Abstract
Ce/Sb/Mn different borate glass systems based PbO in concentrations of 50, 35, and 15 mol%, respectively, were prepared by the melting–annealing method. Wide chemical, structural, and radiation shielding characterizations were studied before and after 120 kGy of gamma radiation to test the possible use of glasses for immobilizing radioactive wastes. The results showed suitable density values ranging from 3.34 to 5.30 g/cm3 increased by irradiation. FTIR spectra revealed high structural stability against irradiation correlated to the trigonal BO3, tetrahedral BO4 groups, high polarizable Pb2+ ions, and the doped metal ions. Unexpectedly, the chemical durability after in situ leaching process in H2O, 0.1 N HCl, and 0.1 N NaOH for ~ 3 months revealed clear improving after irradiation e.g., enhanced by ~ 25% for Ce-lead borate glass. Scanning electron microscope (SEM) images of the glass surfaces revealed more smooth and homogenous surfaces after irradiation. Shielding parameters by Monte Carlo code (MCNP5) and Phy-X/PSD software were studied, e.g., mass and linear attenuation coefficients (MAC and LAC), effective atomic number (Zeff), radiation protection efficiency (RPE%), half and tenth value layers (HVL and TVL), and heaviness%. Comparing the shielding behavior of the three glasses revealed that Ce-lead borate glass has the highest values of LAC, MAC, Zeff, heaviness%, and RPE% and the lowest values of HVL, TVL, and MFP, referring to the best shielding efficiency. The whole study indicates the desired properties of glasses as immobilizers or containers for radioactive wastes, e.g., nuclear medicine units in hospitals, especially lead borate glass doped Ce ions.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In spite of the great importance of nuclear power in many fields of life, its progressive generation and accompanied nuclear fuel cycle, produce many types of radioactive wastes that become more and more hazardous problem. Therefore, handling radioactive wastes is highly required; however, it is a very dangerous procedure because of the produced radioactive pollution and the negative effects of external exposure [1]. Some strict rules on radiation protection are applied, e.g., dose rates in nuclear waste containers below certain limits through treatment and conditioning routes such as shielding of nuclear medical constructions and facilities by sheltering radioactive materials into containers to diminish radiation exposure to medical employees during radiation therapy. During transportation and storage of radioactive materials, safe separation is also required, where many objectives need to be attained involving the prevention of radiation waste leakages [2].
Disposal of radioactive waste must fulfill many safety and regulatory charities. This process is difficult and very expensive, and the final product should undergo many activities to reduce the cost to the possible low amount [1].
One of the most efficient and highly desirable ways to deal with radioactive waste problem is the production of lightweight materials with a radiation shielding efficiency like glass [1,2,3]. Glass is one of the recent promising light materials that can be used for this purpose since the complicated structure of glass makes it one of the first candidates for immobilizing high-level radioactive wastes (HLW). Glass has a flexible structure to incorporate numerous kinds of waste elements through highly atomic bonding between the glass network and radioactive wastes [4,5,6]. The most common glasses used as nuclear waste immobilizers are borosilicate named Pamela, SON68, and WAK [7].
The bezel efficiency of nuclear waste glass can be determined by its tendency to resist the released ionizing radiation photons and prevent their passages [6]. So, some conditions should be present in this glass, such as high stability to radiation doses, mechanical reliability, thermal and radiation stability, and high chemical durability in different leaching media [6, 8]. The ability of a specific glass to shield radiation depends mainly on its host composition. The amorphous nature of glass structure facilitates its accommodation to different metal ions that provide radiation shielding efficiency. So the choice of each metal oxide and its concentration should be carefully selected. Essentially, heavy lead and bismuth oxides can be used widely in radiation shielding glasses because of their heavy mass, low field strength, and high polarizable effect to absorb radiation photons, e.g., Pb is the main component of radiation protection concrete. B2O3-based glasses are distinctive in their properties because of their cost-effectiveness and ease of preparing and shaping, as well as the tendency of boron to accommodate radiation photons. As known, the main structural borate units are the trigonal BO3 and tetrahedral BO4 units, and introducing of modifier oxide tends to convert BO3 to BO4 units by creating more non-bridging oxygens (NBO) [9]. The structural role of Pb2+ ions suggests significant changes in the glass network concerned with its local environment and coordination to form some possible building units, e.g., PbO3, PbO4, and PbO6 [10]. Lead borate glasses can be widely used in radiation protection of observers or experimenters in a wide range of healthcare, industrial environments, veterinary, and dentistry [11,12,13]. Introducing of rare earth ions or transition metal ions reinforces the radiation absorbing efficiency of the main host glasses [14,15,16,17] due to their capability to alter their coordination and dealing with the defect color centers [18,19,20,21].
Several experiments and tests should be carried out on the selected glass system to verify attenuation of gamma activity to its minimum level, e.g., the glass chemical durability in the presence of heat, pressure, radiation, different pH media, long-term stability of glass constituents, and many of the glasses shielding parameters. Different heavy metal ions provide outstanding radiation shielding properties to their host glasses because of their surprising mass attenuation coefficient related to such large atomic numbers [14].
The present work aims to prepare three different lead borate glasses based on different metal ions, Ce, Sb, and Mn ions, and study many of their chemical, structural, and shielding properties to evaluate their possible use as immobilizers or storage containers for radioactive wastes. A simulation process was used in this manner to represent the surrounding environment of radioactive wastes either by ionizing radiation or by different leaching media. For these purposes, the in situ chemical durability, structural study by FTIR, scanning surfaces by SEM, and some of the glass’s physical parameters were examined. Also, many of their shielding parameters using a NaI (Tl) detector, Phy-X/PSD software with an energy of 0.015 to 15 MeV, and simulation code MCNP5 were also examined, for example, linear attenuation coefficient (LAC in cm−1), mass attenuation coefficient (MAC in cm2/g), effective atomic number (Zeff), half-value layer (HVL), tenth-value layer (TVL), mean-free path (MFP), and radiation protection efficiency (RPE%). Finally the best glass composition candidate can be determined for immobilizing radioactive wastes.
