Abstract
Type 1 diabetes is a complex, chronic disease in which the insulin-producing beta cells in the pancreas are sufficiently altered or impaired to result in requirement of exogenous insulin for survival. The development of type 1 diabetes is thought to be an autoimmune process, in which an environmental (unknown) trigger initiates a T cell-mediated immune response in genetically susceptible individuals. The presence of islet autoantibodies in the blood are signs of type 1 diabetes development, and risk of progressing to clinical type 1 diabetes is correlated with the presence of multiple islet autoantibodies. Currently, a “staging” model of type 1 diabetes proposes discrete components consisting of normal blood glucose but at least two islet autoantibodies (Stage 1), abnormal blood glucose with at least two islet autoantibodies (Stage 2), and clinical diagnosis (Stage 3). While these stages may, in fact, not be discrete and vary by individual, the format suggests important applications of precision medicine to diagnosis, prevention, prognosis, treatment and monitoring. In this paper, applications of precision medicine in type 1 diabetes are discussed, with both opportunities and barriers to global implementation highlighted. Several groups have implemented components of precision medicine, yet the integration of the necessary steps to achieve both short- and long-term solutions will need to involve researchers, patients, families, and healthcare providers to fully impact and reduce the burden of type 1 diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
A typical definition of ‘precision medicine’ includes the concept of targeting disease prevention and treatment based upon individual characteristics, including genetics, environments (exposures) and lifestyles–the “right treatment to the right person at the right time”. The precision medicine approach is contrasted to the “one size fits all” or “average patient” concept that, in truth, may not be the typical practice of modern medicine. Nonetheless, precision medicine forms a framework for integrating many sources of information to better guide approaches for the improvement of individual and public health.1
The implementation of precision medicine in type 1 diabetes will be a function of several key components. First, there are advances in medical science that include genomics, imaging, miniaturization, drug delivery, and development of biomarkers and therapeutics. Second, the field of data science has incorporated mining of health care data from electronic health record systems with advanced analytics, including artificial intelligence, machine and deep learning approaches, and neural networks. Third, there is recognition of the impact of lifestyle and social inequities as a major driver of health and disease. Finally, national policies on health care and vast variation in global wealth and public health approaches on precision medicine has significant impact on how readily and effectively precision medicine can be implemented. Given the appropriate framework of implementation of precision medicine, the underlying heterogeneity of type 1 diabetes and overlap with other forms of disease will need to be addressed before true benefits of science and medical care can be applied globally.
2 Type 1 Diabetes
Type 1 diabetes is considered to be an autoimmune disease in which the etiology is due to destruction of insulin-producing pancreatic beta cells by the host immune system in response to a foreign antigen triggering a response in a genetically susceptible individual.2 Although type 1 diabetes is often viewed as a disease of children and young adults, it can occur at any age and in people of diverse genetic ancestry. Factors that influence the risk of type 1 diabetes include genetic predisposition, with genetic factors accounting for ~ 50% of the overall risk.3 While recent research has discovered the majority of genetic factors contributing to risk and many of their putative functional targets,4,5 these findings are based predominantly on studies in European ancestry with disease occurring during childhood. Nearly one-half of the genetic risk is assigned to genes in the human Major Histocompatibility Complex (MHC), primarily the HLA class II and class I genes6,7; however, within these key risk genes there is allelic variation associated with risk across populations, even when restricted to European ancestry groups.8 There is evidence that there are ancestry-specific loci and variants contributing to type 1 diabetes genetic risk, making risk prediction dependent upon site and potentially limiting transferability of genetic risk score derived from one population to another.8,9
The development of type 1 diabetes in most individuals can be missed, despite symptoms that include increased thirst and urination, increased hunger, blurred vision, fatigue, and unexpected weight loss. Many of these symptoms are initially mild, and can masquerade as common diseases of childhood.2 In children, the signs of type 1 diabetes result from the increase in glucose in the blood, triggering both osmotic removal of fluid from tissue and osmotic diuresis (which in turn increases thirst) and loss of insulin that is critical for entry of glucose into the cells of the body where it can be metabolized into energy (fatigue). As insulin deficiency progresses, the liver produces alternate fuels, the ketones, that do not require insulin for entry into cells but are acidic leading to potentially deadly diabetic ketoacidosis (DKA) . Typically, ~ 5% of those who develop type 1 diabetes have a parental history of the disease, although the rates vary by population, with the 9% occurring in the high prevalence Finnish population.10 Across multiple populations of European ancestry, the fathers with type 1 diabetes more frequently transmit disease to offspring than mothers10,11; however, there is no family history and often no family awareness of the early symptoms of type 1 diabetes in 90–95% of childhood cases. Children are first recognized with the development of DKA in ~ 40% of new onset cases, but precision prediction of type 1 diabetes followed by intensive monitoring for symptoms represents a critical area for mitigation of life-threatening illness. Reduction of DKA to nearly 0% is possible with early recognition and monitoring and with potential to preserve beta cell function and delay disease onset.12,13,14
Implementation of precision medicine in type 1 diabetes is tied necessarily to the natural history of the disease. Type 1 diabetes is thought to consist of an initiation of the immune system attacking, modifying, impairing, and (ultimately) destroying, the insulin-producing beta cells,15,16,17 with the “triggering event” unknown at this time but could include a variety of environmental factors. It should be noted, however, that the progression of the attack (as measured by presence of islet autoantibodies) differs across individuals, in terms of the rate of beta cell loss in the “pre-diabetic” period.18 Thus, the decline in beta cell mass can be represented as “waves” of effector and regulatory T cells mediating beta cell destruction, increasing in intensity over time, with the overall progression in any individual determined by numerous factors and their effect on the immune system.18 As shown in the TEDDY study, the progression from a single islet autoantibody to multiple autoantibodies (and clinical disease) is associated with a number of factors, including which islet autoantibody appears first.19
Since interventions in type 1 diabetes are dependent upon knowledge of initiation and progression to clinical disease, a major barrier for immune intervention in the application of precision medicine to type 1 diabetes is the accurate prediction of risk. From a population perspective, the prediction of risk involves both genetic and islet autoantibody screening, with the fundamental question of whom to screen (and when to screen and whom to follow for islet autoantibodies).
3 Principles of Precision Medicine
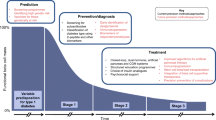
The joint ADA/EASD Precision Medicine in Diabetes Initiative (PMDI) considered application and gaps in knowledge related to precision medicine “pillars” across multiple forms of diabetes,20 precision diagnosis, precision therapeutics, precision prevention, precision prognostics and precision monitoring (Fig. 1). Each of these areas was defined with identification of barriers to implementation and research gaps, with clarity on the differences in barriers and gaps relevant to type 1 diabetes as well as type 2 diabetes, monogenic forms of diabetes and gestational diabetes . These “pillars” differ in complexity across the forms of diabetes; for example, the genetic architecture of monogenic forms of diabetes is more advanced than for that of type 1 diabetes, and less advanced for type 2 diabetes. Within each form of diabetes, there remains heterogeneity in etiology, natural history and acknowledged differences that reflects incomplete knowledge of disease. Recent publications have further addressed precision medicine in specific applications21,22,23,24,25 as well as gaps in knowledge. It should be noted that many of the gaps in knowledge at the biostatistical and epidemiology perspective,26 such as spectrum bias, absence of appropriate biomarkers of risk, different levels of complexity, and individual variation in prognosis, treatment response and diagnostic accuracy, are applicable to all medical conditions.
