Abstract
Soils with high cadmium (Cd) content can be decontaminated with phytoremediation. In this work, we aimed at adding nitrogen (N) fertilizer for enhancing the vitality of our test plant, oregano (Origanum vulgare). In a pristine soil, we added Cd at 0, 20, and 40 mg kg−1 soil (thereafter Cd0, Cd20, and Cd40) and two N rates at 0 and 340 kg N ha−1 (thereafter N0 and N1) in a 75-day pot experiment. We found that oregano dry weight increased significantly from 4.95 at control to 7.34 g pot−1 at Cd40N1 (a 148% increase). This indicated that the benefit of added N surpassed the negative effects of the Cd-borne stress. All other treatments had non-significant differences compared to the control. Cadmium content in oregano aerial biomass was zero in the unamended treatments, raised to 0.35 mg kg−1 at Cd40N0, and to 1.11 mg kg−1 at Cd40N1. Further, we assessed the plant’s performance in regard to its potential use as a phytoremediation species. We calculated the Cd soil-to-plant transfer coefficient (TC; maximum was 0.042) and the root-to-shoot translocation factor (TF; maximum 0.171). Both indices exhibited the failure of oregano as a potential hyperaccumulator. However, our findings rather confirmed our scientific hypotheses that N addition to soil boosted plant’s ability to accumulate Cd (as indicated in the significant twofold increase in shoot content of Cd and the 7.8-fold increase in TF at Cd40N1 compared to Cd40N0). We conclude that boosted vigor by added N is a promising method for enhancing phytoremediation of Cd-contaminated soils, but further field tests are necessary regarding oregano to verify those findings.
Similar content being viewed by others
1 Introduction
Cadmium is a mobile metal in the soil–plant interface; hence, it can be bioaccumulated at high concentrations in plant species (Zhao et al. 2021; Yang et al. 2023). Thus, the toxicity symptoms in plants are related to the distraction of essential physiological functions, such as the reduced photosynthetic rate, alteration in protein structure, and adverse effects in water uptake (Dong et al. 2019; Zhao et al. 2021; Ali et al. 2022; El Rasafi et al. 2022).
Cadmium has lower solubility in alkaline soils than in acidic; however, even in alkaline pH values, it can still be readily absorbed by plants (Kicinska et al. 2022). Factors that may affect Cd uptake by plants include genotype, plant age, and vigor (Usman et al. 2023; Haider et al. 2021). Plants in order to alleviate Cd stress develop defense strategies which may be either exclusion mechanisms (to decrease Cd uptake) or tolerance mechanisms (Huang et al. 2020; Haider et al. 2021). A plant that exhibits the ability to translocate significant concentrations of Cd from roots to aerial parts may be a good candidate as a phytoremediation species. Oregano (Origanum vulgare L.) is a species known for its tolerance in abiotic stresses. Hence, it has been used in the recent past as a candidate species for the phytoremediation of soils contaminated with Pb (Thalassinos et al. 2023) and Cr(VI) (Levizou et al. 2019; Thalassinos et al. 2022). For the further examination of its behavior toward Cd uptake, some factors affecting this process should be studied and evaluated—especially factors that may increase the plant’s ability to absorb soil Cd without affecting the metal’s chemical mobility in the soil–plant interface although there are still elements to be elucidated. One such factor may be the addition of ample quantities of N in soil. Cd uptake by the test plant may thus be affected by two mechanisms: (a) plant vigor is increased and this may increase Cd uptake; (b) Cd geochemical fractionation may be altered, its mobility may be increased, and thus Cd uptake by the test plant. There is a research gap regarding which of the two mechanisms would be more effective in the interaction of Cd in oregano. For the evaluation of this second mechanism, there should be an examination of the geochemical fractionation of Cd.
The level of contamination by potentially toxic metals (among which, Cd) is mainly referred to total (or pseudo-total) concentrations. However, this cannot safely give an indication of the environmental risks toxic elements may have because their phyto-availability is dependent upon their fractionation among various availability “pools” in soil. Some of these pools permit Cd to be highly phyto-available (e.g., the soluble and exchangeable species). Thus, the method of sequential extraction is often used to investigate the fate of soil Cd (Silveira et al. 2006; Vollprecht et al. 2020). The sequential extraction which is widely used includes the soluble/exchangeable Cd (fraction 1; F1), carbonate-bound (F2), Fe/Mn oxides-bound (F3), organic matter-bound (F4), and residual (F5) (Tessier et al. 1979; Liang et al. 2014; Shaheen and Rinklebe 2015).
Hence, we hypothesize that the added N to Cd-contaminated soil could have an effect in the alteration of the geochemical fractionation and phytoavailability of Cd due to the fact that the changes in forms of N are rapid and result in, or caused by, alterations of soil characteristics. Also those changes in Cd behavior with added N could be triggered by the increased plant vitality boosted by the amended macronutrient. For example, soil pH is depressed in nitrification, i.e., during the oxidation of N−IIIH4-N toward NVO3-N, a process creating 2 mol H+ per mol of oxidized N, and is expected in normal aerated soils within weeks of N addition. Also, the added N could help to boost the plant’s vigor and ability to accumulate Cd, which presumably may lead to enhanced Cd phytoextraction. To the best of our knowledge, the two described potential mechanisms we test as part of our hypothesis have not been thoroughly investigated and thus need to be further elucidated, a fact that stresses the novelty of our study. For example, in a review by Kulsum et al. (2023), N was not among the nutrients studied as those having a known interaction with Cd in the soil–plant interface. To test our hypotheses and fill the research gab, we aimed in this study to (a) test the effect of added N in Cd geochemical behavior in soil; (b) evaluate the effect of Cd in relation to added N to oregano physiological responses and uptake; (c) examine oregano as a potential candidate plant species for the phytoremediation of Cd-contaminated soils.
2 Materials and Methods
2.1 Experimental Design
For the experiment, soil from the University Farm at Velestino was used. The soil was loamy (sand = 45.2%, clay = 16.0%), had a pH of 7.79, electrical conductivity (EC) of 673 μS cm−1, equivalent CaCO3 = 10.4%, and organic carbon = 1.5%. Details on the characterization analyses are reported in Thalassinos et al. (2021). Also pseudo-total content of Cd was 0.61 mg kg−1.
The soil was divided in three equal parts, which were added with increasing doses of Cd: the first was left unamended (thereafter named “Cd0”); the second was amended with 20 mg Cd kg−1 soil (“Cd20”), and the remaining soil with 40 mg Cd kg−1 (“Cd40”). Cadmium was added as its nitrate salt (Cd(NO3)2.4H2O): For the spiking, 10.97 g of this Cd salt was solubilized in 500 mL of d. H2O, and 2.5 mL of this spiking solution was added to 1 kg of soil to form Cd20 and 5 mL to 1 kg of soil to form Cd40. Spiking was done once. The spiked soils were placed into plastic bags, moisture was also added equal to 2/3 of the soil’s water holding capacity, and they were thoroughly mixed every day for 1 month.
