Abstract
Based on municipal solid waste (MSW) ash as a main raw material, three novel borosilicate glasses with the composition of 70 waste + 20 borax + 10 Na2O + x ZrO2, where x = 0, 0.1 or 0.3 (wt. %),were prepared by the traditional melting-annealing technique. The prepared glasses were analyzed by EDX analysis, revealing the rich compositions of the prepared glasses correlated to the MSW ash used by 70 wt.% in preparing glasses. Some optical, chemical and radiation shielding properties of the prepared glasses were investigated. Either Zr addition or 80 kGy of gamma radiation revealed improvement of the glasses optical transmittance and chemical durability in neutral dis H2O, alkaline 0.1 N NaOH and acidic 0.1 N HCl leaching media for 70 days. Electron spin resonance (ESR) revealed the same spectra before and after irradiation, referring to the prevention of free radical formation by irradiation.
The shielding parameters were measured by the experimental gamma spectroscopy (NaI detector) and the theoretical Phy-X/PSD software e.g., linear attenuation coefficients (LAC) and the findings revealed high unanimity among them at photon energies 0.662, 1.173 and 1.333 MeV. Another shielding parameters were also studied e.g., mass attenuation coefficients (MAC), effective atomic number (Zeff), effective electron density (Neff) and effective conductivity (Ceff). Presence of various metal oxides and the host trigonal BO3 and tetrahedral BO4 and SiO4 units, and ZrO2 provide the glasses compactness and effectual stability against ionizing irradiation. The prepared borosilicate glasses have highly strong and compacted structures that can inhibit the passage of radiation photons, because of the variety of many glass network formers, intermediates and modifiers present in the used waste ash. The results indicate the highly economic benefit of the prepared glasses, where the useless MSW ash are used mainly by 70 wt.% to produce effective borosilicate glass systems for promising radiation shielding purposes, especially 0.3 Zr borosilicate glass that has the best radiation shielding properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
One of the most popular problems in countries is the accumulation of municipal solid wastes (MSWs) especially countries with high populations, because only about 40% of the waste total volume can be recycled (Abou Hussein 2019; El-Alaily et al. 2018a). So, managing municipal solid wastes (MSWs) becomes extremely urgent and important challenge at least to diminish their total accumulated volume and get rid of the resultant pollution. In developing countries, landfilling is the lowest-cost and familiar way used to deal with MSWs in spite of pollution and toxic materials getting behind in air, in soil and in ground water (Park and Heo 2002). On the other side, incineration is the common method followed by most European countries for lessening the waste volume as well as recovering thermal energy (Alexander et al. 2003). Either landfilling or incineration methods leave residue ashes behind them signifying another environmental problem by the polluting or useless accumulated materials (Alexander et al. 2003). Some ways can be used to deal with these useless waste ashes e.g. vitrification and cementation. They represent highly useful and commercial methods to use the useless polluting ashes for producing new industrial and beneficial products. Vitrification process is an efficient way including fabrication of commercial and applicable glasses or glass ceramics (Abou Hussein 2019; Barbieri et al. 2000; Maximina and Jesus 1999) such as nuclear waste glass that is used to immobilize radioactive wastes e.g. SON68, WAK, Pamela and calcium-borosilicate glass–ceramics (Abou Hussein 2019; El-Alaily et al. 2018a, 2014; Roth and Weisenburger 2000; Kim and Heo 2015). Because of the presence of several metal oxides in MSW ashes, many glass forming metal oxides are present specially SiO2 and B2O3, so borosilicate glass system can be obtained. Borosilicate glassy network has a complex structure containing trigonal BO3 and tetrahedral SiO4 and BO4 groups. This specific structure acquires borosilicate glass many desirable properties e.g. precise optical and mechanical properties, high chemical durability, thermal stability, and good radiation shielding ability (Abou Hussein 2019).
Recently, ionizing radiation has linked directly to a number of biological and industrial negative impacts such as the negative impacts on human body and living organisms as well as the failure of several electronic functions in aeronautical, industrial and medical devices. Hence, shield ionizing radiation to avoid its harmful effects is highly needed. Glasses used for radiation shielding purposes have remarkable tension because of their significant importance in many industrial and medical institutions deal with radiation (such as radiation cancer therapy units, dentistry and veterinary), in order to protect specialists and patients from the harmful radiation dangers (Abou Hussein et al. 2021a, b, c, 2022; Wagh et al. 2017; Alrowaili et al. 2021, 2023; Alzahrani et al. 2022; Abou Hussein and Madbouly 2023). Depending upon the rich composition of MSW ashes by containing many glass forming, intermediate and modifying ions, they can be used to produce borosilicate glass systems with promising radiation shielding features. For instance, Al3+ ions can form strong covalent bonds of tetrahedral and/or octahedral; AlO4 and AlO6 structural units that enhance radiation shielding competence of the prepared glasses (Abou Hussein 2019; Rupesh Kumar et al. 2013). Additionally the modifying Ca2+ and Ba2+ ions develop some of the glasses mechanical and chemical properties e.g. microhardness and chemical durability (Abou Hussein 2019; El-Alaily et al. 2018b). Ti4+, Cu2+, Zn2+ and Fe3+ ions as transition metal (TM) ions, can also provide the glasses shielding efficiency by changing their outer valence sates and healing defects caused by irradiation like negative electron defects and/or positive hole trapping sites (Abou Hussein et al. 2021b, d; Batal et al. 2020; Marzouk and Abou Hussein 2019). Certain concentrations of specific metal oxides should be added to acquire the prepared glass precise properties required for the wanted application. Flux materials like NaCO3 and borax can be added to ease the preparation process of glasses at relatively low melting temperature (Baydogan and Tugrul 2012), additionally, Zr4+ ions can develop the glass stability as well (Moustafa and Hassaan 2017). ZrO2 has the ability to form homogeneous glassy matrix and insertion of ZrO2 even in small amount significantly improves the glass physical properties e.g., density, viscosity, and glass transition temperature, as well as enhancing the glass thermal stability and chemical durability not only in neutral pH conditions, but also in alkali-rich environments (Fisher et al. 2005; Xiaonan et al. 2018). So zirconia (ZrO2) plays an important role in the development of radiation shielding and nuclear waste glass compositions, because of its tendency to provide the glasses compactness and effectual stability against ionizing irradiation (Du et al. 2006). Furthermore, zirconium ion was found mostly in a six-fold coordinated and the [ZrO6] octahedral groups that can connect with other network formers such as the tetrahedral [SiO4] by corner-sharing. In borosilicate glass system, ZrO2 works to polymerize the glassy network by attracting alkali cations to compensate the charge of six-fold coordinated zirconium ion and strength the glassy network structure (Shreif et al. 2023, Xiaonan et al. 2018; Du et al. 2006). ZrO2 inclines to yield more stable or relaxed structure attributable to the possible back conversion of BO4 → BO3 so it facilitates the formation of BO3 more than BO4 (Moustafa and Hassaan 2017), revealing the helpful effect of Zr4+ ions in the glassy structure as they contribute as network modifiers entering the glass interstices and forming stronger and more closed systems able to shield the passage of ionizing radiation photons (Abou Hussein and Rammah 2023).
