Abstract
This work aimed to prepare calcium-doped zinc oxide (ZC) ceramic nanoparticles in this formula (1-x)ZnO–xCaO with (x = 0, 0.10 and 0.90 mol.%) which are effective against the emerging multidrug-resistant Candida auris for the first time to our knowledge using the sol–gel method. Three different calcination temperatures (Tc) (500, 550 and 600 °C) were employed here. The prepared samples were characterized by XRD, SEM, and Zeta sizer. Also, their antimicrobial activity was assessed. All the prepared samples that were calcined at 600 °C showed particle size at nanometer range. All ZC ceramic samples showed negative zeta potential with higher magnitude indicating the stability of the produced nanoparticles. On increasing, calcium oxide doped in ZC10 and ZC90 ceramic samples, the particle size was decreased with regular hexagonal shape in SEM images. Finally, the prepared ZC ceramic nanoparticles exhibited excellent inhibitory activity against the emerging multidrug-resistant C. auris. Additionally, the prepared nanoparticles were active against both gram-positive Staphylococcus auris (ATCC 25923) and gram-negative E. coli (ATCC 25922). Collectively, ZC ceramic nanoparticles can be used to combat the emerged drug-resistant C. auris instead of applying the current antifungal drugs that exhibited minimum activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, nanocrystalline material has wide attention due to their unique properties and their widespread application in various aspects such as catalysis, optoelectronic materials to sensors, remediation, and biomedicine [1].
Ceramic oxides in nanometer particle size range like Magnesiumoxide (MgO), Calcium oxide (CaO), Titanium oxide (TiO2), and Zinc Oxide (ZnO) were found to have antibacterial activity [2]. Particularly, the usage of ZnO ceramics has attracted attention due to their distinct properties and characteristics. ZnO can be used in various applications like photocatalysis and as an antibacterial substance, in addition to its wide safety profile thus making them exciting commodities for industries [3, 4]. According to many literatures, the fabrication of ZnO and CaO in nanoparticles (NPs) is very attractive as it is antibacterial and safe, inexpensive, and an environment friendly material. Also, zinc and calcium are necessary elements to our health [3,4,5]. Additionally, the nanostructure of zinc oxide is characterized by massive catalytic activity and powerful adsorption capability [6]. Many methods were recorded to prepare ZnO nanoparticles like sol–gel method, thermal decomposition, chemical vapor decomposition (CVD), and alloy evaporation-deposition [7,8,9,10,11].
The sol–gel method is an attractive preparation method that can control the product purity, with a high surface area and the products can be prepared at a low crystallization temperature, which implies low cost [12, 13]. Sol–gel technique makes molecular precursor transformation onto a stable condensed oxide network that is explained by several stages, hydrolysis, and polymerization for the formation of the sol precursor followed by condensation, dehydration, nucleation, and growth, which is achieved through annealing [14, 15].
Doping with calcium in zinc oxide has a great interest in optical [16], sensing [17], and biological applications [4, 18]. Despite the studies made on Zn1−xCaxO system being scarce, the aforementioned system is very interesting because the calcium element has a higher ionic size than the zinc element and consequently calcium oxide doping is very effective for lattice stabilization and can increase the ionicity of chemical bonds in ZnO system [19]. On the other hand, the studies made on ceramic oxides like ZnO-CaO system and measuring the antimicrobial activity are very rare so our study focus on usage of this composition with different concentrations and studying the availability in biological application.