Preparation and characterization techniques
Preparation of glasses
Three different lead borate glasses were prepared by the common melting–annealing process. Their compositions are listed in Table 1, where the following chemical materials from Sigma-Aldrich company (purity 99.9%) are used in preparing glasses: H3BO3, lead oxide (PbO), K2CO3, Na2CO3, Al2O3, CeO2, Sb2O3, and MnO2. Accurately weighed three batches according to each glass composition listed in Table 1 were diversified sensibly and ground into adequate powders and melted in an electric muffle furnace at temperature range 950–1100 °C in porcelain crucibles for 90–130 min with a cautious stirring at each 30-min time intervals for homogeneity. Then, the homogenized melts were flattened onto preheated stainless steel molds and transferred immediately to a muffle furnace regulated at 350–400 °C for annealing and eliminating stress and thermal strain residues in the glass samples. The annealing muffle was then switched off after 1 h leaving samples in, for cooling gradually and slowly to room temperature with a rate of 30 °C h−1. After that, bulk glass samples with a thickness = 2.98 ± 0.01 mm or powered samples were designed for characterization processes.
Characterization techniques
The glasses were examined via X-ray diffraction (XRD) (Shimadzu XRD-6000, Japan) in the 2θ range 4–90°. FTIR spectra of the prepared glasses were examined before and after exposing them to 120 kGy in a wavenumber range of 400–4000 cm−1 using a VERTEX 70, FT/IR-430 spectrometer type at room temperature.
The in situ chemical leaching experiments were conducted by immersing the glass samples in 150-ml beaker Falcon tubes with a flip-top cap in order to cover the whole glass sample surface with the leachant during the irradiation process (dose = 120 kGy). The leaching solutions used are dis. H2O, 0.1 N HCl, and 0.1 NaOH, and the corrosion process was continued for nearly 3 months. Weight loss% was taken at continual time intervals up to the end of the corrosion process. After particular immersion time, the samples were carefully washed with ethanol and dis. H2O then dried at 40 °C and accurately weighed three times for obtaining the weight loss percentage and recording data. During leaching tests, the temperature and humidity rate in the laboratory were nearly constants ⁓ 25 ± 1 °C and 43 ± 5% RH, respectively. To avoid effects by the volumetric differences, the relation between surface area (S) of the sample and volume (V) of the immersion solutions was kept constant as S/V = ̴ 0.023 cm−1. An Orion Research (601A) pH meter with accuracy measurements ± 0.01pH unit was used to measure the pH values of the final leaching solutions after immersing the fresh and irradiated glass samples for 3 months in the leachate at 25 ± 2 °C. Using an inductively coupled plasma (ICP) analysis, the concentrations of the released ions (mg/L) were measured for each leaching solution at the end of corrosion process (after 3 months).
Accurate and clear images of the glass surfaces before and after the in situ corrosion processes were taken using scanning electron microscope (SEM) technique, type ZEISS EVO15 and resolution 20 μm, Mag = 1.00 KX, EHT = 25 kV, Signal A = SE1, and WD = 8.30 mm. The used gamma radiation source was 60Co gamma cell (2000 Ci) with dose rate = 0.968 kGy/h at 25 ± 5 °C. Density (ρ) of the prepared glasses was calculated according to the following relation:
where (ρ) is the density of the glass specimen, a and b are the weights of the glass specimen in air and xylene, respectively, and 0.86 is the xylene density at 20 °C. Density was measured three times to confirm the accuracy of measuring with uncertainty values ± 0.021 g/cm3.
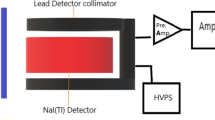
The used gamma-ray spectrometer system consists of NaI (Tl) scintillation detector (Canberra model), a 3′ × 3′ amplifier, and a 16k multichannel analyzer (MCA). During the experiment, the examined glass shields were placed between the detector and the radiation source as modeled in Fig. 1. Attenuation of the glass sample was dignified using narrow beam gamma-ray transmission geometry. Figure 1 describes the experimental geometry setup. The source was placed in a lead container with a 0.5-cm face aperture. Each sample was placed on a sample holder 5 cm away from the source. The distance between the source and the detector was retained constant at 10 cm. The detector was accurately shielded with lead to prevent the scattered radiation from nearby objects to reach the detector. The detector was covered by lead bricks and kept away from the room’s walls to protect it from secondary radiation (background, bremsstrahlung, and fluorescence). Genie 2000 software was used to record and analyze the spectra. To keep the statistical error below 1%, the detector’s actual time was fixed to 600 s for each measurement. The background counts were recorded for the same amount of time (600 s) and utilized to adjust measurements. The experimental LAC values were determined as the arithmetic mean of the experimentally observed LAC values.
The spectra obtained from MAC were analyzed using Genie software, and the gamma energies of 662, 1173, 1275, and 1333 keV were generated by radioactive point sources of 137Cs, 60Co, and 22Na (where their activity is 5 μCi), respectively.
For capturing MCNP5 simulation data, Tally F4, F4 m, F5, and F5 m were utilized. Simulations were performed with 10,000,000 histories, and all results simulated by the MCNP-5 code were reported with less than 0.1% error.
Results and discussion
Figure 2 reveals hump and amorphous peaks without any obvious crystalline peaks in the three studied samples, indicating their amorphous glassy natures, where atoms are neither systematically allied nor identical in the glass structures opposite to the ordered structure in crystals.
FTIR absorption spectra
FTIR spectroscopy is considered the most reliable tool used for analyzing the structure of crystalline or non-crystalline materials. It can be used for identifying various structural groups of the material, in addition to the structural changes carried out by different factors, e.g., composition change and irradiation process [15]. Not only FTIR defines the structure of the glass but it also explains how the various metal oxides are located and interacted with the neighboring ions inside the glass structure [14]. The essential structural building units in borate structure are the trigonal BO3 units that can be connected to form six-membered boroxol rings and tetrahedral BO4 groups. Introducing network modifier oxides, e.g., alkali ions, transition metal ions, rare earth ions, works on the rupture of B–O–B bonds to give more NBO, causing the transformation of some triangular planar sp2 BO3 units into tetrahedral sp3 BO4 groups with penta-, tetra-, tri-, and di-borate groups. This manner depends on the type and concentration of the interleaved modifier ion and its behavior with the other surrounding metal ions in the glassy network. Lead borate glass has a complex and interesting structural system as the constitutional structure of lead borate glass is expected to be different from alkali borate glasses. Unlike the alkali oxides, lead oxide (PbO) can contribute to the glass composition as both a network modifier and a network former [10].