Many other glass systems based different waste materials were prepared and studied for shielding applications (Al-Buriahi et al. 2022a; Khadijah Mohammed et al. 2022; Kurtuluş et al. 2022). The aim of the present work is to use municipal waste ashes as useless and harmful materials (the MSW ashes), to fabricate new industrial and useful glass product. Three new glass systems of un-doped and Zr doped borosilicate glasses were prepared using the MSW ashes as a main raw material (70 wt. %). Additionally many characterizing techniques were used to examine the glasses optical, chemical and radiation shielding properties to evaluate their hopeful uses as radiation shielding candidates.
2 Experimental procedures
2.1 Preparation process
Glass samples having composition of 70 waste + 20 borax + 10 Na2O + x ZrO2, where x = 0, 0.1 or 0.3, all wt. %, were prepared by the traditional melting annealing process. The MSW used in the preparing process as a main raw material was collected from waste landfilling region in eastern Cairo. Primarily, MSW ashes were burned at 750 °C for the volatility of undesirable organic materials. Specific weights of three homogenous batches were perfectly weighed by a four digits sensitive balance according to the composition of each glass sample. MSW ash, borax (Na2B4O7·10H2O), Na2CO3 and ZrO2 were used in the preparation process. The three weighed batches were sensibly mixed and grinded using a gate mortar. In platinum crucibles the three batches were melted in an electric muffle furnace at 1400–1450 °C for 3 h, and rotated at time intervals for achieving homogeneity and eliminating air bubbles in the prepared samples. The melts were then casted onto heated stainless steel molds having dimensions of 1 × 1 × 0.2 cm3. After that, the casted samples were transferred immediately to a muffle furnace at 550 °C for annealing. The muffle was switched off after 1 h leaving the samples inside it until the temperature reduced gradually to the room temperature with cooling rate ± 25 °C /h. Polished glass samples or fine powdered samples were prepared for the required characterization techniques.
2.2 Measuring techniques
-
a.
The Municipal solid wastes were chemically analyzed by using Philips sequential x-ray spectrometer-2400 (XRF technique). A solid sample was ground to very fine powder and mixed with H3BO3 as a binder for the ease of pressing process.
-
b.
The prepared glass samples were chemically analyzed using electron dispersive X- ray analyzer EDX, model; ZEISS—EVO 15.
-
c.
The optical UV–visible transmittance was measured using UV–visible spectroscopy in UV–visible range 200–900 nm by recording double-beam spectrophotometer (Type JASCO Corp, v-570, Rel-100 Japan). The samples were measured twice to approve the accuracy of the recorded peaks.
-
d.
Electron spin resonance (ESR) technique was measured using X-band EMX spectrometer with a rectangular cavity of the standard Bruker ER 4102, Germany. The measured samples were injected in ESR tube and dignified by the operating conditions; microwave power (0.796 mV), time constant (81.92 ms), modulation amplitude (5 Gauss) and conversion time (20.48 ms). All ESR measurements were carried out at the lab-temperature in a single scan (25 ± 2 °C).
-
e.
Testing chemical durability of the glasses were carried out in three leaching solutions; neutral dis H2O, alkaline 0.1 N NaOH and acidic 0.1 N HCl by inserting specific weighed glass samples in 200 ml leaching solutions in Falcon conical tubes with a flipped top cap to cover totally the glass surface by the solution. After definite time intervals up to 70 days, the samples were washed by ethanol and distilled H2O and dried in a drying oven at 40–50 °C for two hours. Then the dried samples were weighed carefully to calculate the weight loss percent (Wt. loss %) according to the following relation;
$${\text{Wt}}.{\text{ loss }}\% \, = \, \left( {\left( {{\text{Wt}}._{{{\text{before}}}} - {\text{ Wt}}._{{\text{after corrosion time}}} } \right)/{\text{Wt}}._{{{\text{before}}}} } \right){\text{ x 1}}00$$(1) -
f.
Scanning electron microscope (SEM) technique; Type, ZEISS, EVO15 and Resolution; 20 µm, Mag = 1.00 KX, EHT = 25 kV, Signal A = SE1, WD = 8.30 mm, was used to examine the glass surface morphology before and after irradiation and corrosion process.
-
g.
Optical UV–visible transmittance, ESR measurements, chemical durability tests and SEM photographs were carried out for both the fresh and gamma irradiated samples using 60Co (2000 Ci) gamma cell as gamma ray source with dose rate = 0.686 kGy/h at 25 ± 5 ◦C.
-
h.
For evaluating radiation shielding efficiency of the prepared glasses, a gamma-ray spectrometer system of NaI (Tl) scintillation detector (Canberra model) was used, with 3’ × 3’amplifier and 16 k multichannel analyzer. The detector was used for the experimental calculations of linear attenuation coefficient (LAC) of the studied glass samples. Experimentally, the tested glass shields were placed between the radiation source and the detector as shown in Fig. 1, where gamma-ray transmission geometry was used for measuring attenuation of samples.
