Key summary points
To evaluate the effect of a transitional care intervention among older medical inpatients.
AbstractSection FindingsThe follow-home transitional care intervention did not have an impact on readmission and mortality rates.
AbstractSection MessageIn a real-world context, several aspects make it difficult to measure the true impact of a multicomponent intervention.
Abstract
Purpose
To evaluate the effect of a transitional care intervention (TCI) on readmission among older medical inpatients.
Methods
This non-randomised quasi-experimental study was conducted at Horsens Regional Hospital in Denmark from 1 February 2017 to 31 December 2018. Inclusion criteria were patients ≥ 75 years old admitted for at least 48 h. First, patients were screened for eligibility. Then, the allocation to the intervention or control group was performed according to the municipality of residence. Patients living in three municipalities were offered the hospital-based intervention, and patients living in a fourth municipality were allocated to the control group. The intervention components were (1) discharge transportation with a home visit, (2) a post-discharge cross-sectorial video conference and (3) seven-day telephone consultation. The primary outcome was 30-day unplanned readmission. Secondary outcomes were 30- and 90-day mortality and days alive and out of hospital (DAOH).
Results
The study included 1205 patients (intervention: n = 615; usual care: n = 590). In the intervention group, the median age was 84.3 years and 53.7% were females. In the control group, the median age was 84.9 years and 57.5% were females. The 30-day readmission rates were 20.8% in the intervention group and 20.2% in the control group. Adjusted relative risk was 1.00 (95% confidence interval: 0.80, 1.26; p = 0.99). No significant difference was found between the groups for the secondary outcomes.
Conclusion
The TCI did not impact readmission, mortality or DAOH. Future research should conduct a pilot test, address intervention fidelity and consider real-world challenges.
Trial registration
Clinical trial number: NCT04796701. Registration date: 24 February 2021.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transitions between hospital and primary care settings are identified as high-risk scenarios for patient safety that often result in adverse events, such as unplanned readmissions [1]. The share of unplanned hospital readmissions among somatic patients was 11.7% in Denmark in 2018 [2]. Approximately 20% of all hospitalised older medical patients are readmitted within 30 days after discharge [3]. Among other reasons, readmissions are caused by poor quality of care after discharge [4, 5], insufficient discharge planning or insufficient communication between hospital and home care or general practitioners (GPs) [6, 7].
Transitional care interventions (TCIs) are one strategy for preventing readmissions. Transitional care is defined as ‘a set of actions designed to ensure the coordination and continuity of healthcare as patients transfer between different locations or different levels of care within the same location. Representative locations include (but are not limited to) hospitals, subacute and post-acute nursing facilities, the patient’s home, primary and specialty care offices, and assisted living and long-term care facilities’ [8]. Over the past few decades, TCIs have been widely examined. TCIs are delivered by various healthcare professions, such as nurses, pharmacists, physiotherapists, GPs and/or geriatricians [9,10,11,12]. Some studies have focused on either the pre- or post-discharge periods, while others have focused on both. Other studies have targeted specific diagnoses or patient groups [10, 13,14,15]. The effects of TCIs on readmission rates vary. However, most studies have reported positive findings [16,17,18,19].
Despite the diversity of studies, we have yet to examine the effects on readmissions among an unselected medical patient group in a regional setting where the demographics, geographical distances and healthcare services differ from large urban settings.
The objective of this study was to examine the effect of a hospital-based TCI on readmissions among older medical patients admitted to a general medical ward at a regional hospital. We hypothesised that cross-sectorial collaboration with a follow-home function, post-discharge cross-sectorial video conferences and telephone consultations would reduce the number of all-cause unplanned readmissions compared to patients who received usual care without this cross-sectorial collaboration.
Methods
Design and setting
This study was a non-randomised quasi-experimental study conducted at Horsens Regional Hospital from 1 February 2017 to 31 December 2018. Horsens is a public hospital in the Central Denmark Region with 21,746 acute hospital admissions in 2017 and a total of 240 beds. The hospital receives patients primarily from the following four municipalities: Odder, Hedensted, Skanderborg and Horsens. The total number of inhabitants in 2017 was 218,286. Of these, 15,907 (7.3%) were older than 75 years, and of those, 57% were women. The share of inhabitants ≥ 75 years old was 7.1% in Horsens municipality, 6.4% in Skanderborg, 8.1% in Hedensted and 8.9% in Odder [20]. The Horsens Medical Department is divided into three subspecialised medical wards. However, the study was conducted in Medical Ward 2, a 27-bed ward with the following specialities: internal medicine, geriatric medicine and endocrinology.
Population
Patients were eligible for inclusion if they met the following criteria: ≥ 75 years old, living in the municipalities of Odder, Skanderborg, Hedensted or Horsens and had been admitted to Medical Ward 2 for at least 48 h. Each weekday morning, patients were screened for inclusion criteria. If the patient was eligible for inclusion, the municipality of residence was determined. Then, allocation was conducted according to the patients’ municipality of residence.
Intervention group
In addition to usual care, patients were offered the follow-home TCI if they lived in the municipalities of Hedensted, Odder or Skanderborg at the time of the index admission. All patients in the intervention group signed an informed consent form. Eligible patients were consecutively approached and enrolled.
Control group
Patients living in the municipality of Horsens at the time of the index admission received the usual care. The control group was identified through the CROSS-TRACKS register [21] during the post-intervention analysis process. According to The Data Protection Act §10, informed consent was not needed to retrieve data from the register.