On the other hand, following the Covid-19 pandemic, antimicrobial resistance was raised by 15% according to the Centers for Disease Control and Prevention (CDC) [20]. Candida auris is an emerging multidrug resistant microorganism that was first isolated in Japan in 2009 [21]. According to the CDC, C. auris cases were raised by 60% during the Covid-19 pandemic between 2019 and 2020 [22]. Recently, C. auris showed great ability to acquire resistance to several classes of antifungal drugs once they were exposed to the antifungal drug at sub-MIC level [4]. Combating antimicrobial resistance requires the constant need for novel antimicrobials. Metal nanoparticles have been considered a new approach in combating antimicrobial resistance due to their unique mechanisms, as they can disrupt the membrane of the microbial cell and generate reactive oxygen species (ROS) in-addition to the ability to inhibit biofilm formation [4, 23]. Several types of metal nanoparticles have shown antimicrobial activity against Candida such as silver nanoparticles (AgNPs), iron nanoparticles (IONPs), ZnONPs, CaONPs, Copper nanoparticles (CuONPs), and titanium dioxide nanoparticles (TiO2NPs) [4, 24]. For instance, AgNPs showed potent synergistic activity with fluconazole against Candida albicans as shown by Jia and Sun study [25]. IONPs were functionalized with chitosan and the produced nanoparticles showed eightfold reduction in C. albicans growth compared to the antifungal drug miconazole [26]. On the other hand, CuNPs completely managed to inhibit C. albicans growth at a concentration of 150 µg/mL [27]. TiO2NPs with diameter of 26 nm managed to prevent C. albicans adhesion on the polymethylmethacrylate denture base [28]. Finally, ZnO NPs enhanced caspofungin activity against C. auris [4]. Despite several reports that highlighted the application of metal nanoparticles against C. albicans, limited study evaluated the bioactivity of metal nanoparticles against C. auris.
Hence, in the presented study, calcium-doped zinc oxide samples in this formula (1-x)ZnO–xCaO (x = 0, 0.10 and 0.90 mol.%) were fabricated in nanoparticle size by sol–gel method for the first time to our knowledge, and the possible application of this composition as an antimicrobial against the emergent multidrug-resistant C. auris was evaluated. The prepared samples were characterized by X-ray diffraction (XRD), scanning electron microscope (SEM), and Zetasizer. The antimicrobial activity was also evaluated on other bacterial strains such as E. coli and Staphylococcus aureus for validation. The produced nanoparticles can be used to combat the emerged drug-resistant C. auris instead of applying the current antifungal drugs that exhibited minimum activity.
Material and methods
Materials
The starting materials used in this study were zinc acetate dehydrate (Aldrich, USA) and calcium chloride (anhydrous) (Alpha Chemicka, India). Polyethylene glycol and citric acid were supplied by (Merk-Schuchardt, Germany), and (Aldrich, USA).
Nanoparticles preparation by sol–gel (citrate route) method
Three compositions of (1-x)ZnO–xCaO system (x = 0, 0.10 and 0.90 mol.% Ca) were stoichiometrically prepared by sol–gel method. The samples were designed as ZC0, ZC10, and ZC90, as shown in Table 1 where the last two digits indicate the ratio of calcium oxide. The gel was prepared using citric acid C6H8O7•H2O/polyethylene glycol 60:40 mass% as a crosslinking agent. Starting, the citric acid was dissolved in deionized water for 30 min. Then, stoichiometric batches were weighed as required for compositions of (1-x)ZnO–xCaO (x = 0, 0.10 and 0.90 mol.%) by mixing of Zn(CH3COO)2.2H2O and CaCl2 stoichiometrically (mathematically calculated proportions) in deionized water with continuous stirring. After mixing the salts with complete dissolution in citric acid, the polyethylene glycol was added to the solution. The reaction mixture was heated at 110 °C for 1 h with continuous stirring to evaporate most water in the gel phase until gel formation. After that, this solution was dried at 60℃ for 24 h to obtain nanoparticle ZC0, ZC10, and ZC90 samples as seen in Fig. 1. The dried gel were calcined at different calcination temperature (Tc) (500, 550 and 600 °C) for 1 h under air [29].