The container of the source has a lead face aperture of 0.5 cm. The detector was shielded from secondary radiations (such as background, bremsstrahlung, and fluorescence) by a lead insulator on the walls of the chamber. The glass sample was positioned on a specimen holder 5 cm from the source, with a 10 cm gap maintained between the source and the detector. 137Cs and 60Co radioactive point sources, with an activity of 5 Ci, produced the gamma energies of 662, 1173, and 1333 keV that were employed.
The data of multichannel analysis were analyzed using the Genie software, while the arithmetic mean of the observed LAC-values was used to determine the experimental LAC value.
3 Results and discussion
Table 1 displays XRF analysis of the burned MSW ash where there are variety of different metal oxides facilitate the preparation of multicomponent glass systems e.g. glass forming metal oxide e.g. SiO2, glass intermediate metal oxide e.g. Al2O3, glass modifying metal oxides e.g. Na2O, MgO and CaO, as well as many transition metal oxides e.g. TiO2, Cr2O3, MnO, Fe2O3 and NiO. The EDX analysis of the prepared glasses is tabulated in Table 2 and their picture is presented in Fig. 2.
3.1 Optical UV–visible transmittance spectra
Optical UV–visible spectra of the glasses were measured for identifying the chemical environment around the glassy metal ions and changes happened in the glass lattice structure (Abou Hussein 2023). Transition metal ions cause electronic transfer mechanisms because of their variable coordination states even they were present in very few concentrations (e.g. p.p.m). This electronic transfer includes an electron transition from coordinated oxygen atomic orbital to metal ion orbital generating obvious UV bands (Abou Hussein and El-Alaily 2018). Figure 3 shows UV–visible optical transmittance spectra of the three prepared un-doped and Zr doped borosilicate glasses before and after gamma irradiation with 80 kGy. As obviously shown, there are similar optical spectra for the three glasses correlated to the similarity of composition and the few additions of Zr ions. The observed low transmittance in the glasses can be correlated to the high content of TM oxides that can yield strong field strength in the glassy structure causing a contraction of the network and a decrease in the optical transmittance of the host glass (Morsi et al. 2015). Quite UV bands e.g. the small peaks appeared before 400 nm at 200–330, 370, and 395 nm in the three glasses spectra, can be directly correlated to the amorphous nature of the glass (Abou Hussein and Abdel-Galil 2023) and absorbance of ferric ions (Fe3+) convoyed either to the waste as a main raw material or chemicals used in preparation process (even in p.p.m concentrations). Many authors assumed that the strong UV peaks of iron trace impurities tend to reduce the transmittance of the optical glasses like phosphate, fluorophosphate and borosilicate glasses that would be used in many optical applications like lenses or laser glasses (Batal et al. 2020). So they recommended the necessity for using ultrapure chemical materials for preparing such special optical glasses. Duffy (1997) has assumed the strong UV peaks to the electron charge transfer mechanism of the present transition metal ions e.g. Fe3+ and Cr6+ ions even in ppm level.
Figure 3 reveals also obvious small peaks at about 410 and 430 nm in the un-doped glass and cutoff peaks in Zr glasses at about 400 nm correlated to the valence state of the present transition metal ions as listed in Table 2 e.g. Fe2+/Fe3+, Ti4+, Cu2+, Zn2+ and Zr4+. The slight blue shift to slightly lower wavelength with the addition of Zr4+ ions indicates the conversion of non-bridging oxygen bonds (NBOs) to bridging oxygen bonds (BO), thus the transformation of four coordination boron [BO4]− to the three coordinated boron units BO3 (El-Diasty et al. 2014). This manner can discuss the obvious increase in transmittance with the addition of ZrO2 by comparing the optical spectra of the un-doped and Zr-doped glasses. Some authors assumed that introducing of ZrO2 causes BO4 → BO3 back conversion as the existence of Zr4+ ions in the glassy structure facilitates the formation of trigonal BO3 more than the tetrahedral BO4 or the appearance of ‘‘loose’’ BO4− tetrahedral, causing some changes in the coordination borate matrix and converting BO4 to BO3 (Moustafa and Hassaan 2017).
The effect of 80 kGy gamma irradiation is also shown in Fig. 3, where there is a slight increase in the glasses optical transmittance indicating the positive effect of such ionizing radiation on the prepared glasses. This performance can be correlated to the possible optical modifications that may be caused by irradiation. Sometimes ionizing radiation makes healing of the color defect centers present in the glass e.g. hole centers which cause hole trapping and decrease the glass optical transmittance (El-Zaiat et al. 2016). So, we can predict a healing positive effect of irradiation on the optical properties of the glasses especially those with such complicated structure rich in many transition metal ions, where many photochemical reactions can be expected e.g. photo-oxidation and/or photo-reduction by changing NBO number and extrinsic defects former “color defect centers” (Abou Hussein et al. 2021b).
3.2 Electron spin resonance ESR
ESR is a non-destructive technique used to study chemical species that have one or more free electrons. So it helps in identifying the irradiation-induced changes regarding the paramagnetic defects e.g. centers with unpaired electrons (holes and/or electrons trapped at different sites in the glass). Accordingly, ESR spectra are considered as fingerprint of paramagnetic centers for detecting structural changes taken place in the amorphous or crystalline materials (Abou Hussein 2019; El-Alaily et al. 2016). Figure 4 reveals ESR spectra of the un-doped and Zr doped borosilicate glasses before and after 80 kGy of gamma irradiation. As obviously shown each of the three investigated glasses has the same ESR spectra before and after irradiation either in the same position or intensity. Furthermore, the spectra appear fake and broad peaks, indicating the negligible forming of free radicals by irradiation (El-Alaily et al. 2016). The stable ESR spectra can be correlated also to the optical spectra where the positive effect of irradiation on the optical transmittance indicates non formation of free radicals or breaking in bonds. Mainly, the highly strong structure of borosilicate building units; trigonal BO3 and tetrahedral SiO4 and BO4, provide the glass structure strength towards the effect of radiation by blocking the glass network and inhibit photons passage through the material. Therefore, the identical ESR spectra before and after irradiation demonstrate the high electronic stability of the glasses against irradiation where generation mechanism may overcome destruction and the amplitude of ESR spectra of defect centers may come up to highly saturated level giving the produced stable spectrum (Mahmud et al. 2014), consequently the high resistance of the investigated glasses to ionizing radiation and then their possible use for radiation shielding purposes.
