Abstract
Sarcopenia is a syndrome described as a progressive and generalized loss of muscle mass and strength, with decrease in physical performance. It is related to an increased risk of many adverse events, such as falls, fractures, osteoporosis, major postoperative complications, loss of quality of life, prolonged hospital stay, disability, and even death. Although sarcopenia can also be assessed using a handheld dynamometer and a short physical performance battery (SPPB); it has lower accuracy, sensitivity, and specificity. Previous studies confirmed that imaging methods can serve as an important tool in the assessment of muscle mass and quality, and can even detect microscopic changes in muscle, achieving an early diagnosis of sarcopenia. Therefore, this article reviews the advantages and disadvantages of clinical and imaging assessment methods, specific applications, and the development of imaging techniques for the assessment of sarcopenia, including the currently unresolved problems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sarcopenia has currently received an increasing interest in the research field, due to its higher prevalence with aging and people’s growing awareness of sarcopenia. The concept of sarcopenia was first defined in 1988, by American scholar Irwin Rosenberg, as an age-related loss of skeletal muscle mass and function [1]. The widely used definition of sarcopenia, which refers to a syndrome associated with progressive and general loss of skeletal muscle mass and strength with a risk of adverse outcomes, was developed by the European Working Group on Sarcopenia in Older People (EWGSOP) in 2010 [2]. EWGSOP has emphasized that decreased muscle function is essential to the diagnosis of sarcopenia. Sarcopenia is now formally considered a muscle disease (muscle failure) rooted in adverse muscle changes that accrue across a life [3]. Sarcopenia is a multifactorial disorder involving a reduction of physical activity, protein intake, vitamin D levels [4], and anabolic hormonal activity (especially testosterone, estrogen, and growth hormone) [5] Besides, it is also related to pro-inflammatory status [6]. Previous studies found the prevalence of sarcopenia among hospitalized older adults (14–33%) is higher than that in community-dwelling older adults (1–29%), and the global prevalence of sarcopenia is expected to increase from 50 million in 2010 to 200 million in 2050 [8]. Therefore, the country will spend higher healthcare costs regarding days of hospitalization, nursing home placement, and ambulatory care [7, 8]. In addition to increasing social and economic costs [9], sarcopenia is associated with adverse outcomes in patients, mainly including falls, fractures, osteoporosis, major postoperative complications, loss of quality of life, prolonged hospital stay, disability, and mortality [3, 10,11,12]. Therefore, using relevant clinical and imaging assessment enables to the achievement of early diagnosis of sarcopenia, which can lead to early intervention, treatment, and a delay of adverse outcomes in patients with sarcopenia improving the quality of life and reducing national health care costs. The most commonly used imaging techniques to evaluate sarcopenia are dual X-ray absorptiometry (DXA), computed tomography (CT), magnetic resonance imaging (MRI), and ultrasonography (US). In addition, we also briefly introduce the application of Peripheral Quantitative Computed Tomography (pQCT), High-resolution peripheral quantitative computed tomography (HR-PQCT) in sarcopenia in the review. The imaging techniques mentioned above are mainly used to measure body composition to assess muscle mass and quality in patients with sarcopenia. Combined clinical evaluation and imaging indicators can improve the sensitivity and specificity of sarcopenia diagnosis [13]. Nowadays, imaging methods in diagnosis of sarcopenia have received growing attention, so this article mainly reviews the currently used and latest imaging techniques that can detect muscle changes in sarcopenia and serve as fundamental tools in the diagnosis of sarcopenia.
Assessment methods of sarcopenia
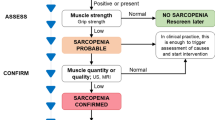
According to the recently updated diagnostic criteria by EWGSOP [2], the assessment of muscle strength and muscle mass is essential to the diagnosis of sarcopenia. Measuring muscle strength with a handheld dynamometer is a simple and easy method and is easily applied in the community and clinical environment. However, for patients with upper limb disability, this test cannot be performed. EWGSOP also recommends that chair standing tests can be used to assess muscle strength. Physical performance can be assessed using the short physical performance battery (SPPB) and gait speed [2]. Bioelectrical impedance analysis (BIA) and imaging methods such as DXA, CT, MRI, and US can be used to measure muscle mass (Table 1). According to the latest consensus of EWGSOP and AWGSOP, BIA is considered the main clinical evaluation method for sarcopenia because it is cost-effective and portable [14]. However, BIA measurement is susceptible to various factors such as age, race, gender, electrode position, fat content, and hydration status of the patient [15], so its value is not accurate. However, imaging biomarkers measured by imaging methods are relatively objective and are rarely affected by acute disease and cognitive dysfunction [16]. Especially, CT and MRI, as the gold standard for quantifying muscle mass and visceral fat area or volume [17], can improve the sensitivity and specificity of the diagnosis of sarcopenia. Notably, Faron et al. have confirmed that CT and MRI have good consistency in the assessment of muscle mass, so CT and MRI have interchangeability [18]. The following sections describe in detail the main applications and recent advances of imaging techniques for the assessment of sarcopenia.
Imaging assessment methods and the latest development of sarcopenia
Dual-energy X-ray absorptiometry
Characteristics, principles, and application fields of DXA
DXA is more favored by clinicians and working groups as compared to other imaging methods for the quantitative analysis of muscle and is commonly used for body composition analysis at the molecular level. It can be widely used in clinical environments because of lower radiation, cost effectiveness, simple operation, short scanning time, and high accuracy [19]. Messina et al. have demonstrated lean mass (LM) and fat mass(FM) measured by DXA are strongly correlated with LM and FM by CT and MRI [20]. The measurement of bone mineral density (BMD) was performed using DXA [21]. Tegola et al. have proposed that combined appendicular skeletal muscle mass index (ASMMI) with BMD of the femoral neck to assess the risk of fracture in the elderly [22]. With the development of technology, it has also been gradually applied in the measurement of muscle mass. The principle of DXA is the transmission and attenuation of energy when an X-ray penetrates tissues of the human body at two different energy levels (40 kv and 70 kv) [7, 8, 23]. Based on the above principles, DXA can be used to indirectly measure focal and total body composition. Commonly used indicators include appendicular lean mass, appendicular lean mass index (ALMI), appendicular skeletal muscle mass (ASMM), ASMMI, fat mass index, and Android/Gynoid ratio (A/G ratio). A multicenter and large cohort study confirmed that DXA measured ALMI was strongly associated with the risk of mortality in patients with sarcopenia [24].