Characterization of the samples
The prepared dried samples of ZC0, ZC10, and ZC90 were characterized via different techniques to study the formed phases and their morphologies as follows:
Zeta potential and particle size measurement
The zeta potential and the particle size of the prepared NPs were measured by a Zetasizer (Malvern, Cambridge, UK). Initially, particles were diluted with distilled water then the particle size was assessed by the Dynamic Light Scattering (DLS) mode at 25 °C, while the Zeta potential (mV) was evaluated by applying Laser Doppler Velocimetry (LDV) mode [30].
X-ray diffraction analysis (XRD)
The X-ray diffraction analysis was used to study the formed phases of the prepared calcined ZC sample at 600 °C for 1 h using D8 advance diffractometer (Bruker, Germany) with secondary monochromatic beam CuKα radiation at 40 kV and 40 mA to identify the crystalline phases of formed samples in nanoparticle range at 600 °C temperature. Intensity data were collected over the range of 2θ from 10 to 60°.
Scanning electron microscopy analysis (SEM)
The surface morphology and the particle size were explored by applying SEM as follows, the calcined ZC samples at 600 °C for 1 h were sputter-coated with gold then they were scanned and photomicrographed using the Philips XL30 scanning electron microscopy (SEM) model, with an accelerating voltage of 30 kV and magnification of up to 400,000 × .
Determination of the antimicrobial activity
The antimicrobial activities of the prepared nanoparticles against C. auris (CDC-CAU9), C. auris (CDC-B11903), E. coli (ATCC 25922) and S. auris (ATCC 25923) were evaluated by liquid broth media according to modified Clinical and Laboratory Standards Institute (CLSI) and as described before [4]. Initially, several concentrations of the prepared nanoparticles were prepared and suspended in growth broth media for the tested microbes. For C. auris, Sabaroud dextrose broth media was used while Miller Hinton broth media was employed for E. coli (ATCC 25922), and S. aureus (ATCC 25923). The microbial culture was prepared (104 CFU/mL) and incubated with the tested nanoparticles into 96-well microplates at 37 °C for 48 h. The microbial growth was evaluated by measuring the turbidity at OD600 with a microplate reader (LT-4500, Labtech, Pocklington, York, UK). Colisitin (Cat# C4461, Sigma-Aldrich) and Amphotricin-B (Cat#46,006, Sigma-Aldrich) were used as positive controls while cultures broth media only were employed as negative controls and each test was performed in triplicate. Approval for exempt from ethical review No.: EX0030052023.
Statistical analysis
The data was collected and graphed using GraphPad Prism 8.02 for windows (GraphPad Inc., La Jolla, CA, USA). One-way analysis of variance (ANOVA) using Tukey’s Multiple Comparison Test was used to analyze the data. A P-value < 0.05 was considered as significant.
Results and discussion
Particle size of synthesized samples
Particle size can be affected in several ways: sonification, adding a stabilizer, and dispersant [31]. Initially, we measured the particle size for all zinc calcium oxide compositions fired at 500 °C, 550 °C and 600 °C for 1 h. Our data showed that the particle size for all zinc calcium oxide compositions fired at 500 °C and 550 °C were in the micrometer range while the zinc calcium oxide composition that was fired at 600 °C showed particle size in the desired nanometer range. Hence, the zinc calcium oxide ceramic samples fired at 600 °C were selected for further characterization. The decrease in particle size with raising the calcination temperature like another report by Ramadan et al. [32] and can be attributed to the ability of temperature to eliminate the ZnO-CaO NPs traces reaction with completely removing the moisture from the internal network of the produced ZC NPs crystal. The calcined ZC ceramic samples at 600 °C as illustrated in Fig. 2 showed that the particle size of ZC samples were 367 ± 50.99, 226.9 ± 29.18, and 137.3 ± 8.16 nm for ZC0, ZC10, and ZC90, respectively. The particle size reduced by increasing the doping of calcium oxide in ZC10 and ZC90 samples may be due to the saturation occurred by calcium doping and this explanation agreed with those recorded by various authors [33, 34].