3.3 Chemical durability
Testing chemical durability of glass is very impressive indicator to expect the long term behavior of the glass surface against different leaching media. Therefore, the required chemical properties of the glass can be estimated for a definite application. As known, glass is chemically strong material, however it can be affected when it contacted to water vapor or liquid (Abou Hussein 2019). The glass chemical durability can be deliberated generally by two mechanisms; leaching and etching. Leaching or ion-exchange mechanism can take place at relatively low pH e.g. acidic or aqueous solutions, where H+ or H3O+ work as the attacking ions of the leaching solution. This process includes ion exchanging between mobile cationic ions on the glass surface e.g. Na+, K+ or Li+ (M+) and ions of the attacking leaching solution e.g. H+ or H3O+. Continuing of ion exchange process leads to the formation of leached alkali depleted layers accumulated on the glass surface as shown in the following chemical reactions in silicate and borate glasses, respectively.
The progressive ion exchange process makes the formed depleted layers thicker enough to diminish the rate of diffusion of cationic mobile ions. Etching mechanism takes place in higher pH solution e.g. alkaline solution, it can be nominated also as dissolution reaction since the formed OH− groups because of the progressive diffusion of attacking solution, can cause dissolving or breaking down of the main glassy network (Clark et al. 1979). The following reactions explain dissolution of silicate, borate and borosilicate glass systems in alkaline media, respectively.
Consequently, chemical durability of the glass depends mainly on the composition of the host glass, in addition to other factors like pH of the leaching solution (neutral, acidic or alkaline), glass surface area and time of leaching.
Figures 5, 6, and 7 show the corrosion behavior of the three prepared un-doped and Zr doped borosilicate glasses in neutral (dis. H2O), acidic (0.1 N HCl) and alkaline (0.1 N NaOH) leaching solutions for 70 days, before and after being irradiated with gamma rays (80 kGy). As shown the three glasses reveal high chemical durability either before or after irradiation where the weight loss % dose not exceed 6% before irradiation and 1% after irradiation. The noticed stable chemical behavior after 40 days’ time of corrosion either before or after irradiation can be correlated to the possible formation of precipitated layers on the glass surfaces that eventually prevent the normal progression of glass leaching e.g. Al(OH)3, Ba(OH)2 and Ca(OH)2.
The promising chemical durability of the prepared borosilicate glasses depends mainly on the strong structure of borosilicate network. Borosilicate network consists of three structural units trigonal BO3 and tetrahedral BO4 and SiO4 groups, where the trigonal BO3 groups likes to be more soluble than tetrahedral BO4 or SiO4 which firmly attached in four directions, in addition to the exceptional compositions of the three prepared glasses shown in Table 2.
Depending upon the glass compositions, pH of the leaching media and irradiation process, chemical durability results can be interpreted according to three factors;
3.3.1 Effect of leaching solution
As obviously shown the alkaline NaOH solution is highly corrosive than the acidic HCl and neutral H2O, where the glasses reveal their highest durability in the neutral medium. From EDX analysis of each glass compositions (Table 2), it can be noticed that the concentration of the mobile alkali ions Na+ and K+ ions are small enough to lessen the inter-diffusion process between the glass surface and H+/ H3O+ of the attacking acidic or neutral solutions. This is because Na+ ions work to enhance the glass alterability by disrupting the glass structure through creating percolation channels defined by NBO at the network regions ends that ionically bonded to alkalis (Abou Hussein 2019; Ojovan and Lee 2005). Therefore, alkaline NaOH solution appears more corrosive to make dissolution in borosilicate network or relative breaking down in Si-O-B bonds as shown in equation (6).
3.3.2 Effect of glass composition
Comparing the corrosion behavior of the three un-doped and Zr doped borosilicate glasses reveals that 0.3 Zr is more durable than 0.1 Zr than the un-doped glass. This performance can be interpreted by data of EDX analysis shown in Table 2 according to the following points:
-
(a)
0.3 Zr glass contains higher silica content (12.21) than 0.1 Zr and un-doped glass (6.63). As known SiO2 is the most common strong glass network former. Silicate network attached firmly by the tetrahedral SiO4 groups, where the increase of SiO2 content in the glassy network produces highly compacted and interconnected structure that retards the progress of corrosion process causing the observed high chemical durability of 0.3 Zr with the higher silica content (Abou Hussein 2019; Alexander et al. 2003).
-
(b)
Presence of the intermediate Al3+ ions in acceptable content in the three investigated glasses works to lengthen the glassy network through the aggregation of the working range by the strong AlO6/AlO4 octahedral and tetrahedral units. Additionally presence of higher Ti4+ ions content in 0.3 Zr glass, can act as intermediate ions participated in the glassy network in four, five or six-fold coordination’s to improve the glass viscosity and chemical resistance.
-
(c)
Ca2+ ions are present obviously in higher content in 0.3 Zr glass (11.84) than the other two glasses (0.57 and 1.09 for the un-doped and 0.1 Zr glasses, respectively). Ca2+ ions are characterized by their large ionic radii so that they can block the passage of mobile ions diffusion or inhibit the ion exchange leaching process giving higher chemical durability to the glass with higher Ca content (Abou Hussein 2019; El-Alaily et al. 2018a).
-
(d)
Ca2 and Ba2+ modifier ions can enhance the glass chemical durability by stabilizing the glassy structure, and transition metals ions e.g. Cu2+, Zn2+, Ti4+ and Fe3+ work to develop the glasses chemical durability by changing their variable coordination’s (Abou Hussein 2023; Abou Hussein and Barakat 2022).
3.3.3 Effect of gamma irradiation
Generally ionizing radiation impacts the glass structure causing the formation of induced defect centers or non- bridging hole centers, the following reaction describes the effect of radiation on silicate glass system.