Specific application and limitation of DXA in sarcopenia
The ASMM is the most widely used parameter. However, because ASMM is susceptible to the body size, most guidelines recommend the use of ASMM adjusted for height, which is also known as ASMM index (ASMMI) [20]. When ASMMI is lower than the cut-off point defined by relevant guidelines (Table 2) [16], the low muscle mass can be diagnosed. In addition to the diagnosis of sarcopenia, DXA can be used for follow-up of patients, because repeatable assessment can be performed using the same equipment and cut-off point in a short time [3]. Kim et al. demonstrated using the AWGS ASMMI can predict perioperative complication risk of malignant tumor in recent study [25]. Therefore, we can distinguish the subjects with “physiological” loss of muscle mass from those with “pathological” impoverishment due to the short-term changes in muscle mass [26]. Furthermore, the assessment of resting energy expenditure (REE) by DXA is a better tool for the management of sarcopenic patients [27]. Osteosarcopenia can be diagnosed and the risk of fracture can be assessed combined BMD, the trabecular bone score (TBS) and the Bone Strain Index (BSI) in recent years [28]. DXA also has some limitations. (1) Although current new software provides the possibility to evaluate subcutaneous fat and visceral fat, DXA still cannot assess intramuscular fat, so qualitative analysis of skeletal muscle cannot be performed [4, 9]; (2) the values of relevant parameters measured by different devices are inconsistent, so standardized measurement protocols are required [3]; (3) DXA is susceptible to body thickness, hydration status, and diseases that can cause edema, such as DXA may overestimate muscle mass in patients with edema and ascites and underestimate muscle mass in obese people [5]. (4) There is no consensus on the assessment equation of muscle quantity such as ASM [29, 30]. Therefore, radiologists and radiographers should have standardized training for DXA examination acquisition, including patient positioning, demographics collection, and image analysis to improve the accuracy of outcomes [31]. And orientation of future researches should focus on the above limitations.
CT and pQCT
CT
Characteristics, principles, levels, and muscles assessed in sarcopenia about CT
Computed tomography (CT), as the gold standard for evaluation of body composition, is currently a promising modality for quantitative and qualitative evaluation of muscles [32], which is widely used for tumor patients. The principle is to distinguish different tissue densities based on different X-ray attenuation values, so it can provide more detailed anatomical information. Muscle mass and muscle fat infiltration CT measured can be used in the diagnosis of sarcopenia and muscle fat infiltration that could reflect the quality of skeletal muscle. Park et al. have confirmed the accuracy of CT to be higher than that of MRI for the assessment of muscle mass [33]. At present, muscle mass and quality are mostly measured at the level of the L3 vertebra due to less motion artifacts [34]; and previous studies have confirmed that a more stable measurement level is the inferior endplate level of L3 [17]. Other levels that are used include the L4 level; the L1 level which has good consistency of measurement results with the L3 level and the measurement results are associated with prognosis [35, 36]; T4 and T12 levels [37, 38]; and the mid-thigh level [39]. The most commonly assessed cross-sectional area (CSA) of muscles are all muscles at the level of the L3 vertebra (Fig. 1A), including psoas major, paraspinal muscles, and abdominal muscles, which have the best correlation with total body muscle mass and fat infiltration [40]. Other involved muscles include the psoas muscle at the L3 and L4 levels; the latissimus dorsi muscle or all muscles based on the chest CT level [41]; the mid-thigh muscle group and the hip muscle group [42].
Axial CT and MRI images assessing muscle mass and quality in sarcopenic patient. A 31-year-old woman. Axial CT image of the abdomen at the L3 level shows region of interest (ROI) delineation of all truncal muscles along muscle boundary to CSA evaluation. B A 67-year-old man. An axial MRI image of the abdomen at the L3 level is commonly used to measure the CSA of the psoas major muscle (yellow) by ROI delineation along the muscle fascia. C 67-year-old-man, Axial T2-weighted MRI image of the abdomen at the L3 level showing the segmentation of fatty tissue(red) of the psoas major muscle based on the grayscale signal by ImageJ software
CT biomarkers in the assessment of muscle mass and quality in sarcopenia
Muscle volume, CSA, and skeletal muscle index (SMI) are commonly used in the assessment of muscle mass. SMI (TAMA/Height2) at the L3 vertebral level is the most commonly used biomarker in most studies based on the following reasons: (1) total abdominal muscle area at this level can reflect muscle mass of the whole body [21]; (2) TAMA at this level is more reliable than total psoas muscle are [33]; (3) SMI can predict the mortality of patients with chronic liver diseases rather than psoas muscle index (PMI) [43]. The biomarkers commonly used for muscle quality assessment include muscle attenuation value, low-density lean muscle tissue, and intermuscular adipose tissue [44,45,46]. Kim et al. suggested that normal attenuation muscle is divided by total abdominal muscle area which can also be used for the assessment of muscle fat infiltration, and the cut-off point of diagnosis was obtained [45]. IMAT is a commonly used biomarker, because it can reflect changes in muscle strength, which is associated with poor patient prognosis [47]. The threshold method is commonly used for muscle segmentation and measurement of IMAT [48]. The muscle threshold is further divided into normal muscle thresholds of 30–150Hu and Low Muscle Threshold of − 29 to 29Hu, and the fat threshold is − 30 to 190 Hu. The cut-off point of diagnosing sarcopenia is also different in different age groups and scanning levels (Table 3) [49]. For the protocols of image acquisition, the enhanced images may affect the CT value of the muscle, and the KV peak value and the scanning slice thickness will also affect the measurement results [50, 51], therefore, extensive research is needed to standardize the scanning protocols in the future.
CT can provide more detailed anatomical information and distinguish subcutaneous fat and visceral fat and is more suitable for measuring muscle mass in the clinical environment. Moreover, in addition to accuracy and repeatability of measurement, SMI and IMAT measured by CT can also predict adverse outcomes [52]. However, due to the larger radiation dose, higher cost, complex post-processing and technical requirements, it is currently mainly used for the diagnosis of sarcopenia in patients with diseases that require CT examination, which is known as opportunistic examination. It is mainly aimed for tumor patients, patients undergoing major surgery, and trauma patients [53, 54].
Peripheral QCT (pQCT) and HR-pQCT
Application, strength, and limitation of pQCT in sarcopenia
Peripheral Quantitative Computed Tomography (pQCT) was primarily used to diagnose osteoporosis and to identify the risk of fracture through the measurement of BMD, bone content, and bone strength [55]. It is increasingly used for CSA measurement of appendicular muscles, muscle density, and IMAT with the application of software, which can distinguish different compositions based on different density thresholds. Compared with CT, it is portable and has a smaller radiation dose and lower cost [55]. CSA of muscles measured by pQCT has a strong correlation with measurements conducted by MRI [56], but the accuracy is not comparable to MRI. The most common acquisition sites are 2/3 (66%) of the full length of the tibia and 65% of the full length of the radius [57, 58]. Previous studies have demonstrated that low muscle mass can be determined when the CSA of the muscles is less than 83.3 cm2 for males and less than 62.6 cm2 for women of the tibia two-thirds along its length (starting from the tibiotarsal joint) [59]. Muscle CSA and muscle density obtained through pQCT measurement are related to adverse prognosis of sarcopenia such as mortality, and frailty [60], and studies also have demonstrated that muscle size is related to bone size and bone strength [58]. The pQCT evaluation of skeletal muscles still has certain limitations, (1) there are still no standard scanning conditions, and different scanning conditions and muscle segmentation methods may affect the accuracy of the measurement results [61]. (2) Although the tibia and radius are most common site, there are still no standard scanning sites and cut-off points. (3) pQCT has lower accuracy for the assessment of IMAT [35].