3.1 Precision Diagnosis
Precision diagnosis also incorporates the individual characteristics with predictors of disease (classical laboratory tests, novel biomarkers, genetic risk) that provides a temporal likelihood of risk, recognizing that a disease “evolves” over time and may be altered by other co-existing conditions/factors that also change over time. From a clinical perspective, precision diagnosis also could generate disease “subtypes”, especially in those situations that a subtype of a disease may have a preferred therapy or prognosis. In type 1 diabetes, the population characteristics include factors such as ancestry, age, the presence of islet autoantibodies, and genetic risk (particularly HLA genotype) coupled with absence of C-peptide provides important clinical information for diagnosis.19,27 However, these predictors may not apply to all cases as (Fig. 1): there are cases diagnosed in adulthood (with a more indolent clinical course); the genetic risk score has low predictive value (given the low disease prevalence); there are differences in outcome associated with which islet autoantibody first emerges and the age at which it occurs; and there are those who have clinical feature of type 1 diabetes without islet autoantibodies or with “protective” genetic risk scores.28 Although type 1 diabetes is often considered as a homogeneous disease (complete destruction of beta cells leading to life-long insulin therapy), it is likely heterogeneous in etiology, clinical course, and factors that require subtyping for optimal (future) intervention and therapies.
3.2 Precision Prevention
Precision prevention utilizes individual characteristics, coupled with their environment and individual preferences, to optimize outcomes of interventions for disease or disease risk. Interventions in diabetes can be varied, often pharmacological and behavioral (for type 2 diabetes) that can focus on reduced exposure to risk factors. Prevention is dependent, in part, on the presence of an intervention that is available, one that is economically accessible, with minimal off-target effects and globally available to ensure health equity.
In type 1 diabetes, the majority of prevention efforts involve development of immune interventions, including those involving beta cell preservation with T cell targets (teplizumab/anti-CD3, and abatacept/anti-CD80 and anti-CD86),29 B cell targets (rituximab/anti-CD20), and inflammatory cytokines (anti-TNF-alpha, anti-IL-6R).29,30 In addition, oral insulin or peptides.31,32 as well as a number of other targets as alternatives to islet transplantation have been investigated. A promising future intervention involves use of stem cells to augment residual beta cell reserve.33 Other approaches that include targeting processes to reduce inflammation involved in beta cell destruction through cytokine and free fatty acid sensitivity have been proposed, with protection of those cells from immune-mediated rejection.34
3.3 Precision Therapeutics (or Precision Treatment)
Precision therapeutics utilizes the individual characteristics, including that of the disease state, to “tailor” treatment while minimizing adverse responses to the treatment.20 While there have been efforts to use genomic data (pharmacogenomics) to improve and target therapies, the primary approach to precision therapeutics is based on specific treatment guidelines based upon a diagnosis. Although much has been accomplished in pharmacogenetics and pharmacodynamics in diabetes, the focus has been primarily on type 2 diabetes and monogenic forms of diabetes, in which specific genotypes account for variability in response to a variety of treatment options.24
In type 1 diabetes, the only treatment option is insulin, yet there can be variation in the amount of insulin delivery, formulation of insulin, and monitoring of insulin and its physiologic effects through technological innovation; however, a pharmacogenomic approach to assess genotype-informed decision making on treatment has yet to be adopted, despite evidence that DNA sequence variants influence gene expression response to insulin via kinase modulators.35 While much is known about the genetic risk of developing type 1 diabetes, little is known about the genetic variation that modifies the response to insulin treatment; in addition, the genetic architecture that defines development of type 1 diabetes may not overlap with the ones that control insulin therapy. Greater understanding of the effect of insulin on signaling pathways and genetic modulation of effects may be important in tailoring optimal insulin administration guidelines.
3.4 Precision Prognosis
Precision prognosis relates to risk of diabetes-related outcomes (complications) given an individual’s form (or subtype) of diabetes with their combination of biological (genetic), lifestyle, societal and cultural features. Implementation of precision prognosis includes prevalence of the type of diabetes in the population, availability and cost of diagnostic testing, understanding personal preferences for management and compliance, and risk of complications through predication and monitoring of systems. Prognosis, or prediction, of type 1 diabetes outcomes include optimizing quality of life and minimizing risk of complications (retinopathy, nephropathy, neuropathy, cardiovascular and cerebrovascular diseases).
In type 1 diabetes, there is increasing use of genetics (that accounts for ~ 50% of the risk) with genetic risk scores to distinguish type 1 diabetes from type 2 diabetes.36 as well as improved prediction in European ancestry populations.37,38 However, the genetic basis of complications of type 1 diabetes appears to be distinct from those variants associated with risk of type 1 diabetes itself, and the extent of heritability of the complications may vary. In most cases, presence of diabetes, of any form, is viewed as an independent risk factor for retinopathy, nephropathy, neuropathy, cardiovascular disease (myocardial infarction) and cerebrovascular disease (stroke). Further, glycemic control plays a major role in defining risk of complications from diabetes, particularly for retinopathy whether in type 1 diabetes.39 or type 2 diabetes,40 with little role for modification by genetic factors; however, gene set enrichment analyses of genome-wide association studies in type 2 diabetes identified important biological pathways (lipid catabolism, digestion, mobilization and transport; nitric oxide biosynthesis; apoptosis; retinal ganglion cell degeneration) as targets for research.41 Recent results suggest that both glycemic and non-glycemic factors (HbA1c and BMI, psychological stress and cardiac autonomic neuropathy) are important in risk of neuropathy in type 1 diabetes.42 In contrast, the risk for nephropathy in type 1 diabetes43 or in combined type 1 and type 2 diabetes44 has been shown to have a strong genetic component. Further, a polygenic risk score for eGFR has been shown to be associated with incident kidney diseases and proteins related to kidney function.45
Cardiovascular and cerebrovascular diseases present the major causes of morbidity and mortality in type 1 diabetes.46,47 Although intensive glycemic control has been implicated as a primary risk reduction factor in type 1 diabetes,48 other factors also contribute to risk, including family history of cardiovascular and cerebrovascular disease, dyslipidemia, high blood pressure, smoking, and obesity.49 Even in absence of the traditional risk factors, cardiovascular disease, and particularly heart failure, is increased in type 1 diabetes, with rates greater than those observed in type 2 diabetes.50 Thus, accurately identifying those who are likely to progress to either single or multiple complications of type 1 diabetes could optimize management and decision making to treat risk factors of complications, rather than the specific complication. Other complications of type 1 diabetes include hypoglycemia and fatty liver disease, each with specific risk factor interventions and monitoring efforts to affect outcome.
