Key summary points
Frailty appears to be an important risk factor for COVID-19-related deaths, but studies to data have important limitations. The aim of this study was to describe the relationship between COVID-19, frailty risk and mortality in older people.
AbstractSection FindingsThis study examined outcomes for all older people hospitalised with COVID-19 between March 2020 and July 2021 and showed that mortality risk was increased with higher Hospital Frailty Risk Scores.
AbstractSection MessageAny level of elevated frailty risk should be considered an important prognostic marker for older people hospitalised with COVID-19.
Abstract
Introduction
Frailty has emerged as an important construct to support clinical decision-making during the COVID-19 pandemic. However, doubts remain related to methodological limitations of published studies.
Methods
Retrospective cohort study of all people aged 75 + admitted to hospital in England between 1 March 2020 and 31 July 2021. COVID-19 and frailty risk were captured using International Classification of Disease-10 (ICD-10) diagnostic codes. We used the generalised gamma model to estimate accelerated failure time, reporting unadjusted and adjusted results.
Results
The cohort comprised 103,561 individuals, mean age 84.1, around half female, 82% were White British with a median of two comorbidities. Frailty risk was distributed approximately 20% low risk and 40% each at intermediate or high risk. In the unadjusted survival plots, 28-day mortality was almost 50% for those with an ICD-10 code of U071 (COVID-19 virus identified), and 25–35% for those with U072 (COVID-19 virus not identified). In the adjusted analysis, the accelerated failure time estimates for those with intermediate and high frailty risk were 0.63 (95% CI 0.58–0.68) and 0.67 (95% CI 0.62–0.72) fewer days alive respectively compared to those with low frailty risk with an ICD-10 diagnosis of U072 (reference category).
Conclusion
In older people with confirmed COVID-19, both intermediate and high frailty risk were associated with reduced survival compared to those with low frailty risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The COVID-19 pandemic has had a disproportionate impact upon older people, especially those with multiple comorbidities, such as chronic obstructive pulmonary disease, diabetes and dementia [1,2,3]. In addition to addressing comorbidities, an emerging feature of the clinical response has been to use the frailty construct to refine estimates of the risk of poor outcomes in the heterogeneous population of older people [4,5,6]. Frailty is a state of increased vulnerability to poor resolution of homoeostasis after a stressor event, which increases the risk of adverse outcomes in the acute care context, including delirium, disability and death [7,8,9].
Emerging evidence suggests frailty may be a predictor of poor outcomes in older people hospitalised with COVID-19 [10, 11]. However, the majority of studies that have examined the link between frailty and COVID-19 focussed upon selected samples (typically non-consecutive or convenience samples, or only those with frailty data available) of older people with COVID-19, rather than all older people admitted to hospital with suspected COVID-19 (the exception being Kundi et al. [12] from Turkey). This raises the possibility of selection bias. In addition, there were wide variations in the use of prognostically important covariates for risk adjustment: age and sex were used commonly, but comorbidities, ethnicity, socioeconomic status, previous hospitalisation, illness severity and time since the start of the pandemic (to reflect evolving treatments) were used in only a minority of reports [13]. This risks estimation bias. Moreover, many studies only captured in-hospital mortality, risking outcome bias. These limitations have important implications for clinicians using frailty as part of their ongoing COVID-19 risk assessment processes, as suggested by the National Institute of Health and Care Excellence [14].
Given these limitations with existing studies, and the potential impact upon clinical decision-making, we examined the relationship between frailty risk and 28-day mortality for all older people admitted to hospital in England and tested for COVID-19 between 2020 and 2021. To assess frailty risk, we employed the Hospital Frailty Risk Score (HFRS), which uses International Classification of Disease (ICD) codes generated following a hospital admission to estimate the risk of frailty and associated mortality. Although the HFRS is not a frailty index, it is moderately correlated with other frailty scales [15], includes ICD-10 codes that capture common frailty syndromes, such as falls, fractures, dementia and delirium, and has the advantage that it can be constructed for nearly everyone 75 years or older admitted to hospital.