High-resolution peripheral quantitative computed tomography (HR-pQCT)
High-resolution peripheral quantitative computed tomography (HR-pQCT) is the main method used for quantifying in bone microstructure, and it is also used to measure parameters related to muscles and tendons [62]. Hildebrand et al. suggested that CSA of skeletal muscle and muscle density measured by pQCT and HR-pQCT had a significant positive correlation and higher accuracy [63], but for the specific application in sarcopenia, it still needs further research to confirm.
Magnetic resonance imaging (MRI)
Regular application of MRI in sarcopenia
MRI can generate contrast between fat and non-fat tissue based on emission and adsorption of radiofrequency energy. It is also the gold standard for the study of body composition [64]. MRI has more advantages than CT, with no radiation, higher soft-tissue contrast, and the ability to detect changes in muscle microstructure, including edema, fibrosis, inflammation, and fatty infiltration [65]. Additionally, MRI is more advantageous in the assessment of muscle quality, because it can distinguish intramuscular and intermuscular adipose tissue and can detect early fatty infiltration and quantitative measurement through post-processing software (Fig. 1B, C). However, MRI has some limitations; longer acquisition time, the interference of metal or motion artifacts, and high cost and the lack of a standardized evaluation [55]. Sarcopenia is now routinely measured through cross-sectional imaging of the mid-thigh and the L4 and L5 vertebral body levels [66]; and most common biomarkers include muscle CSA, volume, and muscle thickness. The other levels include the superior mesenteric artery level [16], the L2 and L3 levels [67], and the C3 level [68]. The common biomarkers include TAMA, TPA, and CSA of the erector spine or the quadriceps [69,70,71]. Temporalis muscle thickness can be measured to assess sarcopenia in neurosurgical oncology, which has been shown to correlate with the CSA of the psoas major [72].
At present, with the application of MRI water-fat separation imaging (Dixon sequence) and proton spectroscopy imaging, the accuracy and sensitivity of muscle quality measurement have been improved. The most common site is the mid-thigh muscle, and the most common biomarker is the proton density fat fraction (PDFF) [73]. Studies have confirmed that PDFF measured by MRI has a significant correlation with muscle strength [74]. Other commonly used indicators include fat-free muscle area, intermuscular fat volume, intramuscular adipose tissue, T2 relaxation time, proton density water fraction, apparent diffusion coefficient (ADC), and diffusion coefficient [7, 75].
Common advanced MR technology for skeletal muscle measurement
Dixon and magnetic resonance spectroscopy (MRS) are chemical shift-based imaging methods separating water and fat (Dixon) based on the difference in proton resonance frequencies between water and fat. Grimm et al. confirmed muscle fat quantification using a 6-point Dixon has a significant correlation with MRS [76], which promotes their clinical application. The main method for the muscle lipid composition assessment is 1H-MRS, which can precisely measure the percentage of fat content. Chemical shift resonance frequency of water protons is at 4.7 ppm, extramyocellular lipids (EMCL) at 1.5 ppm and intramyocellular lipids (IMCL) at 1.3 ppm (Fig. 2B) [77]. EMCL is the main factor of muscle fat infiltration and decreased function [78]. Since the separation of (IMCL) and (EMCL) is orientation-dependent; we often select the spindle-shaped muscles in which the orientation of fibers is consistent with the long axis of the muscle, such as the vastus intermedius and the tibialis anterior muscle [79]. Even though MRS can distinguish between IMCL and EMCL, the inhomogeneous distribution of EMCL makes accurate quantification of EMCL challenging [80]. Surov et al. suggested that the accuracy of measurements can be increased through the selection of fat-selective imaging technique [53]. Further research on new sequences or algorithms is required to improve the precision of repositioning when repeatedly measured, because muscle fibers are easily deformed during measurement. Dixon magnetic resonance imaging can acquire two or more echoes at different echo times, obtaining images of water, lipid, in-phase, and out-phase images to measure PDFF (Fig. 2A) and PDWF. PDWF is varied due to the presence of muscle edema and fibrosis. Compared with the 2-point Dixon imaging, the multi-point Dixon can correct the inhomogeneity of the magnetic field, the attenuation of T2*, and increase the accuracy of the PDFF measurement [81]. Compared with MRS imaging, the Dixon sequence provides images of fat distribution and corrects for magnetic field inhomogeneity. Alexandra Grimm et al. confirm that the Dixon sequence has a higher repeatability to PDFF measurement [73], which supports its application in the future to predict mobility disorders and to evaluate the efficacy of therapeutic interventions for sarcopenia. However, it remains unclear for the most representative anatomical region and level for fat infiltration assessment of sarcopenia.
Dixon and magnetic resonance spectroscopy images to evaluate PDFF, IMCL, and EMCL of psoas muscles. A Region of interest (ROI) delineation of psoas major muscle in the Dixon proton density fat fraction images of abdomen at the L3 level to evaluate the PDFF of psoas major muscle. B The volume of interest (VOI) for MR spectroscopy measurement was placed in the region of without macroscopic fatty infiltration of left psoas major muscle without macroscopic fatty infiltration on the sagittal, coronal, and axial orientation at the L3 level and obtained corresponding typical muscle spectrum with numbered peaks
Rare advanced MR technology for skeletal muscle measurement
DTI
Advanced magnetic resonance techniques, such as diffusion tensor imaging (DTI), diffusion-weighted imaging (DWI), T1 mapping, T2 mapping, and strain rate tensor imaging, can also be used to evaluate muscle changes [32]. In addition to identifying early pathological changes in muscles that are difficult to detect on T1- and T2-weighted images, DTI could quantitatively evaluate myo-fibrosis, fat infiltration, and the anisotropy of muscle fiber orientation (Fig. 3A) [82]. These changes can be described through radial and axial diffusivity, mean diffusivity (MD), and fractional anisotropy (FA) (Fig. 3B). Scheel et al. have demonstrated that FA can predict the proportion of muscle fiber types, which Type I of fiber increased with FA value increased [83]. Current research about DTI focuses on muscle injury, muscle disease (Duchenne muscular dystrophy), and denervation changes, ischemia/reperfusion injury. DTI is expected to be used to evaluate the relation between muscle properties and external muscle strength in sarcopenia [84]
DWI, T1 mapping, and T2 mapping
Diffusion-weighted imaging (DWI) is an imaging technique based on the degree of motion of water molecules in tissues that are associated with their interaction with cell membrane macromolecules. Asides brain imaging, Apparent Diffusion Coefficient (ADC) value is helpful to evaluate the pathological changes of oncological tissues. Myositis and pathological muscle show increased ADC values compared to normal muscle, which suggests that ADC value can reflect the pathological status of muscle [85]. Surov et al. have confirmed that the ADC value has a significant association with end-stage liver disease in patients with cirrhosis [86]. The general skeletal muscle can also be measured using T1mapping and T2mapping. T1 mapping can measure muscle fat infiltration and is associated with the PDFF measured by Dixon [87]. T2 mapping can measure the increased T2 value (T2W) in sarcopenic patients with myo-fatty infiltration [88], which is sensitive to muscle changes and can assess acute and chronic muscle changes.