Some authors [10] have attributed that the ratio (R) of PbO in the glass system determines its contribution to the glassy network. They assumed that lead oxide enters the glassy network originally as a modifier at 0 ≤ R ≤ 0.33 mol%. Each Pb2+ ion can act as a dual charge balance to provide the positive charge needed for the formation of two tetrahedral borate (BO4)− units. At R = 0.33, the role of cations begins to change, providing the behavior of PbO as a former oxide. Lead ions contribute with more covalent arrangements forming compact structural groups, e.g., PbO4 and/or PbO3 pyramidal units. The contribution of lead cations and their associated oxygens in the pyramidal units diminishes the accessibility of ionic charge balance, decreasing the rate of forming the four coordinated borons [10].
Figures 3, 4, and 5 depict the FTIR absorption spectra of the prepared lead borate glasses, where some common structural units in the three investigated lead borate structures can be detected.
Herein, the interpretation of the common essential structural groups in the three investigated glasses according to their FTIR absorption spectra shown in Figures 3, 4, and 5.
-
1.
A characteristic band observed in far-IR region 445–460 cm−1 centered at 445, 457, and 448 cm−1 for G1, G2, and G3, respectively. This band can be attributed to the presence of the heavy metal Pb2+ ions as building units in the glass network [13, 22].
-
2.
An observed small broad band at the region 570–610 cm-1 at 587, 608, and 577cm-1, that can be directly correlated to vibrational motions of Ce, Sb, or Mn cations in their network sites, respectively [14].
-
3.
An observed absorption band centered in the medium region at ~ 680–708 cm−1 at 687, 708, and 694 cm−1 identified for the bending vibration of triangular borate linkages (B–O–B) [23].
-
4.
High sharp bands at 940, 960, and 950 cm−1 assigned to the asymmetric stretching vibration of the B–O bonds of tetrahedral BO4 units [15].
-
5.
Quit broad bands with high intensity at ~ 1200–1400 cm−1 correlated to the stretching vibration of the trigonal BO3 units; a high peak at 1311 cm−1 for G1 and two high distinctive peaks at 1250–1380 cm−1 for G2 and 1370–1380 cm−1 for G3 correlated to vibrations of di-borate rings between B3O6-BO3 links and stretching vibration of tetra-borate or penta-borate rings [15, 24]. The obvious splitting of the band at ~ 1200–1400 cm−1 in G2 and G3 glasses can be correlated to the slight aggregation of NBO concentration, where the last separated bands are attributed to B–O–B bending vibrations in penta-borate and/or the asymmetric stretching vibrations of BO3 units with non-bridging oxygens (BØ2O−). This performance is concerned with the different doped metal ions where Sb2O3 can modify borate structure to give BO4 units in limited levels, and Mn ions as transition metal ions can easily change their coordination giving various structural units (e.g., MnO4− and MnO42−) through modifying NBO concentration, so the main BO3/ BO4 structural units appear more connected in G1 glass than in G2 and G3 glasses.
-
6.
The observed peaks in the region 1600–4000 cm−1 correlated to vibrations of H-bonding, e.g., broad bands around 2000–2300 cm−1 as well as vibrations of molecular H2O, OH, and B–OH units around 2890 and 2990 cm−1 [14, 15].
After irradiating the investigated glasses with a high dose of gamma radiation (120 kGy), the inspected FTIR spectra display almost no major changes in the vibrational bands either in their positions or intensities, revealing the highly stable structure of lead borate glasses and their compacted structures. Such connected structures are provided by the high polarizable heavy metal ions (Pb2+) and their effective role in blocking the passage of gamma photons in the glass network, in addition to the special effect of the different induced metal ions, so that an excellent shielding effect against ionizing radiation can be observed. The special effect of the introduced metal ions can be discussed according to the following points:
-
1.
Cerium (Ce) ion is a rare earth ion that has desirable properties recognized by its surface electronic configuration by sieving electrons in the outer 6s2 5d1 and 4f1 orbitals [14]. Cerium ions can occupy the glassy network in tri- and/or tetravalent states in an equilibrium redox reaction (Ce3+ ↔ Ce4+) for balancing negative charges on the adjacent tetrahedrons; however, the absolute ratio of Ce4+ can be frequently enhanced. As observed in Fig. 3, the decrease in absorbance intensity after irradiation especially in bands around 800 cm−1 indicates the formation of more bridging or connected bonds and then a more stable structure after irradiation. So, it can be predicted that a relaxation process in the glass structure may take place including the liberation of the extra stored energy and equilibrium between Ce3+ and Ce4+ giving then more relaxed structure. The high polarizability of heavy Pb2+ ions that are present in high concentration (50 mol%) and the special effect of cerium ions on absorbing radiation photons and defect color centers (e.g., commercially, CeO2 is extensively used as a decolorizing agent) produce a highly relaxed and stable glass structure with excellent radiation shielding effects.
-
2.
Sb2O3 likes PbO as a glass network intermediate oxide. Sb2O3 can modify borate structure (Sb2O3–B2O3) forming BO4 units in certain quiet levels, while the rest can form mainly stable SbO3 trigonal pyramids. A slight decrease in absorbance intensity is the clear change on FTIR spectra after irradiation as shown in Fig. 4. The presence of the two heavy massive polarizable Pb2+ and Sb3+ ions, helps to inhibit the free passage of liberated electrons caused by irradiation, leaving the main structure nearly constant after irradiation [15, 16].
-
3.
Mn2+ ions are transition metal ions that can exist in many oxidation states: Mn2+, Mn3+, Mn4+, MnO4−, and MnO42−, or a mixture of them [25]. Mn2+ ions occupy the glassy network mainly as distorted octahedral or tetrahedral units, so they can participate as network modifiers or formers depending upon their concentration and the possible redox equilibrium of Mn2+/Mn3+ in borate network [25]. As obvious in Fig. 5, only a decrease in FTIR absorbance intensity is detected after irradiation. This relatively stable behavior depends on some factors correlated to the glass composition tabulated in Table 1 such as the highly polarizable Pb ions (PbO3/PbO4), the compacted AlO4 groups, and Mn2+/Mn3+ ions that have the ability to change their valence states to absorb defect centers produced by irradiation (−ve electrons/+ve holes). Therefore, a highly compacted and stable glass structure with high efficiency to shield ionizing radiation is obtained.