With these amendments, a small amount of NO3-N was unavoidably added, equivalent to 40 kg N ha−1 (or 10 mg N kg−1 soil). This was properly accounted for in the subsequent step of treatment preparations. Each of the three soils was further divided into two parts: the first of these was left without any amendments (thereafter named “N0”), while the second was added with N at a rate equivalent to 300 kg N ha−1 (or 120 mg N kg−1 soil; thereafter named “N1”). In order to make sure that all N0 treatments would account for the same amount of N which was added in the highest Cd treatments, we also added N so that all N0 treatments would have 40 kg N ha−1. In all the treatments with the added N, we had 340 kg N ha−1.
Amendments are shown in detail in Table S1 (Supplementary material). This resulted in the following 6 treatments: (a) Cd0N0 (no added Cd, no added N); (b) Cd0N1 (no Cd, added N at 340 kg N ha−1); Cd20N0 (added Cd at 20 mg kg−1, no N); Cd20N1 (added Cd at 20 mg kg−1, added N at 340 kg ha−1); Cd40N0 (added Cd at 40 mg kg−1, no N); and Cd40N1 (added Cd at 40 mg kg−1, added N at 340 kg ha−1). The N amendment was done with the application of a solution of NH4NO3 (further details are shown in Table S1). In this two-factor design (one factor being added Cd, and the other added N), there were 10 replicates, and mixtures were placed in 60 plastic bags (2 N rates × 3 Cd rates × 10 replicates) containing 1 kg of soil each.
The bags were mixed thoroughly, and then soils were placed in pots, where they were brought to a moisture of ca. 2/3 of their water holding capacity and left to equilibrate for one month, before plants were established. In the meantime, oregano (Origanum vulgare) seeds were placed in seed beds prepared with the unamended soil. When soil mixtures in pots had sufficient time to equilibrate, plants had grown to a height of 7 cm. They were then transplanted to the 60 pots, one plant per pot. That day, the 11th of April 2019, was considered the beginning of the experiment. Plants were left to grow for 75 days until the 24th of June 2019. The pots were left in open-air conditions for the course of the experiment, watered at regular intervals so that they maintain their moisture content, and translocated regularly in order to compensate for any differences in ambient conditions (temperature and light).
On Day 75, aerial biomass of the test plant in the pots was harvested by being cut at 2 cm above soil surface. Biomass was immediately (i.e., while fresh) separated into leaves and shoots, weighed (so that fresh weight may be recorded), and placed into preweighed paper bags. Then, soils in the pots were sampled by taking 3 cores of soil per pot. The cores removed soil from the whole depth of the pots so that sample uniformity may be ensured. The three subsamples per pot were mixed into one composite sample, placed in paper bags and taken to air-dry. Immediately after soil sampling, roots were carefully removed from pots and delicately washed under running tap water in order to make sure that there were no adhered soil particles to them. Roots were then washed with d. H2O and placed into pre-weighed paper bags. All bags containing the collected plant material (leaves, shoots, and roots) were placed into a forced-draught oven at 70 °C for 48 h until no further weight loss. After that, they were weighed (so that dry weight may be recorded), ground to fine powder using a non-metallic mill and placed into plastic containers ready for analysis. Soils, when air-drying was complete, were sieved through a 2-mm sieve and placed into plastic containers ready for analysis.
2.2 Measurements and Analyses
2.2.1 Soil Analyses
We measured pH (at a soil suspension of a ratio of 1-to-2.5 with d. H2O) (pH Meter pH 526 WTW) and EC (at a soil suspension of a ratio of 1-to-5 with d. H2O) (Metrohm 712 Conductometer). Also the pseudo-total Cd concentrations were determined with aqua regia (1 of soil digested with 15 mL of concentrated HCl:HNO3 at a ratio of 1:3 for 5 h at 140 °C). Analyses were performed according to Rowel (1994). Samples were also subjected to a 5-step sequential extraction to fractionate Cd in the soil into five geochemical forms, where fraction 1 (F1) was the soluble and exchangeable Cd, F2 was the carbonate-bound Cd, F3 was the Fe/Mn oxides-bound Cd, F4 the organically bound Cd, and F5 was the residual fraction of Cd. The protocol was performed according to the method of Tessier et al. (1979), as modified by Sánchez-Martín et al. (2007) and used by others (e.g., Shaheen and Rinklebe 2015). The full details of the five-step chemical fractionation procedure are shown in the supplementary material (Table S2).
2.2.2 Plant Analyses
The photosynthetic pigments were extracted from 15 mature leaves per treatment (one sample per plant) just before the final harvest. The extraction was performed in 80% acetone, followed by a 10 min at 4000 rpm centrifugation, and finally the absorbance of the supernatant was read with a dual-beam spectrophotometer (SHIMATZU, UV-1900) at 720, 663, 646 and 470 nm. The equations of Lichtenthaler and Wellburn (1983) were used to calculate the concentrations of chlorophyll a and b, as well as the carotenoids.
The same sampling scheme was followed for the determination of leaf concentration of malondialdehyde (MDA). Fresh leaves (0.4 g) were homogenized in 5 mL of 0.1% (w/v) trichloroacetic acid and then centrifuged at 5000 rpm for 10 min. The supernatant (0.5 mL) was mixed with 4 mL of 20% trichloroacetic acid containing 0.5% thiobarbituric acid, heated in a water bath at 95 °C for 30 min, and subsequently cooled in ice. After a second centrifugation at 5000 rpm for 10 min, the supernatant was subjected to absorbance measurements at 532 nm (for MDA) and 600 nm (for nonspecific absorption). The latter was subtracted from the 532 nm values and then the MDA concentration was calculated with the use of 155 mM−1 cm−1 extinction coefficient (Fu and Huang 2001).
From the ground plant material, 0.5 g was weighed into porcelain crucibles and dry-ashed at 500 °C for 5 h, and the ash was extracted with 20 mL 20% HCl to a final volume of 50 mL (Jones 1991). The levels of Cd were determined in all extracts (both in soil and plant) with atomic absorption spectrometry (Perkin Elmer A3300).
2.2.3 Plant Indices
We determined the following indices, as per Antoniadis et al. (2017):
where TC = Transfer coefficient (unitless).
where TF = Translocation factor (unitless).
