Abstract
The convergence of advances in medical science, human biology, data science and technology has enabled the generation of new insights into the phenotype known as ‘diabetes’. Increased knowledge of this condition has emerged from populations around the world, illuminating the differences in how diabetes presents, its variable prevalence and how best practice in treatment varies between populations. In parallel, focus has been placed on the development of tools for the application of precision medicine to numerous conditions. This Consensus Report presents the American Diabetes Association (ADA) Precision Medicine in Diabetes Initiative in partnership with the European Association for the Study of Diabetes (EASD), including its mission, the current state of the field and prospects for the future. Expert opinions are presented on areas of precision diagnostics and precision therapeutics (including prevention and treatment) and key barriers to and opportunities for implementation of precision diabetes medicine, with better care and outcomes around the globe, are highlighted. Cases where precision diagnosis is already feasible and effective (i.e. monogenic forms of diabetes) are presented, while the major hurdles to the global implementation of precision diagnosis of complex forms of diabetes are discussed. The situation is similar for precision therapeutics, in which the appropriate therapy will often change over time owing to the manner in which diabetes evolves within individual patients. This Consensus Report describes a foundation for precision diabetes medicine, while highlighting what remains to be done to realise its potential. This, combined with a subsequent, detailed evidence-based review (due 2022), will provide a roadmap for precision medicine in diabetes that helps improve the quality of life for all those with diabetes.
Similar content being viewed by others





Rationale for precision medicine in diabetes
The practice of medicine centres on the individual. From the beginning, the physician has examined the patient suffering from illness, ascertained his/her signs and symptoms, related them to the medical knowledge available at the time, recognised patterns that fit a certain category and, based on the practical wisdom accumulated via empirical trial and error, applied a given remedy that is best suited to the situation at hand. Thus, the concept of precision medicine, often defined as providing the right therapy, for the right patient at the right time, is not novel. What has changed radically is our ability to characterise and understand human biological variation through [1] assessment of the genetic and metabolic state, [2] leveraging data to inform disease categories, and [3] science-guided preventive and treatment decisions tailored to specific pathological conditions. Coupling these with detailed information about lifestyle and environment, available through digital devices and technologies that collect those measures, as well as data abstracted from electronic medical records, present unparalleled opportunities to optimise diabetes medicine.
Diabetes mellitus is diagnosed by the presence of hyperglycaemia that is higher than a threshold blood glucose concentration which predisposes to microvascular end-organ complications. However, hyperglycaemia is the end-product of numerous pathophysiological processes that often emerge over many years and converge on the inability of the pancreatic beta cells to secrete enough insulin to meet the demands of target tissues. In clinical practice, absolute insulin deficiency can be detected from the autoimmune destruction of beta cells in type 1 diabetes, which represents ~10% of all diabetes cases. Making the diagnosis of type 1 diabetes is critical for survival, given the therapeutic requirement of exogenous administration of insulin. However, less commonly, hyperglycaemia might derive from an inherited or de novo loss of function in a single gene (e.g. monogenic diabetes, comprising 2–3% of all diabetes diagnosed in children or young adults). Diabetes can also appear after pancreatitis or organ transplantation, during pregnancy or as a result of cystic fibrosis. Most individuals with diabetes, however, are likely to be diagnosed with type 2 diabetes, which includes defects in one or (more often) multiple physiological pathways (e.g. beta cell insufficiency, fat accumulation or miscompartmentalisation, inflammation, incretin resistance, dysfunctional insulin signalling).
Our modern capacity to comprehensively interrogate diverse axes of biology has facilitated the approach of studying an individual to infer general principles, from which a discrete treatment plan is selected. These axes include developmental/metabolic context, genomic variation, chromatin signals that mark genes as active or repressed in tissues, expressed transcripts, biomarkers of disease and increased knowledge of lifestyle/environmental risk factors. Parallel advances in computational power and analytical methods required to appropriately interrogate ‘big data’ are driving insights that may radically transform the practice of medicine. Yet, at this time, the individual physician often lacks the time and training needed to incorporate these insights into medical decision making. Thus, the translation of the rapidly accumulating new knowledge into practice requires careful evaluation and translational strategies involving specialist training, education and policy considerations.