All relevant legal approvals to collect data on the intervention and control groups were obtained. For details, see Declarations.
The study is reported according to the ‘Guidelines for Reporting Non-Randomised Studies’ [22] and ‘Conducting and Reporting Trials for Older People’ [23].
Follow-home intervention
‘The Template for Intervention Description and Replication (TIDieR) Checklist and Guide’ [24] has been used to ensure a transparent and thorough description of the intervention.
Intervention development
The intervention was originally developed in an urban setting in Denmark [25]. It was adjusted to a regional context with different demographics, geography and budget by a hospital-based expert panel in 2016. The panel consisted of healthcare professionals from Horsens Regional Hospital: the head consultant, the head nurse, the nurse manager, registered nurses from the Medical Department and the project leader of Safer Patient Flow. The services should bridge and ensure continuity of care and treatment between sectors. This should be assured by close, comprehensive and timely communication with patients, relatives and healthcare collaborators.
Delivering the intervention
Three healthcare professionals from Medical Ward 2 were dedicated as project workers. Two were registered nurses with four and 12 years of job experience, respectively, and one was a social and healthcare assistant with 17 years of experience.
Intervention components
Overall, the hospital-based intervention consisted of three components covering the pre-discharge and post-discharge phases.
-
1.
Pre-discharge phase: discharge transportation and home visit
On the day of discharge, patients were physically escorted home from the hospital by a project worker, either in a project car or by bedridden-patient transportation, if they were physically or mentally incapable of sitting in a car. Whichever means of transportation, a physical home visit was conducted immediately after the patient’s return home or on the first weekday afterwards. During the visit, the project worker ensured that the patient felt as safe as possible, basic human needs were met, a medication reconciliation was conducted, the patient’s nutritional status was assessed, fall prevention efforts were initiated and challenges and concerns were discussed and resolved whenever possible. A video conference for the following working day was arranged during the visit.
-
2.
Post-discharge phase: cross-sectional video conference and telephone consultations
A video conference was conducted on the following working day. The conference aim was to bridge the gap between hospital and primary healthcare professionals by ensuring (1) a smooth transition with timely and sufficient communication; (2) that questions, barriers and worries were handled; and (3) continuity of care and treatment plans were arranged. The project worker participated from the hospital, while the patient, district nurse and relatives participated from the patient’s home. If a video conference was not possible, a telephone conference was held instead. To facilitate the conference, one computer or tablet was provided for the project worker, and one was provided for the district nurses in each of the three participating municipalities.
Telephone consultations with the project workers or geriatricians were offered for up to seven days after discharge. Patients, relatives, district nurses and GPs were given a direct phone number that was answered by either a project worker or a nurse coordinator 24/7. Among other things, questions about hospital-based treatment and care, modifications of the treatment and care plans and disease deterioration could be discussed during these consultations.
Usual care
The usual care was provided equally to all patients in the intervention and control group, thus all patients receive the same level of standard care. At Horsens Regional Hospital, planning of the discharge begins no later than the second day after hospital admission. Overall, the nurses are responsible for the daily assessments of functionality and needs and for care plans and digital communication with the home care staff and practical matters in relation to the discharge. On admission day, a medication reconciliation is conducted by a hospital doctor, and a nurse begins the nursing discharge status document and checklist.
A nursing care plan is started the day after admission and digitally sent to the home care staff as a preliminary plan. This plan includes information about the expected discharge date, functional ability, need for home healthcare services after discharge and coordination in relation to the discharge. Families or other caregivers are only automatically involved in the plan if the patients explicitly want them to be involved in complex discharge situations. During admission, nurses assess and act on the need for assistive technology after discharge and the need for post-discharge follow-up from outpatient clinics, GPs, home care services, district dieticians and specialised services for specific patient groups.
The nurse sends the updated nursing care plan to home healthcare before 12 p.m. the day before discharge. On the day of discharge, plans and lists are updated, the hospital doctor draws up a medication reconciliation, and prescriptions are written. If specialised care or treatment is needed, the nurse contacts the district nurse by phone as a supplement to the digital plan.
Finally, the hospital doctor writes a discharge letter and digitally send it to the patient’s GP and district nurse. After discharge, the GP and district nurse are responsible for treatment and care. Online Resource 1 illustrates the components of the intervention and usual care.
Variables and data sources
Outcome measures
The primary outcome was the all-cause 30-day unplanned readmission rate. The secondary outcomes were (1) the all-cause mortality rate measured at 30 and 90 days after discharge and (2) days alive and out of hospital (DAOH) and without GP visits (DAOHGP).
Readmission was defined as any unplanned hospital admission within four hours and 30 days after discharge [26]. The readmission could not be caused by accidents, violence or suicidal attempts. No specific diagnostic relations were drawn between the index admission and readmission [27]. The outcome assessment was not blinded. This, however, was not a problem or potential bias since all outcome data were retrieved from a register.
Data sources
Data were obtained from CROSS-TRACKS [21]. These data cover home care services, pre-hospital services, prescriptions, hospital services, laboratory tests, socioeconomics data and data from the National Health Insurance Service Register, the Civil Registration System and the National Patient Register.
Statistical analysis
The power analysis was calculated based on readmission rates of 22% in the control group and 12% in the intervention group [28]. The analysis found that 694 patients should be included in the study (347 in each group) to achieve a 5% significance level and a power of 90%, allowing for a 10% dropout rate.
Data management and statistical analysis plans were conducted prior to the study.