3.5 Precision Monitoring
Precision monitoring is based upon the collection of biological, behavioral and environmental data that can reflect the temporal status of the individual, their disease state and their response to intervention/treatment. Early monitoring activities were restricted to measuring blood glucose levels and HbA1c as surrogates for the individual’s changing physiology and response to insulin injections. With advancement in technology, these characteristics of glucose homeostasis and other physiologic characteristics can be obtained through digital applications, sensors, assays and novel technologies. Digital technologies have the potential to provide accurate and clinically relevant information to improve glycemic control, with significant efficacy shown by use of continuous glucose monitoring in both type 1 diabetes and type 2 diabetes.51
4 The Increasing Global Burden of Type 1 Diabetes
Although type 1 diabetes accounts for a relatively small proportion of all forms of diabetes (including the majority, type 2 diabetes, but also monogenic/neonatal forms, gestational, and atypical diabetes), the incidence of type 1 diabetes appears to be increasing globally.52 The increase in type 1 diabetes is not likely due to genetic factors, as the rates of change of frequency of risk alleles that account for the observed increase, even under the most favorable modes of inheritance, would take hundreds of generations, not the few generations observed. The SEARCH for Diabetes in Youth study reported that the adjusted annual incidence of type 1 diabetes across five centers in the USA increased by 1.8% per year in those 0–19 years of age.53 The increase in incidence, however, was not uniform across genetic ancestries, with the adjusted annual increase in non-Hispanic whites (1.4%) less than non-Hispanic black (2.2%), Asian/Pacific Islander (3.7%) or Hispanic (4.2%) participants. The increase in annual incidence in non-Hispanic whites (1.4%) was less than the 3.4% annual rate of increase in type 1 diabetes reported by the larger EURODIAB study (84,000 children aged 0–14 years from 22 countries) over a longer observation period (1989–2013).54
In an updated, global analysis and modeling of prevalence and incidence rates estimated from 94 countries as mined from the literature, it was shown that high-income countries (with 17% of the global population) accounted for nearly one-half of the incident cases.55 Asia (with 60% of the global population) had the largest number of incident cases (32% of the total) while Europe (with 10% of the global population) had 27% of the total incident cases.55 The distribution of incident cases varied by country, as well as by age. Together, these and other studies.56 suggest that genetic factors, while important in defining risk of type 1 diabetes, is not driving the observed increase in incidence (and prevalence) globally. More likely, the increase in type 1 diabetes incidence could be accounted by improvement in clinical diagnosis/detection, and the interaction of genetic risk with changing environmental exposure that manifest in epigenetic modification. Thus, application of precision medicine approaches to type 1 diabetes should account for these factors, and address the gaps in knowledge across genetic ancestry, access to health care, and differences in non-genetic factors that influence risk of developing type 1 diabetes and risk of complications.20
5 Implementing Precision Medicine in Type 1 Diabetes
The approaches to precision medicine in type 1 diabetes are evolving with integration of technology, decision support systems, regulatory guidelines and patient engagement, all aspects that have been noted in the development of precision medicine in type 2 diabetes.57 Both type 1 diabetes and type 2 diabetes share aspects of genetic contribution to disease risk, although through quite different gene sets and fundamental etiology. The incomplete contribution of genetics to risk necessarily limits the utility of screening, as does the lack of diversity in genetic studies.58; in addition, there is limited information globally on the natural history of type 1 diabetes, autoantibody development and temporality, and progression to clinical disease in low prevalence and under-represented populations. However, knowledge of genetics provides significant insights into disease heterogeneity and potential for subtyping into more homogeneous groups of patients that can be used for improved treatment and prediction of disease progression. Ultimately, the implementation of “precision medicine” approaches, versus “classical medicine” approaches, involves using data analytics to enhance early prediction of islet autoimmunity, detection of those at high genetic and immunologic risk, and matching novel immune (or non-immune) interventions to delay or prevent disease; in those progressing to disease, precision monitoring would greatly reduce the occurrence of DKA at onset (Fig. 2).
5.1 Screening
In type 1 diabetes, with genetic variation accounting for ~ one-half of the risk, screening that includes presence of autoantibodies has improved prediction of disease initiation and progression to clinical diabetes. Together, the concept of “staging” h as been proposed and embraced in type 1 diabetes,59 emerging from natural history studies of type 1 diabetes that extends the original concept that recognized the features of initiation, progression and clinical diagnosis.15 The concept of staging has been utilized as an approach for population screening,60 with specific recommendations that include use of genetic markers only in research and limited to HLA class II typing (that accounts for ~ 50% of genetic risk, or 25% of total risk), autoantibody screening in children < 10 years of age, and metabolic testing for estimation of the first phase insulin response. Screening in Germany has been highly informative for implementing an initial autoantibody screen,61 recruitment through an established health care system,62 and determining cost.63 In a parallel study in the USA, a pediatric screening program for celiac disease and type 1 diabetes has provided experience in a clinic setting, with both non-Hispanic white and Hispanic children involved,64 and experience gained in these and other studies.65 can provide guidance for approaches in more diverse and less-resourced populations. It should be noted, however, that screening costs currently vary across countries and, in particular, are dependent on specific health care delivery systems; thus, costs for screening may not be viable for most countries.66 although, in theory, screening could be expected to lower subsequent health care costs from reduced rates and severity of complications.
5.2 Intervention
A major barrier in type 1 diabetes prevention is the availability of an appropriate intervention, where “appropriate” includes safety, affordability, quality of life, and absence of secondary effects; thus, the role of precision medicine in type 1 diabetes focused on identifying the target group of individuals who are likely to benefit from a specific intervention.67 A growing number of interventions at the islet autoimmunity stage (prior to clinical onset, or Stage 3) of type 1 diabetes have been developed and being used in clinical trials.30 It is likely that multiple intervention approaches will be required, as response to an intervention will depend upon the stage of beta cell destruction, the immune regulatory landscape, concurrent physiologic status and genetic variation, with the “endophenotype” grouping providing the appropriate interventions.
Progress in immune intervention has been led by the Fc receptor nonbinding anti-CD3 monoclonal antibody, teplizumab, in relatives of individuals with type 1 diabetes and high risk of disease.68 In a phase 2, randomized, placebo-controlled, double-blind trial (NCT01030861), a single 14-day course resulted in a delay (by ~ 24 months) in progression to clinical (Stage 3) disease, with annualized rates of type 1 diabetes occurrence reduced from ~ 36% in the placebo group to ~ 15% in the teplizumab group.68 In an extended 923-day median follow-up, the difference in time to diagnosis remained similar in the placebo group (~ 27 months) but was extended in the teplizumab group (~ 60 months), with 50% of the teplizumab group remaining disease-free.69 Of importance for future trials and applications of precision prevention are those characteristics that determine success in similar groups, as well as extensions to diverse at-risk populations in order to optimize improvements in metabolic responses and delay, or development, of type 1 diabetes with immune therapy.
5.3 Prognostics and Monitoring
Key factors for prediction of outcomes for those with type 1 diabetes include traditional “biomarkers”, such as clinical characteristics of disease state, glycemic control, genetic propensity (whether based upon DNA or other ‘omic evidence or family history), social/cultural and physical environment, individual behavioral characteristics and access to care. Implicit in developing a precision medicine approach to prediction of outcomes (prognostics) is monitoring the state of disease at intervals. Improvements in monitoring, such as seen in continuous glucose monitoring, can have a major impact on prediction of development of type 1 diabetes.70 as well as prediction of subsequent risk of complications using metrics.51,71 As complications of type 1 diabetes represent the primary outcomes associated with treatment satisfaction, quality of life, morbidity and mortality, the availability of dynamic and immediate data on an individual’s physiologic state from wearables and other devices can be used to monitor health as well as guide active treatment.
6 Global Implementation of Precision Medicine to Type 1 Diabetes
While great advances are being made in understanding the genetic architecture of type 1 diabetes,4,5 development of immune-focused interventions,68,69 and miniaturization of wearables for monitoring,71 the translation of many advances in precision medicine applied to type 1 diabetes will require aspects not directly related to either basic science or clinical medicine (Fig. 3). Results from basic science, clinical and population sciences will need to be adopted by a heterogenous and fundamentally diverse process that will necessarily be tailored to each country (and, within countries, regional and local agencies).
Features of implementation of precision medicine in type 1 diabetes will necessarily involve patient engagement, educational systems that target healthcare delivery (doctors, nurses, support staff) as well as patients, regulatory agencies (for those countries with reimbursement protocols), and mechanisms that will ensure health equity at a global level. Many of these issues have been noted previously.20; however, implementation will be dependent upon many societal and economic factors.
One of the “pillars” of precision medicine is precision diagnosis. In type 1 diabetes, it is thought that a portion of adults with type 1 diabetes may be misdiagnosed as having type 2 diabetes. A number of algorithms have been developed to aid in reducing misdiagnosis using standard clinical features with or without genomic information.36,72,73 In a retrospective study, a machine learning algorithm was employed using ambulatory electronic medical records that included age, demographics, risk factors, symptoms, treatments, procedures, vital signs and available laboratory values.74 The machine learning algorithm identified age, BMI/weight, therapy history and HbA1c/blood glucose as the primary predictors of misdiagnosis. While these data are suggestive of machine learning approaches being useful in diagnosis, the available data and context is critical in assessing performance. Recent results from a study in Uganda,75 a less-resourced country, characterized lean versus non-lean individuals with “new-onset type 2 diabetes” with socio-demographic, clinical, biophysical and metabolic features with screening for islet autoantibodies. In this setting, 32% of subjects were lean (with diagnosis of type 2 diabetes), yet 6.4% of these had an autoimmune form (type 1 diabetes) based upon islet autoantibodies. Thus, translation of advanced (machine learning) approaches for precision diagnosis will be dependent upon the context of the population as well as the resources and data available.