Zeta potential of nano-sized samples
The prepared zinc calcium oxide ceramic samples in nanometer range were further characterized by measuring the zeta potential. The obtained data showed that all the measured samples have negative zeta potential with values equal to − 12 ± 5.63, − 14.4 ± 4.95, and − 16.8 ± 4.22 mV, for ZC0, ZC10, and ZC90, respectively as shown in Fig. 3. Zeta potential reflects the surface charge of the developed nanoparticles. The stability of ZnO-NPs is mainly affected by the zeta potential of the prepared nanoparticles. The lowest stability of ZnO NPs is observed at the isoelectric point when the zeta potential is equal to zero, as the particles tend to coagulate [6]. In contrast, the greater magnitude of zeta potential will result in higher repulsion forces and more stable particle suspension will be produced [35]. On the other hand, negatively charged nanoparticles have several biomedical applications as highlighted in several studies [30, 36,37,38]. For instance, Smeets et al. showed that nanoparticles with negative Zeta potential were favorable over the other charges for osseointegration of dental implants and bone regeneration [37]. Additionally, the negative charged nanoparticles can establish hydrophobic interactions with bacteria as indicated by the recent study of Mahmud and his colleagues [39]. Our fabricated ZC ceramic nanoparticles showed negative zeta potential with higher magnitude, indicating the stability of the produced nanoparticles ceramic samples and their potential biomedical application.
Phase compositions (XRD)
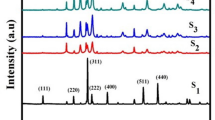
Figure 4 represents the XRD pattern of calcined zinc calcium oxide ceramic nanoparticles samples at 600 °C for 1 h. Generally, the XRD pattern for all ZC samples shows strong and sharp peaks that confirm the presence of hexagonal wurtzite structure of ZnO as the main phase with good crystallinity [32]. The pure zinc oxide sample for ZC0 was composed of pure zincite ZnO phase without any other phases according to ICDD card (01–089-1397) which revealed the ZnO synthesized in nanocrystal size with high purity as shown in Fig. 4. Interestingly, the doping with calcium oxide in ZC10 and ZC90 ceramic samples increases the intensity of peaks and indicates the presence of pure zincite ZnO as the main phase with zinc hydroxyl Zn5(OH)8Cl2H2O as secondary phase according to ICDD card (01–074-3156) which is formed from a chemical reaction between zinc and calcium chloride during synthesis as seen in Fig. 4. This result is like that recorded by Rosset et al. [9] that detects the same phase in XRD observation by using CaCl2 as precursor in preparation for Ca-alloyed ZnO nanoparticles by sol–gel method. Also, traces amount of calcium carbonate as the calcite phase appeared with increasing calcium oxide content in ZC10 and ZC90 samples. A slight shift of the XRD peaks can be noted, which may be due to the difference in ion size between zinc (1.42 Å) and calcium (1.94 Å). Furthermore, the increasing of calcium oxide doping in ZC samples is more effective for the stabilization of lattice systems which increases the chemical bonds ionicity in ZnO system [40].
Microstructure (SEM)
Figure 5 shows the SEM image of zinc calcium oxide ceramic nanoparticles samples calcined at 600 °C for 1 h. The SEM image was taken at × 12,000 and × 24,000 magnification. Figure 5(A–B) represents pure zinc oxide ZC0 sample which appears as nanoflake-like structures in shape and the size of the particles around ≈300 nm. On increasing calcium oxide doped in ZC10 and ZC90 ceramic samples occurs a decrease in particle size in grain with regular hexagonal shape. These particles have a size 226 nm, 148 nm; respectively, as seen in Fig. 5(C–F). This reduction in particles size may be attributed to the distortion in the host ZnO lattice by incorporating with Ca2+ in ZnO NPs surface area that occurred in the reduction in nucleation process resulting in a decrease in particle size as shown in Fig. 5(E–F). This behavior is in agreement with that reported by Hameed et al. [18].