However, radiation effect on borosilicate glass is more complicated than vitreous silicate or borate glasses. Since the collaboration between the two main SiO2 and B2O3 provide the glass ability to resist the effect of radiation. As obviously shown in Figs. 5, 6, and 7, there is unexpected improving in the glasses chemical durability after being irradiated with 80 kGy, where the weight loss% of irradiated glasses are decreased near to 0 %, indicating the positive effect of radiation on the glass chemical properties. This performance can be attributed to the possible modifications in the glass structure due to the effect of irradiation where some bonding rearrangements may take place causing healing of the present defect centers present in the glassy network, then the release of the extra energy stored in the network, thus more relaxed and more chemically stable structures obtained (Ahmad et al. 2020).
The glass surface morphology can be estimated to study the progress of corrosion process and examine the leached layers formed after immersing glasses in different leaching solutions. Figure 8 depicts SEM photographs of 0.3 Zr glass surfaces in disH2O, 0.1 N HCl and 0.1 N NaOH for 70 days, before and after irradiation with 80 kGy. The images illustrate rougher and more corrosive surfaces for the fresh samples than those of the irradiated samples, indicating a positive chemical effect of irradiation on the glass where the irradiated surfaces appeared more homogenous. Additionally the existence of some insoluble hydroxide layers deposited on the glass surfaces especially in NaOH solution that appeared more corrosive leaching medium than H2O and HCl, whereas the etching mechanism would be expected in the alkaline medium with higher pH.
3.4 Radiation shielding parameters
The attenuation of low-energy gamma or X-rays, that are predominate in medical X-ray equipment’s especially those used for diagnostic purposes, can be determined by detecting LAC and MAC values, Thus shielding capabilities of various materials can be inspected.
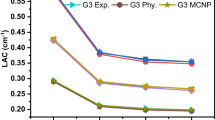
LAC is a helpful test for assessing how well glassy materials attenuate gamma rays and shield them. Glass samples were evaluated with photon energies of 662, 1173, and 1333 keV, Fig. 9 displays the experimental LAC values and the theoretical values by Phy-X / PSD software. As shown from the figure, LAC values of all glass samples decrease with rising photon energy and the varied LACs values can be correlated to the various densities and compositions of the glasses. Additionally, Fig. 9 reveals an excellent agreement between the experimental data of LACs and the Phy-X/PSD theoretical results verifying the accuracy of Phy-X/PSD software program. Phy-X/PSD software was used also to display MAC values in the energy range 0.040—2 MeV as shown in Fig. 10.
As seen in Fig. 10, MAC values in the energy range of 0.040—2 MeV, which include also the gamma energies 0.662, 1.1173, and 1.333 MeV, were displayed using the Phy-X/PSD software.
As obviously shown MAC values are closely similar in the three examined glasses however, the highest MAC value is found for 0.3 Zr glass near 1.055 cm2/g at 0.040 MeV, while with the energy increase, the MAC is declined e.g. at 2 MeV, it reaches its lowest value 0.044 cm2/g.
For the studied glasses with different amounts of Zr ions, resonant absorption peaks known as photoelectric absorption edges are observed at about 100 keV. After these edges, MAC begins to decrease gradually, where photoelectric absorption becomes more prevalent.
The photoelectric effect (PE) is less likely with the increase of photon energy, whereas it is profitable for medium-energy Compton scattering (CS). As the photon energy increases, the pair production process (PP) is clearly dominated specially in the higher energy range (beyond 1.2 MeV) (Sathish et al. 2020).
The effective atomic number, or Zeff, is another essential factor helps to evaluate the material’s capacity for attenuation. Several studies have revealed that materials with high Zeff provide outstanding radiation shielding behaviors (Mengge et al. 2017). Figure 11 displays Zeff values for the investigated glasses in the energy range from 0.04 to 2 MeV. In spite of the similar behavior of Zeff values in the three studied glasses, the quiet differences can be attributed to variations in the glasses chemical composition at each gamma photon energy ranges (Abou Hussein et al. 2021b). As shown from Fig. 11, larger Zeff values are found at the lowest energies where photoelectric effect is the influential. The modest rise in Zeff at higher energies can be recognized to the pair-production effect, while the minimal values realized at medium energies can be attributed to Compton scattering. As shown in Fig. 11, the un-doped glass has the lowest Zeff values in the examined photon energy range, while 0.3 Zr glass with the highest Zr content (0.3 wt. %) has the highest Zeff values.
Neff and Ceff of the studied glasses are displayed in Fig. 12 in the energy range from 0.04 to 2 MeV. It is evident that with the increase of energy levels, Neff and Ceff values are decreased. At low energies, photoelectric absorption has the governing effect where the excited electrons give a significant drop, however as the energy increases, more photons are released causing the increase of excited electrons and then the observed reduction in Neff and Ceff values. A significant hump of Neff and Ceff values is observed in all the studied samples at 100 keV referring to K-shell absorption edge, followed by a progressive decline with the energy increase, where they continue to exist in a fixed state inside the medium-energy area owing to Compton-scattering mechanism (Abou Hussein et al. 2021d). Ceff is generally based on the anticipated number of collisions between the strong photons and electrons, trailed by their alteration to free electrons so it is directly proportional to Neff (Ravangvong et al. 2020). An electron on a material becomes free when a photon strikes it, so the number of free electrons would control the material's effective conductivity. Depending upon photon density and energy, shielding characteristics of samples with different conductivities would change. Because of the predominant photoelectric absorption in the low-energy range, the greatest effective conductivity values were recorded, and because of the Compton scattering and pair-production processes predominate in the high-energy zone, the change in effective conductivity was decreased.
Half value layer (HVL) and the tenth-value layer (TVL) are important parameters of the shielding attenuation that describe the gamma-ray shielding strength for the studied samples, they were evaluated by Phy-X/PSD software. The results of these factors helped in evaluating the sample thickness required for shielding the half and tenth of the initial photon intensity (Dong et al. 2016). Better shielding materials are those with thinner layers of HVL and TVL (Sayyed et al. 2021).