We used chi-square tests to describe and compare the baseline characteristics. When observations were not normally distributed, Wilcoxon rank-sum or Kruskal–Wallis tests were used.
A binary regression model was used to compare the 30-day readmission risk, 30- and 90-day mortality rates, DAOH and DAOHGP between the two groups. The 30-day readmission analysis was performed based on the first readmission. The relative risk (RR) with a 95% confidence interval (CI) was calculated. The statistical models were adjusted for covariates with a statistically significant difference between the intervention and control groups at baseline.
In this study, selection bias may be present, as the intervention and control groups were identified differently. Furthermore, identifying patients not receiving the allocated treatment was only possible if they were in the intervention group, not if they were in the control group. To mitigate selection bias and ensure the highest level of homogeneity between the groups, the analysis was based on data from all patients who met the inclusion criteria—that is, we analysed the effect of the assignment to the intervention at baseline rather than the effect of adhering to the intervention.Footnote 1
Furthermore, for the primary outcome, analyses were performed in the following sub-groups: age group, gender, housing, civil status, social status, length of stay, Charlson Comorbidity Index (CCI), home care after discharge from index admission, municipality, blood samples at discharge, body mass index, admission year, vital signs at discharge, polypharmacy (five or more daily medicines [29]), number of admissions within one year prior to the index admission and visits to the GP within one year prior to index admission.
The Aalen–Johansen estimator displayed the mortality-adjusted cumulative incidence of 30-day readmissions. Both 30 and 90-day mortality rates were displayed using the Kaplan–Meier survival estimator.
DAOH was calculated by subtracting the days spent in the hospital (in the period within 30 days after discharge from index admission) from 30. DAOHGP was calculated as above, but visits to the GP were also subtracted. DAOH and DAOHGP were analysed as binary variables with cut-offs at ≥ 23 days.
Difference-in-difference (before and after) analysis was calculated based on 30-day readmission rates in patients who met the study inclusion criteria in all four participating municipalities in the pre-intervention period from 2015 to 2016. These data were compared to the 30-day intervention readmission rates. The results are presented as risk difference with standard errors (SEs) and p-values between the groups for 30-day readmissions.
The CCI scores were calculated based on International Classification of Diseases-10 diagnostic codes of 19 conditions [30, 31] and reported in the following categories: no comorbidity (score = 0), low (score = 1), medium (score = 2) and high (score = 3 +).
STATA version 17 [32] was used to conduct the statistical analysis and to display Aalen–Johansen- and Kaplan–Meier estimators and the difference-in-difference analysis. Statistical assumptions were tested prior to the analysis.
Ethics
Approval was obtained from the Danish Data Protection Agency (case no. 1-16-02-105-19). The CROSS-TRACKS case no. was 008-2. Approval by the Central Denmark Region Ethical Committee was not required. Patients in the intervention group could withdraw their consent at any time. The intervention did not harm any patients. All procedures in this study were performed in accordance with the 1964 Declaration of Helsinki and its later amendments.
Patient and public involvement
Neither the patients nor the public were involved in planning or evaluating this study.
Results
Participants
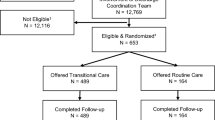
During the study period, 1228 met the inclusion criteria, and 23 were excluded due to unknown reasons. Finally, 1205 patients were included in the study: 615 in the intervention group and 590 in the control group (Fig. 1). In the intervention group, 214 (17%) did not receive the intervention as planned. No patients were lost to follow-up, as we had CROSS-TRACKS data on all outcome variables.
The groups were similar in baseline characteristics, except for social status, type of home healthcare prior to index admission, and visits to the GP and out-of-hour doctor prior to the index admission. The median age was 84.3 years in the intervention group and 84.9 years in the control group. Of the intervention group, 53.7% were female and 46.3% were male, while 57.5% of the control group were female and 42.5% were male. See Table 1 for details.
Outcome data
All analyses were adjusted for the four covariates, with a statistically significant difference between the groups at baseline (see Table 1). The outcome estimates are reported in Table 2.
30-day readmission
In total, 128 patients (20.8%) in the intervention group and 119 (20.2%) in the control group were readmitted within 30 days after discharge from index admission. No difference in the risk of readmissions was found between the groups in the adjusted analysis (RR: 1.00; 95% CI: 0.80, 1.26; p = 0.994).
The sub-group analysis showed a statistically significant difference between the intervention and control groups when looking at the sodium and potassium levels. Patients with abnormal sodium and potassium levels in the intervention group had a lower risk of readmission than patients with abnormal sodium and potassium levels in the control group (RR: 0.60, 95% CI: 0.37, 0.98, and RR: 0.59, 95% CI: 0.35, 1.00, respectively). For details on sub-group analyses, see Online Resource 2.
The mortality-adjusted cumulative incidence of 30-day readmission is displayed in Fig. 2.
30-day mortality
One hundred and fifteen (18.7%) patients from the intervention group and 108 (18.3%) from the control group died within 30 days after discharge. The adjusted risk for 30-day mortality was 1% higher in the intervention group than in the control group (RR: 1.01; 95% CI 0.80, 1.29; p = 0.919).
The Kaplan–Meier survival estimates of 30-day mortality are displayed in Fig. 3.
90-day mortality
In total, 166 (27.0%) in the intervention group and 151 (25.6%) in the control group died within 90 days after discharge, resulting in a slightly higher adjusted risk of 7% (RR: 1.07; 95% CI 0.89, 1.30; p = 0.477).