Strain rate tensor images, non-proton MRI, and MR elastography
Increased extracellular matrix is associated with decreased muscle strength and the magnitude of transient deformation. These changes can be detected through strain rate tensor images, which can provide information about the magnitude and direction of the deformation rate [32]. Besides this, the changes in muscle contractility and elasticity can also be observed in strain rate tensor imaging. The strain rate (SR) and the strain rate fiber angle (SR-fiber angle) are commonly used biomarkers [89]. Sinha et al. suggested that increased muscle stiffness with age may be related to the remodeling of the extracellular matrix [90]. In the future, strain rate tensor imaging may be used to assess the mechanical changes of muscles in sarcopenia, because it can reflect the mechanical properties of age-related muscles. Non-proton MRI techniques can achieve more microscopic assessment for the composition of muscle tissue through 23Na MRI, having strongly correlation with the muscle tissue ion homeostasis and energy balance. The most commonly used biomarker is the tissue sodium concentration, which is a volume-weighted average of intra- and extracellular sodium concentrations. Gerhalter et al. demonstrated 23Na MRI can detect elevated tissue sodium concentration in skeletal muscles of Duchenne muscular dystrophy [91], Besides 23Na MRI, 35Cl, and 39K MRI of skeletal muscle tissue has been developed and conducted in some studies [92, 93]. Pathological changes of muscle, such as fat infiltration, fibrosis, and edema, can affect the muscle biomechanical properties. Magnetic resonance elastography (MRE) is based on the imaging of shear waves and enables quantitative evaluation of biomechanical tissue properties of skeletal muscle. MRE-derived biomechanical properties during skeletal muscle contraction and relaxation can reflect skeletal muscle function [94]. Recent studies demonstrated that MRE was a reliable technique to quantitatively detect muscle stiffness on thigh muscles and paraspinal muscles [95, 96]. Current application of skeletal muscle mainly involves diseases related to neuromuscular dysfunction. Its application potential in sarcopenia is worthwhile to explore future. Although the new sequences mentioned above can reflect the changes in the microstructure and biomechanics of skeletal muscles, it is challenging to use these in clinical practice and some still are limited to the research field.
US
Compared with CT and MRI, ultrasonography provides the advantages of no radiation, simple and portable operation, low cost, good repeatability, and even the ability to measure muscle at the patient’s bedside [97], making it a better choice for primary screening of sarcopenia. The lower extremity muscles are the most commonly measured muscle, because the loss of lower extremity muscles is higher than that of the upper extremities in sarcopenic patients [32]. The quadriceps femoris and gastrocnemius muscles are most often used in the research; and the commonly used indicators are muscle thickness (Fig. 4) and CSA for muscle mass and is echogenicity for muscle quality [98], which increases with the muscle fibrosis and fatty infiltration. Fukumoto et al. demonstrated that the echogenicity of the quadriceps was negatively correlated with muscle thickness and isometric knee extension force [99]. Other often used indicators include fascicle length, and pinnate angle [14]. At present, microvascular changes of muscles can also be assessed by measuring the microvascular blood volume, microvascular blood flow rate, and microvascular blood flow of the muscle through contrast-enhanced ultrasound [100]. The latest technologies include panoramic ultrasound and shear wave elastography. Jessica et al. confirmed that CSA of the quadriceps femoris measured by panoramic ultrasound was a significant correlation with the MRI-measured value [101]. Muscle stiffness can be quantitatively assessed by ultrasound shear wave elastography through measurement of shear wave velocity. However, due to the lack of standardized conditions, critical values, and poor accuracy, it has not been included in the diagnostic guidelines for sarcopenia so far.
Application of artificial intelligence
The most important fields of artificial intelligence in radiology are machine learning and deep learning. In recent years, deep learning architectures is mainly used for image segmentation, reconstruction, recognition, and classification [102]. The machine learning method is mainly to extract lesion features from medical images to obtain valuable information [103]. The application of AI in sarcopenia can reduce time-consuming and increase accuracy. In sarcopenia, AI is mainly used for the automatic segmentation of body composition in images of CT, MRI, and ultrasonography; in addition, AI is helpful for evaluation and analysis of body composition parameters to collect information related to prognosis of sarcopenia or to recommend follow-up and potential treatment interventions [104]. Study of Gu et al. developed an AI segmentation model for a wider range of abdomen to predict sarcopenia and has been demonstrated high accuracy [105]. In the past two years, AI has achieved the transition from 2D to 3D in the automatic segmentation of CT body composition. And Mai et al. confirmed that the new segmental technique has higher feasibility and accuracy [106]. Cohort study of MBBS demonstrated that sarcopenia identified by a deep learning-based segmentation approach significantly affects overall survival in patients with cancer [107]. Although AI can reduce time consumption and decrease inter-evaluator variability, more rigorous guidelines and comparative studies are needed to assess the feasibility of AI segmentation models before into clinical practice.
Role of imaging techniques in sarcopenia
DXA be considered the mostly accept method for the assessment of body composition in clinical practice owing its safety, non-contraindications and clear diagnostic cut-off points. As cross-sectional imaging methods, CT and MRI have more advantages in measuring skeletal muscle fatty infiltration, especially MRI, which has higher accuracy in the assessment of skeletal muscle quality due to the rapid development and innovation of multi-functional and multi-parameter imaging sequences. In addition, CT and MRI also play an important role in treatment, follow-up and prognosis assessment of sarcopenic patients, mainly for patients with tumors and chronic diseases. The decrease in CT-measured PMI is also associated with risk for osteoporosis and vertebral fractures [108]. Recent study confirmed that measured SMI and psoas muscle thickness using routine MRI scans can be used as imaging marker to predict the therapy efficacy and survival of patients with cancer in clinical practice [109, 110]. However, due to CT and MRI lack diagnostic criterion value, high costs and uneven distribution of medical resources, restricting their use on clinical settings. With technology development, ultrasound can also evaluate more microscopic changes in skeletal muscle, including skeletal muscle microvascular changes and muscle stiffness. However, due to the high compliance and low repeatability required by US, its clinical application is limited. AI are mostly focused on the improvement of segmentation efficiency to make easier to screen categories at risk of sarcopenia. The above indicates that various imaging parameters of sarcopenia play their respective advantages in malignant tumors and chronic diseases, but radiologists should choose the best imaging method according to the purpose of research and treatment.