The following two indices are used as per Thalassinos et al. (2023):
where Uptake is in unit of μg Cd in plant per pot or μg Cd in plant per kg soil.
Number of harvests to decrease soil Cd concentration to half of the current concentration (unitless):
where 103 is for unit conversion from μg to mg.
2.3 Statistical Analysis and Quality Control
Data quality control was addressed with the systematic use of blanks (to account for any laboratory contamination) and soil and plant reference materials (to account for required measurement precision). The percentage of recovery of extracted Cd from the soil and plant reference materials ranged between 92 and 108%. All analyses were performed in triplicates and acceptable coefficient of variation was less than 15%. The primary and the secondary (i.e., indices) experimental data underwent a two-way ANOVA (analysis of variance) for the identification of significant differences at the level of 95% (p < 0.05); the first factor was added N and the second added Cd. Also data underwent a post hoc analysis according to Duncan.
3 Results and Discussion
3.1 Effects of Cd/N Levels on Cd Fate and Potential Mobility in Soil
The 5-step Cd fractionation revealed that the unamended control of no added Cd, both with and without added N, had Cd in the residual fraction of F5 (Cd0N0 was 0.88 and Cd0N1 0.81 mg kg−1; no significantly different). In the treatment added with 20 mg kg−1 Cd, the F1 fraction increased significantly compared to the control, and in the 40-mg kg−1 treatment it further increased significantly both in the treatments of added and no added N (Table 1). However, the percentage of the distribution of F1 to the total extractable Cd was non-significantly different between the treatment of 20 mg kg−1 of added Cd (i.e., 9%) and that of 40 mg kg−1 (8%, Table S3, Supplementary material). As for the second fraction, F2 (carbonate-bound Cd), it was the highest along with the residual (F5) among the five extractable Cd species: In all Cd-added treatments, F2 was significantly higher compared to F1, F3, and F4, and similarly high to F5 (i.e., F2 and F5 did not differ significantly).
Indeed, in the treatments added with 20 and 40 mg kg−1, both F2 and F5 accounted for 42–46% of the total Cd—percentages did no differ significantly for F2 and F5 in all four Cd20N0, Cd20N1, Cd40N0, and Cd40N1 treatments (Table S2). Thus, at all added Cd treatments, the fraction of Cd being carbonate-bound (F2) was ca. 45% of the total soil Cd, while the residual (F5) was another 45%, with the rest 10% being distributed at F1, F3, and F4. This means that Cd had a particularly high mobility in soil, and thus phytoavailability, contrary to the case of other heavy metals, where F5 (the most highly inert fraction) is usually the dominant among the 5 evaluated fractions (Rinklebe and Shaheen 2014). The high portion of Cd in the non-residual fractions (55%) indicates its high potential mobility in soil, particularly the anthropogenically contaminated and spiked soils, which is in agreement with other studies (Lair et al. 2008; Rinklebe and Shaheen 2014; Kubier et al. 2019; Lashen et al. 2022). For example, Rinklebe and Shaheen (2014) found that Cd was abundant in the non-residual fractions and accounted for 89.7–96.8% of the total Cd in 39 soil samples collected from 7 soil profiles repressing floodplain soils along the Elbe River in Germany. The high abundance of Cd in the non-residual fractions could be due to its high affinity to carbonates especially in alkaline soils like ours (Kubier et al. 2019). The retention of added Cd in the carbonate fraction is not unexpected in a soil with a very high total CaCO3 content such as this studied here. The presence of added Cd at F2 (and to some extend also in the most highly mobile fraction of F1) shows the increased risk of its uptake by the plant (Devi and Bhattacharyya 2018; Asmoay et al. 2019).
The percentage of Cd found in the residual fraction is not unexpected either, although soil Cd was spiked into soil, as residual does not account for Cd embedded into the primary soil minerals, but rather for Cd transferred to soil pools inaccessible to plants (Ahmadipour et al. 2014; Lima et al. 2023). These could include interlayer areas in clay particles. The one month of soil incubation before the introduction of plants and the commencement of the experiment served the exact purpose of allowing sufficient time for added Cd to be distributed to the various geochemical soil fractions. Over time, metallic elements, such as Cd, tend to be re-distributed in less mobile soil pools. Thus, it is expected that the residual fraction will increase over time. E.g., Verna et al. (2023) reported that over 50% of a multi-element-contaminated soil was found to have Cd in its residual form, none of which was embedded in crystalline structures of primary soil minerals. Likewise, Liu et al. (2023), studying a skarn-type copper tailing, reported that residual Cd was found in its residual form. It is, however, noteworthy, that in studies where Cd has been spiked prior to the experimentation, Cd residual species is lower than when deposited over time: e.g., Dhaliwal et al. (2023) and Hamid et al. (2020) both found that Cd residual form in Cd-spiked soils was ca. 10% of that added. In our work, for comparison, the added Cd was re-distributed in the residual form at higher percentage (45%). There are two possible reasons for this: (a) alkaline soil pH, which reduces Cd solubility by causing enhanced electrostatic attraction of Cd with soil colloids and by encouraging the formation of the insoluble metal hydroxide species; (b) the equilibration time prior to the commencement of the experimentation, which included vigorous soil mixing, which would lead the added Cd to interact more thoroughly with the sol matrix. It must also be added that the satisfactory recovery of Cd as measured in the 5 geochemical species of up to 90% of that added bears evidence of the quality assurance of this finding.
Hence, the fractionation analysis indicates that Cd mobility in soil (which also affected Cd uptake by oregano, as will be discussed later) was not related to any geochemical changes of Cd in the level of the studied soil. This seems to disprove the first hypothesis of this work (i.e., any alterations in Cd mobility are soil-related due to geochemical changes induced by added N) and rather gives evidence to the second hypothesis (i.e., Cd mobility alterations are plant-related). An additional evidence that Cd behavior was not dictated by soil chemical changes is the fact that soil pH was with no significant differences among treatments (Table S4, Supplementary material). Added N could have induced a reduction in pH due to nitrification, and that could possibly cause an increase in Cd mobility. If this was the case, we would expect a re-distribution in the fractionation, an effect that was not actually observed.
The summation of the five fractions (ΣF1-F5) of extracted Cd (Table 1) was very close to the pseudo-total content, as extracted by aqua regia (Table 2), a finding indicating the satisfactory recovery of the used sequential extraction procedure. With aqua regia, the measured Cd was close to that added: it was found to be 18.32 at Cd20N0 and 17.21 mg kg−1 at Cd20N1 (not significantly different between them), and 35.00 (Cd40N0) 34.49 mg kg−1 (Cd40N1) (again, with no differences between the two). It is likely that these findings concerning the distribution of Cd in the 5 soil fractions would be different if tested soil differed in particle size distribution and pH.