The structural stability of the glasses after irradiation can be also confirmed by the obvious disappearance of B–OH vibrations near 3000 cm−1 that appeared before irradiation in the three investigated glasses as shown in Figures 3, 4, and 5. After the irradiation process, the bands at 2890 and 2990 cm−1 are completely diminished, indicating the prevention of forming more NBO and the more connected B–O–B bonds that provide the glass network connectivity and thus the structural stability of irradiated glasses against the path of radiation photons.
The whole FTIR results obtained in the three prepared glasses against irradiation with 120 kGy of gamma rays indicate the high stable and compacted glass matrices to avoid the influence of radiation and their effective radiation shielding effect.
Chemical durability
The chemical durability of glass is a very effective test used to predict the ability of the glass surface to avoid effects of different chemical agents. It is also very important to evaluate the chemical quality of different applicable glasses according to their surrounding environment. For instance, shielding glass used for immobilizing radioactive wastes should be tested under geological repository conditions [6, 26]. Therefore, three different leaching media (dis H2O, 0.1N HCl, and 0.1N NaOH) were used to study the chemical durability of the investigated glasses for ~ 3 months (90 days), during the exposure to 120 kGy of gamma irradiation. Because of the difficult process of dealing with radioactive wastes, the in situ process can be used effectively for simulating conditions, where the high radiation dose (120 kGy) and leaching media represent radiation emitted from radioactive wastes and the chemical nature of the surrounding soil, respectively.
Chemical durability in glassy systems can be described through two probable mechanisms: leaching and etching. The leaching or ion exchange mechanism takes place in the acidic or dilute aqueous solution (at low pH), involving the existence of mobile cations like Na+ or Li+ ions from the glass surface to the leaching solution or the exchange between the alkali ions (M+) from the glass with H+ or H3O+ of the attacking solution, causing the formation of an alkali-depleted layer according to the following equation:
When the formed depleted layer becomes thicker, the diffusion rate of the mobile ions slows down gradually. The second process or etching mechanism takes place at a higher pH medium, e.g., an alkaline solution, and it involves the dissolution of the glass matrix or breaking down of the total glass structure according to the following reaction [27]:
Figures 6, 7, and 8 reveal the leaching process of the three studied glasses before and after the in situ ɣ-irradiation process at 120 kGy in three leaching media 0.1N HCl, 0.1N NaOH, and dis H2O for 90 days. As is obvious, the highest corrosive solution was HCl for Ce and Sb lead borate glasses, while NaOH was more corrosive than HCl for Mn-lead borate glass. HCl solution is considered as a strong mineral acidic solution as it can be dissociated totally, producing high quantities of H+ or H3O+ ions; thus, a higher progression of leaching process can be expected [6, 17].
The obvious relative stability of glass durability at the later stages of the corrosion process can be correlated to the formation of “colloidal layers” or depleted leached layers, e.g., precipitated gelatinous hydroxides on the glass surfaces that cause the inhibition of ion exchange between alkali ions on the glass surface and ions of the attacking solution [6, 17].
Data of chemical durability behavior of the studied glasses before and after irradiation can be discussed according to the following two points:
a- Effect of glass composition
The first is related to the main composition of the glasses which is based mainly on PbO ratios: 50, 35, and 15 mol% for Ce, Sb, and Mn PbO-B2O3 glass systems, respectively. The presence of PbO in high contents with their high polarizable nature and small field strength enables the structure of glass to be more compacted. As assumed, PbO appeared in many structural units, PbO6, PbO4, or PbO3, where the low PbO content encouraged the abundance of oxygen from B2O3 to form PbO6 units, while the high PbO content encourages the formation of PbO3. Anyway, at any of Pb units, the oxygen coordination is adjusted to accomplish the PbO formula meaning that each Pb atom in PbO4 unit needs to link to four oxygen atoms and in PbO3 unit needs to link to three O atoms [28].
According to the obtained results of the in situ leaching processes before irradiation, the glasses with high Pb content reveal a more durable structure where Ce glass (50 mol% PbO) and Sb glass (35 mol% PbO) display quite higher chemical durability than Mn glass (15 mol% PbO). In addition, the presence of mobile alkali ions Na+ and/or boron in Mn glass usually increases the dissolution rate [6] and enhances the ion exchange process relatively more than in Ce and Sb glasses.
The overview effect of the glass composition that provides such high chemical durability depends on two factors: the first is the main role of Pb ions to form a strong and closed glass network, as there is a weak chance for the ion exchanging process to take place giving then more chemically durable glasses. The second is the doped metal ions Ce, Sb, or Mn that have an effective role for enhancing the glass chemical durability: (a) the rare earth Ce ions occupy the glassy network in more than one valence state through an equilibrium redox reaction between tri- and tetravalent states (Ce3+ ↔ Ce4+) to balance the negative charges in the adjacent tetrahedrons. (b) Sb ions are considered as effective intermediate ions and can affect the borate network by participating in the network structure similar to Pb2+ ions; however, Pb2+ has higher affinity to convert BO3 to BO4 units than Sb3+ in borate network; additionally, presence of both heavy massive polarizable Pb2+ and Sb3+ ions in Sb glass helps to inhibit the free passage of liberated electrons caused by irradiation, forming more relaxed structure after irradiation, and then, closed network is formed diminishing the ions leaching from the glass to leaching solution, giving lower weight loss% and higher durable glass. (c) The transition metal Mn ions participate in the glass as network modifiers in a possible redox equilibrium between Mn2+ and Mn3+ in alkali lead borate, so they can deal with the defect centers present in the network by their variable valence states.
The special effect of each doped metal ions enhances the glass connectivity and blocks the glass percolation channels, inhibiting then the passage of ions during the corrosion process.