Conclusion
Sarcopenia, as a silent disease, is rarely noticed in its early stages and people do not seek medical attention until symptoms appear. Imaging measurements of both muscle mass and quality are important diagnostic criteria for sarcopenia, suggesting that imaging techniques play a pivotal role in the evaluation of sarcopenia. Through comprehensive application of imaging methods and clinical assessment in diagnosing sarcopenia, patients can obtain early intervention through diet and exercise; thereby improving the prognosis, quality of life, and survival rates. However, there is a lack of standardization in scan site, diagnostic indicators, and criterion-based cut-off values. In addition, potential imaging biomarkers that can help to screen people at risk of developing sarcopenia at an early stage remain unknown, and there is no consensus about measurement indicators can predict adverse prognosis, restricting their application in the clinical environment. Therefore, future research is needed to solve the above problems.
Data availability
The authors declare data of the review is available.
References
Izzo A, Massimino E, Riccardi G, et al. A narrative review on sarcopenia in type 2 diabetes mellitus: prevalence and associated factors. Nutrients. 2021. https://doi.org/10.3390/nu13010183.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. 2010;394:412–23. https://doi.org/10.1093/ageing/afq034.
Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;481:16–31. https://doi.org/10.1093/ageing/afy169.
Brioche T, Pagano AF, Py G, et al. Muscle wasting and aging: experimental models, fatty infiltrations, and prevention. Mol Aspects Med. 2016;50:56–87. https://doi.org/10.1016/j.mam.2016.04.006.
Marty E, Liu Y, Samuel A, et al. A review of sarcopenia: enhancing awareness of an increasingly prevalent disease. Bone. 2017;105:276–86. https://doi.org/10.1016/j.bone.2017.09.008.
Albano D, Messina C, Vitale J, et al. Imaging of sarcopenia: old evidence and new insights. Eur Radiol. 2020;304:2199–208. https://doi.org/10.1007/s00330-019-06573-2.
Codari M, Zanardo M, di Sabato ME, et al. MRI-derived biomarkers related to sarcopenia: a systematic review. J Magn Reson Imaging. 2020;514:1117–27. https://doi.org/10.1002/jmri.26931.
Guglielmi G, Ponti F, Agostini M, et al. The role of DXA in sarcopenia. Aging Clin Exp Res. 2016;286:1047–60. https://doi.org/10.1007/s40520-016-0589-3.
Mijnarends DM, Luiking YC, Halfens RJG, et al. Muscle, health and costs: a glance at their relationship. J Nutr Health Aging. 2018;227:766–73. https://doi.org/10.1007/s12603-018-1058-9.
Cruz-Jentoft AJ, Sayer AA. Sarcopenia. The Lancet. 2019;39310191:2636–46. https://doi.org/10.1016/s0140-6736(19)31138-9.
Xia L, Zhao R, Wan Q, et al. Sarcopenia and adverse health-related outcomes: an umbrella review of meta-analyses of observational studies. Cancer Med. 2020;921:7964–78. https://doi.org/10.1002/cam4.3428.
Goates S, Du K, Arensberg MB, et al. Economic Impact of Hospitalizations in Us Adults with Sarcopenia. J Frailty Aging. 2019. https://doi.org/10.14283/jfa.2019.10.
Sanz-Requena R, Martínez-Arnau FM, Pablos-Monzó A, et al. The role of imaging biomarkers in the assessment of sarcopenia. Diagnostics. 2020. https://doi.org/10.3390/diagnostics10080534.
Chen LK, Woo J, Assantachai P, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;213:300–7. https://doi.org/10.1016/j.jamda.2019.12.012. (e302).
Gonzalez MC, Barbosa-Silva TG, Heymsfield SB. Bioelectrical impedance analysis in the assessment of sarcopenia. Curr Opin Clin Nutr Metab Care. 2018;215:366–74. https://doi.org/10.1097/mco.0000000000000496.
Lee CM, Kang BK, Kim M. Radiologic definition of sarcopenia in chronic liver disease. Life (Basel). 2021. https://doi.org/10.3390/life11020086.
Baltzer PAT, Park J, Gil JR, et al. Reliable and robust method for abdominal muscle mass quantification using CT/MRI: an explorative study in healthy subjects. PLoS ONE. 2019. https://doi.org/10.1371/journal.pone.0222042.
Faron A, Sprinkart AM, Kuetting DLR, et al. Body composition analysis using CT and MRI: intra-individual intermodal comparison of muscle mass and myosteatosis. Sci Rep. 2020;101:11765. https://doi.org/10.1038/s41598-020-68797-3.
Ponti F, Santoro A, Mercatelli D, et al. Aging and imaging assessment of body composition: from fat to facts. Front Endocrinol. 2020. https://doi.org/10.3389/fendo.2019.00861.
Messina C, Albano D, Gitto S, et al. Body composition with dual energy X-ray absorptiometry: from basics to new tools. Quant Imaging Med Surg. 2020;108:1687–98. https://doi.org/10.21037/qims.2020.03.02.
Guerri S, Mercatelli D, Aparisi Gomez MP, et al. Quantitative imaging techniques for the assessment of osteoporosis and sarcopenia. Quant Imaging Med Surg. 2018;81:60–85. https://doi.org/10.21037/qims.2018.01.05.
Tegola L, Mattera M, Cornacchia S, et al. Diagnostic imaging of two related chronic diseases: sarcopenia and osteoporosis. J Frailty Sarcopenia Falls. 2018;33:138–47. https://doi.org/10.22540/JFSF-03-138.
Tosato M, Marzetti E, Cesari M, et al. Measurement of muscle mass in sarcopenia: from imaging to biochemical markers. Aging Clin Exp Res. 2017;291:19–27. https://doi.org/10.1007/s40520-016-0717-0.
Westbury LD, Beaudart C, Bruyère O, et al. Recent sarcopenia definitions—prevalence, agreement and mortality associations among men: findings from population-based cohorts. J Cachexia Sarcopenia Muscle. 2023;141:565–75. https://doi.org/10.1002/jcsm.13160.
Kim M, Lee C-M, Kang BK, et al. Sarcopenia assessed with DXA and CT increases the risk of perioperative complications in patients with gastrectomy. Eur Radiol. 2023;337:5150–8. https://doi.org/10.1007/s00330-023-09401-w.
Baim S, Wilson CR, Lewiecki EM, et al. Precision assessment and radiation safety for dual-energy X-ray absorptiometry. J Clin Densitometry. 2005;84:371–8. https://doi.org/10.1385/jcd:8:4:371.
Achamrah N, Jésus P, Grigioni S, et al. Validity of predictive equations for resting energy expenditure developed for obese patients: impact of body composition method. Nutrients. 2018. https://doi.org/10.3390/nu10010063.
Gonera-Furman A, Bolanowski M, Jędrzejuk D. Osteosarcopenia—the role of dual-energy X-ray absorptiometry (DXA) in diagnostics. J Clin Med. 2022. https://doi.org/10.3390/jcm11092522.
Shi S, Chen W, Jiang Y, et al. A more accurate method to estimate muscle mass: a new estimation equation. J Cachexia Sarcopenia Muscle. 2023;144:1753–61. https://doi.org/10.1002/jcsm.13254.