Antimicrobial activity
The antimicrobial activity of ZC ceramic samples was evaluated against two strains of the multi-drug resistant yeast C. auris. The data exhibited in Fig. 6 showed that the 3 formula has potent activity against the two tested strains. For C. auris (CDC-B11903), the MIC50 for ZC0, ZC10, ZC90 were 40.93, 77.51, and 44.76 µg/ml respectively. MIC50 is referred to the lowest concentration of the nanoparticles that inhibit 50% of the growing microorganism [4]. Additionally, ZC0, ZC10, ZC90 were effective against C. auris (CDC-CAU9) with MIC50 equal 110.8, 167.6, and 101.9 µg/ml respectively. Furthermore, the prepared nanoparticles exhibited antibacterial activity against E. coli (ATCC 25922), and S. aureus (ATCC 25923) as shown in Fig. 7. ZC0 showed better activity against the two strains with MIC50 87.97 and 87.11 µg/ml against E. coli (ATCC 25922), and S. aureus (ATCC 25923) respectively, while ZC10, ZC90 showed comparable activity against the two strains with MIC50 144.6, 116.7 µg/ml and 132.2, 139.9 µg/ml against E. coli (ATCC 25922), and S. aureus (ATCC 25923) respectively.
The antimicrobial activity is considered the main biological application of metallic nanoparticles. The most favorable metallic nanoparticles for pharmaceutical application are the ZnO-NPs for being safe, cheap, and FDA-approved to be used as pharmaceutical excipients [41, 42]. Our antimicrobial data showed that the prepared nanoparticles produced a potent effect against the multidrug-resistant strain C. auris. The aforementioned strain is causing a major thread in the healthcare setting [43]. It was observed that once C. auris is exposed to antifungal drugs, it can develop several resistance mechanisms to overcome the antifungal pressure [4, 44]. The developed resistance mechanism is mainly dependent on the upregulation/mutation in the antifungal target genes [4]. To combat the aforementioned resistance mechanism, it is advisable to use an antifungal agent that targets multiple biomolecules to hinder the ability of C. auris to develop acquired resistance [45]. Based on that, the prepared nanoparticles in the current study would be the optimal solution to overcome the acquired resistance mechanism developed b C. auris. This is because ZnO in the nano range is passively internalized within the microbial cells and releases Zn2+. The release of Zn2+ generates reactive oxygen species and will induce disequilibrium in the zinc-mediated protein activity. Also, Zn2+ can interact with cellular protein and lipids and induce metabolic pathway disruption [23]. Altogether, it will induce oxidative stress and harmfully affect cellular DNA replication that eventually kills the target microbe [46, 47]. The ability of ZnO NPs to act on multi-biomolecules within C. auris cells, makes it an ideal potential treatment for C. auris infection.
Conclusion
In the presented study, novel zinc calcium oxide (ZC) ceramic nanoparticles in formula (1-x)ZnO–xCaO with (x = 0, 0.10 and 0.90 mol.%) were produced by the sol–gel method. Calcination temperatures (Tc) of 600 °C produced particles at nano size unlike calcination at 500, and 550 °C. Particles were negative in charge indicating the stability of the produced nanoparticles. Also, XRD confirmed the presence of pure zincite ZnO as the main phase with good crystallinity. On increasing, calcium oxide doped in ZC10 and ZC90 ceramic samples, the particle size was decreased with regular hexagonal shape in SEM images. The produced ZC ceramic nanoparticles samples inhibited the growth of the emergent multidrug-resistant Candida auris with MIC50 around 40 µg/ml. The bioactivity of the ZC ceramic nanoparticles was also confirmed against E. coli and Staphylococcus aureus and also ZC0 showed better activity against the two strains with MIC50 87.97 and 87.11 µg/ml against E. coli (ATCC 25922), and S. aureus (ATCC 25923) respectively as compared with ZC10 and ZC90. The produced nanoparticles could replace the current antifungal drugs to combat the emerging multidrug resistant C. auris instead of applying the current antifungal drugs that exhibited minimum activity.