The Kaplan–Meier survival estimates of 90-day mortality are displayed in Fig. 4.
Days alive and out of hospital (DAOH)
In the intervention and control groups, 646 (75.5%) and 453 (76.8%) patients, respectively, were alive and out of hospital for more than 23 days within 30 days after discharge. This corresponds to a slightly lower adjusted risk of 2% in the intervention group compared with the control group (RR: 0.98; 95% CI 0.92, 1.05; p = 0.593).
Days alive and out of hospital and GP visit (DAOHGP)
Almost similar numbers were seen when GP visits were taken into account. In other words, 433 (70.4%) of the patients in the intervention group were alive and out of hospital and had not visited the GP for more than 23 days within 30 days after discharge. The number was 434 (73.6%) for the patients in the control group. Thus, the adjusted risk was 4% lower among the intervention group compared with the control group (RR: 0.96; 95% CI 0.90, 1.03; p = 0.238).
Other analysis
The risk of 30-day readmission two years before the intervention was 20.06% in the intervention group (Hedensted, Odder and Skanderborg) and 24.92% in the control group (Horsens). The risk after the intervention was 20.81% in the intervention group and 20.17% in the control group. Hence, the risk difference was + 0.75% (SE: 2.26%; p = 0.74) in the intervention group and − 4.75% (SE: 2.36%; p = 0.044) in the control group. The risk difference between the groups was 5.51% (SE: 3.27%; p = 0.092). The graphical display of the results can be found in Online Resource 3.
Other results
No significant modifications were made to the intervention during the study period, and no adverse events were reported.
Discussion
The intervention did not have a significant impact on 30-day readmission, 30- and 90-day mortality rates, DAOH or DAOHGP, as all RRs were close to 1. The no evidence of effect is generally caused by weakness in the design, implementation failure or no intervention effect. Plausible explanations to the lack of intervention impact in this study are discussed below.
Comparison with previous research
Unfortunately, the results of no intervention impact on the outcome measures are common in the research field of TCIs. Over the last couple of decades, research has shown diverging effects on readmission and mortality among older medical patients. Some studies have reported positive impacts, while others have reported no or negative impacts [17, 18, 33,34,35,36,37]. Other studies found major implementation bottlenecks due to lack of resources, and information and organisational problems [38, 39]. This is in line with our experience with the TCI implementation. Most implementation barriers are related to information management, clinical uncertainties, sense of competences, perception of liability, expectations, standards of practice, financial disincentives and administrative constrains [40]. Some of these issues were, to different degrees, also present during our implementation.
Design
Our intervention consisted of three components and was, thus, a low-intensity intervention.
A systematic review comparing the effects of different TCIs on readmissions among older medical patients found a noticeable impact among ‘high-intensity’ interventions and in interventions with duration of 1 month or more [17]. The low-intensity and short intervention duration may explain the lack of impact on the study outcomes.
Additionally, the intervention components were delivered merely by registered nurses and a social and healthcare assistant. Previous studies have documented that interventions with an interdisciplinary team positively impact readmission rates [28, 41]. If other healthcare professions had been involved, the intervention may have had a greater impact on the outcomes.
Programme implementation
Some of the intervention components took a long time to implement in the municipalities. Most of the patients, relatives, district nurses and GPs in the three intervention municipalities did not use the seven-day telephone consultation offer in the first period of the study. The degree of usage and implementation could be assumed to have varied in and between municipalities. Additionally, the municipalities should have provided extra resources and time for the included patients to facilitate the post-discharge follow-up visits and video conferences. The video conference was a challenge for the district nurses and the involved patients. Technical difficulties, along with audio and visual challenges faced by patients, may have reduced the effect of the conference. In addition, scheduling conferences was difficult due to the high workloads among district nurses. Hence, some of the video conferences and physical visits were cancelled.
The GPs and district nurses were not thoroughly informed and instructed about the seven-day consultation offer’s purpose, benefit and usage. Thus, it was not fully implemented. This resulted in uncertainty about the post-discharge treatment responsibility among district nurses and GPs, and the consultation offer was not used as often as anticipated. This may have resulted in readmissions where it could have been prevented by communication between the GP, district nurse and the geriatrician.
Unfortunately, intervention fidelity was impossible to assess due to missing data entry. Therefore, we do not know the degree to which the intervention components were delivered as intended. If infidelity was present, it may have resulted in non-significant findings that were not due to the study design but rather to the intervention delivery [42].
The Medical Research Council’s [43] gold standard for developing a complex intervention was not applied when the intervention was adapted and modified. This could have resulted in critical aspects not being addressed. Among others, the council recommends assessing the feasibility prior to implementation to prevent problems of acceptability, compliance and delivery of the intervention [43]. If the gold standard had been followed, the abovementioned implementation challenges could have been reduced or prevented, resulting in a positive impact on outcome measures [44].
Concurrent municipality-based projects and home care services
The initiative ‘Get Home Safe’ in Horsens home care was implemented during the study period. The aims were to (1) ensure the feeling of safe transitions from the hospital to patients’ own homes or community beds and (2) prevent hospital (re)admissions. As Horsens municipality served as our study’s control group, this initiative may have reduced the number of readmissions in the control group, thus resulting in a smaller study impact. In the same period, Odder home care also had an increased focus on improving the quality of transitions between the hospital and the patients’ own homes or community beds. Finally, Hedensted home care also implemented two intermediate beds in a nursing home during the period. These beds were used as an alternative to (re)admissions. Even though the readmission rates in the intervention group seemed constant over the two years, we must consider the possibility that these projects had an impact on the study outcomes.