The failure to adequately understand the diverse molecular and environmental processes that underlie diabetes and our inability to identify the pathophysiological mechanisms that trigger diabetes in individual patients, limit our ability to prevent and treat the disease. Public health strategies have struggled to slow the epidemic, even in countries with the greatest financial and scientific resources. Pharmacological therapies, comprising 12 different drug classes currently approved by the US Food and Drug Administration (FDA), may, at best, control blood glucose and modify disease course, but do not provide a cure or result in the remission of disease. Moreover, these agents are sometimes prescribed based on non-medical considerations (cost, side effects, patient preference or comorbidities), which may overlook the biological mechanism. Thus, more people are developing diabetes worldwide and have disease progressing to complications, incurring a significant healthcare burden and cost.
There are, however, several reasons for hope. First, diabetes caused by single gene defects can be characterised and targeted therapies are particularly effective [1, 2]. Second, islet autoantibody biomarkers and genomic risk have clarified autoimmune diabetes from other forms of the disease [3, 4], thereby facilitating immune-intervention trials and pre-onset monitoring to reduce risk of severe complications and aiding in detection of environmental triggers [5]. Third, multiple biomarkers and genetic variants have been shown to alter risk of type 2 diabetes, revealing previously unsuspected biological pathways and providing new targets. Fourth, type 2 diabetes has been shown to be a complex combination of multiple conditions and processes, defined by process-specific subgroups in which individuals with extreme burdens of risk in particular pathways reside and for whom a specific therapeutic approach may be optimal [6]. Finally, the tools, resources and data now exist to determine the biological and lifestyle/environmental predictors of drug response, as measured by a variety of clinical outcomes [7].
The Precision Medicine in Diabetes Initiative
The idea of precision diabetes medicine is gaining momentum, based upon the promise of reducing the enormous and growing burden of diabetes worldwide. To address this, the Precision Medicine in Diabetes Initiative (PMDI) was launched in 2018 by the American Diabetes Association (ADA), in partnership with the European Association for the Study of Diabetes (EASD). The PMDI has partnered subsequently with other organisations (the US National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK] and JDRF).
The mandate of the PMDI is to establish consensus on the viability and potential implementation of precision medicine for the diagnosis, prognosis, prevention and treatment of diabetes, through expert consultation, stakeholder engagement and systematic evaluation of available evidence. This mandate is pursued in order to realise a future of longer, healthier lives for people with diabetes.
The PMDI is focused on assessing evidence, promoting research, providing education and developing guidelines for the application of precision medicine in diabetes. The 2019 ADA Scientific Sessions (held in June 2019) sponsored a research symposium focused on precision medicine, followed by a PMDI stakeholder meeting (held in October 2019) that was attended by experts in areas germane to precision diabetes medicine from around the world. Future PMDI symposia will extend the themes of precision diabetes medicine during the 2020 ADA Scientific Sessions and EASD Annual Meeting. In the coming years, educational approaches to translate the science into practice will be the target of a series of postgraduate education symposia. A global clinical research network focused on precision diabetes medicine is also being planned, along with other education and information dissemination activities (see Fig. 1 for an overview of key objectives).