References
Kahru, A., Dubourguier, H.C.: From ecotoxicology to nanoecotoxicology. Toxicology 269(2–3), 105–119 (2010)
Jin, T., Sun, D., Su, J.Y., Zhang, H., et al.: Antimicrobial efficacy of zinc oxide quantum dots against Listeria monocytogenes, Salmonella Enteritidis, and Escherichia coli O157:H7. J. Food. Sci. 74(1), M46-52 (2009)
Puckett, S.D., Taylor, E., Raimondo, T., Webster, T.J.: The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials 31(4), 706–713 (2010)
Fayed, B., Jayakumar, M.N., Soliman, S.S.: Caspofungin-resistance in Candida auris is cell wall-dependent phenotype and potential prevention by zinc oxide nanoparticles. J. Med. Mycol. 59(12), 1243–1256 (2021)
Roy, A., Gauri, S.S., Bhattacharya, M., Bhattacharya, J.: Antimicrobial activity of caO nanoparticles. J. Biomed. Nanotechnol. 9(9), 1570–1578 (2013)
Marsalek, R.: Particle size and zeta potential of ZnO. J. APCBEE procedia. 9, 13–17 (2014)
Huang, X., Guo, R., Wu, J., Zhang, P.: Mesoporous ZnO nanosheets for lithium ion batteries. J Mater. Lett. 122, 82–85 (2014)
Li, L.H., Deng, J.C., Deng, H.R., Liu, Z.L., et al.: Synthesis and characterization of chitosan/ZnO nanoparticle composite membranes. Carbohydr. Res. 345(8), 994–998 (2010)
Rosset, A., Djessas, K., Goetz, V., Grillo, S., et al.: Sol-gel synthesis and solar photocatalytic activity of Ca-alloyed ZnO nanoparticles elaborated using different precursors. RSC Adv. 10(43), 25456–25466 (2020)
Zak, A.K., Razali, R., Majid, W.H., Darroudi, M.: Synthesis and characterization of a narrow size distribution of zinc oxide nanoparticles. Int. J. Nanomed. 6, 1399–1403 (2011)
Hajiashrafi, S., Motakef Kazemi, N.: Preparation and evaluation of ZnO nanoparticles by thermal decomposition of MOF-5. Heliyon. 5(9), e02152 (2019)
Dutta, S., Ganguly, B.N.: Characterization of ZnO nanoparticles grown in presence of folic acid template. J. Nanobiotechnol. 10, 29 (2012)
Jokela, S., McCluskey, M.: Structure and stability of O− H donors in ZnO from high-pressure and infrared spectroscopy. J. Phys. Rev. B. 72(11), 113201 (2005)
Ghosh, S.P.: Synthesis and characterization of zinc oxide nanoparticles by sol-gel process. National Institute of Technology, India (2012)
Samuel, E.P., Bhadane, H., Chandra, U., Gautam, D.: Sol gel Spin coated ZnO thin films for biosensing applications. J. Int. J. Eng. Technol. Res. 2, 42–44 (2014)
Li, C., Hu, R., Zhou, T., Wu, H., et al.: Special morphologies of Mg, Ca, and Y-doped ZnO/La2O3 composite for photocatalysis. J. Mater. Lett. 124, 81–84 (2014)
Water, W., Yang, Y.-S.: The influence of calcium doped ZnO films on Love wave sensor characteristics. J. Sensors Actuators A: Phys. 127(2), 360–365 (2006)
Hameed, A.S.H., Karthikeyan, C., Sasikumar, S., Kumar, V.S., et al.: Impact of alkaline metal ions Mg 2+, Ca 2+, Sr 2+ and Ba 2+ on the structural, optical, thermal and antibacterial properties of ZnO nanoparticles prepared by the co-precipitation method. J. Mater. Chem. B. 1(43), 5950–5962 (2013)
Pradana, H.Y., Joni, I.M., Men, L.K., Yuliah, Y., et al.: Synthesis and characterization of ZnO: Ca2+ prepared by simple solution method. in AIP Conference Proceedings. AIP Publishing LLC (2018)
Prevention, CfDCa. COVID-19 & antimicrobial resistance. February 25, 2022]. https://www.cdc.gov/drugresistance/covid19.html.