The aim of this study was to examine the relationship between frailty risk and COVID-19-associated mortality using the Hospital Frailty Risk Score applied to the English population of older people admitted with COVID-19 between 2020 and 2021.
Methods
Design
This was a retrospective cohort study using the NHS England Secondary Uses Service (SUS) electronic database. A full description of the data linkage, security and processing is detailed in the study protocol [16].
The analysis used data relating to individuals’ first emergency presentation during which they received a COVID-19 test, between 1 March 2020 and 31 July 2021. Participants were included if they were 75 years or older and had an ICD-10 diagnostic code of U07.1 ‘COVID-19 virus identified’ or U07.2 ‘COVID-19 virus not identified’.
Sample
The data represent all National Health Service (NHS) patients admitted to hospital with suspected COVID-19 in England. At the end of August 2021, there were 103,561 patients over 75 years of age who had been hospitalised and who had a COVID-19 test available for whom linkage was possible. Participants were only excluded if they did not have information essential to undertake linkage. Missing data were handled using the complete case analysis approach.
Outcome variables
The primary outcome was all-cause mortality captured by the Office for National Statistics (ONS). We reported mortality at 28 days from the date of admission, consistent with NHS practice in England; individuals were censored if they were still alive at 28 days.
Explanatory variables
COVID-19 status was defined by ICD-10 codes U07.1 ‘COVID-19, virus identified’ and U07.2 ‘U07.2 COVID-19, virus not identified’ recorded in the SUS record. U07.1 is used when COVID-19 has been confirmed by laboratory testing and U07.2 when a clinical diagnosis of COVID-19 was suspected, but where laboratory confirmation is inconclusive or not available. These groups were analysed separately in case there was some systematic difference in those with vs. without positive laboratory tests. For example, it is possible that people with delirium were not tested early on in the pandemic, as it took a whilst for delirium to be widely recognised as possible presenting feature of COVID-19 [17].
Frailty risk was measured using the HFRS, which uses ICD-10 codes to assign patients to three categories of low (< 5), intermediate (5–15), and high frailty risk (> 15) [15]. The HFRS uses ICD-10 codes pertaining elective or non-elective hospital admissions to generate a frailty risk score. The HFRS uses diagnostic information in an algorithm that identifies the risk of frailty and outcomes, such as death or unplanned hospital readmissions [15]. In the national validation cohort (n = 1,013,590), compared with the 42% patients with the lowest risk scores, the 20% patients with the highest HFRSs had increased odds of 30-day mortality (odds ratio 1.71; 95% CI 1.68–1.75), long hospital stay (6.03; 5.92–6.10), and 30-day readmission (1.48; 1.46–1.50). The HFRS offers an opportunity to assess frailty risk as a case-mix characteristic; its relative ease of application makes it an ideal tool for use in national datasets to provide a population perspective. We drew on diagnostic information from the patient’s current hospital admission and data from any previous emergency admissions that occurred within two years of the index admission to construct the HFRS [18]. We were principally interested in estimating the rate of death for those with and without confirmed COVID-19 and at different HFRS levels and thus we tested for an interaction between COVID-19 status and HFRS category.
The analyses controlled for differences in individual characteristics, using a broad set of risk adjusters including age, sex, ethnicity, deprivation (Index of Multiple Deprivation (IMD) quintile), Charlson Comorbidity Index [19], number of previous admissions, number of procedures, and Ambulatory Care Sensitive Conditions (ACSCs, defined as per the NHS Digital [20].
Analyses
We used the generalised gamma model to estimate accelerated failure time as the proportional hazards assumption was violated. Estimated coefficients were reported as time ratios, illustrating an acceleration (coefficient > 1 indicating longer survival) or deceleration of survival time (coefficient < 1 indicating shorter survival). Model fit and comparison of accelerated failure time models was assessed using the Akaike Information Criterion (AIC) and Cox–Snell Residuals. Adjusted and unadjusted time ratios were used to compare the rate of death for those with and without confirmed COVID-19, at different levels of HFRS. We tested for an interaction between confirmed COVID-19 and HFRS status. All analyses were fitted in STATA version 16.