3.2 Effects of Cd Levels and N Fertilizer on the Oregano Growth Parameters
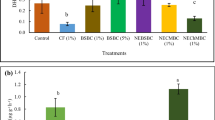
Shoot dry weights did not show any significant differences among treatments, ranging from 2.15 g (at Cd0N0) to 3.02 g (at Cd20N0) (Fig. 1). As for the leaves, all treatments had similar dry weights compared to the control (Cd0N0), ranging from 2.24 to 3.22 g, except for Cd40N1 which was found to be significantly higher than all other treatments (4.61 g, p < 0.05). Root dry biomass was also significantly higher at Cd40N1 compared to all other treatments with 3.05 g, with the only exception of Cd20N0 (2.32 g) and Cd20N1 (2.72 g), which showed no significant differences with Cd40N1. Nitrogen content in plant ranged from 0.63 to 0.82% in the six treatments, but the differences among them were not significant (Table S4; supplementary material).
Dry weights (in g per pot) of shoots, leaves and roots of oregano in the various experimental treatments. Different letters within each plant part denote statistically significant differences among treatments. According to two-way ANOVA results both N addition and Cd levels, as well as their interaction, were statistically significant factors at p < 0.05. Error bars indicate standard error
Thus, in aerial plant biomass, added Cd did not seem to have any particular toxic effects (the factor “Cd” had a non-significant effect; p = 0.737), not even in the highest addition of 40 mg Cd kg−1, a dosage 13 times higher than the maximum allowable Cd concentration according to the Directive 86/278/EC (1986). The increased biomass with added N in the high Cd additions shows that the beneficial effect of N was more dominant than any negative Cd effects, which were not even expressed in the test plant. The result that N-bearing materials may mask any potential adverse effects on the biomass of test plants concurs with findings from the literature, as is the case with Wang et al. (2023).
However, in most such cases, the studied materials that apply N also increase the Cd retention capacity—as in the previously mentioned work, where the added material was biochar. Thus, it is not entirely clear if the increased biomass is caused by added N or by the decreased Cd availability. Similar results have been reported in a maize cultivation, where added N (100 mg of urea kg−1 soil) led to the minimization of toxic effects of Cd (added at 20 mg kg−1 as CdCl2)—a finding recorded in growth rate, chlorophyll content, and photosynthetic rate. In that same work, added N in soil increased Cd translocation to aerial tissues (An et al. 2022). Similar were the results reported for Panicum maximum in an experiment of soilless culture, where N was added as a nutrient solution with a ratio of NH4-to-NO3 of 1 (as was the case in our work), added N at 15 mM and added Cd as high as 1.0 mM: it was reported that Cd upward translocation was increased (de Sousa and Monteiro 2019).
There is another possible mechanism that could explain this plant behavior, i.e., phytohormesis. Shahid et al. (2020) in their review defined phytohormesis as a stimulation in plant growth at additions of otherwise toxic elements, including Cd. Associated plant toxicity reduction mechanisms are shown to include the activation of plant physiological tolerance reactions after Cd-induced “overproduction of reactive oxygen species (ROS), and interplay between phytohormones and Cd-mediated ROS production toward plant growth” (Shahid et al. 2020).
The total chlorophyll content of oregano leaves was found significantly increased in all N-addition treatments compared with Cd0N0, irrespective of the amount of Cd amended to the soil (Fig. 2). Interestingly, Cd40N0 also showed higher values than the other Cd20N0 treatments, but similar to Cd20N1 and Cd40N1. The statistical analysis revealed that N supplementation, Cd levels, and their interaction were significant factors in shaping the chlorophylls profile. The MDA concentration in oregano leaves was kept in the same low level in all treatments. A slightly higher content was obvious in the N-added treatments, yet statistical analysis revealed no significant differences.
The concentration of A total chlorophylls and B malondialdehyde (MDA) of oregano leaves (n = 15). Different letters in (A) denote statistically significant differences among treatments. Absence of post hoc letters (in graph B) indicates that there were no significant differences among treatments. According to two-way ANOVA results both N addition and Cd levels, as well as their interaction were statistically significant factors at p < 0.05. The absence of letters in (B) denote no significant differences. Error bars indicate standard error
The results of the present work demonstrated that N addition to the soil promotes the biosynthesis of photosynthetic pigments in oregano. Total chlorophyll content as well as carotenoid content (data not shown) responded with increased values to N addition irrespective of the presence of Cd in the soil. It is well-documented that Cd interferes and inhibits chlorophyll biosynthesis, among other physiological processes, such as nutrient uptake, water relations, and enzyme activity (Waheed et al. 2022; Das et al. 1997). Consequently, the reduction of the concentration of photosynthetic pigments is a common symptom in plants growing in Cd-enriched soils. Dressler et al. (2014) reported that soil Cd of similar levels to ours lowered total chlorophyll content, and carotenoid content in Echium vulgare, similarly to Eruca sativa (Waheed et al. 2022) and Dittrichia viscosa (Fernández et al. 2013). However, Manousaki and Kalogerakis (2009) found that 20 ppm Cd did not affected the chlorophyll content of Atriplex halimus, a salt-tolerant Mediterranean scrub. On the contrary, an increase was observed under Cd and salinity stress, corroborating our results in the Cd40N0 treatment.
Leaf MDA has been typically used in stress-related studies as a lipid peroxidation marker. The formation of MDA is the product of enzymatic and non-enzymatic lipid peroxidation processes caused by reactive oxygen species, usually maximized by stress conditions (Morales and Munné-Bosch 2019). In the present study, the levels of leaf MDA were low and non-different among treatments. Cadmium stress has been shown to trigger MDA accumulation, as evidenced by the two-fold increase in peppers (Kaya et al. 2023) and Eruca sativa (Waheed et al. 2022) grown under Cd levels similar to ours. Oregano has been reported to respond to high salinity with an increase in MDA content (Tanaka et al. 2018). The results of our study may indicate either the absence of oxidative stress, thus MDA response, or the relaxation phase of MDA increase. Concerning the latter, there is a new concept focusing on the protective role of MDA under stress through the activation of plant defense genes (Morales and Munné-Bosch 2019). In this process, MDA has been found to transiently increase activating the ROS scavengers. Then a relaxation of MDA content occurs as the successful dissipation of ROS disappears the signs of damage (Tounekti et al. 2011). Under this framework, low MDA levels may represent an efficient antioxidant protection of oregano leaf.