Table 3 reports the HVL and TVL values of the examined glasses at selected energies. As shown HVL and TVL values are followed the order of un-doped > 0.1Zr > 0.3Zr. In other words, HVL and TVL appeared the lowest for 0.3Zr glass referring to the best shielding capability. However, the un-doped glass has the highest HVL and TVL referring to the least shielding capability. Additionally, HVL and TVL are increased with the increase of energy as shown in Table 3. Since the high energetic photons can interact with the material atoms less frequently however passing more through the glass matrix. The HVL and TVL values are generally increased in a progressive manner as energy increases, so the studied glasses accomplish their best shielding behavior at lower energies. They are also positively impacted by Zr content, over all the incident gamma energies, 0.3 Zr glass has the lowest thickness needed for HVL and TVL.
It is valuable to compare the shielding parameters of the studied samples with those for several common shields. Table 4 provides a characteristic comparison in mass attenuation coefficient MAC between the studied lead free glass (0.3 Zr) with other lead glass and shielding materials. The results demonstrate that the examined 0.3 Zr glass is more effective as shielding material comparing to other referenced shielding materials (Askin and Dal 2019; Chaiphaksa et al. 2016; Almatari et al. 2019; Abdel Wahab et al. 2022; Al-Buriahi et al. 2022b).
4 Conclusion
The prepared un-doped and Zr doped borosilicate glasses based MSW ashes displayed superior optical, chemical and radiation shielding characteristics before and after gamma irradiation with a dose of 80 kGy. XRF analysis of MSW ash revealed rich by many glass forming, intermediate and modifying metal oxides and EDX analysis of the prepared glasses revealed their various compositions correlated to the used MSW ash. The optical UV–visible transmittance appeared relatively low, due to the high content of TM oxides that can produce strong field strengths in the glass structure and contraction of the network. The glasses revealed high chemical durability in H2O, NaOH and HCl leaching media for 70 days either before or after irradiation; however NaOH solution appeared more corrosive than HCl and H2O. Unexpected improvement in the glasses chemical durability after irradiation was obtained by about 5 weight loss% lesser than before irradiation. The surface morphology of the corroded glass surfaces approved also the positive impact of irradiation on the glasses durability. ESR measurements revealed the same spectra indicating the electronic stable structure of the glasses and the absence of free radicals formed by irradiation. LAC displayed good agreement between the experimental results obtained from NaI detector, and the theoretical data from Phy-X/PSD software. The studied shielding parameters are considerably impacted by Zr concentration. The highest LAC, MAC, Zeff, Neff and Ceff values were obtained for the highest Zr content (0.3 Zr glass). HVL and TVL values were in the order of un-doped > 0.1 Zr > 0.3 Zr giving the lowest values for 0.3Zr glass, referring to the lowest thickness needed for HVL and TVL and the best shielding ability. The shielding parameters impacted positively by Zr ions addition over all the incident gamma energies.
The overall data produced that both Zr addition and irradiation cause an enhancement of the desired glasses properties e.g. an increases in optical transmittance, increase of the glasses chemical durability in the three different pH leaching media and inhibition of forming free radicals. This manner can be associated with the particular various contents of the prepared borosilicate glasses that facilitate a possible healing effect of irradiation that sometimes can absorb the glass defect centers (e.g. positive holes, negative electron centers) and release of extra energy stored in the network, producing more relaxed structure. The high compacted structure of borosilicate consists of; trigonal BO3 and tetrahedral SiO4 and BO4 building units afford the glass structure strength against the influence of radiation by blocking the network and inhibit the photons passage along the glassy material. The overall study approved the economic valuable effect of the prepared glasses as radiation shielding materials voiding the toxic effect of lead, since the useless MSW ash were used mainly by 70 wt. %, to produce effective borosilicate glass systems appropriate for the safe usage as radiation shielding glasses in nuclear medicine units in hospitals or other facilities.
Data availability
Not applicable.
References
Abdel Wahab, E., Al-Baradi, A.M., Sayed, M.A., Ali, A.M., Makhlouf, S.A., Shaaban, K.S.: Crystallization and radiation proficiency of transparent sodium silicate glass doped zirconia. Silicon 14, 8581–8597 (2022)
Abou Hussein, E.M.: Vitrified municipal waste for the immobilization of radioactive waste, preparation and characterization of borosilicate glasses modified with metal oxides. SILICON 11, 2675–2688 (2019)
Abou Hussein, E.M.: The impact of electron beam irradiation on some novel borate glasses doped V2O5; Optical, physical and spectral investigation. Inorg. Chem. Commun. 147, 110232 (2023)
Abou Hussein, E.M., El-Alaily, N.A.: Study on the effect of gamma radiation on some spectroscopic and electrical properties of lithium borate glasses. Inorg. Organomet. Polym. Mater. 28, 1214–1225 (2018)
Abou Hussein, E.M., Barakat, M.A.Y.: Structural, physical and ultrasonic studies on bismuth borate glasses modified with Fe2O3 as promising radiation shielding materials. Mater. Chem. Phy. 290, 126606 (2022)