The purpose of the work underlying the ADA/EASD PMDI consensus reports, of which this is the first, is to define relevant terminology (Text box 1) and review the current status of diagnostics and therapeutics (prevention and treatment) in diabetes, including key areas of opportunity and where further inquiry is needed (Text boxes 2–4). Particular focus is placed on elucidating the aetiological heterogeneity of diabetes, which involves a combination of approaches including contemporaneous measures of risk factors, biomarkers and genomics, as well as lifestyle and pharmacological interventions. Monogenic diabetes is one of few areas where precision diabetes medicine has been proven feasible and is practised (as discussed at a recent Diabetes Care Editors’ Expert Forum; M. C. Riddle, personal communication). This first Consensus Report does not seek to address extensively the role of precision medicine in the complications of diabetes, which is a topic for future evaluation. In addition, we do not discuss diabetes digital device technology, as this is addressed in a joint ADA/EASD consensus report [8, 9]. A second PMDI consensus report will be published documenting the findings of a systematic evidence review, focusing on precision diagnostics and precision therapeutics (prevention and treatment).
An Executive Oversight Committee, comprising representatives from the founding organisations, ADA (LP) and EASD (JJN), and the two co-chairs of the initiative (PWF and SSR), provide PMDI governance. The Executive Oversight Committee is responsible for ensuring that the PMDI activities are executed. Leadership and direction of the PMDI are provided by members of the PMDI Steering Committee, currently comprised of academic leaders in precision diabetes medicine from the USA (WKC, JCF, JMN) and Europe (ATH, MIM, ERP), a representative from NIDDK (CGL) and the Executive Oversight Committee members (LP, JJN, PWF, SSR). The Steering Committee is responsible for providing guidance for PMDI activities and engages in developing precision diabetes medicine education, drafting consensus statements and building interest/working groups to achieve its mission. The Executive Oversight Committee and the Steering Committee work closely together under the banner of the PMDI Task Force. Membership of the Steering Committee will expand to include experts from around the world and across multiple areas of expertise germane to the topic of precision diabetes medicine.
Work for this Consensus Report began at the October 2019 stakeholder meeting in Madrid. The meeting included presentations and roundtable discussions. At the conclusion of the meeting, a writing group meeting attended by the PMDI Task Force and stakeholders was held to determine what should be addressed in the Consensus Report. Following the meeting, consensus was reached by the PMDI Task Force through bimonthly calls and electronic communication. Relevant experts outside of the Task Force were asked to contribute sections as needed. The Consensus Report was then peer-reviewed by experts in the field and by the clinical committees of the founding organisations. The report was then submitted to Diabetes Care and Diabetologia for simultaneous publication.
Precision diabetes medicine: what it is and what it is not
Precision diabetes medicine refers to an approach to optimise the diagnosis, prediction, prevention or treatment of diabetes by integrating multidimensional data, accounting for individual differences (Text box 1). The major distinction from standard medical approaches is the use of complex data to characterise the individual’s health status, predisposition, prognosis and likely treatment response. Precision medicine also focuses on identifying patients who, despite a diagnosis, do not require treatment (or require less than might conventionally be prescribed). These data may stem from traditional sources such as clinical records, as well as from emergent sources of ‘big data’, such as individual medical records from very large cohorts of patients, geomobility patterns obtained from devices, behavioural monitors (e.g. actigraphy for exercise and sleep assessments), ingestible, subcutaneous or wearable sensors (e.g. for blood glucose monitoring) and genomic and other ‘omics’ data. Integration of patient preferences, patient-centred outcomes, cost-effectiveness and shared decision making will guide how precision diabetes medicine is formulated and applied.