McCarthy C, Tinsley GM, Bosy-Westphal A, et al. Total and regional appendicular skeletal muscle mass prediction from dual-energy X-ray absorptiometry body composition models. Sci Reports. 2023. https://doi.org/10.1038/s41598-023-29827-y.
Messina C, Bandirali M, Sconfienza LM, et al. Prevalence and type of errors in dual-energy x-ray absorptiometry. Eur Radiol. 2014;255:1504–11. https://doi.org/10.1007/s00330-014-3509-y.
Tagliafico AS, Bignotti B, Torri L, et al. Sarcopenia: how to measure, when and why. Radiol Med. 2022;1273:228–37. https://doi.org/10.1007/s11547-022-01450-3.
Park J, Gil JR, Shin Y, et al. Reliable and robust method for abdominal muscle mass quantification using CT/MRI: an explorative study in healthy subjects. PLoS ONE. 2019;149: e0222042. https://doi.org/10.1371/journal.pone.0222042.
Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;976:2333–8. https://doi.org/10.1152/japplphysiol.00744.2004.
Faron A, Luetkens JA, Schmeel FC, et al. Quantification of fat and skeletal muscle tissue at abdominal computed tomography: associations between single-slice measurements and total compartment volumes. Abdom Radiol. 2019;445:1907–16. https://doi.org/10.1007/s00261-019-01912-9.
Faron A, Pieper CC, Schmeel FC, et al. Fat-free muscle area measured by magnetic resonance imaging predicts overall survival of patients undergoing radioembolization of colorectal cancer liver metastases. Eur Radiol. 2019;299:4709–17. https://doi.org/10.1007/s00330-018-5976-z.
van Heusden HC, Swartz JE, Chargi N, et al. Feasibility of assessment of skeletal muscle mass on a single cross-sectional image at the level of the fourth thoracic vertebra. Eur J Radiol. 2021;142: 109879. https://doi.org/10.1016/j.ejrad.2021.109879.
Tan L, Ji G, Bao T, et al. Diagnosing sarcopenia and myosteatosis based on chest computed tomography images in healthy Chinese adults. Insights Imaging. 2021;121:163. https://doi.org/10.1186/s13244-021-01106-2.
Kim EY, Kim YS, Park I, et al. Prognostic significance of CT-determined sarcopenia in patients with small-cell lung cancer. J Thoracic Oncol. 2015;1012:1795–9. https://doi.org/10.1097/jto.0000000000000690.
Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 1985;2004(976):2333–8. https://doi.org/10.1152/japplphysiol.00744.2004.
Dabiri S, Popuri K, Cespedes Feliciano EM, et al. Muscle segmentation in axial computed tomography (CT) images at the lumbar (L3) and thoracic (T4) levels for body composition analysis. Comput Med Imaging Graphics. 2019;75:47–55. https://doi.org/10.1016/j.compmedimag.2019.04.007.
Zannoni S, Albano D, Jannone ML, et al. Correlation between muscle mass and quality around the hip and of psoas muscles at L3 level using unenhanced CT scans. Skeletal Radiol. 2020;4910:1649–55. https://doi.org/10.1007/s00256-020-03475-z.
Ebadi M, Wang CW, Lai JC, et al. Poor performance of psoas muscle index for identification of patients with higher waitlist mortality risk in cirrhosis. J Cachexia Sarcopenia Muscle. 2018;96:1053–62. https://doi.org/10.1002/jcsm.12349.
Hamaguchi Y, Kaido T, Okumura S, et al. Impact of skeletal muscle mass index, intramuscular adipose tissue content, and visceral to subcutaneous adipose tissue area ratio on early mortality of living donor liver transplantation. Transplantation. 2017;1013:565–74. https://doi.org/10.1097/tp.0000000000001587.
Kim H-K, Kim KW, Kim EH, et al. Age-related changes in muscle quality and development of diagnostic cutoff points for myosteatosis in lumbar skeletal muscles measured by CT scan. Clin Nutr. 2021;406:4022–8. https://doi.org/10.1016/j.clnu.2021.04.017.
Tan L, Ji G, Bao T, et al. Diagnosing sarcopenia and myosteatosis based on chest computed tomography images in healthy Chinese adults. Insights Imaging. 2021. https://doi.org/10.1186/s13244-021-01106-2.
Aleixo GFP, Shachar SS, Nyrop KA, et al. Myosteatosis and prognosis in cancer: Systematic review and meta-analysis. Crit Rev Oncol Hematol. 2020;145: 102839. https://doi.org/10.1016/j.critrevonc.2019.102839.
Lee K, Shin Y, Huh J, et al. Recent issues on body composition imaging for sarcopenia evaluation. Korean J Radiol. 2019;202:205–17. https://doi.org/10.3348/kjr.2018.0479.
Lenchik L, Boutin R. Sarcopenia: beyond muscle atrophy and into the new frontiers of opportunistic imaging, precision medicine, and machine learning. Semin Musculoskelet Radiol. 2018;2203:307–22. https://doi.org/10.1055/s-0038-1641573.
Morsbach F, Zhang YH, Nowik P, et al. Influence of tube potential on CT body composition analysis. Nutrition. 2018;53:9–13. https://doi.org/10.1016/j.nut.2017.12.016.
Morsbach F, Zhang YH, Martin L, et al. Body composition evaluation with computed tomography: contrast media and slice thickness cause methodological errors. Nutrition. 2019;59:50–5. https://doi.org/10.1016/j.nut.2018.08.001.
Shachar SS, Deal AM, Weinberg M, et al. Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane-based chemotherapy. Clin Cancer Res. 2017;233:658–65. https://doi.org/10.1158/1078-0432.Ccr-16-0940.
Caan BJ, Cespedes Feliciano EM, Prado CM, et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. 2018;46:798–804. https://doi.org/10.1001/jamaoncol.2018.0137.
Wahlen BM, Mekkodathil A, Al-Thani H, et al. Impact of sarcopenia in trauma and surgical patient population: a literature review. Asian J Surg. 2020;436:647–53. https://doi.org/10.1016/j.asjsur.2019.10.010.
Erlandson MC, Lorbergs AL, Mathur S, et al. Muscle analysis using pQCT, DXA and MRI. Eur J Radiol. 2016;858:1505–11. https://doi.org/10.1016/j.ejrad.2016.03.001.
Sherk VD, Bemben MG, Palmer IJ, et al. Effects of filtering methods on muscle and fat cross-sectional area measurement by pQCT: a technical note. Physiol Meas. 2011;3212:N65–72. https://doi.org/10.1088/0967-3334/32/12/N01.
Cesari M, Leeuwenburgh C, Lauretani F, et al. Frailty syndrome and skeletal muscle: results from the Invecchiare in Chianti study. Am J Clin Nutr. 2006;835:1142–8. https://doi.org/10.1093/ajcn/83.5.1142.
Edwards MH, Gregson CL, Patel HP, et al. Muscle size, strength, and physical performance and their associations with bone structure in the Hertfordshire Cohort Study. J Bone Miner Res. 2013;2811:2295–304. https://doi.org/10.1002/jbmr.1972.
Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 1985;2003(955):1851–60. https://doi.org/10.1152/japplphysiol.00246.2003.
Cesari M, Pahor M, Lauretani F, et al. Skeletal muscle and mortality results from the InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2009;643:377–84. https://doi.org/10.1093/gerona/gln031.
Frank-Wilson AW, Johnston JD, Olszynski WP, et al. Measurement of muscle and fat in postmenopausal women: precision of previously reported pQCT imaging methods. Bone. 2015;75:49–54. https://doi.org/10.1016/j.bone.2015.01.016.
Erlandson MC, Wong AKO, Szabo E, et al. Muscle and myotendinous tissue properties at the distal tibia as assessed by high-resolution peripheral quantitative computed tomography. J Clin Densitom. 2017;202:226–32. https://doi.org/10.1016/j.jocd.2016.10.005.
Hildebrand KN, Sidhu K, Gabel L, et al. The assessment of skeletal muscle and cortical bone by second-generation HR-pQCT at the tibial midshaft. J Clin Densitom. 2021;243:465–73. https://doi.org/10.1016/j.jocd.2020.11.001.
Sergi G, Trevisan C, Veronese N, et al. Imaging of sarcopenia. Eur J Radiol. 2016;858:1519–24. https://doi.org/10.1016/j.ejrad.2016.04.009.
Seabolt LA, Welch EB, Silver HJ. Imaging methods for analyzing body composition in human obesity and cardiometabolic disease. Ann N Y Acad Sci. 2015;1353:41–59. https://doi.org/10.1111/nyas.12842.
Seabolt LA, Welch EB, Silver HJ. Imaging methods for analyzing body composition in human obesity and cardiometabolic disease. Ann N Y Acad Sci. 2015;13531:41–59. https://doi.org/10.1111/nyas.12842.
Lemos T, Gallagher D. Current body composition measurement techniques. Curr Opin Endocrinol Diabetes Obes. 2017;245:310–4. https://doi.org/10.1097/MED.0000000000000360.
Zwart AT, Becker JN, Lamers MJ, et al. Skeletal muscle mass and sarcopenia can be determined with 1.5-T and 3-T neck MRI scans, in the event that no neck CT scan is performed. Eur Radiol. 2021;316:4053–62. https://doi.org/10.1007/s00330-020-07440-1.
Rossi A, Zoico E, Goodpaster BH, et al. Quantification of intermuscular adipose tissue in the erector spinae muscle by MRI: agreement with histological evaluation. Obesity (Silver Spring). 2010;1812:2379–84. https://doi.org/10.1038/oby.2010.48.
Jang HD, Won SH, Kim DW, et al. Magnetic resonance imaging characteristics and age-related changes in the psoas muscle: analysis of 164 patients with back pain and balanced lumbar sagittal alignment. World Neurosurg. 2019;131:e88–95. https://doi.org/10.1016/j.wneu.2019.07.048.
Li K, Dortch RD, Welch EB, et al. Multi-parametric MRI characterization of healthy human thigh muscles at 3.0 T—relaxation, magnetization transfer, fat/water, and diffusion tensor imaging. NMR Biomed. 2014;279:1070–84. https://doi.org/10.1002/nbm.3159.
Huq S, Khalafallah AM, Ruiz-Cardozo MA, et al. A novel radiographic marker of sarcopenia with prognostic value in glioblastoma. Clin Neurol Neurosurg. 2021;207: 106782. https://doi.org/10.1016/j.clineuro.2021.106782.
Grimm A, Meyer H, Nickel MD, et al. Repeatability of Dixon magnetic resonance imaging and magnetic resonance spectroscopy for quantitative muscle fat assessments in the thigh. J Cachexia Sarcopenia Muscle. 2018;96:1093–100. https://doi.org/10.1002/jcsm.12343.
Baum T, Inhuber S, Dieckmeyer M, et al. Association of quadriceps muscle fat with isometric strength measurements in healthy males using chemical shift encoding-based water-fat magnetic resonance imaging. J Comput Assist Tomogr. 2016;403:447–51. https://doi.org/10.1097/RCT.0000000000000374.
Sanz-Requena R, Martinez-Arnau FM, Pablos-Monzo A, et al. The role of imaging biomarkers in the assessment of sarcopenia. Diagnostics (Basel). 2020. https://doi.org/10.3390/diagnostics10080534.
Grimm A, Meyer H, Nickel MD, et al. A comparison between 6-point Dixon MRI and MR spectroscopy to quantify muscle fat in the thigh of subjects with sarcopenia. J Frailty Aging. 2019;81:21–6. https://doi.org/10.14283/jfa.2018.16.
Takashima H, Takebayashi T, Ogon I, et al. Evaluation of intramyocellular and extramyocellular lipids in the paraspinal muscle in patients with chronic low back pain using MR spectroscopy: preliminary results. Br J Radiol. 2016. https://doi.org/10.1259/bjr.20160136.
Engelke K, Chaudry O, Gast L, et al. Magnetic resonance imaging techniques for the quantitative analysis of skeletal muscle: state of the art. J Orthop Transl. 2023;42:57–72. https://doi.org/10.1016/j.jot.2023.07.005.
Krssak M, Lindeboom L, Schrauwen-Hinderling V, et al. Proton magnetic resonance spectroscopy in skeletal muscle: Experts’ consensus recommendations. NMR Biomed. 2021;345: e4266. https://doi.org/10.1002/nbm.4266.
Codari M, Zanardo M, di Sabato ME, et al. MRI-derived biomarkers related to sarcopenia: a systematic review. J Magn Reson Imaging. 2019;514:1117–27. https://doi.org/10.1002/jmri.26931.
Grimm A, Meyer H, Nickel MD, et al. Evaluation of 2-point, 3-point, and 6-point Dixon magnetic resonance imaging with flexible echo timing for muscle fat quantification. Eur J Radiol. 2018;103:57–64. https://doi.org/10.1016/j.ejrad.2018.04.011.
Froeling M, Nederveen AJ, Nicolay K, et al. DTI of human skeletal muscle: the effects of diffusion encoding parameters, signal-to-noise ratio and T2 on tensor indices and fiber tracts. NMR Biomed. 2013;2611:1339–52. https://doi.org/10.1002/nbm.2959.
Scheel M, von Roth P, Winkler T, et al. Fiber type characterization in skeletal muscle by diffusion tensor imaging. NMR Biomed. 2013;2610:1220–4. https://doi.org/10.1002/nbm.2938.
Takao S, Kaneda M, Sasahara M, et al. Diffusion tensor imaging (DTI) of human lower leg muscles: correlation between DTI parameters and muscle power with different ankle positions. Jpn J Radiol. 2022;409:939–48. https://doi.org/10.1007/s11604-022-01274-1.
Giraudo C, Cavaliere A, Lupi A, et al. Established paths and new avenues: a review of the main radiological techniques for investigating sarcopenia. Quant Imaging Med Surg. 2020;108:1602–13. https://doi.org/10.21037/qims.2019.12.15.