In precision monitoring and management of type 1 diabetes, particularly in adolescents, blood glucose and insulin administration can be challenging. Data science-driven approaches, such as use of machine learning algorithms, have been tested to identify risk of suboptimal self-management.76 Youth from the Vanderbilt Eskind Pediatrics Diabetes Clinic (13–19 years of age) participated if they had a smartphone and could use a Bluetooth blood glucose meter with implementation of an ecological momentary assessment (EMA) model coupled with a machine learning algorithm. The EMA model integrated with the machine learning algorithm was able to predict those with deviations from appropriate management; however, the major factor in achieving improved self-management behavior of adolescents with type 1 diabetes was related to social determinants of health . The psychosocial component and the methods used to assess appropriate management (wearable technology) may represent another major barrier globally for implementation of precision medicine in type 1 diabetes.
Implementation of precision medicine in type 1 diabetes will involve education at many levels, focused on how the clinician interacts with the patient and the return and action on the available data from a variety of sources. In many western societies, extensive electronic health record systems exist, often with elaborate biobanks of samples that permit extensive use of genomics to characterize disease risk and response to therapeutic agents. The IGNITE network evaluated several clinical decision support systems to aid clinicians and patients interpret and act on genomic data.77 All projects included feedback from stakeholders, identified “local champions”, and included training for clinicians and stakeholders. The IGNITE experience identified several key “lessons” for integrating genomic data into a clinical service including (1) a variety of strategies will be necessary to tailor genomic medicine to a specific practice and environment, and (2) providing patient genotypes in an electronic health record format to guide therapeutic guidance is complicated to implement as it relies on disease-specific and patient-specific needs and preferences. While this process was conducted in the USA, other countries will have different health care systems, tracking and practices that would make the strategies tested either unlikely or cost-prohibitive.
7 Cost Effectiveness of Precision Medicine
The cost of implementing precision (or personalized) medicine and its impact on quality of life is an emerging area of research. This research is challenging due to global differences in burden of disease (whether primary impact is on infectious versus chronic disease), existence and availability of diagnostic tests and treatments, and education/training of health care providers as well as patients. Recent work has focused on systematic reviews of the literature, often in the area of oncology, yet there are consistent findings related to the impact on cost and potential cost savings of precision medicine, as well as barriers, that have emerged.
Much of the focus of precision medicine has been on the use of genomics technology for diagnosis, treatment and evaluation of treatment response, especially in the field of oncology. Although other technologies are being applied to augment genomic profiling in precision medicine, including gene therapy for specific disorders, the concept remains genomic. The assessment of evidence of economic value of precision medicine approaches often has utilized the incremental impact on quality-adjusted life years (QALYs). Using an available cost-effectiveness analysis registry, an analysis of studies found that a majority (72%) of precision medicine tests did provide improved health, but at a higher cost,78 with only 20% of the tests predicted to save money. In this series, primarily based on data from the USA, the tests used genetic or molecular information that is appropriate for testing of germline or somatic mutations, and excluded conditions that have relatively few genetic causes. Although results were based in a high-income country, with extensive data collection systems, the analysis found that only 25% of available tests and 20% of tests with clinical utility had associated cost-utility data,78 illustrating a major gap in knowledge across both high- and low-income countries.
A more recent global evaluation of cost-effectiveness of precision medicine still had the majority of data from countries in Europe (31%) and North America (28%) and focused on cancer (43%) and cardiovascular disease (28%), outcomes of westernized societies.79 Over 70% of the studies analyzed concluded that the cost-effectiveness of precision medicine was equivalent to usual care, although the “willingness to pay” thresholds varied significantly. Thus, the implementation of precision medicine in a country is dependent upon the money per QALY, which is dependent upon the fiscal health and priority of the country. The key factors influencing cost-effectiveness were prevalence of the genetic “condition” in the population, the costs and accuracy of genetic testing and treatment, and the likelihood of complication or mortality from the condition. Using a different metric, the “net monetary benefit” (NMB) of precision medicine, similar outcomes were identified.80 in the large heterogeneity across conditions in upper-middle- or high-income countries, with the greatest benefit in cancer, yet still at a high cost to improve health. As was illustrated in this and other analyses, there is difficulty in assessing cost-effectiveness thresholds in each country, in part due to limited data, difference in perspectives of what the threshold represents (society’s willingness to pay for increases in health versus the cost of health care spending), and how the threshold should be calculated. Again, the results suggest that the health benefits of precision medicine are more costly than, but similar (or slightly lower) than the health benefits of other interventions.
The majority of cost-effectiveness evaluations of precision medicine have been conducted through systematic reviews of published literature that are limited in terms of low-income (as well as middle-income) countries. Although this is due, in part, to the limited economic evaluations of precision medicine available in low-income countries, a recent report on precision medicine in oncology focused on Nigeria and Nepal, providing important insights applicable to other low-income countries and conditions.81 In Nigeria, with Africa’s largest economy and population, ~ 3% of the Gross Domestic Product was spent on healthcare, similar to the ~ 4.5% in Nepal, more than the ~ 2% of India, but much less than ~ 20% of the USA. Specific barriers to implementation of precision oncology in Nigeria and Nepal were identified as both structural (lack of funding health systems for availability, affordability and acceptability of health services to the population) and technological (high cost of equipment and genomic diagnostic devices). In oncology, genomic information on “driver mutations” are known primarily in those of European ancestry, making application to low-income, non-European ancestry populations limit transferability of testing and reduced “precision” of molecular diagnoses. Compounding the structural and technological barriers of implementation of precision medicine is the avoidance of use of precision diagnostic tests because of lack of training in interpretation of test results. Although this factor is not limited to low-income countries, it is more prevalent due to lack of educational infrastructure and experience with new technologies. Together, these factors lead to health care system limitations, physician resistance, and patient unawareness.
The cost-effectiveness of precision medicine in low-income countries has limited data, but significant barriers (particularly in oncology) have been identified. It should be noted that the progress made in precision medicine in oncology has been considered the “role model” for precision medicine in other conditions. The status of diabetes treatment in low- and middle-income countries has been reviewed recently, as ~ 80% of adults with diabetes (typically type 2 diabetes) live in these countries.82 Fewer than 10% of those with diabetes received comprehensive, guideline-recommended treatment, as the proportion of those eligible actually receiving treatment varied with income and region – coverage of glucose-lowering medication was ~ 40% in low-income countries, ~ 45% in lower-middle-income countries, and ~ 64% in upper-middle-income countries. These results highlight the need to improve treatment delivery for glucose lowering and reduction in complications risk among those living with diabetes in low- and middle-income countries. These goals form the basis of diabetes medicine without advanced technology, serving as a first-line approach to improved clinical outcome through “usual care”, prior to the application of precision medicine approaches.
8 Summary
Precision medicine as applied to type 1 diabetes is evolving rapidly along all of the “pillars” of precision medicine. There are advances being made in diagnosing type 1 diabetes accurately through use of standards of care, improved testing and inclusion of novel biomarkers. At the same time, it is being recognized that type 1 diabetes is, itself, heterogeneous and stratification into subclasses of disease, particularly across global populations, may enable improved targeted treatments to improve glycemic control and reduce risk of complications, with improved long-term quality of life. Improvements in monitoring the physiologic state are being applied to all aspects of precision medicine (diagnostics, therapeutics, prevention and prognostics) and serves as a cross-cutting application of technology and data science to improve health. These improvements in monitoring are needed globally, to benefit all populations, and the process to reduce cost and simultaneously provide an optimal decision support system represents a major barrier to implementation. Finally, while insulin is the sole treatment for type 1 diabetes, there have been major advances in developing interventions to delay onset of type 1 diabetes. It is anticipated that multiple interventions will be required, tailored to specific subgroups of at-risk individuals, with the subgroups defined by precision medicine principles. In the future, precision medicine applied to type 1 diabetes would enable early identification of those at risk, classification into subgroups for intervention, and optimization of treatment in those who develop disease to reduce risk of complications. Effectively, one needs to address the needs of the individual and their features of risk (susceptibilities) and target medical intervention before, and not after, disease onset. This effort will necessarily require an integration of teams to develop a plan for the implementation of precision medicine for type 1 diabetes. To ensure this effort is beneficial to all individuals at risk, additional research is needed to improve ancestral and geographic representation into our precision prediction models.