Satoh, K., Makimura, K., Hasumi, Y., Nishiyama, Y., et al.: Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 53(1), 41–4 (2009)
Tanne, J.H.: Covid-19: antimicrobial resistance rose dangerously in US during pandemic, CDC says. British Medical Journal Publishing Group (2022)
Mishra, A., Pradhan, D., Halder, J., Biswasroy, P., et al.: Metal nanoparticles against multi-drug-resistance bacteria. J. Inorg. Biochem. 237, 111938 (2022)
Baptista, P.V., McCusker, M.P., Carvalho, A., Ferreira, D.A., et al.: Nano-strategies to fight multidrug resistant bacteria-“a battle of the titans.” Front. Microbiol. 9, 1441 (2018)
Jia, D., Sun, W.: Silver nanoparticles offer a synergistic effect with fluconazole against fluconazole-resistant Candida albicans by abrogating drug efflux pumps and increasing endogenous ROS. Infect. Genet. Evol. 93, 104937 (2021)
Arias, L.S., Pessan, J.P., de Souza Neto, F.N., Lima, B.H.R., et al.: Novel nanocarrier of miconazole based on chitosan-coated iron oxide nanoparticles as a nanotherapy to fight Candida biofilms. Colloids Surf B Biointerfaces. 192, 111080 (2020)
Padmavathi, A.R., Das, A., Priya, A., et al.: Impediment to growth and yeast-to-hyphae transition in Candida albicans by copper oxide nanoparticles. Biofouling 36(1), 56–72 (2020)
Gad, M.M., Abualsaud, R.: Behavior of PMMA denture base materials containing titanium dioxide nanoparticles: a literature review. Int. J. Biomater. 2019, 6190610 (2019)
Acosta-Humánez, M., Montes-Vides, L., Almanza-Montero, O.J.D.: Sol-gel synthesis of zinc oxide nanoparticle at three different temperatures and its characterization via XRD. IR EPR. Dyna. 83(195), 224–228 (2016)
Hamdy, R., Fayed, B., Hamoda, A.M., Rawas-Qalaji, M., et al.: Essential oil-based design and development of novel anti-Candida azoles formulation. J. Mol. 25(6), 1463 (2020)
Kolekar, T., Yadav, H., Bandgar, S., Deshmukh, P.: Synthesis by sol–gel method and characterization of ZnO nanoparticles. Indian Streams Res. J. 1(1), 1–4 (2011)
Ramadan, M., Nassar, S., Montaser, A., El-Khatib, E., et al.: Synthesis of nano-sized zinc oxide and its application for cellulosic textiles. J. Egypt J. Chem. 59(4), 523–535 (2016)
Torres-Ramos, M.I., Martín-Camacho, U.J., González, J.L., Yañez-Acosta, M.F., et al.: A study of Zn-Ca nanocomposites and their antibacterial properties. Int. J. Mol. Sci. 23(13), 7258 (2022)
Istrate, A.-I., Nastase, F., Mihalache, I., Comanescu, F., et al.: Synthesis and characterization of Ca doped ZnO thin films by sol–gel method. J. Sol-Gel Sci. Technol. 92, 585–597 (2019)
Pan, H., Marsh, J.N., Christenson, E.T., Soman, N.R., et al.: Postformulation peptide drug loading of nanostructures. In: Methods in enzymology, pp. 17–39. Elsevier (2012)
Fayed, B.E., Tawfik, A.F., Yassin, A.E.B.: Novel erythropoietin-loaded nanoparticles with prolonged in vivo response. J. Microencapsul. 29(7), 650–656 (2012)
Smeets, R., Kolk, A., Gerressen, M., Driemel, O., et al.: A new biphasic osteoinductive calcium composite material with a negative Zeta potential for bone augmentation. Head Face Med. 5, 13 (2009)
Teng, N.C., Nakamura, S., Takagi, Y., Yamashita, Y., et al.: A new approach to enhancement of bone formation by electrically polarized hydroxyapatite. J. Dent. Res. 80(10), 1925–1929 (2001)