Abou Hussein, E.M., Abdel-Galil, A.: Synthesis, optical, chemical and electrical characterizations of γ-irradiated transition metal ions reinforced borate glasses. J. Non-Crys. Solids 610, 122302 (2023)
Abou Hussein, E.M., Madbouly, A.M.: Chemical and radiation shielding effectiveness of some heavy metal oxide glasses for immobilizing radioactive wastes. J. Aust. Ceram. Soc. (2023). https://doi.org/10.1007/s41779-023-00951-2
Abou Hussein, E.M., Rammah, Y.S.: Fabrication of zirconium borosilicate glasses using municipal waste ash for radiation shielding applications; optical and structural investigations. Phys. Chem. Solids 183, 111633 (2023)
Abou Hussein, E.M., Madbouly, A.M., Ezz Eldin, F.M.: Characterization of some radiation shielding, optical, and physical properties of fluorophosphate glasses modified by Sm3+. Mater. Sci. Mater. Electron. 32, 25933–32595 (2021a)
Abou Hussein, E.M., Madbouly, A.M., Ezz Eldin, F.M., El-Alaily, N.A.: Evaluation of physical and radiation shielding properties of Bi2O3–B2O3 glass doped transition metals ions. Mater. Chem. Phys. 261, 124212 (2021b)
Abou Hussein, E.M., Maksoud, M.I.A.A., Fahim, R.A., Awed, A.S.: Unveiling the gamma irradiation effects on linear and nonlinear optical properties of CeO2–Na2O–SrO–B2O3 glass. Opt. Mater. 114, 111007 (2021c)
Abou Hussein, E.M., Madbouly, A.M., El-Alaily, N.A.: Gamma ray interaction of optical, chemical, physical behavior of bismuth silicate glasses and their radiation shielding proficiency using Phy-X / PSD program. J. Non-Cryst. Solids 570, 121021 (2021d)
Abou Hussein, E.M., El-Agawany, F.I., Rammah, Y.S.: CuO reinforced lithium-borate glasses: Fabrication, structure, physical properties, and ionizing radiation shielding competence. J. Aust. Ceram. Soc. 58(1), 157–169 (2022)
Ahmad, Z., Ali, S., Ahmad, H., Hayat, K., Iqbal, Y., Zulfiqar, S., Zaman, F., Rooh, G., Kaewkhao, J.: Radio-optical response of cerium-doped lithium gadolinium bismuth borate glasses. J. Lumin. 224, 117341 (2020)
Al-Buriahi, M.S., Kavas, T., Kavaz, E., Kurtulus, R., Olarinoye, I.O.: Recycling potential of cathode ray tubes (CRTs) waste glasses based on Bi2O3 addition strategies. Waste Manag. 148, 43–49 (2022a)
Al-Buriahi, M.S., Alrowaili, Z.A., Eke, C., Alzahrani, J.S., Olarinoye, I.O., Sriwunkum, C.: Optical and radiation shielding studies on tellurite glass system containing ZnO and Na2O. Optik 257, 168821 (2022b)
Alexander, K., Mario, P., Alessandro, H.: Sintered glass-ceramics from municipal solid waste-incinerator fly ashes-part I: The influence of the heating rate of the heating rate on the sinter-crystallization. Eur. Ceram. Soc. 23, 827–832 (2003)
Almatari, M., Issa, S.A.M., Dong, M.G., Sayyed, M.I., Ayad, R.: Comparison between MCNP5, Geant4 and experimental data for gamma rays attenuation of PbO–BaO–B2O3 glasses. Heliyon 5, e02364 (2019)
Alrowaili, Z.A., Taha, T.A., Ibrahim, M., Saron, K.M.A., Sriwunkum, C., Al-Baradi, A.M., Al-Buriahi, M.S.: Synthesis and characterization of B2O3-Ag3PO4-ZnO-Na2O glasses for optical and radiation shielding applications. Optik 248, 168199 (2021)
Alrowaili, Z.A., Yılmaz, E., Çalışkan, F., Öztürk, B., Olarinoye, I.O., Arslan, H., Al-Buriahi, M.S.: Radiation shielding performance of a newly synthesized bismuth borate glass system. Radiat. Phys. Chem. 204, 110711 (2023)
Alzahrani, J.S., Alrowaili, Z.A., Eke, C., Boukhris, I.: Optical properties and photon-shielding performance of B2O3-based glasses. Optik 262, 169343 (2022)
Askin, A., Dal, M.: Investigation of the gamma ray shielding behaviour of (90–x)TeO2— xMoO3—10ZnO glass system using geant4 simulation code and WinXCOM database. Cumhuriyet Sci. J. 40, 742–752 (2019)
Barbieri, L., Bonamartini, A.C., Lancellotti, I.: Alkaline and alkaline-earth silicate glasses and glass-ceramics from municipal and industrial wastes. Eur. Ceram. Soc. 20, 2477–2483 (2000)
Baydogan, N., Tugrul, A.B.: Borosilicate glass for gamma irradiation fields. Solid State Sci. 14, 1692–1697 (2012)
Chaiphaksa, W., Limkitjaroenporn, P., Kim, H.J., Kaewkhao, J.: The mass attenuation coefficients, effective atomic numbers and effective electron densities for GAGG: Ce and CaMoO4 scintillators. Prog. Nucl. Energy 92, 48–53 (2016)
Clark, D.E., Pantano, C.G., Hench, L.L.: Corrosion of glass. Books for industry, New York (1979)
Dong, M., Xue, X., Yang, H., Liu, D., Wang, C., Li, Z.: A novel comprehensive utilization of vanadium slag: As gamma ray shielding material. Hazard Mater. 318, 751–757 (2016)
Du, J., Devanathan, R., Corrales, L.R., Weber, W.J., Cormack, A.N.: Short- and medium-range structure of amorphous zircon from molecular dynamics simulations. Phys. Rev. B 74, 214204 (2006)
Duffy, J.A.: Charge transfer spectra of metal ions in glass. Phys. Chem. Glasses 38, 289–292 (1997)
El-Alaily, N.A., Abou-Hussein, E.M., Abdel-Monem, Y.K., Abd Elaziz, T.D., Ezz-Eldin, F.M.: Vitrified municipal waste as a host form for high-level nuclear waste. Radioanal. Nucl. Chem. 299, 65–73 (2014)
El-Alaily, N.A., Abou Hussein, E.M., Saad, E.A.: Bismuth silicate glass containing heavy metal oxide as a promising radiation shielding material. Radiat. Eff. Defects Solids 171, 1–12 (2016)
El-Alaily, N.A., Abou Hussein, E.M., Ezz ElDin, F.M.: Chemical and optical degradation of some glass formulated from common municipal solid waste, decorated glass. SILICON 10, 2031–2042 (2018a)
El-Alaily, N.A., Abou Hussein, E.M., Ezz Eldin, F.M.: Gamma irradiation and heat treatment effects on barium borosilicate glasses doped titanium oxide. Inorg. Organomet. Polym. Mater. 28, 2662–2676 (2018b)