Ethical and regulatory considerations
This study was conducted according to the current revision of the Declaration of Helsinki and ICH Guidelines for Good Clinical Practice. Fulham Research Ethics Committee (part of the UK Health Regulatory Authority) reviewed the application and issued a Favourable Opinion (reference 289267). The University of Leicester acted as study sponsor (reference 0804 COVID & Frailty).
Results
Our sample comprised 103,561 observations with descriptive data summarised in Table 1.
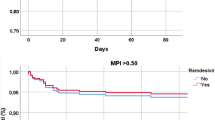
Figure 1 shows the time to death by HFRS categories, stratified by COVID ICD-10 codes. In the unadjusted survival plots, 28-day mortality was almost 50% for those with an ICD-10 code of U071, and 25–35% for those with U072.
Table 2 shows the unadjusted and adjusted results, in which lower time ratios indicate a shorter survival time. An ICD-10 diagnosis code of U071 was associated with reduced survival time, estimated in the unadjusted analysis as 12.5% (time ratio (TR):0.88 (95% CI 0.78–0.98) fewer days alive, and 16% (TR: 0.84 (95% CI 0.76–0.93)) fewer days alive in the adjusted analysis.
In the unadjusted analysis, those with an ICD-10 diagnosis code of U072 and intermediate frailty risk, had 18% (TR: 0.82 (95% CI 0.78–0.85)) fewer days alive, whilst those with high frailty risk had 11% (TR: 0.90 (95% CI 0.86–0.94)) fewer days alive compared to those with low frailty risk. In the adjusted analysis, the intermediate frailty risk appeared to have 15% (TR: 0.85 (95% CI 0.81–0.89)) fewer days alive compared to those with low frailty risk, whilst the estimated difference was not statistically significant for those with high frailty risk (TR: 0.99 (95% CI 0.95–1.03)) compared to those with low frailty risk (Fig. 2).
In the unadjusted analysis, those with either intermediate (TR: 0.62 (95% CI 0.57–0.67)) or high (TR: 0.62 (95% CI 0.58–0.67)) frailty risk with an ICD-10 diagnosis code of U071 had 38% fewer days alive, compared to those with low frailty risk with an ICD-10 diagnosis code of U072 (Fig. 2). In the adjusted analysis, the estimates for those with intermediate and high frailty risk were 37% (TR: 0.63 (95% CI 0.58–0.68)) and 33% (TR: 0.67 (95% CI 0.62–0.72)) fewer days alive compared to those with low frailty risk with an ICD-10 diagnosis of U072, respectively (Fig. 2).
As regards the influence of the adjustment factors, survival time was lower for older people, for women, for those from more deprived communities, and for people of non-White ethnicity. Survival time was higher for those who had more hospital procedures and if admitted with an Ambulatory Care Sensitive Condition (Table 2).
In view of the possible collinearities between the Charlson score and previous admissions with the HFRS, we undertook sensitivity analyses excluding these separately and in combination. The findings were robust to these sensitivity analyses; approximately 550 people had some missing IMD data. The distribution of the HFRS was approximately in keeping with previous reports [15, 21].
Figure 2 shows the time to death by HFRS categories, stratified by COVID ICD-10 codes. In the unadjusted survival plots, 28-day mortality was almost 50% for those with an ICD-10 code of U071, and 25–35% for those with U072.
Table 2 shows the unadjusted and adjusted results, in which lower time ratios indicate a shorter survival time. An ICD-10 diagnosis code of U071 was associated with reduced survival time, estimated in the unadjusted analysis as 12.5% (time ratio (TR):0.88 (95% CI 0.78–0.98) fewer days alive, and 16% (TR: 0.84 (95% CI 0.76–0.93)) fewer days alive in the adjusted analysis.
