatis Agriculturae Et Silviculturae Mendelianae Brunensis, 70, 283–294. https://doi.org/10.11118/actaun.2022.021
Öztürk, İ, & Korkut, K. Z. (2018). Relation of stomatal frequency and size on wheat yield and morpho-physiological traits under varied drought stresses conditions. Journal of International Scientific Publications: Agriculture & Food, 6, 368–380.
Per, T. S., Khan, N. A., Reddy, P. S., Masood, A., Hasanuzzaman, M., Khan, M. I. R., & Anjum, N. A. (2017). Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant Physiology and Biochemistry, 115, 126–140. https://doi.org/10.1016/j.plaphy.2017.03.018
Podkówka, Z., & Podkówka, L. (2011). Chemical composition and quality of sweet sorghum and maize silages. Journal of Central European Agriculture, 12, 294–303. https://doi.org/10.5513/JCEA01/12.2.915
Pokharel, D., and Pandey, M. (2012). Genetic Variability of Drought Adaptive Traits in Nepalese Wheat (Triticum aestivum L.) Germplasm. Hydro Nepal: Journal of Water, Energy and Environment. https://doi.org/10.3126/hn.v11i1.7208
Queiroz, M. S., Oliveira, C. E. S., Steiner, F., Zuffo, A. M., Zoz, T., Vendruscolo, E. P., Silva, M. V., Mello, B. F. F. R., Cabral, R. C., and Menis, F. T. (2019). Drought stresses on seed germination and early growth of Maize and Sorghum. Journal of Agricultural Science. https://doi.org/10.5539/jas.v11n2p310
Quilambo, O. A. (2004). Proline content, water retention capability and cell membrane integrity as parameters for drought tolerance in two peanut cultivars. South African Journal of Botany, 70, 227–234. https://doi.org/10.1016/S0254-6299(15)30239-8
Romm, J. (2015). Climate Change: What Everyone Needs to Know. Oxford University Press.
Sah, S. K., Reddy, K. R., and Li, J. (2016). Abscisic acid and abiotic stress tolerance in crop plants. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2016.00571
Schittenhelm, S., & Schroetter, S. (2014). Comparison of drought tolerance of Maize, Sweet Sorghum and Sorghum-Sudangrass hybrids. Journal of Agronomy and Crop Science, 200, 46–53. https://doi.org/10.1111/jac.12039
Shavrukov, Y., Kurishbayev, A., Jatayev, S., Shvidchenko, V., Zotova, L., Koekemoer, F., de Groot, S., Soole, K., and Langridge, P. (2017). Early flowering as a drought escape mechanism in plants: how can it aid wheat production? Frontiers in Plant Science. https://doi.org/10.3389/fpls.2017.01950
Sholihah, R. I., Trisasongko, B. H., Shiddiq, D., La Ode, S. I., Kusdaryanto, S., & Zanuju, D. R. (2016). Identification of agricultural drought extent based on vegetation health indices of landsat data: case of Subang and Karawang, Indonesia. Procedia Environmental Sciences, 33, 14–20. https://doi.org/10.1016/j.proenv.2016.03.051
Shrestha, U. B., Gautam, S., Bawa, K. S., and Bohrer, G. (2012). Widespread climate change in the Himalayas and associated changes in local ecosystems. PLoS One. https://doi.org/10.1371/journal.pone.0036741
Soltys-Kalina, D., Plich, J., Strzelczyk-Żyta, D., Śliwka, J., & Marczewski, W. (2016). The effect of drought stress on the leaf relative water content and tuber yield of a half-sib family of ‘Katahdin’-derived potato cultivars. Breeding Science, 66, 328–331. https://doi.org/10.1270/jsbbs.66.328
Středa, T., Středová, H., Chuchma, F., Kučera, J., & Rožnovský, J. (2019). Smart method of agricultural drought regionalization: A winter wheat case study. Contributions to Geophysics and Geodesy, 49, 25–36. https://doi.org/10.2478/congeo-2019-0003
Taiz, L., Zeiger, E. (2010). Plant physiology. 5th. USA: Sinauer Associates
Vijn, I., & Smeekens, S. (1999). Fructan: More Than a Reserve Carbohydrate? 1st. Plant Physiology, 120, 351–360. https://doi.org/10.1104/pp.120.2.351
Wahyuni, Y., Miyamoto, T., Hartati, H., Widjayantie, D., Windiastri, V. E., Sulistyowati, Y., Rachmat, A., Hartati, N. S., Ragamustari, S. K., Tobimatsu, Y., Nugroho, S., and Umezawa, T. (2019). Variation in lignocellulose characteristics of 30 Indonesian sorghum (Sorghum bicolor) accessions. Industrial Crops and Products. https://doi.org/10.1016/j.indcrop.2019.111840
Waterland, N. L., Campbell, C. A., Finer, J. J., & Jones, M. L. (2010). Abscisic acid application enhances drought stress tolerance in bedding plants. HortScience, 45, 409–413. https://doi.org/10.21273/HORTSCI.45.3.409
Xu, H., Twine, T. E., Girvetz, E., Wang, W. (2016). Climate change and Maize yield in Iowa. PLOS One. https://doi.org/10.1371/journal.pone.0156083
Xu, Z., & Zhou, G. (2008). Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. Journal of Experimental Botany, 59, 3317–3325. https://doi.org/10.1093/jxb/ern185
Yamada, M., Morishita, H., Urano, K., Shiozaki, N., Yamaguchi-Shinozaki, K., Shinozaki, K., & Yoshiba, Y. (2005). Effects of free proline accumulation in petunias under drought stress. Journal of Experimental Botany, 56, 1975–1981. https://doi.org/10.1093/jxb/eri195
Zambrano, F., Lillo-Saavedra, M., Verbist, K., and Lagos, O. (2016). Sixteen years of agricultural drought assessment of the BioBío region in chile using a 250 m resolution Vegetation Condition Index (VCI). Remote Sensing. https://doi.org/10.3390/rs8060530
Zardin, P. B., Velho, J. P., Jobim, C. C., Alessio, D. R. M., Haygert-Velho, I. M. P., Conceição, G. M., and Almeida, P. S. G. (2017). Chemical composition of corn silage produced by scientific studies in Brazil - A meta-analysis. Semina: Ciências Agrárias. 038, 503–512. https://doi.org/10.5433/1679-0359.2017v38n1p503
Acknowledgements
The authors thank the co-workers at the Experimental Field Station in Žabčice for their assistance during the field trial, especially Jan Syrový, Pavel Kirch, Monika Kirchová, Tomáš Rábek and Vilém Sitte.
Funding
Open access publishing supported by the National Technical Library in Prague. This experiment was financially supported by the project AF-IGA2022-IP-009.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare declared that no competing interest exists.
Ethics Approval
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Frantová, N., Rábek, M., Porčová, L. et al. Monitoring Drought Tolerance Mechanisms of Sorghum and Maize Under Unevenly Distributed Precipitation. Int. J. Plant Prod. 18, 49–67 (2024). https://doi.org/10.1007/s42106-023-00278-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42106-023-00278-9