Additionally, the healthcare services offered differ among the municipalities. For instance, acute care teams are the first to visit discharged patients in one municipality, while a social and healthcare helper or a social and healthcare assistant conducts the first visits in another municipality. One could speculate that these differences in knowledge and professional competencies may have affected the outcome estimates.
Study population
The inclusion criteria in this study were few and broad, resulting in a rather unselected and heterogeneous population. Thus, the group comprised patients representing various of conditions, diseases and severities. This unselected patient group could lead to a null effect. A systematic review found a pronounced positive intervention impact among patients categorised as ‘high risk’ compared to unselected patients [17].
Older medical patients are a complex population characterised by severe diseases, comorbidity, polypharmacy, impaired nutritional status, reduced physical or mental functionality and limited ability to care for oneself [3]. In addition, this population has the highest utility of healthcare services compared to the general population [45]. One may argue that it is difficult to target and address the diversity of challenges and needs to prevent readmissions in this patient group.
The sub-group analysis found a weak statistical significant association between abnormal sodium and potassium levels and the risk of 30-day readmission. However, we would need further research to explain a possible causal relationship.
It is also important to remember that not all readmissions are preventable, as they are an expected part of a patient trajectory in this ageing patient group. It has previously been estimated that 23–36% of all readmissions are preventable, depending on the timespan between discharge and readmission [46, 47].
Readmission and other outcomes
The difference in readmission rates between the groups was smaller after the intervention than before. The decreased risk difference over time was attributed to the control group. The rate decreased in the control group, while it was constant over time in the intervention group. Therefore, it seems that the intervention did not impact readmissions over time. This analysis suggests that a time trend did not affect the intervention effect.
To our knowledge, DAOH has not previously been used to analyse TCI impacts in this specific population. The outcomes have primarily been used to evaluate the effects in patients with heart conditions [48,49,50,51]. Hence, directly comparing our results with previous studies is difficult.
Strengths and limitations
Our study has several strengths. Using CROSS-TRACKS’s wide range of data is a major strength. This enables detailed descriptions of the study population and a wide variety of sub-group analyses.
The heterogeneous population of general medical patients reflects a real-world population in regional hospital settings. This is also a strength, as most regional hospitals have general medical wards with a wide variety of medical conditions. This underlines the external validity. Compared to other similar studies, the sample size is rather large, which gives the study high power. Furthermore, the long study period prevented seasonal variation among the outcome measures, contributing to more valid results. Detection bias is not present in this study, as outcomes were identified through the CROSS-TRACKS register and assessed in all patients. Moreover, this study reports on DAOH and DAOHGP, which are upcoming outcomes within this research field. This adds a new perspective on the impact of a TCI.
Nevertheless, the study also has several limitations. First, the absence of frailty status and, thus, frailty sub-group analysis make it difficult to assess the intervention effect in this group. However, it would be impossible to screen the participants in the control group with a frailty tool, as they were identified through a register. However, we performed several sub-group analyses with variables included in different frailty tools (e.g. CCI, cohabiting and home healthcare prior to admission). Our sub-group analysis did not find any difference in the effect among the sub-groups.
Second, potential selection bias may threaten the internal validity, as the allocation was conducted according to the municipality of residence. In addition, the allocation process was untraditional, as one group was identified during hospitalisation and the other through a data register, thus preventing it from being a contemporary control group. This concern and possible attrition bias were handled in the analysis. The data were analysed based on the allocated intervention rather than the received intervention. Third, we conducted a difference-in-difference analysis to address the potential effect on readmission over a long study period. This showed no effect of time on readmissions in the intervention group. Fourth, performance bias may be present, as the healthcare services offered and provided were heterogeneous across the four municipalities. Fifth, the intervention fidelity was not calculated, thus making it difficult to assess whether the lack of intervention impact was affected by implementation failure. Hence, as with many similar studies [17], we could not identify essential components from unimportant ones. In addition, we did not conduct a process evaluation, which is often requested in these kinds of studies [52]. Sixth, the applicability of the results to other healthcare institutions should be carefully considered. The context, healthcare services, the financial payment schemes and patient characteristics may differ significantly nationally versus internationally. Sixth, the study’s expenses related to tablets, computers and a project-specific car are practical limitations to its applicability.
Lastly, we adjusted for covariates that were statistically significantly different between the groups in the statistical models. However, we did not have access to data on functional disability, socioeconomic status, prior falls and method of referral, which are known risk factors. This may have had a small impact on the outcome estimates.
Clinical and research implications
This study illustrates the ‘real-world’ challenges in planning, implementing and evaluating a TCI in an ever-changing setting, with a complex study population and a long study period. To address this, it is essential to plan the study thoroughly based on previous research findings and to conduct pilot tests prior to implementation to ensure feasibility. Designing an interdisciplinary intervention is also essential to achieve a greater impact. In addition, future research should register implementation fidelity to provide a clear picture of whether the effects are caused by implementation failure or success, the study design or are solely due to the intervention. Future research should also address the importance of the implementation context and consider conducting a process evaluation. Finally, we recommended to screen participants for frailty to assess the intervention effect in this patient group.
Conclusion
This modified TCI consisting of a discharge transportation, home visit, cross-sectorial video conference and seven-day telephone showed no evidence of effect. We do not know whether this was caused by implementation failure, weakness in the study design, lack of intervention effect or uncontrollable factors in the primary healthcare. The real-life implementation of this TCI was challenging and it seem crucial to be adherent the Medical Research Council’s recommendations and to evaluate the delivery of intervention components to assess the true effect of TCIs.