In the unadjusted analysis, those with an ICD-10 diagnosis code of U072 and intermediate frailty risk, had 18% (TR: 0.82 (95% CI 0.78–0.85)) fewer days alive, whilst those with high frailty risk had 11% (TR: 0.90 (95% CI 0.86–0.94)) fewer days alive compared to those with low frailty risk. In the adjusted analysis, the intermediate frailty risk appeared to have 15% (TR: 0.85 (95% CI 0.81–0.89)) fewer days alive compared to those with low frailty risk, whilst the estimated difference was not statistically significant for those with high frailty risk (TR: 0.99 (95% CI 0.95–1.03)) compared to those with low frailty risk (Fig. 2).
In the unadjusted analysis, those with either intermediate (TR: 0.62 (95% CI 0.57–0.67)) or high (TR: 0.62 (95% CI 0.58–0.67)) frailty risk with an ICD-10 diagnosis code of U071 had 38% fewer days alive, compared to those with low frailty risk with an ICD-10 diagnosis code of U072 (Fig. 2). In the adjusted analysis, the estimates for those with intermediate and high frailty risk were 37% (TR: 0.63 (95% CI 0.58–0.68)) and 33% (TR: 0.67 (95% CI 0.62–0.72)) fewer days alive compared to those with low frailty risk with an ICD-10 diagnosis of U072, respectively (Fig. 2).
As regards the influence of the adjustment factors, survival time was lower for older people, for women, for those from more deprived communities, and for people of non-White ethnicity. Survival time was higher for those who had more hospital procedures and if admitted with an Ambulatory Care Sensitive Condition (Table 2).
In view of the possible collinearities between the Charlson score and previous admissions with the HFRS, we undertook sensitivity analyses excluding these separately and in combination. The findings were robust to these sensitivity analyses.
Discussion
After taking account of the interaction between HFRS and U071, intermediate and high frailty risk were associated with reduced survival compared to those with low frailty risk. However, there was minimal difference between intermediate and high-risk frailty in patients with a U071 diagnosis code. Other variables associated with reduced survival included an ICD-10 diagnosis code of U071, age, female sex, increasing comorbidities and non-White British ethnicity. Those undergoing procedures or presenting with ambulatory care sensitive conditions had increased survival compared to those without.
These findings support the previous studies of selected samples of older people with COVID-19 that show frailty is associated with reduced survival in people with COVID-19 [10, 11, 22]. However, an important finding from this analysis is that there appears to be relatively little separation in terms of survival when comparing those at intermediate and high risk of frailty, which supports some previous reports [23, 24] but not all [10, 22]. This has important implications for clinical practice, in which the presence of severe frailty (broadly HFRS scores > 15) is considered to be a marker of poor outcomes, and used to guide more supportive or palliative approaches, whereas those with moderate frailty (broadly HFRS scores between 5 and 15) would usually be considered for restorative care (should that be something that they value) [14]. Ambulatory Care Sensitive Conditions were associated with better survival, which has not previously been shown—these represent conditions that are considered preventable or could be treated in the community or an outpatient setting. These data could be used to identify cohorts suitable for home-based management of COVID, for example in the COVID virtual wards.
Important strengths of this study include the capture of all people aged 75 year or more admitted to all NHS hospitals with suspected COVID-19, using Routinely Collected data (RCD). RCD collected under real-world circumstances maximises representativeness of the study population and generalisability of findings, maximises resource efficiencies and allows the capture of information in large populations with continuously collected clinical events across long time periods [25]. We included a wide range of prognostically important covariates in the analysis to isolate the influence of frailty risk on mortality. We were able to report upon all-cause mortality both in-hospital and in the community, limiting the risk of outcome bias. It is possible that not all deaths reported here are directly related to COVID-19, particularly in the latter stages of the reporting period. However, in a range of sensitivity analyses looking at the impact of time from the start of the pandemic, we did not observe and significant changes in the rate of death nor the ability of the HFRS to predict deaths. We also included a wide range of covariates in our models that have not been widely reported in other studies yet are known to be important predictors of mortality: deprivation, previous admissions, and Ambulatory Care Sensitive Conditions. As with all studies that employ routine data, there is a possibility of coding error. However, especially given the heightened sensitivity about COVID-19 diagnoses and the medical examiner scrutiny that is part of the death certification process in England, this is unlikely to be a major threat to internal validity.