Data availability
There are no primary data included in this manuscript; it is a “perspective” and summarizes the current status of precision medicine in type 1 diabetes.
References
Ashley EA (2015) The precision medicine initiative: a new national effort. JAMA 313:2119–2120. https://doi.org/10.1001/jama.2015.3595
Type 1 Diabetes. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes/type-1-diabetes. Accessed 27 December 2022
Rich SS (1990) Mapping genes in diabetes genetic epidemiological perspective. Diabetes 39:1315–1319. https://doi.org/10.2337/diab.39.11.1315
Robertson CC, Inshaw JRJ, Onengut-Gumuscu S, Chen WM, Santa Cruz DF, Yang H, Cutler AJ, Crouch DJM, Farber E, Bridges SL Jr, Edberg JC, Kimberly RP, Buckner JH, Deloukas P, Divers J, Dabelea D, Lawrence JM, Marcovina S, Shah AS, Greenbaum CJ, Atkinson MA, Gregersen PK, Oksenberg JR, Pociot F, Rewers MJ, Steck AK, Dunger DB, Wicker LS, Concannon P, Todd JA, Rich SS, Type 1 Diabetes Genetics Consortium (2021) Fine-mapping, trans-ancestral and genomic analyses identify causal variants, cells, genes and drug targets for type 1 diabetes. Nat Genet 53:962–971. https://doi.org/10.1038/s41588-021-00880-5
Chiou J, Geusz RJ, Okino ML, Han JY, Miller M, Melton R, Beebe E, Benaglio P, Huang S, Korgaonkar K, Heller S, Kleger A, Preissl S, Gorkin DU, Sander M, Gaulton KJ (2021) Interpreting type 1 diabetes risk with genetics and single-cell epigenomics. Nature 594:398–402. https://doi.org/10.1038/s41586-021-03552-w
Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, Mychaleckyj JC, Todd JA, Bonella P, Fear AL, Lavant E, Louey A, Moonsamy P, Type 1 Diabetes Genetics Consortium (2008) HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes 57:1084–1092. https://doi.org/10.2337/db07-1331
Hu X, Deutsch AJ, Lenz TL, Onengut-Gumuscu S, Han B, Chen WM, Howson JM, Todd JA, de Bakker PI, Rich SS, Raychaudhuri S (2015) Additive and interaction effects at three amino acid positions in HLA-DQ and HLA-DR molecules drive type 1 diabetes risk. Nat Genet 47:898–905. https://doi.org/10.1038/ng.3353
Noble JA (2015) Immunogenetics of type 1 diabetes: a comprehensive review. J Autoimmun 64:101–112. https://doi.org/10.1016/j.jaut.2015.07.014
Onengut-Gumuscu S, Chen WM, Robertson CC, Bonnie JK, Farber E, Zhu Z, Oksenberg JR, Brant SR, Bridges SL Jr, Edberg JC, Kimberly RP, Gregersen PK, Rewers MJ, Steck AK, Black MH, Dabelea D, Pihoker C, Atkinson MA, Wagenknecht LE, Divers J, Bell RA, Erlich HA, Concannon P, Rich SS, Search for Diabetes in Youth; Type 1 Diabetes Genetics Consortium (2019) Type 1 diabetes risk in African-ancestry participants and utility of an ancestry-specific genetic risk score. Diabetes Care 42:406–415. https://doi.org/10.2337/dc18-1727
Parkkola A, Härkönen T, Ryhänen SJ, Ilonen J, Knip M, Register FPD, Finnish Pediatric Diabetes Register (2013) Extended family history of type 1 diabetes and phenotype and genotype of newly diagnosed children. Diabetes Care 36:348–354. https://doi.org/10.2337/dc12-0445
Warram JH, Krolewski AS, Gottlieb MS, Kahn CR (1984) Differences in risk of insulin-dependent diabetes in offspring of diabetic mothers and diabetic fathers. N Engl J Med 311:149–152. https://doi.org/10.1056/NEJM198407193110304
Larsson HE, Vehik K, Bell R, Dabelea D, Dolan L, Pihoker C, Knip M, Veijola R, Lindblad B, Samuelsson U, Holl R, Haller MJ, TEDDY Study Group; Swediabkids Study Group; DPV Study Group; Finnish Diabetes Registry Study Group (2011) Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care 34:2347–2352. https://doi.org/10.2337/dc11-1026
Steck AK, Larsson HE, Liu X, Veijola R, Toppari J, Hagopian WA, Haller MJ, Ahmed S, Akolkar B, Lernmark Å, Rewers MJ, Krischer JP, TEDDY Study Group (2017) Residual beta-cell function in diabetes children followed and diagnosed in the TEDDY study compared to community controls. Pediatr Diabetes 18:794–802. https://doi.org/10.1111/pedi.12485
Wentworth JM, Oakey H, Craig ME, Couper JJ, Cameron FJ, Davis EA, Lafferty AR, Harris M, Wheeler BJ, Jefferies C, Colman PG (2022) Harrison LC (2022) Decreased occurrence of ketoacidosis and preservation of beta cell function in relatives screened and monitored for type 1 diabetes in Australia and New Zealand. Pediatr Diabetes. https://doi.org/10.1111/pedi.13422
Atkinson MA, Eisenbarth GS (2001) Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 358:221–229. https://doi.org/10.1016/S0140-6736(01)05415-0
Bluestone JA, Herold K, Eisenbarth GS (2010) Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 464:1293–1300. https://doi.org/10.1038/nature08933
Atkinson MA, Eisenbarth GS, Michels AW (2014) Type 1 diabetes. Lancet 383:69–82. https://doi.org/10.1016/S0140-6736(13)60591-7
von Herrath M, Sanda S, Herold K (2007) Type 1 diabetes as a relapsing-remitting disease? Nat Rev Immunol 7:988–994. https://doi.org/10.1038/nri2192
Krischer JP, Liu X, Lernmark Å, Hagopian WA, Rewers MJ, She JX, Toppari J, Ziegler AG, Akolkar B, TEDDY Study Group (2022) Predictors of the initiation of islet autoimmunity and progression to multiple autoantibodies and clinical diabetes: The TEDDY Study. Diabetes Care 45:2271–2281. https://doi.org/10.2337/dc21-2612
Chung WK, Erion K, Florez JC, Hattersley AT, Hivert MF, Lee CG, McCarthy MI, Nolan JJ, Norris JM, Pearson ER, Philipson L, McElvaine AT, Cefalu WT, Rich SS, Franks PW (2020) Precision medicine in diabetes: A Consensus Report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 43:1617–1635. https://doi.org/10.2337/dci20-0022
Krook A, Mulder H (2022) Pinpointing precision medicine for diabetes mellitus. Diabetologia 65:1755–1757. https://doi.org/10.1007/s00125-022-05777-4
Deutsch AJ, Ahlqvist E, Udler MS (2022) Phenotypic and genetic classification of diabetes. Diabetologia 65:1758–1769. https://doi.org/10.1007/s00125-022-05769-4
Herder C, Roden M (2022) A novel diabetes typology: towards precision diabetology from pathogenesis to treatment. Diabetologia 65:1770–1781. https://doi.org/10.1007/s00125-021-05625-x
Florez JC, Pearson ER (2022) A roadmap to achieve pharmacological precision medicine in diabetes. Diabetologia 65:1830–1838. https://doi.org/10.1007/s00125-022-05732-3
Carr ALJ, Evans-Molina C, Oram RA (2022) Precision medicine in type 1 diabetes. Diabetologia 65:1854–1866. https://doi.org/10.1007/s00125-022-05778-3
Griffin S (2022) Diabetes precision medicine: plenty of potential, pitfalls and perils but not yet ready for prime time. Diabetologia 65:1913–1921. https://doi.org/10.1007/s00125-022-05782-7