Surov A, Paul L, Meyer HJ, et al. Apparent diffusion coefficient is a novel imaging biomarker of myopathic changes in liver cirrhosis. J Clin Med. 2018. https://doi.org/10.3390/jcm7100359.
Marty B, Coppa B, Carlier PG. Monitoring skeletal muscle chronic fatty degenerations with fast T1-mapping. Eur Radiol. 2018;2811:4662–8. https://doi.org/10.1007/s00330-018-5433-z.
Chianca V, Albano D, Messina C, et al. Sarcopenia: imaging assessment and clinical application. Abdom Radiol (NY). 2021. https://doi.org/10.1007/s00261-021-03294-3.
Malis V, Sinha U, Csapo R, et al. Relationship of changes in strain rate indices estimated from velocity-encoded MR imaging to loss of muscle force following disuse atrophy. Magn Reson Med. 2018;792:912–22. https://doi.org/10.1002/mrm.26759.
Sinha U, Malis V, Csapo R, et al. Age-related differences in strain rate tensor of the medial gastrocnemius muscle during passive plantarflexion and active isometric contraction using velocity encoded MR imaging: Potential index of lateral force transmission. Magn Reason Med. 2015;735:1852–63. https://doi.org/10.1002/mrm.25312.
Gerhalter T, Gast LV, Marty B, et al. 23Na MRI depicts early changes in ion homeostasis in skeletal muscle tissue of patients with duchenne muscular dystrophy. J Magn Reson Imaging. 2019;504:1103–13. https://doi.org/10.1002/jmri.26681.
Umathum R, Rösler MB, Nagel AM. In Vivo39K MR imaging of human muscle and brain. Radiology. 2013;2692:569–76. https://doi.org/10.1148/radiol.13130757.
Nagel AM, Lehmann-Horn F, Weber M-A, et al. In Vivo35Cl MR imaging in humans: a feasibility study. Radiology. 2014;2712:585–95. https://doi.org/10.1148/radiol.13131725.
Strijkers GJ, Araujo ECA, Azzabou N, et al. Exploration of new contrasts, targets, and MR imaging and spectroscopy techniques for neuromuscular disease—a workshop report of working group 3 of the biomedicine and molecular biosciences COST action BM1304 MYO-MRI. J Neuromuscular Dis. 2019;61:1–30. https://doi.org/10.3233/jnd-180333.
Hsieh T-J, Chou M-C, Chen Y-C, et al. Reliability of gradient-echo magnetic resonance elastography of lumbar muscles: phantom and clinical studies. Diagnostics. 2022. https://doi.org/10.3390/diagnostics12061385.
Ambrósio CE, Chakouch MK, Charleux F, et al. Quantifying the elastic property of nine thigh muscles using magnetic resonance elastography. PLoS ONE. 2015. https://doi.org/10.1371/journal.pone.0138873.
Sconfienza LM, Albano D, Allen G, et al. Clinical indications for musculoskeletal ultrasound updated in 2017 by European Society of Musculoskeletal Radiology (ESSR) consensus. Eur Radiol. 2018;2812:5338–51. https://doi.org/10.1007/s00330-018-5474-3.
Tagliafico AS, Bignotti B, Torri L, et al. Sarcopenia: how to measure, when and why. Radiol Med (Torino). 2022;1273:228–37. https://doi.org/10.1007/s11547-022-01450-3.
Jacobson JA. Sarcopenia: relevance and assessment. Acad Radiol. 2021;284:524–5. https://doi.org/10.1016/j.acra.2020.08.034.
Mitchell WK, Phillips BE, Williams JP, et al. Development of a new Sonovue™ contrast-enhanced ultrasound approach reveals temporal and age-related features of muscle microvascular responses to feeding. Physiol Reports. 2013. https://doi.org/10.1002/phy2.119.
Scott JM, Martin DS, Ploutz-Snyder R, et al. Panoramic ultrasound: a novel and valid tool for monitoring change in muscle mass. J Cachexia Sarcopenia Muscle. 2017;83:475–81. https://doi.org/10.1002/jcsm.12172.
Mazurowski MA, Buda M, Saha A, et al. Deep learning in radiology: an overview of the concepts and a survey of the state of the art with focus on MRI. J Mag Reason Imaging. 2018;494:939–54. https://doi.org/10.1002/jmri.26534.
Erickson BJ, Korfiatis P, Akkus Z, et al. Machine learning for medical imaging. Radiographics. 2017;372:505–15. https://doi.org/10.1148/rg.2017160130.
Santhanam P, Nath T, Peng C, et al. Artificial intelligence and body composition. Diabet Metab Syndrome. 2023. https://doi.org/10.1016/j.dsx.2023.102732.
Gu S, Wang L, Han R, et al. Detection of sarcopenia using deep learning-based artificial intelligence body part measure system (AIBMS). Front Physiol. 2023. https://doi.org/10.3389/fphys.2023.1092352.
Mai DVC, Drami I, Pring ET, et al. A systematic review of automated segmentation of 3D computed-tomography scans for volumetric body composition analysis. J Cachexia Sarcopenia Muscle. 2023;145:1973–86. https://doi.org/10.1002/jcsm.13310.
Nandakumar B, Baffour F, Abdallah NH, et al. Sarcopenia identified by computed tomography imaging using a deep learning–based segmentation approach impacts survival in patients with newly diagnosed multiple myeloma. Cancer. 2022;1293:385–92. https://doi.org/10.1002/cncr.34545.
Zhang Y, Dilixiati Y, Jiang W, et al. Correlation of psoas muscle index with fragility vertebral fracture: a retrospective cross-sectional study of middle-aged and elderly women. Int J Endocrinol. 2022;2022:1–7. https://doi.org/10.1155/2022/4149468.
Ding PA, Yang P, Yang L, et al. Impact of skeletal muscle loss during conversion therapy on clinical outcomes in lavage cytology positive patients with gastric cancer. Front Oncol. 2022. https://doi.org/10.3389/fonc.2022.949511.
Bannangkoon K, Hongsakul K, Tubtawee T, et al. Association of myosteatosis with treatment response and survival in patients with hepatocellular carcinoma undergoing chemoembolization: a retrospective cohort study. Sci Reports. 2023. https://doi.org/10.1038/s41598-023-31184-9.
Funding
This work is supported by the National Natural Science Foundation of China (No. 82260338, 82302131); the Yunnan Fundamental Research Projects (No. 202301AS070016, 202301AY070001203, 202301AU70163).
Author information
Authors and Affiliations
Contributions
BH: designed the study and conceived the report. CL and YH: wrote the draft of the manuscript and revised it critically. HW and JL: created the figures. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article is a review, so it does not require ethics approval with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, C., Huang, Y., Wang, H. et al. Application of imaging methods and the latest progress in sarcopenia. Chin J Acad Radiol 7, 15–27 (2024). https://doi.org/10.1007/s42058-024-00142-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42058-024-00142-3