There seem to be, among others, three main plant defense mechanisms against Cd toxicity, i.e., root exudation, Cd exclusion, and Cd speciation. The first is related to the process under which tolerant plants release substances from their roots that can help in Cd exclusion and detoxification. The second to plants that can limit the uptake of Cd into their cells through the use of transition metal transporters. And the former to plants that bind Cd with carboxylic acids or metal binding peptides like phytochelatins (Sebastian et al. 2018). Also plants can boost their defense by regulating endogenous Ca2+ levels, which helps in enhancing Cd tolerance due to the chemical similarity of the two metals (Cheng et al. 2021).
3.3 Effects of Cd Levels and N Fertilizer on Cd Accumulation and Translocation by Oregano (Phytoremediation Indices)
Cadmium content in leaves was zero in the treatments with no added Cd (Cd0N0 and Cd0N1), and increased in the treatments of added Cd at 20 mg kg−1 (0.61 at Cd20N0 and 0.65 mg kg−1 at Cd20N1), although the differences were not significant. At Cd40N1, Cd content further increased to 1.11 mg kg−1, exhibiting significant difference (p < 0.05) with all the other experimental treatments. This rather novel finding reveals that added N increased plant capacity to absorb Cd to aerial biomass. In the literature, there are reports where increased plant vigor through various practices (chemical or biological, e.g., Labudda et al. 2022) lead to increased tolerance toward toxic metals, but our work, to the best of our knowledge, is the first to report increased Cd concentration with added N.
In order to further assess the accumulation of Cd to our test plant, we measured Cd content in leaves per fresh biomass. We aimed to assess if fresh leaves were safe to be consumed by humans by making comparisons with the maximum allowable concentrations as set by the Directive EC/2006/1881 (2006): Under the category “herbs”, (the closest relative category for oregano) is 0.050 mg Cd kg−1 of fresh weight, equivalent to 0.50 mg Cd kg−1 dry weight for a moisture content of 90%. This limit was overcome even in the treatment of added Cd at 20 mg kg−1, while at Cd40N1 Cd was twice as high as the limit value. On the other hand, added N seemed to have the opposite effect in Cd concentration, as Cd at Cd40N1 was lower than at Cd40N0 (actually, it was almost halved). This likely means that the presence of N assisted Cd in its upward translocation—a discussion that will be related to TF.
As for the soil-to-plant transfer (quantified by the TC), it was calculated in order to assist us in categorizing our test plant as a potential phytoremediation species. Although there is not a clear threshold set in the literature, usually hyperaccumulators are expected to have TC close to, or even higher than, unity. In our case, TC was much lower than that. In the treatments without N addition, TC decreased with added Cd, a trend that was not unexpected, as explained by Antoniadis et al. (2017): Α toxic element added to soil cannot be absorbed by a plant linearly when applied at high concentrations, as plant tends to decrease its uptake ability gradually with increased exposure. On the other hand, it is noteworthy that TC increased at Cd40N1. Actually, it was nearly doubled, and it was retained without significant differences compared to the high values of Cd20N0 and Cd20N1. The TC values were higher at Cd20N0 than at Cd40N0, and at Cd20N1 than at Cd40N1, but Cd20N0 (TC = 0.028) did not differ from Cd20N1 (0.042); similarly, Cd40N0 (0.008) did not differ from Cd40N1 (0.032).
Also comparing the treatments added with Cd at 20 and at 40 mg kg−1, both with and without N, differences were found to be non-significant. The highest value of TC was that of Cd20N1, which was significantly different from Cd40N0 and the two control treatments without added Cd. In similar works, the trend is rather the opposite when factors that increase ion exchange capacity of the soil are added (Usman et al. 2023). This shows that added N makes the plant capable of retaining its ability for high Cd uptake even at high additions through soil. This is likely associated to the increased vitality of the test plant due to amble nutrition.
As for the TF, in the non-added-N regime, in the treatment with 40 mg kg−1 added Cd (Cd40N0 = 0.022), it was not different from that at Cd20N0 (0.112). However, the N-added treatment (Cd40N1 = 0.171) had a significantly different TF compared to Cd40N0 (0.022). In order for a plant to be characterize as a hyperaccumulator, TF must be higher than unity—i.e., the test plant should utilize Cd as a nutrient rather than as a toxic substance. Thus, oregano was far from achieving this threshold. As in the case of TC, it is noteworthy that at Cd40N1 added N led to significant increase (a ca. fivefold increase) compared to the treatment without added N (Cd40N0). This confirms the significant acceleration of the upward translocation of Cd from roots to the aerial biomass. It must also be added that the requirement for TF > 1 is rarely ever reported for plants tested in growth experiments with soil (Wang et al. 2022), as was the case here.
Uptake (amount of Cd in plant per pot, i.e., μg of plant Cd pot−1) increased from zero (in the two Cd-unamended treatments) to its highest value of 3.022 μg Cd at Cd40N1. This value was significantly different from the two unamended treatments and from Cd40N0 (1.276), but not different from Cd20N0 (1.764) and Cd20N1 (1.926). In our evaluation related to the number of harvests needed to halve the current soil Cd concentration, we found that in the treatment with Cd added at 40 mg kg−1, the addition of N caused a significant fivefold decrease in the number of necessary harvests (from ca. 51,000 to 10,000), confirming the beneficial effect of added N, as also explored earlier in the discussion. However, there must be added that the numbers are so high that rather make the option of phytoremediation through the use of oregano a non-attractive option. In another similar work, Antoniadis et al. (2021), regarding 12 wild species in Germany, found that the amount of harvests required to halve current soil Cd were one-to-two orders of magnitude lower than those reported here. This comes rather as a confirmation that oregano is not to be treated as a Cd hyperaccumulator.
Compared to other tested plant species, oregano indeed did not fare well. For example, Min et al. (2022) tested cotton and found a TC of 1.10, while Chen et al. (2015) measured TF for the same species above 1, although added Cd was at low rates of 1.26 mg kg−1. Also, soybean was tested by El-Esawi et al. (2020) and Li et al. (2019). Both works found that TC was high enough to qualify their tested species as a hyperaccumulator, but it was reduced when soils were amended with stabilizing materials. Moreover, Li et al. (2022) tested rapeseed and found that, when intercropped with wheat, its TC of 0.97 decreased considerably leading to the phyto-stabilization of Cd. Similarly, Shahid et al. (2019) tested flax, which was found to decrease its TC by 31%.