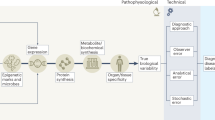
There are several terms sometimes used interchangeably with precision medicine, including ‘personalised medicine’, ‘individualised medicine’ and ‘stratified medicine’. The 2020 ADA Standards of Medical Care in Diabetes (ADA SOC) places considerable emphasis on the personalisation of diabetes medicine, highlighting that ‘clinicians care for patients and not populations’ (page S2 of [10]). This reflects the appreciation of individual differences with respect to symptomatology, presentation, behaviours, preferences, social circumstances, response to treatment, comorbidities or clinical course. For precision diabetes medicine to be effective, it must be tailored to the individual. Thus, the ADA SOC instructs the clinician to adapt guidelines to each patient’s characteristics, circumstances and preferences, including the patient’s food security, housing and financial stability. In the context of the PMDI, this is not considered to be precision medicine; rather, this final step in the process of translating knowledge into practice is personalised (or individualised) medicine. In contrast, precision (or stratified) medicine emphasises tailoring diagnostics or therapeutics (prevention or treatment) to subgroups of populations sharing similar characteristics, thereby minimising error and risk while maximising efficacy. Included within precision diabetes medicine is the monitoring of disease progression using advanced technologies or considering how patient features affect the reliability of assays. The application of precision diabetes medicine may substantially reduce errors in diagnostic (Fig. 2), therapeutic (Fig. 3) and prognostic (Fig. 4) processes. For example, the interrogation of large sets of longitudinal clinical data could identify disease subtypes and match the patient to others with a similar disease profile; through knowledge of treatment efficacy and outcomes, more precise prognosis and optimisation of therapies for this patient by concordance to similar subgroups would emerge (Text box 1, Figs 3 and 4).
Precision diagnostics
What are the requirements for precision diagnosis?
Precision diagnostics (Text box 2) employs methods to subclassify patients to enable the successful application of precision medicine approaches (Fig. 2). This will facilitate matching precise prevention strategies and treatments to individuals either at risk for or diagnosed with diabetes. Ideally, a precision diagnostic test should be: (1) robust (high test–retest reliability within and between laboratories); (2) able to define a discrete subgroup, giving insights into disease aetiology, prognosis and treatment response; (3) widely available; (4) easily performed, with accepted norms for interpretation; (5) inexpensive (or at least cost-effective); and (6) approved by regulatory authorities.
Precision diagnosis can be conceptualised as a pathway that moves through stages, rather than as a single step. The diagnostic stages include assessing the:
-
expected prevalence based on epidemiology, including age, or age at diagnosis of diabetes, sex and ancestry,
-
probable clinical diagnosis using clinical features and other data, and
-
modification by diagnostic tests that are interpreted in the light of prevalence and diagnosis.
A diagnosis in precision medicine is a probability-based decision, typically made at a point in the natural history of a disease, reflecting neither an absolute truth nor a permanent state. Presenting the degree of uncertainty in a manner that is intuitive to the patient and practitioner is critical if the precision diagnosis is to be effective.
Precision diagnosis in clinical practice
Interpreting HbA1c in diagnosis and monitoring
Data and outcomes from the widespread use of HbA1c, rather than blood glucose levels, for diagnosis has led to a precision approach for the diagnosis of diabetes. The level of HbA1c will depend on factors that impact haemoglobin and red cell stability as well as average glucose values [10]. Genetic testing can reveal unsuspected variants that alter HbA1c. Thus, knowledge of the patient’s ancestry and specific genetic information can guide interpretation of assay results for diagnosis and the monitoring of blood glucose.
Diagnosing type 1 vs type 2 diabetes
Currently, the most common step towards precision diagnosis that is made in clinical diabetes medicine is the classification of type 1 vs type 2 diabetes, the two most prevalent subcategories with different aetiologies and different treatment requirements. Part of the diagnostic dilemma is that neither type 1 nor type 2 diabetes are monolithic entities and robust ‘gold standards’ are not universally agreed. Diagnostic issues arise when expected clinical features are discordant from established norms (e.g. people diagnosed with diabetes who are young and have obesity, or old and slim, or who are a rare subtype in that clinical setting) [11]. Islet autoantibody positivity varies by clinical setting (e.g. in people without diabetes, individuals diagnosed with probable type 1 diabetes as children, individuals with clinical features of type 2 diabetes), resulting in an altered prior probability of type 1 diabetes that reflects the different prevalence in these diverse settings. The best diagnosis depends on integrating all diagnostic modalities, as demonstrated in predicting long-term C-peptide negativity in individuals diagnosed with diabetes between 20 and 40 years of age, where an integrated model outperformed diagnosis based on clinical features, circulating antibodies or genetics used in isolation [3]. The frequency of misdiagnosis of type 1 and type 2 diabetes in middle-aged and elderly adults [11, 12] suggests that precise diagnostic approaches are needed, especially as failure to recognise insulin-deficient states can be fatal.