El Batal, H.A., Abou Hussein, E.M., El-Alaily, N.A., EzzEldin, F.M.: Effect of different 3d transition metal oxides on some physical properties of γ-Irradiated Bi2O3- B2O3 glasses: A comparative study. J. Non-Cryst. Solids 528, 119733 (2020)
El-Diasty, F., Moustafa, F.A., Abdel-Wahab, F.A., Abdel-Baki, M., Fayad, A.M.: Role of 4p–3d orbital hybridization on band gap engineering of heavy metal glass for optoelectronic applications. J. Alloy. Compd. 605, 157–163 (2014)
El-Zaiat, S.Y., Medhat, M., Mona, F., Marwa, A.: Effect of UV exposure on photochromic glasses doped with transition metal oxides. J. Optics Commun. 370, 176–182 (2016)
Fisher, J.G., James, P.F., Parker, J.M.: Soda lime zirconia silicate glasses as prospective hosts for zirconia-containing radioactive wastes. J. Non Cryst. Solids 351, 623–631 (2005)
Katubi, Khadijah Mohammed, Kurtulus, Recep, Alrowaili, Z.A., Kavas, Taner, Kavaz, E., Al-Buriahi, M.S.: Optical properties, elastic moduli, and radiation shielding performance of some waste glass systems treated by bismuth oxide. Optik 266, 169567 (2022)
Kim, M., Heo, J.: Calcium-borosilicate glass-ceramics waste forms to immobilize rare-earth oxide wastes from pyro-processing. Nucl. Mater. 467, 224–228 (2015)
Kurtuluş, R., Buriahi, M.S., Shams Issa, A.M., Tekin, H.O., Kavas, T., Kavaz, E.: Physical, structural, mechanical and radiation shielding features of waste pharmaceutical glasses doped with Bi2O3. Optik 261, 169108 (2022)
Mahmud, H.H., Mansour, A., Ezz-Eldin, F.M.: Generation and bleaching of E′-centers induced in a-SiO2 by γ-irradiation. Radioanal. Nucl. Chem. 302, 261–272 (2014)
Marzouk, M.A., Abou Hussein, E.M.: Induced defects by gamma irradiation doses on the structure and optical behavior of undoped and TiO2, Cr2O3, or MnO-doped heavy metal borate glasses. Appl. Phys. A 125, 140 (2019)
Maximina, R., Jesus, V.M.: Surface and bulk crystallization of glass-ceramic in the Na2O-CaO-ZnO-PbO-Fe2O3-Al2O3- SiO2 system derived from a goethite waste. Am. Ceram. Soc. 82, 1313–1317 (1999)
Mengge, D., Xiangxin, X., He, Y., Zhefu, L.: Highly cost-effective shielding composite made from vanadium slag and boron-rich slag and its properties. Radiat. Phys. Chem. 141, 239–244 (2017)
Morsi, R.M., El-Ghany, S.I., Morsi, M.M.: Electrical properties of silicate glasses of low level gadolinium oxide doping including dielectric and infrared measures. J. Mater. Sci. Mater. Electron. 26, 1419–1426 (2015)
Moustafa, M.G., Hassaan, M.Y.: Optical and dielectric properties of transparent ZrO2–TiO2–Li2B4O7 glass system. J. Alloy. Compd. 710, 312–322 (2017)
Ojovan, M.I., Lee, W.E.: Immobilization of radioactive wastes in glass. In: ElsevierScience (ed.) An Introduction to Nuclear Waste Immobilization. Elsevier, Amesterdam (2005)
Park, Y.J., Heo, J.: Vitrification of fly ash from municipal solid waste incinerator. J. Hazard. Mater. B 91, 83–93 (2002)
Ravangvong, S., Sriwongsa, K., Chaiyoc, P., Glumglomchit, P.: Calculation of fast neutron removal cross-sections for lithium borate glasses system doped lutetium. Mater. Sci. Appl. Energy 9, 473–477 (2020)
Roth, G., Weisenburger, S.: Vitrification of high-level liquid waste: Glass chemistry, process chemistry and process technology. Nucl. Eng. Des. 202, 197–207 (2000)
Rupesh Kumar, A., Rao, T.G.V.M., Neeraja, K., Rami Reddy, M., Veeraiah, N.: Gamma ray induced changes on vibrational spectroscopic properties of strontium alumino-borosilicate glasses. Vib. Spectrosc. 69, 49–56 (2013)
Sathish, K.V., Manjunatha, H.C., Seenappa, L., Sridhar, K.N., Nagaraj, N., Alfred Cecil Raj, S.: Specific absorbed fraction of energy of silicon-boron alloys. Indian J. Pure Appl. Phys. 58, 213–217 (2020)
Sayyed, M.I., Albarzan, B., Almuqrin, A.H., El-Khatib, A.M., Kumar, A., Tishkevich, D.I., Trukhanov, A.V., Elsafi, M.: Experimental and theoretical study of radiation shielding features of CaO-K2O-Na2O-P2O5 glass systems. Materials 14, 3772 (2021)
Shreif, A., Farag, M.A., El-Sherbiny, M.A., Hassaan, M.Y.: Effect of Zr ions on the structure and optical properties of lithium borosilicate molybdate glass system. Ceram. Int. 49, 8709–8717 (2023)
Wagh, A., Raviprakash, Y., Kamath, S.D.: Gamma rays interactions with Eu2O3 doped lead fluoroborate glasses. J. Alloy. Compd. 695, 2781–2798 (2017)
Xiaonan, Lu., Deng, Lu., Kerisit, Sebastien, Jincheng, Du.: Structural role of ZrO2 and its impact on properties of boroaluminosilicate nuclear waste glasses. npj Mater. Degrad. 19, 1–10 (2018)
Acknowledgements
We are gratefully acknowledging financial support from The Science, Technology & Innovation Funding Authority (STDF) under grant number 45983, and Egyptian Atomic Energy Authority for their fruitful assistance in completing this work.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This work was supported by Science, Technology & Innovation Funding Authority (STDF), Grant number 45983.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [E.M. Abou Hussein1] and [A.M. Madbouly3]. The first draft of the manuscript was written by [E.M. Abou Hussein1], [S.E. Shaban2], [A.M. Madbouly3] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.”
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hussein, E.M.A., Shaban, S.E. & Madbouly, A.M. Chemical durability and shielding study of borosilicate glass systems from solid municipal waste ash for radiation shielding applications. Opt Quant Electron 56, 543 (2024). https://doi.org/10.1007/s11082-023-06180-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-023-06180-y