Data availability
All data relevant to the study are included in the article or uploaded as supplementary information.
Notes
This method of analysis was supported by the statistician Morten Frydenberg, who is one of the authors of the book An Introduction to Stata for Health Researchers, 5th Edition, Stata Press, 2022.
References
World Health Organization (2016) Transitions of care. Technical Series on Safer Primary Care. https://apps.who.int/iris/bitstream/handle/10665/252272/9789241511599-eng.pdf;jsessionid=26E25FF7F1204F9C337806BBADA984FB?sequence=1. Accessed April 2022
Danske Regioner (2022) Nationale mål: Flere områder udvikler sig positivt (National goals: more areas are developing positively). https://www.regioner.dk/media/22206/noegletal-for-nationale-maal-juni-2022-til-hjemmeside.pdf. Accessed Nov 2022.
Sundheds- og Ældreministeriet (2016)Styrket indsats for den ældre medicinske patient. National handlingsplan 2016 (Improved effort for the elderly medical atient. National action plan), Sep 2021.
KL, Danske Regioner, Finansministeriet, Økonomi- og Indenrigsministeriet, Ministeriet for Sundhed og Forebyggelse (2015) Sammenhængende indsats i sundhedsvæsenet - Synlige resultater, (Coherent effort in the Healthcare System -Visible results, 2015): Ministeriet for Sundhed og Forebyggelse; 2015. https://www.welfaretech.dk/media/2993/2015_05_18_sammenhaengende-indsats-i-sundhedsvaesnet-2015ashx.pdf. Accessed Jan 2022.
Sundhedsdatastyrelsen. Indikatorbeskrivelse. 30-dages akutte somatiske genindlæggelser. Version 1.0 (Indicator description. 30-days acute somatic readmissions. Version 1.0) 2018. https://www.esundhed.dk/-/media/Files/Publikationer/Emner/Patienter-og-sygehuse/Sundhedsaftaler/Indikator_beskriv_soma_genind_v1_0_090318.ashx?la=da&hash=DC7BB9889C6F322FF7D8A9E9A27C8BFD. Accessed Jan 2022.
Jencks SF, Williams MV, Coleman EA (2009) Rehospitalizations among patients in the medicare fee-for-service program. N Engl J Med 360(14):1418–1428. https://doi.org/10.1056/NEJMsa0803563
Mesteig M, Helbostad JL, Sletvold O, Rosstad T, Saltvedt I (2010) Unwanted incidents during transition of geriatric patients from hospital to home: a prospective observational study. BMC Health Serv Res 10:1. https://doi.org/10.1186/1472-6963-10-1
Coleman EA, Boult C (2003) Improving the quality of transitional care for persons with complex care needs. J Am Geriatr Soc 51(4):556–557. https://doi.org/10.1046/j.1532-5415.2003.51186.x
Daliri SA-O, Hugtenburg JG, Ter Riet G, van den Bemt BJF, Buurman BM, Scholte Op Reimer WJM et al (2019) The effect of a pharmacy-led transitional care program on medication-related problems post-discharge: a before-after prospective study. PLoS ONE 14(3):e0213593. https://doi.org/10.1371/journal.pone.0213593
Markle-Reid MA-O, McAiney C, Fisher K, Ganann R, Gauthier AP, Heald-Taylor G et al (2021) Effectiveness of a nurse-led hospital-to-home transitional care intervention for older adults with multimorbidity and depressive symptoms: a pragmatic randomized controlled trial. PLoS ONE. https://doi.org/10.1371/journal.pone.0254573
Rose T, Frith K, Zimmer R (2021) Transitional care following a skilled nursing facility stay: utilization of nurse practitioners to reduce readmissions in high risk older adults. Geriatr Nurs 42(6):1594–1596. https://doi.org/10.1016/j.gerinurse.2021.06.024
Nielsen LM, Maribo T, Kirkegaard H, Petersen KS, Lisby M, Oestergaard LG (2018) Effectiveness of the ‘elderly activity performance intervention’ on elderly patients’ discharge from a short-stay unit at the emergency department: a quasi-experimental trial. Clin Interv Aging 13:737–747. https://doi.org/10.2147/CIA.S162623[doi]
Liss DT, Ackermann RT, Cooper A, Finch EA, Hurt C, Lancki N et al (2019) Effects of a transitional care practice for a vulnerable population: a pragmatic, randomized comparative effectiveness trial. J Gen Intern Med 34(9):1758–1765. https://doi.org/10.1007/s11606-019-05078-4
Finlayson K, Chang AM, Courtney MD, Edwards HE, Parker AW, Hamilton K et al (2018) Transitional care interventions reduce unplanned hospital readmissions in high-risk older adults. BMC Health Serv Res 18(1):956. https://doi.org/10.1186/s12913-018-3771-9
Verhaegh KJ, MacNeil-Vroomen JL, Eslami S, Geerlings SE, de Rooij SE, Buurman BM (2014) Transitional care interventions prevent hospital readmissions for adults with chronic illnesses. Health Aff 33(9):1531–1539. https://doi.org/10.1377/hlthaff.2014.0160
Weeks LE, Macdonald M, Martin-Misener R, Helwig M, Bishop A, Iduye DF et al (2018) The impact of transitional care programs on health services utilization in community-dwelling older adults: a systematic review. JBI Database System Rev Implement Rep 16(2):345–384. https://doi.org/10.11124/JBISRIR-2017-003486
Rasmussen LF, Grode LB, Lange J, Barat I, Gregersen M (2021) Impact of transitional care interventions on hospital readmissions in older medical patients: a systematic review. BMJ Open 11(1):e040057. https://doi.org/10.1136/bmjopen-2020-040057
Lee JY, Yang YS, Cho E (2022) Transitional care from hospital to home for frail older adults: a systematic review and meta-analysis. Geriatr Nurs 43:64–76. https://doi.org/10.1016/j.gerinurse.2021.11.003
Hansen TK, Pedersen LH, Shahla S, Damsgaard EM, Bruun JM, Gregersen M (2021) Effects of a new early municipality-based versus a geriatric team-based transitional care intervention on readmission and mortality among frail older patients: a randomised controlled trial. Arch Gerontol Geriatr 97:104511. https://doi.org/10.1016/j.archger.2021.104511
Danmark Statistik. Folketal 1 januar 2017 efter tid, kommune, alder og køn (Population January 1st. 2017 after time, municipality, age and sex) 2022. https://www.statistikbanken.dk/statbank5a/default.asp?w=1536. Accessed Jan 2022.