Redondo MJ, Gignoux CR, Dabelea D, Hagopian WA, Onengut-Gumuscu S, Oram RA, Rich SS (2022) Type 1 diabetes in diverse ancestries and the use of genetic risk scores. Lancet Diabetes Endocrinol 10:597–608. https://doi.org/10.1016/S2213-8587(22)00159-0
Cefalu WT, Andersen DK, Arreaza-Rubín G, Pin CL, Sato S, Verchere CB, Woo M, Rosenblum ND, Rosenblum N, Cefalu W, Andersen DK, Arreaza-Rubín G, Dhara C, James SP, Makarchuk MJ, Pin CL, Sato S, Verchere B, Woo M, Powers A, Estall J, Hoesli C, Millman J, Linnemann A, Johnson J, Pin CL, Hawkins M, Woo M, Gloyn A, Cefalu W, Rosenblum N, Huising MO, Benninger RKP, Almaça J, Hull-Meichle RL, MacDonald P, Lynn F, Melero-Martin J, Yoshihara E, Stabler C, Sander M, Evans-Molina C, Engin F, Thompson P, Shalev A, Redondo MJ, Nadeau K, Bellin M, Udler MS, Dennis J, Dash S, Zhou W, Snyder M, Booth G, Butte A, Florez J, Symposium planning committee, moderators, and speakers (2022) Heterogeneity of diabetes: b-cells, phenotypes, and precision medicine: proceedings of an international symposium of the Canadian Institutes of Health Research’s Institute of Nutrition, metabolism and diabetes and the U.S. National Institutes of Health’s National Institute of Diabetes and Digestive and Kidney Diseases. Diabetes 71:1–22. https://doi.org/10.2337/db21-0777
Jacobsen LM, Bundy BN, Greco MN, Schatz DA, Atkinson MA, Brusko TM, Mathews CE, Herold KC, Gitelman SE, Krischer JP, Haller MJ (2020) Comparing beta cell preservation across clinical trials in recent-onset type 1 diabetes. Diabetes Technol Ther 22:948–953. https://doi.org/10.1089/dia.2020.0305
von Scholten BJ, Kreiner FF, Gough SCL, von Herrath M (2021) Current and future therapies for type 1 diabetes. Diabetologia 64:1037–1048. https://doi.org/10.1007/s00125-021-05398-3
Krischer JP, Schatz DA, Bundy B, Skyler JS, Greenbaum CJ, Writing Committee for the Type 1 Diabetes TrialNet Oral Insulin Study Group (2017) Effect of oral insulin on prevention of diabetes in relatives of patients with type 1 diabetes: a randomized clinical trial. JAMA 318:1891–1902. https://doi.org/10.1001/jama.2017.17070
Ziegler AG, Achenbach P, Berner R, Casteels K, Danne T, Gündert M, Hasford J, Hoffman VS, Kordonouri O, Lange K, Larsson HE, Lundgren M, Snape MD, Szypowska A, Todd JA, Bonifacio E, GPPAD Study group (2019) Oral insulin therapy for primary prevention of type 1 diabetes in infants with high genetic risk: the GPPAD-POInT (global platform for the prevention of autoimmune diabetes primary oral insulin trial) study protocol. BMJ Open 9(6):e028578. https://doi.org/10.1136/bmjopen-2018-028578
Sintov E, Nikolskiy I, Barrera V, Hyoje-Ryu Kenty J, Atkin AS, Gerace D, Ho Sui SJ, Boulanger K, Melton DA (2022) Whole-genome CRISPR screening identifies genetic manipulations to reduce immune rejection of stem cell-derived islets. Stem Cell Rep 17:1976–1990. https://doi.org/10.1016/j.stemcr.2022.08.002
Corkey BE, Kilpatrick LE, Evans-Molina C (2022) Hypothesis: Induction of autoimmunity in type 1 diabetes – a lipid focus. Diabetes 71:2067–2074. https://doi.org/10.2337/db22-0240
Wang IX, Ramrattan G, Cheung VG (2015) Genetic variation in insulin-induced kinase signaling. Mol Syst Biol 11:820. https://doi.org/10.15252/msb.20156250
Oram RA, Patel K, Hill A, Shields B, McDonald TJ, Jones A, Hattersley AT, Weedon MN (2016) A type 1 diabetes genetic risk score can aid discrimination between type 1 and type 2 diabetes in young adults. Diabetes Care 39:337–344. https://doi.org/10.2337/dc15-1111
Sharp SA, Rich SS, Wood AR, Jones SE, Beaumont RN, Harrison JW, Schneider DA, Locke JM, Tyrrell J, Weedon MN, Hagopian WA, Oram RA (2019) Development and standardization of an improved type 1 diabetes genetic risk score for use in newborn screening and incident diagnosis. Diabetes Care 42:200–207. https://doi.org/10.2337/dc18-1785
Ferrat LA, Vehik K, Sharp SA, Lernmark Å, Rewers MJ, She JX, Ziegler AG, Toppari J, Akolkar B, Krischer JP, Weedon MN, Oram RA, Hagopian WA, TEDDY study group (2020) A combined risk score enhances prediction of type 1 diabetes among susceptible children. Nat Med 26:1247–1255. https://doi.org/10.1038/s41591-020-0930-4
Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C, Diabetes control and complications trial research group (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986. https://doi.org/10.1056/NEJM199309303291401
UK Prospective Diabetes Study (UKPDS) Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–853. https://doi.org/10.1016/S0140-6736(98)07019-6
Sobrin L, Susarla G, Stanwyck L, Rouhana JM, Li A, Pollack S, Igo RP Jr, Jensen RA, Li X, Ng MCY, Smith AV, Kuo JZ, Taylor KD, Freedman BI, Bowden DW, Penman A, Chen CJ, Craig JE, Adler SG, Chew EY, Cotch MF, Yaspan B, Mitchell P, Wang JJ, Klein BEK, Wong TY, Rotter JI, Burdon KP, Iyengar SK, Segrè AV (2022) Gene set enrichment analyses identify pathways involved in genetic risk for diabetic retinopathy. Am J Ophthalmol 233:111–123. https://doi.org/10.1016/j.ajo.2021.06.014
Martin CL, Trapani VR, Backlund JYC, Lee P, Braffett BH, Bebu I, Lachin JM, Jacobson AM, Gubitosi-Klug R, Herman WH, DCCT/EDIC Research Group (2022) Physical function in middle-aged and older adults with type 1 diabetes: long-term follow-up of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study. Diabetes Care 45:2037–2045. https://doi.org/10.2337/dc21-2119
Salem RM, Todd JN, Sandholm N, Cole JB, Chen WM, Andrews D, Pezzolesi MG, McKeigue PM, Hiraki LT, Qiu C, Nair V, Di Liao C, Cao JJ, Valo E, Onengut-Gumuscu S, Smiles AM, McGurnaghan SJ, Haukka JK, Harjutsalo V, Brennan EP, van Zuydam N, Ahlqvist E, Doyle R, Ahluwalia TS, Lajer M, Hughes MF, Park J, Skupien J, Spiliopoulou A, Liu A, Menon R, Boustany-Kari CM, Kang HM, Nelson RG, Klein R, Klein BE, Lee KE, Gao X, Mauer M, Maestroni S, Caramori ML, de Boer IH, Miller RG, Guo J, Boright AP, Tregouet D, Gyorgy B, Snell-Bergeon JK, Maahs DM, Bull SB, Canty AJ, Palmer I, Stechemesser L, Paulweber B, Weitgasser R, Sokolovska J, Rovīte V, Pīrāgs V, Prakapiene E, Radzeviciene L, Verkauskiene R, Panduru NM, Groop LC, McCarthy MI, Gu HF, Möllsten A, Falhammar H, Brismar K, Martin F, Rossing P, Costacou T, Zerbini G, Marre M, Hadjadj S, McKnight AJ, Forsblom C, McKay G, Godson C, Maxwell AP, Kretzler M, Susztak K, Colhoun HM, Krolewski A, Paterson AD, Groop PH, Rich SS, Hirschhorn JN, Florez JC, SUMMIT Consortium, DCCT/EDIC Research Group, GENIE Consortium (2019) Genome-wide association study of diabetic kidney disease highlights biology involved in glomerular basement membrane collagen. J Am Soc Nephrol 30:2000–2016. https://doi.org/10.1681/ASN.2019030218