This interpretation can be also approved by density results shown in Table 1, where the glass with higher PbO content has higher density values indicating the participation of PbO as a former oxide in the glassy network. Moreover, the replacement of the lighter boron atoms B by the heavy lead atoms Pb enhances the density of the glass structure, since density is defined mainly as the weight per unit volume and depends on the atomic weights of the participating ions and how firmly they attach to each other’s in the glassy network [14,15,16,17].
b-Effect of irradiation
The second is correlated to the unexpected effect of irradiation on improving the durability of the glasses contrary to its usual behavior of creating more NBO and giving less durable glasses. As shown in Figs. 6, 7, and 8, a great improvement in the chemical durability of the glasses has obviously obtained after the in situ corrosion at 120 kGy. This behavior may be correlated to the possible rearrangements of bonds that enhance relaxation of the glass structure. This behavior helps in releasing the extra energy stored in the network plus the disordered nature of the glass structure that assists this process [15]. As obviously shown from the figures, the chemical durability of the investigated Ce, Sb, and Mn glasses is enhanced by a rate of ~ 25, 4, 6–10%, respectively, correlating to Pb content in each glass composition and the dopant ions as shown in Table 1. Accordingly, Ce glass without mobile alkali ions reveals higher chemical improvement after irradiation than Mn glass with lower Pb content, mobile Na+ ions, and Sb glass with leachable K+ ions.
The special effect of each doped metal ion works to improve the desired chemical durability of the glasses by dealing with the defects that already present in the glassy network and healing them to strengthen the network connectivity and retard ion exchanging mechanism.
Improving chemical durability by the in situ irradiation process till the end of examined corrosion time (~ 90 days) can be directly correlated to FTIR spectra that approve the glass connectivity after irradiation as well as density results shown in Table 1 that reveal enhancing behavior after irradiation. Irradiation tends to make some modifications in the glass structures by absorbing radiation photons, so more compacted or closed systems can be obtained giving slightly higher density values (see Table 1), furthermore making blocking of percolation channels responsible for ion exchanging. Therefore, glasses with higher chemical durability or promising chemical properties are obtained, serving the present application as glass immobilizers or containers for radioactive wastes.
Table 2 displayed the pH values of the final leaching solution after immersing samples for 3 months in the leachate. By following the change in pH values, an obvious decrease in pH values can be noticed after the in situ radiation process. This indicates the inhibition of leaching ions from the glass by irradiation, where pH values of the leaching solution increase with the increase of the leaching ion concentrations. Table 3 shows the concentration of the released ions (mg/L) in the leaching solutions at the end of corrosion process (after ⁓ 3 months). As is obvious, the released ions depend on the concentration of elements in each glass composition (Table 1) and rate of leaching (Figs. 6, 7, and 8). The noticeable absences of releasing Pb2+ and Al3+ ions refer to their participations in the network forming positions and the blocking of ion exchanging or leaching process.
Characterizing of the glass surface morphology by scanning electron microscopy (SEM) is an important parameter to identify the leached surface of the glass and make a comparison study before and after the in situ leaching and irradiation processes. Figure 9 displays the leached glass surfaces before and after the in situ corrosion and irradiation (120 kGy) in the same leaching medium (dis. H2O). As shown, there is an agreement with the corrosion results shown in Figs. 6, 7, and 8. Before irradiation, the glass surfaces appear slightly rough with some non-uniformities because of forming some cavities and precipitated leached layers and/or boron-rich layers holding the hydrated micro-pores [6, 17]. On the other side, the irradiated glass surfaces reveal more smoothness and homogeneity, indicating the positive role of the irradiation process in improving the chemical durability of the investigated lead borate glasses.
Interpretation of radiation shielding parameters
LAC is a useful parameter used to determine the gamma-ray attenuation shielding of the glass samples. Figure 10 displays the LAC values of the glass samples experimentally, simulating by MCNP5, and theoretically by Phy-X/PSD software with photon energies of 662, 1173, 1275, and 1333 keV. According to Fig. 10, it is obvious that the values of LAC decrease with increasing photon energy. Due to the obvious differences in densities of the glasses as shown in Table 1, LAC values are also variable. Comparing the determined values of LAC obtains very good agreement between the results obtained by Monte Carlo simulation (MCNP5) and Phy-X/PSD software. The purpose of this comparison is to verify the results of simulation MCNP5 and Phy-X/PSD software program, where the last program is important for determining other parameters and the simulated code for calculating the exposure dose rate.
Figure 11 shows the MAC values for the energy range 0.015–15 MeV using the Phy-X/PSD program. Among the examined glasses, sample G1 (Ce glass) has the greatest MAC value of 75 cm2/g at 0.015 MeV. However, as the energy increases, the MAC value drops to 0.043 cm2/g. Because of the dominant photoelectric absorption edges at about 100 keV for all glass composites with various concentrations of PbO, MAC appears very high and lessens after these edges [29]. By determining these values, the shielding ability of different PbO glasses can be detected, and the range of photoelectric effects responsible for the attenuation can be determined, e.g., gamma or X-rays that are central in medical X-ray machines, particularly for diagnostic purposes. With increasing photon energy, the probability of the photoelectric effect (PE) drops, with the advantage of medium-energy Compton scattering (CS). Additionally, the cross-section decreases with the increase in photon energy, and the pair production process (PP) exhibits an evident dominance in the upper energy domain (beyond 1.2 MeV) [30].
Because of the highest PbO content in G1 (50 mol%), it displays the greatest values of MAC at all energies, compared to the other two glass samples with lower PbO content G2 and G3. Attenuation essentially depends on concentration, the density of induced elements, and the energy of the incident photons [31]. Different heavy metal ions provide outstanding radiation shielding properties for their host glasses because of the surprising mass attenuation coefficient related to their large atomic numbers [14].
Z eff or effective atomic number is another important parameter that aids in determining the attenuation capabilities of materials. Materials with high Zeff values offer great radiation-protection capability in several studies [32]. The Zeff values of all examined glasses in the energy range 0.05–15 MeV are shown in Fig. 12. It is clearly obvious that photon energy and variations in the glass chemical composition control Zeff values [32]. Larger Zeff values are observed at the lowest energy areas, where the photoelectric effect predominates in the three studied glasses. In contrast, due to the Compton scattering command, minimum values are recorded in the medium-energy region, while the slight increase in Zeff at higher energies can be correlated to the impact of the pair production phenomenon. In the specified photon energy range, it is observed that G3 glass with the lowest PbO content (15 mol%) has the lowest Zeff; however, G1 glass with the highest PbO content (50 mol%) has the greatest Zeff values, as shown in Fig. 12.