The HFRS is not a frailty score or scale, but a score to identify older people in whom frailty is likely to be prevalent. In the original validation study, the HFRS showed fair overlap with dichotomised Fried and Clinical Frailty Scale (CFS) (kappa scores 0.22, 95% CI 0.15–0.30 and 0.30, 0.22–0.38, respectively) and moderate agreement with the Frailty Index (Pearson's correlation coefficient 0.41, 95% CI 0.38–0.47). This is consistent with the performance of other frailty scores, with agreement between frailty ratings tending to be fair or moderate; kappa coefficients comparing Fried to Rockwood for example range from 0.3 to 0.5, depending on the measurement approach [26]. In the context of COVID-19, other studies have found a fair correlation between the CFS and the HFRS (Spearman r = 0.34) [27]. The benefits of using the HFRS is that it can be constructed for all those admitted to hospital using up-to-date diagnostic information from the current admission and previous admissions to generate frailty risk scores [18]. However, it is possible that in individuals with comorbidities for whom this was their first presentation to hospital, frailty risk might be underestimated. This might explain some of the overlap in survival between the intermediate and high frailty risk groups.
We demonstrated an interaction between frailty and an ICD-10 code of U071 that significantly increased the rate of mortality. However, there is little difference in the rate of mortality between high and intermediate HFRS. This may be related to changes in the nature of the population presenting as a consequence of the national lockdown [28], or the availability of testing, which changed over the course of the pandemic. Indeed, we observed both changing populations presenting to acute care over the course of the pandemic (in part related to the imposition and subsequent lifting of lockdowns) and changing outcomes (perhaps related to changing treatment effects, such as the introduction of Dexamethasone [29]). Other studies using population-level, representative datasets have confirmed the dynamic nature of the population presenting during the pandemic, and changes in treatments that have an important effect [21], which has not been addressed in the majority of studies reporting upon frailty and COVID-19 outcomes.
Intermediate or high frailty risk are similarly associated with reduced survival in older people with hospitalised with COVID-19; this extends the use of the frailty construct to alert clinicians to possible adverse outcomes. Frailty is likely to be a useful addition to risk prediction when used alongside other COVID-19 specific tools (e.g. 4C score [30]). Whichever risk scoring system is used, decisions about treatment should always be individualised, and incorporate shared decision-making involving patients and their advocates [14].
Change history
09 August 2022
A Correction to this paper has been published: https://doi.org/10.1007/s41999-022-00684-8
References
Harrison SL et al (2020) Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: a federated electronic medical record analysis. PLoS Med 17(9):e1003321–e1003321
Ioannou GN et al (2020) Risk factors for hospitalization, mechanical ventilation, or death among 10,131 US veterans with SARS-CoV-2 infection. JAMA Netw Open 3(9):e2022310
Atkins JL et al (2020) Preexisting comorbidities predicting COVID-19 and mortality in the UK Biobank community cohort. J Gerontol Ser A 75(11):2224–2230
Maltese G et al (2020) Frailty and COVID-19: a systematic scoping review. J Clin Med 9(7):2106
Mitnitski AB, Song X, Rockwood K (2004) The estimation of relative fitness and frailty in community-dwelling older adults using self-report data. J Gerontol Ser A Biol Sci Med Sci 59(6):M627–M632
Rockwood K et al (2005) A global clinical measure of fitness and frailty in elderly people [see comment]. CMAJ Can Med Assoc J 173(5):489–495
Clegg A et al (2013) Frailty in elderly people. Lancet (Lond, Engl) 381:752–762
Hubbard RE et al (2017) Frailty status at admission to hospital predicts multiple adverse outcomes. Age Ageing 46:801–806
Wou F et al (2013) The predictive properties of frailty-rating scales in the acute medical unit. Age Ageing 42:776–781
Kastora S et al (2021) Clinical frailty scale as a point of care prognostic indicator of mortality in COVID-19: a systematic review and meta-analysis. EClinicalMedicine 36:100896