4 Conclusions
Oregano in this study did not have the characteristics of a typical Cd hyperaccumulator plant, as judged by TC and TF. However, the application through soil of generous doses of N led to a significant increase in Cd uptake, and also increased translocation to plant aerial parts, without any decline in the rate of uptake with added Cd from 20 to 40 mg kg−1. The hypothesis that this increased uptake capacity of plant could be related to alteration of the soil geochemical Cd behavior was examined with the fractionation of Cd. This evaluation revealed that no significant changes in soil Cd pools were found, indicating that Cd-boosted uptake with added N was entirely a plant-related effect. In conclusion, our findings open the possibility of exploring the effect of increased plant vitality (as assessed by plant growth and plant physiological parameters) and vigor as part of a technique to boost the uptake of PTEs, accelerating thus the phytoremediation process, without necessarily altering their geochemical behavior in soil. Thus, enhanced plant vitality through N fertilization can function toward materializing phytoremediation efforts. However, the viability of phytoremediation is doubtful given the high amount of harvests of oregano required to decrease soil Cd content. It is possible that due to the practical difficulty of such scheme, a paradigm shift may be considered toward phytomanagement of contaminated soils with the use of non-edible crops, rather than phytoremediation.
Availability of Data and Materials
Not applicable.
References
Ahmadipour F, Bahramifar N, Ghasempouri SM (2014) Fractionation and mobility of cadmium and lead in soils of Amol area in Iran, using the modified BCR sequential extraction method. Chem Spec Bioavailab 26:31–36. https://doi.org/10.3184/095422914X13884321932037
Ali B, Gill RA (2022) Editorial: Heavy metal toxicity in plants: recent insights on physiological and molecular aspects, volume II. Front Plant Sci 13:1016257. https://doi.org/10.3389/fpls.2022.1016257
An T, Wu Y, Xu B, Zhang S, Deng X, Zhang Y, Siddique KHM, Chen Y (2022) Nitrogen supply improved plant growth and Cd translocation in maize at the silking and physiological maturity under moderate Cd stress. Ecotoxicol Environ Saf 230:113137. https://doi.org/10.1016/j.ecoenv.2021.113137
Antoniadis V, Levizou E, Shaheen SM, Ok YS, Sebastian A, Baum C, Prasad MNV, Wenzel WW, Rinklebe J (2017) Trace elements in the soil-plant interface: phytoavailability, translocation, and phytoremediation—a review. Earth-Sci Rev 171:621–645. https://doi.org/10.1016/j.earscirev.2017.06.005
Antoniadis V, Shaheen SM, Stark HJ, Wennrich R, Levizou E, Merbach I, Rinklebe J (2021) Phytoremediation potential of twelve wild plant species for toxic elements in a contaminated soil. Environ Int 146:106233. https://doi.org/10.1016/j.envint.2020.106233
Asmoay ASA, Salman SA, El-Gohary AM, Sabet HS (2019) Evaluation of heavy metal mobility in contaminated soils between Abu Qurqas and Dyer Mawas Area, El Minya Governorate. Upper Egypt Bull Nat Res Center 43:88. https://doi.org/10.1186/s42269-019-0133-7
Chen Z, Zhao Y, Fan L, Xing L, Yang Y (2015) Cadmium (Cd) localization in tissues of cotton (Gossypium hirsutum L.), and its phytoremediation potential for Cd-contaminated soils. Bull Environ Contam Toxicol 95:784–789. https://doi.org/10.1007/s00128-015-1662-x
Cheng Y, Wang N, Liu R, Bai H, Tao W, Chen J (2021) Cinnamaldehyde facilitates cadmium tolerance by modulating Ca2+ in Brassica rapa. Water Air Soil Pollut 232:19. https://doi.org/10.1007/s11270-020-04952-w
Commission Directive 2006/1881/EC of 19 (2006) Setting maximum levels for certain contaminants in food stuffs. Official Journal of the European Communities:L364–5–24
Council Directive 86/278/EEC of 12 June 1986 on the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture
Das P, Samantaray S, Rout GR (1997) Studies of cadmium toxicity in plants: a review. Environ Pollut 98:29–36. https://doi.org/10.1016/S0269-7491(97)00110-3
de Sousa LT, Monteiro FA (2019) Nitrogen form regulates cadmium uptake and accumulation in Tanzania guinea grass used for phytoextraction. Chemosphere 236:124324. https://doi.org/10.1016/j.chemosphere.2019.07.055
Devi U, Bhattacharyya KG (2018) Mobility and bioavailability of Cd Co, Cr, Cu, Mn and Zn in surface runoff sediments in the urban catchment area of Guwahati. India Appl Water Sci 8:18. https://doi.org/10.1007/s13201-018-0651-8
Dhaliwal SS, Sharma V, Shukla AK, Taneja PK, Lovedeep K, Verma V, Kaur M, Kaur J (2023) Exploration of Cd transformations in Cd spiked and EDTA-chelated soil for phytoextraction by Brassica species. Environ Geochem Health 45:8897–8909. https://doi.org/10.1007/s10653-022-01260-6
Dong Q, Fang J, Huang F, Cai K (2019) Silicon amendment reduces soil Cd availability and Cd uptake of two Pennisetum species. Int J Environ Res Public Health 16:1624. https://doi.org/10.3390/ijerph16091624
Dresler S, Bednarek W, Wójcik M (2014) Effect of cadmium on selected physiological and morphological parameters in metallicolous and non-metallicolous populations of Echium vulgare L. Ecotoxicol Environ Saf 104:332–338. https://doi.org/10.1016/j.ecoenv.2014.03.019
El Rasafi Τ, Oukarroum Α, Haddioui Α, Song Η, Kwon ΕE, Bolan Ν, Tack FMG, Sebastian A, Prasad MNV, Rinklebe J (2022) Cadmium stress in plants: a critical review of the effects, mechanisms, and tolerance strategies. Crit Rev Environ Sci Technol 52:675–726. https://doi.org/10.1080/10643389.2020.1835435
El-Esawi MA, Elkelish A, Soliman M, Elansary HO, Zaid A, Wani SH (2020) Serratia marcescens BM1 enhances cadmium stress tolerance and phytoremediation potential of soybean through modulation of osmolytes, leaf gas exchange, antioxidant machinery, and stress-responsive genes expression. Antioxidants 9:43. https://doi.org/10.3390/antiox9010043
Fernández R, Bertrand A, Reis R, Mourato MP, Martins LL, González A (2013) Growth and physiological responses to cadmium stress of two populations of Dittrichia viscosa (L.) Greuter. J Hazard Mater 244–245:555–562. https://doi.org/10.1016/j.jhazmat.2012.10.044