Mahmud, K.M., Hossain, M.M., Polash, S.A., Takikawa, M., et al.: Investigation of antimicrobial activity and biocompatibility of biogenic silver nanoparticles synthesized using Syzigyum cymosum Extract. ACS Omega 7(31), 27216–27229 (2022)
Ghiloufi, I., El Ghoul, J., Modwi, A., El Mir, L.: Preparation and characterization of Ca-doped zinc oxide nanoparticles for heavy metal removal from aqueous solution. MRS Adv. 1(53), 3607–3612 (2016)
El-Megharbel, S.M., Alsawat, M., Al-Salmi, F.A., Hamza, R.Z.: Utilizing of (zinc oxide nano-spray) for disinfection against “SARS-CoV-2” and testing its biological effectiveness on some biochemical parameters during (COVID-19 pandemic)—“ZnO nanoparticles have antiviral activity against (SARS-CoV-2).” J Coatings. 11(4), 388 (2021)
Hamdy, R., Fayed, B., Mostafa, A., Shama, N.M.A., et al.: Iterated virtual screening-assisted antiviral and enzyme inhibition assays reveal the discovery of novel promising anti-SARS-CoV-2 with dual activity. Int. J. Mol. Sci. 22(16), 9057 (2021)
Bhattacharya, S., Holowka, T., Orner, E.P., Fries, B.C.: Gene duplication associated with increased fluconazole tolerance in Candida auris cells of advanced generational age. Sci. Rep. 9(1), 5052 (2019)
Chowdhary, A., Prakash, A., Sharma, C., Kordalewska, M., et al.: A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–17) in India: role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J. Antimicrob. Chemother. 73(4), 891–899 (2018)
Sánchez-López, E., Gomes, D., Esteruelas, G., Bonilla, L., et al.: Metal-based nanoparticles as antimicrobial agents: an overview. Nanomaterials 10(2), 292 (2020)
Shen, C., James, S.A., de Jonge, M.D., Turney, T.W., et al.: Relating cytotoxicity, zinc ions, and reactive oxygen in ZnO nanoparticle-exposed human immune cells. Toxicol. Sci. 136(1), 120–130 (2013)
Song, W., Zhang, J., Guo, J., Zhang, J., et al.: Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicol. Lett. 199(3), 389–397 (2010)
Acknowledgements
This work was supported by the National Research Centre, Giza, Egypt.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
A.E. Reda contributed to the study conception and design. Material preparation, data collection and analysis were performed by A.E. Reda. B. Fayed contributed to study the antimicrobial activity. The first draft of the manuscript was written by A.E. Reda. The final draft of the manuscript was written by B. Fayed. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable: the research did not involve human participants and/or animals.
Approval for exempt from ethical review No.: EX0030052023.
Consent to participate
All authors have agreed to participate in this research.
Consent for publication
The article was written by the named authors, who are all aware of its content and have given their permission for it to be published.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reda, A.E., Fayed, B. The synthesis of calcium doped zinc oxide ceramic nanoparticles via sol–gel effective against the emerging multidrug-resistant Candida auris. J Aust Ceram Soc 59, 1315–1323 (2023). https://doi.org/10.1007/s41779-023-00912-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-023-00912-9