Cosco TD et al (2021) What is the relationship between validated frailty scores and mortality for adults with COVID-19 in acute hospital care? A systematic review. Age Ageing 9:2106
Kundi H et al (2020) The role of frailty on adverse outcomes among older patients with COVID-19. J Infect 28:28
Cosco T, Davis D, Conroy S (2020) Frailty and mortality outcomes for patients with COVID-19: a rapid systematic review and meta-analysis of hospitalised cohorts. PROSPERO 2020 [cited 2020 18/10/2020]; CRD42020200445: [Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020200445
National Institute for Clinical Excellence (2020) COVID-19 rapid guideline: critical care in adults. NICE guideline ([NG159])
Gilbert T et al (2018) Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet 391(10132):1775–1782
Konstant-Hambling R et al (2020) COVID-19 related outcomes for hospitalised older people at risk of frailty. medRxiv 2020.11.16.20232447
Zazzara MB et al (2020) Probable delirium is a presenting symptom of COVID-19 in frail, older adults: a cohort study of 322 hospitalised and 535 community-based older adults. Age Ageing 36:1
Street A et al (2021) The use of linked routine data to optimise calculation of the Hospital Frailty Risk Score on the basis of previous hospital admissions: a retrospective observational cohort study. Lancet Healthy Longevity 2(3):e154–e162
Charlson ME et al (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
NHS Digital (2020) CCG outcome indicator set: unplanned hospitalisation for chronic ambulatory care sensitive conditions. [cited 2022 29.04.2022]; Available from https://files.digital.nhs.uk/8E/57574F/CCG_2.6_I00757_S.pdf
Navaratnam AV et al (2021) Patient factors and temporal trends associated with COVID-19 in-hospital mortality in England: an observational study using administrative data. Lancet Respir Med. https://doi.org/10.2139/ssrn.3706052
Pei H et al (2021) Association of frailty status with adverse clinical outcomes in patients with COVID-19: protocol for a systematic review and dose-response meta-analysis. BMJ Open 11(5):e046980
Miles A et al (2020) Outcomes from COVID-19 across the range of frailty: excess mortality in fitter older people. medRxiv 2020.05.22.20110486
Owen RK et al (2020) Comparing associations between frailty and mortality in hospitalised older adults with or without COVID-19 infection: a retrospective observational study using electronic health records. Age Ageing 50(2):307–316
Todd OM et al (2020) New Horizons in the use of routine data for ageing research. Age Ageing 49(5):716–722
Aguayo GA et al (2017) Agreement between 35 published frailty scores in the general population. Am J Epidemiol 186:420–434
Hägg S et al (2020) Age, frailty, and comorbidity as prognostic factors for short-term outcomes in patients with coronavirus disease 2019 in geriatric care. J Am Med Directors Assoc 21(11):1555
Mak JKL et al (2021) Frailty and comorbidity in predicting community COVID-19 mortality in the UK Biobank: the effect of sampling. J Am Geriatr Soc 45:1270
Horby P et al (2021) Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 384(8):693–704
Knight SR et al (2020) Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: development and validation of the 4C Mortality Score. BMJ 370:m3339
Funding
Laia Maynou is funded by the Spanish Ministry of Science, Innovation and Universities (PID2019-104319RB-I00).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Informed consent
No informed consent was required.
Ethical approval
This study was conducted according to the current revision of the Declaration of Helsinki and ICH Guidelines for Good Clinical Practice. Fulham Research Ethics Committee (part of the UK Health Regulatory Authority) reviewed the application and issued a Favourable Opinion (reference 289267). The University of Leicester acted as study sponsor (reference 0804 COVID & Frailty).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The Funding information section was missing from this article and should have read ‘Laia Maynou is funded by the Spanish Ministry of Science, Innovation and Universities (PID2019-104319RB-I00)’.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maynou, L., Owen, R., Konstant-Hambling, R. et al. The association between frailty risk and COVID-19-associated all-mortality in hospitalised older people: a national cohort study. Eur Geriatr Med 13, 1149–1157 (2022). https://doi.org/10.1007/s41999-022-00668-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41999-022-00668-8