Riis AH, Kristensen PK, Petersen MG, Ebdrup NH, Lauritsen SM, Jorgensen MJ (2020) Cohort profile: CROSS-TRACKS: a population-based open cohort across healthcare sectors in Denmark. BMJ Open 10(10):e039996. https://doi.org/10.1136/bmjopen-2020-039996
Reeves BC, Gaus W (2004) Guidelines for reporting non-randomised studies. Forsch Komplementarmed Klass Naturheilkd 11(Suppl 1):46–52. https://doi.org/10.1159/000080576
Witham MD, Stott DJ (2017) Conducting and reporting trials for older people. Age Ageing 46(6):889–894. https://doi.org/10.1093/ageing/afx153
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D et al (2014) Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 348:g1687. https://doi.org/10.1136/bmj.g1687
Hansen FR, Spedtsberg K, Schroll M (1992) Geriatric follow-up by home visits after discharge from hospital: a randomized controlled trial. Age Ageing 21(6):445–450. https://doi.org/10.1093/ageing/21.6.445
Sundhedsstyrelsen, MMT. (2009) Genindlæggelser af ældre i Danmark 2008 - Nye tal fra Sundhedsstyrelsen (Readmissions of older people i Denmark 2008 - New numbers from the Danish Health Authority), Dec 2021.
Sundheds og Ældreministeriet. Beskrivelse af indikatorer-de nationale mål (Description of indicators-the national goals) 2018. https://sum.dk/Media/637643698982390066/Beskrivelse%20af%20indikatorer%20-%20de%20nationale%20m%C3%A5l.pdf. Accessed Dec 2021.
Pedersen LH, Gregersen M, Barat I, Damsgaard EM (2016) Early geriatric follow-up after discharge reduces readmissions: a quasi-randomised controlled trial. Eur Geriatr Med 7(5):443–448. https://doi.org/10.1016/j.eurger.2016.03.009
Masnoon NA-O, Shakib S, Kalisch-Ellett L, Caughey GE (2017) What is polypharmacy? A systematic review of definitions. BMC Geriatr 17(1):230. https://doi.org/10.1186/s12877-017-0621-2
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383. https://doi.org/10.1016/0021-9681(87)90171-8
Thygesen SK, Christiansen CF, Fau-Christensen S, Christensen S, Fau-Lash TL, Tl L, Fau-Sørensen HT, Sørensen HT (2011) The predictive value of ICD-10 diagnostic coding used to assess charlson comorbidity index conditions in the population-based danish national registry of patients. BMC Med Res Methodol 11:83. https://doi.org/10.1186/1471-2288-11-83
StataCorp. (2021) Stata statistical software: release 17. StataCorp LLC, College Station, TX
O’Donnell R, Savaglio M, Skouteris H, Banaszak-Holl J, Moranl C, Morris H et al (2021) The effectiveness of transition interventions to support older patients from hospital to home: a systematic scoping review. J Appl Gerontol 40(11):1628–1636. https://doi.org/10.1177/0733464820968712
Morkisch N, Upegui-Arango LD, Cardona MI, van den Heuvel D, Rimmele M, Sieber CC et al (2020) Components of the transitional care model (TCM) to reduce readmission in geriatric patients: a systematic review. BMC Geriatr 20(1):345. https://doi.org/10.1186/s12877-020-01747-w
Le Berre M, Maimon G, Sourial N, Gueriton M, Vedel I (2017) Impact of transitional care services for chronically ill older patients: a systematic evidence review. J Am Geriatr Soc 65(7):1597–1608. https://doi.org/10.1111/jgs.14828
Allen J, Hutchinson AM, Brown R, Livingston PM (2014) Quality care outcomes following transitional care interventions for older people from hospital to home: a systematic review. BMC Health Serv Res 14:346. https://doi.org/10.1186/1472-6963-14-346
Kim H, Thyer BA (2015) Does transitional care prevent older adults from rehospitalization? A review. J Evid Inf Soc Work 12(3):261–271. https://doi.org/10.1080/15433714.2013.827140
Fakha A, Leithaus M, de Boer B, van Achterberg T, Hamers JP, Verbeek H (2022) Implementing four transitional care interventions for older adults: A retrospective collective case study. Gerontologist. https://doi.org/10.1093/geront/gnac128
McAiney C, Markle-Reid M, Ganann R, Whitmore C, Valaitis R, Urajnik DJ et al (2022) Implementation of the community assets supporting transitions (cast) transitional care intervention for older adults with multimorbidity and depressive symptoms: a qualitative descriptive study. PLoS ONE 17(8):e0271500. https://doi.org/10.1371/journal.pone.0271500
Richards DA, Hallberg IR (2015) Complex interventions in Health. Routledge, An overview of research methods, p 408
Leppin AL, Gionfriddo MR, Kessler M, Brito JP, Mair FS, Gallacher K et al (2014) Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med 174(7):1095–1107. https://doi.org/10.1001/jamainternmed.2014.1608[doi]
Horner S, Rew L, Fau-Torres R, Torres R (2006) Enhancing intervention fidelity: a means of strengthening study impact. J Spec Pediatr Nurs 11(2):80–89. https://doi.org/10.1111/j.1744-6155.2006.00050.x
Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM et al (2021) A new framework for developing and evaluating complex interventions: update of medical research council guidance. BMJ 374:n2061. https://doi.org/10.1136/bmj.n2061
Durlak JA, DuPre EP (2008) Implementation matters: a review of research on the influence of implementation on program outcomes and the factors affecting implementation. Am J Community Psychol 41(3–4):327–350. https://doi.org/10.1007/s10464-008-9165-0
Sundheds-og Ældreministeriet. (2018) Sundheds-og Ældreøkonomisk Analyse. Kontaktmønstre på tværs af sektorer blandt befolkningen, kronikere og ældre medicinske patienter (Health- and Eldereconomic Analysis. Contact patterns across sectors among the population, chronically ill and older medical patients). https://sum.dk/Media/0/4/Sundheds-og-aeldreoekonomisk-analyse-okt-2018.pdf. Accessed Nov 2021
Auerbach AD, Kripalani S, Vasilevskis EE, Sehgal N, Lindenauer PK, Metlay JP et al (2016) Preventability and causes of readmissions in a national cohort of general medicine patients. JAMA Intern Med 176(4):484–493. https://doi.org/10.1001/jamainternmed.2015.7863
Graham KL, Auerbach AD, Schnipper JL, Flanders SA, Kim CS, Robinson EJ et al (2018) Preventability of early versus late hospital readmissions in a national cohort of general medicine patients. Ann Intern Med 168(11):766–774. https://doi.org/10.7326/M17-1724
Freund Y, Cachanado M, Delannoy Q, Laribi S, Yordanov Y, Gorlicki J et al (2020) Effect of an emergency department care bundle on 30-day hospital discharge and survival among elderly patients with acute heart failure: the ELISABETH randomized clinical trial. JAMA 324(19):1948–1956. https://doi.org/10.1001/jama.2020.19378
Martín-Sánchez FJ, Parra Esquivel P, Llopis García G, González Del Castillo J, Rodríguez Adrada E, Espinosa B et al (2021) Thirty-day outcomes in frail older patients discharged home from the emergency department with acute heart failure: effects of high-risk criteria identified by the DEED FRAIL-AHF trial. Emergencias 33(3):165–173
García-Blas SA-O, Bonanad C, Fernández-Cisnal A, Sastre-Arbona C, Ruescas-Nicolau MA-OX, González D’Gregorio J et al (2021) Frailty scales for prognosis assessment of older adult patients after acute myocardial infarction. J Clin Med 10(18):4278. https://doi.org/10.3390/jcm10184278
Shah AI, Alabaster A, Dontsi M, Rana JS, Solomon MD, Krishnaswami A (2022) Comparison of coronary revascularization strategies in older adults presenting with acute coronary syndromes. J Am Geriatr Soc 70(8):2235–2245. https://doi.org/10.1111/jgs.17794
Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W et al (2015) Process evaluation of complex interventions: medical research council guidance. BMJ 350:h1258. https://doi.org/10.1136/bmj.h1258
Acknowledgements
We would like to thank the included patients, the primary healthcare in Hedensted, Odder and Skanderborg municipalities and healthcare professionals at Horsens Regional Hospital for their assistance during this study.
Funding
This work was supported by (1) the Fund for Advancement of Health Research in the Central Denmark Region (grant number N/A) and (2) The National Pool Fund ‘Continuity of care for the frail elderly’ (grant number N/A).
Author information
Authors and Affiliations
Contributions
All authors listed have contributed to the conception or design, or the acquisition, analysis or interpretation of data. The corresponding first author, LFR, drafted the work, and all co-authors critically revised the work. All authors approved the final version and are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
All authors have signed the ICMJE Disclosure Statement, and the authors have no relevant financial or non-financial interests to disclose.
Consent to participate
Informed consent was obtained from all participants included in the intervention group.
Ethical approval
Approval was obtained from the Danish Data Protection Agency (case no. 1-16-02-105-19). The CROSS-TRACKS case no. was 008-2. Approval by the Central Denmark Region Ethical Committee was not required. Patients in the intervention group could withdraw their consent at any time. The intervention did not harm any patients. All procedures in this study were performed in accordance with the 1964 Declaration of Helsinki and its later amendments.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rasmussen, L.F., Barat, I., Riis, A.H. et al. Effects of a transitional care intervention on readmission among older medical inpatients: a quasi-experimental study. Eur Geriatr Med 14, 131–144 (2023). https://doi.org/10.1007/s41999-022-00730-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41999-022-00730-5