Sandholm N, Cole JB, Nair V, Sheng X, Liu H, Ahlqvist E, van Zuydam N, Dahlström EH, Fermin D, Smyth LJ, Salem RM, Forsblom C, Valo E, Harjutsalo V, Brennan EP, McKay GJ, Andrews D, Doyle R, Looker HC, Nelson RG, Palmer C, McKnight AJ, Godson C, Maxwell AP, Groop L, McCarthy MI, Kretzler M, Susztak K, Hirschhorn JN, Florez JC, Groop PH, GENIE Consortium (2022) Genome-wide meta-analysis and omics integration identifies novel genes associated with diabetic kidney disease. Diabetologia 65:1495–1509. https://doi.org/10.1007/s00125-022-05735-0
Yu Z, Jin J, Tin A, Köttgen A, Yu B, Chen J, Surapaneni A, Zhou L, Ballantyne CM, Hoogeven RC, Arking DE, Chatterjee N, Grams ME, Coresh J (2021) Polygenic risk scores for kidney function and their associations with circulating proteome, and incident kidney diseases. J Am Soc Nephrol 32:3161–3173. https://doi.org/10.1681/ASN.2020111599
Secrest AM, Becker DJ, Kelsey SF, Laporte RE, Orchard TJ (2010) Cause-specific mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes. Diabetes 59:3216–3222. https://doi.org/10.2337/db10-0862
Gagnum V, Stene LC, Jenssen TG, Berteussen LM, Sandvik L, Joner G, Njølstad PR, Skrivarhaug T (2017) Causes of death in childhood-onset type 1 diabetes: long-term follow-up. Diabet Med 34:56–63. https://doi.org/10.1111/dme.13114
Nathan DM, Cleary PA, Backlund JYC, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B, Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group (2005) Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353:2643–2653. https://doi.org/10.1056/NEJMoa052187
Bebu I, Braffett BH, Pop-Busui R, Orchard TJ, Nathan DM, Lachin JM, DCCT/EDIC Research Group (2017) The relationship of blood glucose with cardiovascular disease is mediated over time by traditional risk factors in type 1 diabetes: the DCCT/EDIC study. Diabetologia 60:2084–2091. https://doi.org/10.1007/s00125-017-4374-4
Ohkuma T, Komorita Y, Peters SAE, Woodward M (2019) Diabetes as a risk factor for heart failure in women and men: a systematic review and meta-analysis of 47 cohorts including 12 million individuals. Diabetologia 62:1550–1560. https://doi.org/10.1007/s00125-019-4926-x
Phillip M, Bergenstal RM, Close KL, Danne T, Garg SK, Heinemann L, Hirsch IB, Kovatchev BP, Laffel LM, Mohn V, Parkin CG, Battelino T (2021) The digital/virtual diabetes clinic: the future is now- recommendations from an international panel on diabetes digital technology introduction. Diabetes Technol Ther 23:146–154. https://doi.org/10.1089/dia.2020.0375
Gregory GA, Robinson TIG, Linklater SE, Wang F, Colagiuri S, de Beaufort C, Donaghue KC, Magliano DJ, Maniam J, Orchard TJ, Rai P, Ogle GD, International Diabetes Federation Atlas Type 1 Diabetes in Adults Special Interest Group (2022) Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol 10:741–760. https://doi.org/10.1016/S2213-8587(22)00218-2
Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, Imperatore G, Linder B, Marcovina S, Pettitt DJ, Pihoker C, Saydah S, Wagenknecht L, SEARCH for Diabetes in Youth Study (2017) Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Eng J Med 376:1419–1429. https://doi.org/10.1056/NEJMoa1610187
Patterson CC, Harjutsalo V, Rosenbauer J, Neu A, Cinek O, Skivarhaug T, Rami-Merhar B, Soltesz G, Svensson J, Parslow RC, Castell C, Schoenle E, Bingley P, Dahlquist G, Jarosz-Chobot PK, Marčiulionytė D, Roche EF, Rothe U, Bratina N, Ionescu-Tirgoviste C, Weets I, Kocova M, Cherubini V, Putarek NR, deBeaufort CE, Samardzic M, Green A (2019) Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989–2013: a multicentre prospective registration study. Diabetologia 62:408–417. https://doi.org/10.1007/s00125-018-4763-3
Green A, Hede SM, Patterson CC, Wild SH, Imperatore G, Roglic G, Beran D (2021) Type 1 diabetes in 2017: global estimates of incident and prevalent cases in children and adults. Diabetologia 64:2741–2750. https://doi.org/10.1007/s00125-021-05571-8
Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ (2010) Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am 39:481–497. https://doi.org/10.1016/j.ecl.2010.05.011
Fitipaldi H, McCarthy MI, Florez JC, Franks PW (2018) A global overview of precision medicine in type 2 diabetes. Diabetes 67:1911–1922. https://doi.org/10.2337/dbi17-0045
Martin AR, Gignoux CR, Walters RK, Wojcik GL, Neale BM, Gravel S, Daly MJ, Bustamante CD, Kenny EE (2017) Human demographic history impacts genetic risk prediction across diverse populations. Am J Hum Genet 100:635–649. https://doi.org/10.1016/j.ajhg.2017.03.004
Insel RA, Dunne JL, Atkinson MA, Chiang JL, Dabelea D, Gottlieb PA, Greenbaum CJ, Herold KC, Krischer JP, Lernmark Å, Ratner RE, Rewers MJ, Schatz DA, Skyler JS, Sosenko JM, Ziegler AG (2015) Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 38:1964–1974. https://doi.org/10.2337/dc15-1419
Bingley PJ, Bonifacio E, Ziegler AG, Schatz DA, Atkinson MA, Eisenbarth GS (2001) Immunology of Diabetes Society (2001) Proposed guidelines on screening for risk of type 1 diabetes. Diabetes Care 24:398. https://doi.org/10.2337/diacare.24.2.398
Raab J, Haupt F, Scholz M, Matzke C, Warncke K, Lange K, Assfalg R, Weininger K, Wittich S, Löbner S, Beyerlein A, Nennstiel-Ratzel U, Lang M, Laub O, Dunstehimer D, Bonifacio E, Achenbach P, Winkler C, Ziegler AG, Fr1 da Study Group (2016) Capillary blood islet autoantibody screening for identifying pre-type 1 diabetes in the general population: design and initial results. BMJ Open 6:e011144. https://doi.org/10.1136/bmjopen-2016-011144
Ziegler AG, Kick K, Bonifacio E, Haupt F, Hippich M, Dunstheimer D, Lang M, Laub O, Warncke K, Lange K, Assfalg R, Jolink M, Winkler C, Achenbach P, Fr1 da Study Group (2020) Yield of a public health screening of children for islet autoantibodies in Bavaria, Germany. JAMA 323:339–351. https://doi.org/10.1001/jama.2019.21565
Karl FM, Winkler C, Ziegler AG, Laxy M, Achenbach P (2022) Costs of public health screening for presymptomatic type 1 diabetes in Bavaria, Germany. Diabetes Care 45:837–844. https://doi.org/10.2337/dc21-1648
Stahl MG, Geno Rasmussen C, Dong F, Waugh K, Norris JM, Baxter J, Yu L, Steck AK, Frohnert BI, Liu E, Rewers MJ, ASK Study Group (2021) Mass screening for celiac disease: The Autoimmunity Screening for Kids study. Am J Gastroenterol 116:180–187