The half-value layer (HVL) and tenth-value layer (TVL) are common factors used for radiation protection studies [33] and can be evaluated using Phy-X/PSD software. The lower the HVL, the more space is saved and the better the radiation shielding effectiveness [34]. Figure 13 plots the HVL and TVL of the examined glasses at selected energies where HVL and TVL values follow the order of G1 < G2 < G3. In other words, HVL and TVL reveal their lowest values for G1 glass, referring to the best shielding ability; however, G3 glass reveals the highest HVL and TVL referring to the least desirable shielding ability. As is obvious, HVL and TVL increase with increasing energy where HVL of G1 increases from 0.02 to 1.2, 1.8, 1.9, and 3.1 cm and TVL increases from 0.06 to 3.9, 6.0, 6.5, and 10.3 cm for energies of 0.04, 0.662, 1.173, 1.333, and 15 MeV, respectively. This trend of progressive increase with energy occurs because the high-energy photons tend to collide with atoms in the material less frequently and pass more through the glass matrix, causing an increase in HVL and TVL values. So the investigated glasses would have their highest gamma protection efficiency at lower energies. Additionally, the concentration of PbO significantly influences the HVL and TVL values, where G1 (50% PbO) has the lowest thickness required for HVL and TVL over all the incident gamma energies. At energy of 662 keV, the HVL and TVL values of G1 decrease by 26.5% and 49.5% compared to G2 and G3, respectively.
Figure 14 displays the mean-free path (MFP) of the studied glasses with the specified photon energy (0.04 to 15 MeV). For G1 glass, the MFP values range from 0.025 to 4.45 cm; for G2, they range from 0.031 to 6.05 cm and for G3 from 0.12 to 11.59 cm. As is obvious from Fig. 14, the lowest MFP values occur at the lowest energies and increase gradually with the photon energy increase. This ascending trend occurs because the high-energy radiation can easily penetrate the incident matter. At higher energies, the predominance of the photoelectric effect is diminished compared to Compton interactions [35], because Compton interactions are weakly dependent on energy (E) and atomic numbers, and it happens only between the incoming photons and the outer shell electrons of the glass network atoms. This change causes a reduction in photon attenuation. As is obvious, G1 has the lowest MFP at all energies; however, G3 has the highest ones, e.g., at 0.662 MeV, the MFP for G1 is 1.7 cm, for G2, it equals 2.3 cm, and for G3, it equals 2.4 cm. At the energy of 1.173 MeV, the MFPs of G1, G2, and G3 are 2.6, 3.5, and 4.7 cm, and at 1.333 MeV, the MFPs are 2.8, 3.7, and 5.0 cm, respectively. Also, HVL and TVL influence the MFP values as they all depend significantly on the concentration of PbO content.
These results are also directly related to the density of each glass composition because photons interact more with atoms of the denser material, causing an increase in attenuation. In other words, increasing the density of the glass causes an increase in the chance of interaction between the incident radiation and the glass shield.
Radiation protection efficiency (RPE%) is one of the most important properties used to indicate the ability of the material to protect radiation. Figure 15 shows the RPE% of the studied glasses at an energy range of 0.4–3 MeV by the simulated code. For all the tested energies, RPE% decreases with energy increase [12]. For example, with increasing photon energy from 0.04 MeV to 3 MeV, the RPE% decreases from 63 to 22% for G1, from 50 to 17% for G2, and from 34 to 13% for G3. Moreover, the values of RPE% at 662 keV increase by 9.4% for G1 more than G2 and by 18.8% for G1 more than G3. When RPE% is evaluated with a single energy, it gives the highest value for G1, which is more effective against low-energy photons because it contains the highest PbO content. So, G1 is ideal for radiation shielding applications.
In practical applications, it is worthwhile to compare the protective properties of the prepared glasses with those of some standard radiation shields. Table 4 shows a typical comparison of key shielding parameters including HVL, MFP, MAC, and Zeff for the investigated glasses and some other shielding materials. The results reveal that the prepared glasses have a high shielding efficiency which is notable compared to other shielding materials.
To verify the heaviness% of the glass samples, lead is assumed as standard and normalized to 100%. With reference to lead, the heaviness% of the studied glasses can be evaluated as shown in Fig. 16. As obvious, the investigated glasses appear lighter than lead with the following order of heaviness%: Pb > G1 > G2 > G3.
Conclusion
Ce/Sb/Mn lead borate glasses reveal many interesting structural, chemical, and shielding properties against the effect of gamma radiation. The obtained data can be concluded according to the following points:
-
1.
FTIR absorption spectra show main absorption bands before 500 cm−1 attributed to vibration of Pb2+ ions as building units in the glass network, bands in the range 570–610 cm−1 correlated to vibrational motions of Ce, Sb, or Mn cations in their network sites, and the main bands of borate at 800–1200 cm−1 correlated to vibration of tetrahedral BO4 units as well as bands at 1200–1400 cm−1 correlated to the stretching vibration of the trigonal BO3 units.
The overall FTIR spectra of the glasses before and after irradiation reveal the stability of vibrational bands for the main constitutional structural units of borate, BO3 and BO4, indicating the formation of more bridging bonds due to the highly polarizable Pb2+ ions and their small field strength, in addition to the own behavior of each glass composition as follows:
-
(a)
In Ce-lead borate glass, the presence of rare earth cerium ions that have a tendency to absorb radiation photons (absorbing glass defect color centers) gives glass a highly stable structure.
-
(b)
In Sb-lead borate glass, the heavy massive Sb3+ ions that delay the free passage of liberated electrons formed by irradiation inhibit the formation of more induced defects.
-
(c)
In Mn-lead borate glass, presence of the compacted AlO4 groups strengthens the glass rigidity and the presence of Mn2+ ions as transition metal ions work to change their coordination number and absorb the formed defect centers by irradiation (−ve electrons/+ve holes).
The disappearance of B–OH vibration bands near 3000 cm−1 (2890 and 2990 cm−1) after irradiation, indicates the inhibition of forming more NBO and the high connected B–O–B bonds that provide the glass network connectivity. The three glasses reveal vibrational bands that are almost constant either in their positions or intensities, approving their high structural stability towards 120 kGy of gamma irradiation. Additionally, the higher the Pb content, the more shielded the glass, where PbO participates as a glass former oxide supporting the glass structure compactness in the order of Ce glass (50 mol% PbO) > Sb glass (35 mol% PbO) > Mn glass (15 mol% PbO).
-
2.