Fu J, Huang B (2001) Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ Exp Bot 45:105–114. https://doi.org/10.1016/S0098-8472(00)00084-8
Haider F, Liqun C, Coulter J, Cheema S, Wu J, Zhang R, Wenjun M, Farooq M (2021) Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol Environ Saf 211:111887. https://doi.org/10.1016/j.ecoenv.2020.111887
Hamid Y, Tang L, Hussain B, Usman M, Liu L, Cao X, Ulhassan Z, Khan MB, Yang X (2020) Cadmium mobility in three contaminated soils amended with different additives as evaluated by dynamic flow-through experiments. Chemosphere 261:127763. https://doi.org/10.1016/j.chemosphere.2020.127763
Huang X, Duan S, Wu Q, Yu M, Shabala S (2020) Reducing cadmium accumulation in plants: structure-function relations and tissue-specific operation of transporters in the spotlight. Plants 9:223. https://doi.org/10.3390/plants9020223
Jones JB Jr, Wolf B, Mills HA (1991) Plant analysis handbook: a practical sampling, preparation, analysis, and interpretation guide. Micro-Macro Publishing, Athens, GA
Kaya C, Ashraf M, Alyemeni MN, Rinklebe J, Ahmad R (2023) Trehalose and NO work together to alleviate Cd toxicity in pepper (Capsicum annuum L.) plants by regulating cadmium sequestration and distribution within cells and the antioxidant defense system. Sci Hortic 314:11948. https://doi.org/10.1016/j.scienta.2023.111948
Kicińska A, Pomykała R, Izquierdo-Diaz M (2022) Changes in soil pH and mobility of heavy metals in contaminated soils. Eur J Soil Sci 73:e13203. https://doi.org/10.1111/ejss.13203
Kubier A, Wilkin RT, Pichler T (2019) Cadmium in soils and groundwater: a review. Appl Geochem 108:104388. https://doi.org/10.1016/j.apgeochem.2019.104388
Kulsum PGPS, Khanam R, Das S, Nayak AK, Tack FMG, Meers E, Vithanage M, Shahid M, Kumar A, Chakraborty S, Bhattacharya T, Biswas JK (2023) A state-of-the-art review on cadmium uptake, toxicity, and tolerance in rice: From physiological response to remediation process. Environ Res 220:115098. https://doi.org/10.1016/j.envres.2022.115098
Labudda M, Dziurka K, Fidler J, Gietler M, Rybarczyk-Płonska A, Nykiel M, Prabucka B, Morkunas I, Muszynska E (2022) The alleviation of metal stress nuisance for plants—a review of promising solutions in the face of environmental challenges. Plants 11:2544. https://doi.org/10.3390/plants11192544
Lair GJ, Graf M, Zehetner F, Gerzabek MH (2008) Distribution of cadmium among geochemical fractions in floodplain soils of progressing development. Environ Pollut 156:207–214. https://doi.org/10.1016/j.envpol.2007.12.011
Lashen ZM, Shams MS, El-Sheshtawy HS, Slaný M, Antoniadis V, Yang X, Sharma G, Rinklebe J, Shaheen SM, Elmahdy SM (2022) Remediation of Cd and Cu contaminated water and soil using novel nanomaterials derived from sugar beet processing- and clay brick factory-solid wastes. J Hazard Mater 428:128205. https://doi.org/10.1016/j.jhazmat.2021.128205
Levizou E, Zanni AA, Antoniadis V (2019) Varying concentrations of soil chromium (VI) for the exploration of tolerance thresholds and phytoremediation potential of the oregano (Origanum vulgare). Environ Sci Pollut Res 26:14–23. https://doi.org/10.1007/s11356-018-2658-y
Li X, Wang X, Chen Y, Yang X, Cui Z (2019) Optimization of combined phytoremediation for heavy metal contaminated mine tailings by a field-scale orthogonal experiment. Ecotoxicol Environ Saf 168:1–8. https://doi.org/10.1016/j.ecoenv.2018.10.012
Li X, Li Y, Kang X, Yu J, Gao S, Zhang J, Wang H, Pan H, Yang Q, Zhuge Y, Lou Y (2022) Effective utilization of weak alkaline soils with Cd-contamination by wheat and rape intercropping. Ecotoxicol Environ Saf 248:114335. https://doi.org/10.1016/j.ecoenv.2022.114335
Liang S, Wang X, Li Z, Gao N, Sun H (2014) Fractionation of heavy metals in contaminated soils surrounding non-ferrous metals smelting area in the North China Plain. Chem Spec Bioavail 26:59–64. https://doi.org/10.3184/095422914X13885123689811
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592. https://doi.org/10.1042/bst0110591
Lima JZ, Nauerth IMR, da Silva EF, Pejon OJ, Rodrigues VGS (2023) Competitive sorption and desorption of cadmium, lead, and zinc onto peat, compost, and biochar. J Environ Manage 344:118515. https://doi.org/10.1016/j.jenvman.2023.118515
Liu B, Jiang S, Guan D-X, Song X, Li Y, Zhou S, Wang B, Gao B (2023) Geochemical fractionation, bioaccessibility and ecological risk of metallic elements in the weathering profiles of typical skarn-type copper tailings from Tongling. China Sci Total Environ 894:164859. https://doi.org/10.1016/j.scitotenv.2023.164859
Manousaki E, Kalogerakis N (2009) Phytoextraction of Pb and Cd by the Mediterranean saltbush (Atriplex halimus L.): Metal uptake in relation to salinity. Environ Sci Pollut Res 16:844–854. https://doi.org/10.1007/s11356-009-0224-3
Min T, Luo T, He H, Qin J, Wang Y, Cheng L, Ru S, Li J (2022) Dissolved organic matter–assisted phytoremediation potential of cotton for Cd-contaminated soil: a relationship between dosage and phytoremediation efficiency. Environ Sci Pollut Res 29:84640–84650. https://doi.org/10.1007/s11356-022-21485-3
Morales M, Munné-Bosch S (2019) Malondialdehyde: facts and artifacts. Plant Phys 180:1246–1250. https://doi.org/10.1104/pp.19.00405
Rinklebe J, Shaheen SM (2014) Assessing the mobilization of cadmium, lead, and nickel using a seven-step sequential extraction technique in contaminated floodplain soil profiles along the Central Elbe River Germany. Water Air Soil Pollut 225:2039. https://doi.org/10.1007/s11270-014-2039-1
Rowell DL (1994) Soil science: methods and applications. Prentice Hall, Harlow, UK
Sánchez-Martín MJ, García-Delgado M, Lorenzo LF, Rodríguez-Cruz MS, Arienzo M (2007) Heavy metals in sewage sludge amended soils determined by sequential extractions as a function of incubation time of soils. Geoderma 142:262–273. https://doi.org/10.1016/j.geoderma.2007.08.012
Sebastian A, Nangia A, Prasad MNV, Rattanapolsan L, Nakbanpote W (2018) Cadmium toxicity and tolerance in micro- and phytobiomes. In: Hasanuzzaman M, Prasad MNV, Fujita M (eds) Cadmium toxicity and tolerance in plants: from physiology to remediation. Elsevier, pp 19–46