Sims EK, Besser REJ, Dayan C, Geno Rasmussen C, Greenbaum C, Griffin KJ, Hagopian W, Knip M, Long AE, Martin F, Mathieu C, Rewers M, Steck AK, Wentworth JM, Rich SS, Kordonouri O, Ziegler AG, Herold KC, NIDDK Type 1 Diabetes TrialNet Study Group (2022) Screening for type 1 diabetes in the general population: a status report and perspective. Diabetes 71:610–623. https://doi.org/10.2337/dbi20-0054
Meehan C, Fout B, Ashcraft J, Schatz DA, Haller MJ (2015) Screening for T1D risk to reduce DKA is not economically viable. Pediatr Diabetes 16:565–572. https://doi.org/10.1111/pedi.12313
den Hollander NHM, Roep BO (2022) From disease and patient heterogeneity to precision medicine in type 1 diabetes. Front Med (Lausanne) 9:932086. https://doi.org/10.3389/fmed.2022.932086
Herold KC, Bundy BN, Long SA, Bluestone JA, DiMeglio LA, Dufort MJ, Gitelman SE, Gottlieb PA, Krischer JP, Linsley PS, Marks JB, Moore W, Moran A, Rodriguez H, Russell WE, Schatz D, Skyler JS, Tsalikian E, Wherrett DK, Ziegler AG, Greenbaum CJ, Type 1 Diabetes TrialNet Study Group (2019) An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 381:603–613. https://doi.org/10.1056/NEJMoa1902226
Sims EK, Bundy BN, Stier K, Serti E, Lim N, Long SA, Geyer SM, Moran A, Greenbaum CJ, Evans-Molina C, Herold KC, Type 1 Diabetes TrialNet Study Group (2021) Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals. Sci Transl Med 13:eabc980. https://doi.org/10.1126/scitranslmed.abc8980
Steck AK, Dong F, Geno Rasmussen C, Bautista K, Sepulveda F, Baxter J, Yu L, Frohnert BI, Rewers MJ, Rewers M, Barbour A, Bautista K, Baxter J, Felipe-Morales D, Dong F, Dormond-Brooks P, Driscoll K, Frohnert B, Rasmussen CG, Gesualdo P, Hoffman M, Karban R, Sepulveda F, Shorrosh H, Simmons K, Steck A, Taki I, Waugh K, Yu L, Liu E, Gallant M, McQueen RB, Norris JM, ASK Study Group (2022) CGM metrics predict progression to type 1 diabetes: autoimmunity Screening for Kids (ASK) study. Diabetes Care 45:365–371. https://doi.org/10.2337/dc21-0602
Montaser E, Fabris C, Kovatchev B (2022) Essential continuous glucose monitoring metrics: the principal dimensions of glycemic control in diabetes. Diabetes Technol Ther 24:797–804. https://doi.org/10.1089/dia.2022.0104
Patel KA, Oram RA, Flanagan SE, De Franco E, Colclough K, Shepherd M, Ellard S, Weedon MN, Hattersley AT (2016) Type 1 diabetes genetic risk score: a novel tool to discriminate monogenic and type 1 diabetes. Diabetes 65:2094–2099. https://doi.org/10.2337/db15-1690
Davis WA, Peters KE, Makepeace A, Griffiths S, Bundell C, Grant SFA, Ellard S, Hattersley AT, Paul Chubb SA, Bruce DG, Davis TME (2018) Prevalence of diabetes in Australia: insights from the Fremantle Diabetes Study Phase II. Intern Med J 48:803–809. https://doi.org/10.1111/imj.13792
Cheheltani R, King N, Lee S, North B, Kovarik D, Evans-Molina C, Leavitt N, Dutta S (2022) Predicting misdiagnosed adult-onset type 1 diabetes using machine learning. Diabetes Res Clin Pract 191:110029. https://doi.org/10.1016/j.daibres.2022.110029
Kibirige D, Sekitoleko I, Lumu W, Jones AG, Hattersley AT, Smeeth L, Nyirenda MJ (2022) Understanding the pathogenesis of lean non-autoimmune diabetes in an African population with newly diagnosed diabetes. Diabetologia 65:675–683. https://doi.org/10.1007/s00125-021-05644-8
Sperber NR, Dong OM, Roberts MC, Dexter P, Elsey AR, Ginsburg GS, Horowitz CR, Johnson JA, Levy KD, Ong H, Peterson JF, Pollin TI, Rakhra-Burris T, Ramos MA, Skaar T, Orlando LA (2021) Strategies to integrate genomic medicine into clinical care: evidence from the IGNITE network. J Pers Med 11:657. https://doi.org/10.3390/jpm11070647
Zhang P, Fonnesbeck C, Schmidt DC, White J, Kleinberg S, Mulvaney SA (2022) Using momentary assessment and machine learning to identify barriers to self-management in type 1 diabetes: observational study. JMIR Mhealth Uhealth 10:e21959. https://doi.org/10.2196/21959
Phillips KA, Sakowski JA, Trosman J, Douglas MP, Liang SY, Neumann P (2014) The economic value of personalized medicine tests: What we know and what we need to know. Genet Med 16:251–257. https://doi.org/10.1038/gim.2013.122
Kasztura M, Richard A, Bempong NE, Loncar D, Flahault A (2019) Cost-effectiveness of precision medicine: a scoping review. Int J Pub Health 64:1261–1271. https://doi.org/10.1007/s00038-019-01298-x
Vellekoop H, Versteegh M, Huygens S, Ramos IC, Szilberhorn L, Zelei T, Nagy B, Tsiachristas A, Koleva-Kolarova R, Wordsworth S, Mölen MR, HEcoPerMed consortium (2022) The net benefit of personalized medicine: a systematic literature review and regression analysis. Value Health 25:1428–1438. https://doi.org/10.1016/j.jval.2022.01.006
Adeniji AA, Dulal S, Martin MG (2021) Personalized medicine in oncology in the developing world barriers and concepts to improve status quo. World J Oncol 12:50–60. https://doi.org/10.14740/wjon1345
Flood D, Seiglie JA, Dunn M, Tschida S, Theilmann M, Marcus ME, Brian G, Norov B, Mayige MT, Gurung MS, Aryal KK, Labadarios D, Dorobantu M, Siler BK, Bovet P, Jorgensen JMA, Guwatudde D, Houehanou C, Andall-Brereton G, Quesnel-Crooks S, Sturua L, Farzadfar F, Moghaddam S, Atun R, Vollmer S, Bärnighausen TW, Davies JI, Wexler DJ, Geldsetzer P, Rohloff P, Ramírez-Zea M, Heisler M, Manne-Goehler J (2021) The state of diabetes treatment coverage in 55 low-income and middle-income countries: a cross-sectional study of nationally representative, individual-level data in 680,102 adults. Lancet Healthy Longev 2:e340–e351. https://doi.org/10.1016/s2666-7568(21)00089-1
Acknowledgements
The work was supported by the University of Virginia Strategic Investment Fund Project #88, the Leona M. and Harry B. Helmsley Charitable Trust project #2204-05134, and the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases grant R01 DK122586.
Funding
Funding was provided by National Institute of Diabetes and Digestive and Kidney Diseases (Grant no. R01 DK122586) and Leona M. and Harry B. Helmsley Charitable Trust (Grant no. #2204-05134).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests that are directly or indirectly related to the work submitted for publication. The study funders had no role in the design or conduct of the research: collection, management, analysis, and interpretation of the data; or preparation of the manuscript. All graphics were created with BioRender.com
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Michalek, D.A., Onengut-Gumuscu, S., Repaske, D.R. et al. Precision Medicine in Type 1 Diabetes. J Indian Inst Sci 103, 335–351 (2023). https://doi.org/10.1007/s41745-023-00356-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41745-023-00356-x