The chemical durability of the three glasses reveals excellent and surprising performance during the in situ corrosion process in H2O, 0.1 N HCl, and 0.1 N NaOH for ~ 3 months at 120 kGy of gamma radiation. This performance is correlated to the possible modifications by irradiation and relaxation of the glass structures, as well as the highly absorbing rates of radiation photons either by the role of polarizable Pb2+ ions or because of the effect of induced ions. So, more compacted glassy systems with higher density values and blocked percolation channels (where the ion exchange process takes place) are obtained, referring to the enhancement of chemical durability of the glasses after the in situ radiation and corrosion processes, e.g., the chemical durability of Ce-lead borate glass is enhanced by ~ 25% after irradiation as cleared by SEM images of the glass surfaces.
-
3.
The studied shielding parameters of the three investigated glasses such as LAC, MAC, HVL, TVL, MFP, Zeff, RPE%, and heaviness% reveal many promising radiation shielding results especially Ce glass which gives the highest LAC, MAC, Zeff, heaviness%, and RPE% but gives the lowest values of HVL, TVL, and MFP referring to the best shielding efficiency. At energy of 662 keV, RPE% values increase by 9.4% for G1 more than G2 and by 18.8% for G1 more than G3, while HVL and TVL values of G1 decrease by 26.5% and 49.5% compared to G2 and G3, respectively.
The outstanding structural stability and high radiation shielding efficiency, in addition to the surprising chemical durability in the simulated corroded environments (at different pH and 120 kGy of ɣ rays), recommend all the promising and safe usage of the studied glasses as immobilizers or containers for radioactive wastes, especially from nuclear medicine units in hospitals or other facilities.
References
Saleh, H.M., Bondouk, I.I., Salama, E., Esawii, H.A.: Consistency and shielding efficiency of cement-bitumen composite for use as gamma-radiation shielding material. Prog. Nucl. Energy. 137, 764 (2021)
Craeye, B., De Schutter, G., Vuye, C., Gerardy, I.: Cement-waste interactions: hardening self-compacting mortar exposed to gamma radiation. Prog. Nucl. Energy. 83, 212–219 (2015)
Beden, S.J., Abd, S.M., Halboot, A., Shams, F., Ahmed, H., Hassan, J.: Hypothetical method for gamma dose rate assessment to conditioned radioactive waste container. Iraqi J. Sci. 59, 476–481 (2018)
Donald, I.W., Metcalfe, B.L., Taylor, R.N.J.: Review: the immobilization of high level radioactive waste using ceramics and glasses. Mater. Sci. 32, 5851–5887 (1997)
Compton, K.L., Bennert, D.M., Bickford, D.F.: Regulatory issues in vitrification research: a case study of circuit board reclamation. Am. Ceram. Soc. 39, 3–12 (1993)
Abou Hussein, E.M.: Vitrified municipal waste for the immobilization of radioactive waste: preparation and characterization of borosilicate glasses modified with metal oxides. Silicon. 11(6), 2675–2688 (2019)
Roth, G., Weisenburger, S.: Vitrification of high-level waste: glass chemistry, process chemistry and process technology. Nucl. Eng. Des. 202, 197–207 (2000)
El-Alaily, N.A., Abou-Hussein, E.M., Abdel-Monem, Y.K., Abd Elaziz, T.D., Ezz-Eldin, F.M.: Vitrified municipal waste as a host form for high-level nuclear waste. J. Radioanal. Nucl. Chem. 299, 65–73 (2014)
Abouhaswa, A.S., Rammah, Y.S., Ibrahim, S.E., El Mallawany, R.: Optical and electrical properties of lead borate glasses. J. Electron. Mater. 48, 5624–5631 (2019)
ElBatal, H.A., Abdelghany, A.M., Ali, I.S.: Optical and FTIR studies of CuO-doped lead borate glasses and effect of gamma irradiation. J Non-Crys Solids. 358, 820–825 (2012)
Schwarz, C.M., Kang, M., Altemose, Q., Raichle, K., Schnable, B., Grabill, C., Rice, J., Truman, M., Pantano, C., Mingareev, I., Sisken, L., Rivero-Baleine, C., Richardson, A., Kuebler, M.: Processing and properties of novel ZnO-Bi2O3-B2O3 glass-ceramic nanocomposites. J. Alloys Compd. 820, 153173 (2020)
Abou Hussein, E.M., Madbouly, A.M., Ezz Eldin, F.M., ElAlaily, N.A.: Evaluation of physical and radiation shielding properties of Bi2O3–B2O3 glass doped transition metals ions. Mater. Chem. Phys. 261, 124212 (2021)
Marzouk, M.A., Abou Hussein, E.M.: Induced defects by gamma irradiation doses on the structure and optical behavior of undoped and TiO2-, Cr2O3, or MnO-doped heavy metal borate glasses. Appl. Phys. A Mater. Sci. Process. 125, 140 (2019)
Abdel Maksoud, M.I.A., Abou Hussein, E.M., Kassem, S.M., Fahim, R.A., Awed, A.S.: Effect of CeO2 addition on structural, optical, and radiation shielding properties of B2O3–Na2O–SrO glass system. Mater. Sci. Mater. Electron. 32(14), 18931–18950 (2021)
Abou Hussein, E.M., Abdel Maksoud, M.I.A., Fahim, R.A., Awed, A.S.: Unveiling the gamma irradiation effects on linear and nonlinear optical properties of CeO2–Na2O–SrO–B2O3 glass. Opt. Mater. 114, 111007 (2021)
El Batal, H.A., Abou Hussein, E.M., El Alaily, N.A., EzzEldin, F.M.: Effect of different 3d transition metal oxides on some physical properties of γ-irradiated Bi2O3- B2O3 glasses: a comparative study. J. Non-Cryst. Solids. 528, 119733 (2020)
Abou Hussein, E.M., Madbouly, A.M., El Alaily, N.A.: Gamma ray interaction of optical, chemical, physical behavior of bismuth silicate glasses and their radiation shielding proficiency using Phy-X/PSD program. Non-Cryst. Solids. 570, 121021 (2021)
Sayyed, M.I., Al-Hadeethi, Y., AlShammari, M.M., Ahmed, M., Al-Heniti, S.H., Rammah, Y.S.: Physical, optical and gamma radiation shielding competence of newly boro-tellurite based glasses: TeO2–B2O3–ZnO–Li2O3–Bi2O3. Ceram. Int. 47, 611–618 (2021)