Shaheen SM, Rinklebe J (2015) Impact of emerging and low cost alternative amendments on the (im)mobilization and phytoavailability of Cd and Pb in a contaminated floodplain soil. Ecol Eng 74:319–326. https://doi.org/10.1016/j.ecoleng.2014.10.024
Shahid M, Javed MT, Masood S, Akram MS, Azeem M, Ali Q, Lindberg S (2019) Serratia sp. CP-13 augments the growth of cadmium (Cd)-stressed Linum usitatissimum L. by limited Cd uptake, enhanced nutrient acquisition and antioxidative potential. J Appl Microbiol 126:1708–1721. https://doi.org/10.1111/jam.14252
Shahid M, Niazi NK, Rinklebe J, Bundschuh J, Dumat C, Pinelli E (2020) Trace elements-induced phytohormesis: a critical review and mechanistic interpretation. Critical Rev Environ Sci Tech 50:1984–2015. https://doi.org/10.1080/10643389.2019.1689061
Silveira ML, Alleoni LRF, O’Connor GA, Chang AC (2006) Heavy metal sequential extraction methods—a modification for tropical soils. Chemosphere 64:1929–1938. https://doi.org/10.1016/j.chemosphere.2006.01.018
Tanaka H, Yamada S, Masunaga T, Yamamoto S, Tsuji W, Murillo-Amador B (2018) Comparison of nutrient uptake and antioxidative response among four Labiatae herb species under salt stress condition. Soil Sci Plant Nutr 64:589–597. https://doi.org/10.1080/00380768.2018.1492334
Tessier A, Campbell PCG, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851. https://doi.org/10.1021/ac50043a017
Thalassinos G, Nastou E, Petropoulos S, Antoniadis V (2021) Nitrogen effect on growth-related parameters and evaluation of Portulaca oleracea as a phytoremediation species in a Cr(VI)-spiked soil. Horticulturae 7:192. https://doi.org/10.3390/horticulturae7070192
Thalassinos G, Nastou E, Petropoulos S, Antoniadis V (2022) Soil dynamics of Cr(VI) and responses of Portulaca oleracea grown in a Cr(VI)-spiked soil under different nitrogen fertilization regimes. Environ Sci Pollut Res 29:14469–14478. https://doi.org/10.1007/s11356-021-16413-w
Thalassinos G, Petropoulos SA, Antoniadis V (2023) The response of purslane (Portulaca oleracea) to soil-added Pb: is it suitable as a potential phytoremediation species? Toxics 11:153. https://doi.org/10.3390/toxics11020153
Tounekti T, Vadel AM, Oñate M, Khemira H, Munné-Bosch S (2011) Salt induced oxidative stress in rosemary plants: damage or protection? Environ Exp Bot 71:298–305. https://doi.org/10.1016/j.envexpbot.2010.12.016
Usman M, Zia-ur-Rehman M, Rizwan M, Abbas T, Ayub MA, Naeem A, Alharby HF, Alabdallah NM, Alharbi BM, Qamar MJ, Ali S (2023) Effect of soil texture and zinc oxide nanoparticles on growth and accumulation of cadmium by wheat: a life cycle study. Environ Res 216:114397. https://doi.org/10.1016/j.envres.2022.114397
Verna A, Yadar S, Kumar R (2023) Geochemical fractionation, bioavailability, ecological and human health risk assessment of metals in topsoils of an emerging industrial cluster near New Delhi. Environ Geochem Health 45:9041–9066. https://doi.org/10.1007/s10653-023-01536-5
Vollprecht D, Riegler C, Ahr F, Stuhlpfarrer S, Wellacher M (2020) Sequential chemical extraction and mineralogical bonding of metals from Styrian soils. Int J Environ Sci Technol 17:3663–3676. https://doi.org/10.1007/s13762-020-02694-0
Waheed A, Haxim Y, Islam W, Ahmad M, Ali S, Wen X, Khan KA, Ghramh HA, Zhang Z, Zhang D (2022) Impact of cadmium stress on growth and physio-biochemical attributes of Eruca sativa Mill. Plants 11:2981. https://doi.org/10.3390/plants11212981
Wang X, Wang L, Zhang Q, Liang T, Li J, Hansen HCB, Shaheen SM, Antoniadis V, Bolan N, Rinklebe J (2022) Integrated assessment of the impact of land use types on soil pollution by potentially toxic elements and the associated ecological and human health risk. Environ Pollut 299:118911. https://doi.org/10.1016/j.envpol.2022.118911
Wang G, Yu G, Chi T, Li Y, Zhang Y, Wang J, Li P, Li J, Yu Z, Wang Q, Wang M, Sun S (2023) Insights into the enhanced effect of biochar on cadmium removal in vertical flow constructed wetlands. J Hazard Mater 443:130148. https://doi.org/10.1016/j.jhazmat.2022.130148
Yang X, Wen E, Ge C, El-Naggar A, Yu H, Wang S, Kwong EE, Song E, Shaheen SM, Wang H, Rinklebe J (2023) Iron-modified phosphorus- and silicon-based biochars exhibited various influences on arsenic, cadmium, and lead accumulation in rice and enzyme activities in a paddy soil. J Hazard Mater 443:130203. https://doi.org/10.1016/j.jhazmat.2022.130203
Zhao H, Guan J, Liang Q, Zhang X, Hu H, Zhang J (2021) Effects of cadmium stress on growth and physiological characteristics of sassafras seedlings. Sci Rep 11:9913. https://doi.org/10.1038/s41598-021-89322-0
Funding
Open access funding provided by HEAL-Link Greece. Not applicable.
Author information
Authors and Affiliations
Contributions
Georgios Thalassinos & Georgia Florokapi: Performing the experiments. Georgios Thalassinos: investigation, analysis, data collection, methodology, and writing the draft manuscript. Efi Levizou: Coordination, experimental guiding, editing, proof reading. Jörg Rinklebe & Sabry M. Shaheen: Editing, proof reading for the entire manuscript. Vasileios Antoniadis: Supervision, conceptualization, research idea, experimental guiding, technical facilities, foundation, review, editing and corresponding author.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no potential conflict of interest.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent to Publish
Authors are responsible for correctness of the statements provided in the manuscript. The publication has been approved by all co-authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thalassinos, G., Levizou, E., Florokapi, G. et al. Nitrogen Fertilizer Enhanced the Vitality of Oregano (Origanum vulgare) Plants and Boosted Their Ability to Accumulate Soil Cadmium: Agro-environmental Implications. Earth Syst Environ (2024). https://doi.org/10.1007/s41748-024-00383-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41748-024-00383-3