Abou Hussein, E.M., Barakat, M.A.Y.: Structural, physical and ultrasonic studies on bismuth borate glasses modified with Fe2O3 as promising radiation shielding materials. Mater. Chem. Phys. 290, 126606 (2022)
Abou Hussein, E.M., Abdel-Galil, A.: Synthesis, optical, chemical and electrical characterizations of γ-irradiated transition metal ions reinforced borate glasses. J Non-Crys Solids. 610, 122302 (2023)
Abou Hussein, E.M., El-Agawany, F.I., Rammah, Y.S.: CuO reinforced lithium-borate glasses: fabrication, structure, physical properties, and ionizing radiation shielding competence. J. Aust. Ceram. 58, 157–169 (2022)
Wen, H., Tanner, P.A., Cheng, B.: Optical properties of 3dN transition metal ion-doped lead borate glasses. Mat. Sci. Mat. Res. Bull. (2016). https://doi.org/10.1016/J.MATERRESBULL.2016.06.032
Gao, G., Hu, L., Fan, H., Wang, G., Li, K., Feng, S., Fan, S., Chen, H.: Effect of Bi2O3 on physical, optical and structural properties of boron silicon bismuthate glasses. Opt. Mater. 32, 159–163 (2009)
Ahmad, Z., Ali, S., Ahmad, H., Hayat, K., Iqbal, Y., Zulfiqar, S., Zaman, F., Rooh, G., Kaewkhao, J.: RADIO-OPTICAL response of cerium-doped lithium gadolinium bismuth borate glasses. J. Lumin. 224, 117341 (2020). https://doi.org/10.1016/j.jlumin.2020.117341
Wen, H., Tanner, P.A.: Optical properties of 3d transition metal ion-doped sodium borosilicate glass. J. Alloys Compd. (2014). https://doi.org/10.1016/j.jallcom.2014.11.094
De Echave, T., Tribet, M., Jollivet, P., Marques, C., Gin, S., Jégou, C.: Effect of clayey groundwater on the dissolution rate of SON68 simulated nuclear waste glass at 70 °C. Nucl. Mater. 503, 279–289 (2018)
Clark, D.E., Pantano, C.G., Hench, L.L.: Corrosion of glass. Books for industry, New York (1979)
Doweidar, H., El-Egili, K., Ramadan, R., Al-Zaibani, M.: Structural investigation and properties of Sb2O3–PbO– B2O3 glasses. J. Non-Cryst. Solids. https://doi.org/10.1016/j.jnoncrysol.2018.01.025
Abou-Laila, M.T., EL-Zayat, M.M., Madbouly, A.M., Abdel-Hakim, A.: Gamma irradiation effects on styrene butadiene rubber/Pb3O4: mechanical, thermal, electrical investigations and shielding parameter measurements. Radiat. Phys. Chem. 192, 109897 (2022)
Sathish, K.V., Manjunatha, H.C., Seenappa, L., Sridhar, K.N., Nagaraj, N., Alfred Cecil, S., Raj.: Specific absorbed fraction of energy of silicon-boron alloys. Indian J. Pure. Appl. Phys. 58, 213–217 (2020)
Mridula Dogra, K.J., Singh, K.K., Anand, V., Kaur, P.: Gamma ray shielding and structural properties of Bi2O3-B2O3-Na2WO4 glass system. Universal Phys. App. 11, 190–195 (2017)
Mengge, D., Xiangxin, X., He, Y., Zhefu, L.: Highly cost-effective shielding composite made from vanadium slag and boron-rich slag and its properties. Radiat. Phys. Chem. 141, 239–244 (2017)
Dong, M., Xue, X., Yang, H., Liu, D., Wang, C., Li, Z.: A novel comprehensive utilization of vanadium slag: as gamma ray shielding material. J. Hazard. Mater. 318, 751–757 (2016)
Sayyed, M.I., Albarzan, B., Almuqrin, A.H., El-Khatib, A.M., Kumar, A., Tishkevich, D.I., Trukhanov, A.V., Elsafi, M.: Experimental and theoretical study of radiation shielding features of CaO-K2O-Na2O-P2O5 glass systems. Materials. 14, 3772 (2021)
Mhareb, M.H.A.: Physical, optical and shielding features of Li2O–B2O3–MgO–Er2O3 glasses co-doped of Sm2O3. Appl. Phys. A Mater. Sci. Process. 126, 71 (2020)
Almatari, M., Issa, S.A.M., Dong, M.G., Sayyed, M.I., Ayad, R.: Comparison between MCNP5, Geant4 and experimental data for gamma rays attenuation of PbO–BaO–B2O3 glasses. Heliyon. 5, e02364 (2019)
Salama, E., Maher, A., Youssef, G.M.: Gamma radiation and neutron shielding properties of transparent alkali borosilicate glass containing lead. J. Phys. Chem. Solids. 131, 139–147 (2019)
Ehab, M., Salama, E., Ashour, A., Attallah, M., Saleh, H.M.: Optical properties and gamma radiation shielding capability of transparent barium borosilicate glass composite. Sustainability. 14, 13298 (2022)
Humaid, M., Asad, J., Aboalatta, A., Shaat, S.K.K., Musleh, H., Ramadan, K., Alajerami, Y., Aldahoudi, N.: Gamma and neutron shielding properties of lead-borosilicate shielded glass; novel technique of solid waste recycling. Constr. Build. Mater. 375, 130896 (2023)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
E. M. Abou Hussein: conceptualization, methodology, software, visualization, data curation, writing—original draft, visualization, investigation, supervision, and writing—review and editing; A. M. Madbouly: methodology, software, visualization, data curation, and writing and editing
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. Ce/Sb/Mn lead borate glasses display stable structures against 120 kGy of ɣ rays.
2. Unexpectedly, improving of glasses chemical durability in simulated corroding environments for 3 months.
3. Polarizable Pb2+ ions and Ce/Sb or Mn ions provide the compacted glassy structures against irradiation.
4. MCNP5 and Phy-X/PSD software approve the glass shielding efficiency.
5. Promising usage of glasses is recommended as immobilizers or containers for radioactive wastes.
6. Ce glass exhibits the most favorable radiation shielding properties.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hussein, E.M.A., Madbouly, A.M. Chemical and radiation shielding effectiveness of some heavy metal oxide glasses for immobilizing radioactive wastes. J Aust Ceram Soc 60, 127–142 (2024). https://doi.org/10.1007/s41779-023-00951-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-023-00951-2