Monogenic diabetes
A Diabetes Care Editors’ Expert Forum (M. C. Riddle, personal communication) has concluded recently that a monogenic diabetes diagnosis is closest to meeting all criteria for a perfect diagnostic test as it defines a discrete subgroup giving insights into aetiology, prognosis and treatment response [1, 2]. Most cases of monogenic diabetes remain misdiagnosed. Perhaps the best example of precision diabetes medicine is the excellent and long-lasting glycaemic response to oral sulfonylureas in insulin-dependent infants diagnosed with neonatal diabetes caused by abnormalities in the beta cell potassium channel [13,14,15,16,17]. In GCK-MODY (MODY2), it is established that patients do not require [18], or respond to, oral medication [19]. Other MODY diagnoses (HNF1A [MODY3], HNF4A [MODY1] and ABCC8 [MODY12]) are acutely sensitive to the glucose-lowering effects of sulfonylureas [20,21,22]; however, unless the diagnosis is precise, these therapeutic benefits are lost. With the clear benefits of precision diagnosis of monogenic diabetes, it is important to reduce barriers to its implementation. For example, the cost of performing molecular genetic testing is high and universal testing is not cost-effective. It is thus necessary to limit testing to those most likely to have a monogenic diagnosis. Moreover, identification protocols require prescreening based on clinical features (e.g. family history, age at onset, phenotype including syndromic features) and non-genetic testing (islet autoantibodies and C-peptide).
One approach for implementing precision medicine in the case of monogenic diabetes would be to:
-
test all infants diagnosed with diabetes in the first 6 months of age, because >80% of neonatal diabetes cases have a monogenic cause
-
use a MODY calculator to identify those whose clinical features suggest a high likelihood of MODY (www.diabetesgenes.org/mody-probability-calculator/) [23]
-
test individuals with paediatric diabetes when at least three islet autoantibodies are antibody negative [24]
The effective use of these pregenetic selection criteria should greatly improve the likelihood of correctly diagnosing monogenic diabetes without the burden of costly genetic screens. Although diagnostic molecular genetic testing utilises robust analysis of germline DNA, which is virtually unchanged throughout life, there are still issues with its implementation. One issue is the incorrect interpretation of the genetic information, leading to inaccurate identification of causal mutations in both clinical practice and in the published research literature [25]. Curation of pathogenic variants for monogenic diabetes is critical and is currently being addressed by international consortia. As a result of technological advances, multiple causes of monogenic diabetes can be tested for in a single next-generation sequencing test. This approach is generally advantageous as it does mean that syndromic monogenic diabetes is diagnosed genetically when the patient presents with isolated diabetes. This will allow other features to be examined and treated appropriately before clinical presentation. Examples of this are neonatal diabetes [2], HNF1B-MODY (MODY5) [26], WFS1 (Wolfram syndrome) [27] and mitochondrial diabetes [28]. For these patients, the genetic diagnosis of diabetes will have implications far beyond the prognosis and care of diabetes, as the patient with certain types of monogenic diabetes will also be at high risk of developmental delay, neurological disease, developmental kidney disease, liver failure, deafness and cardiomyopathy.
Diagnosing latent autoimmune diabetes in adults
Latent autoimmune diabetes in adults (LADA) is not currently recognised by the ADA as a formal subtype of diabetes. Nevertheless, LADA reveals some of the difficulties in diabetes subtyping. It was shown that the presence of GAD autoantibodies in patients with type 2 diabetes was associated with progression to early insulin therapy [29]; yet, controversy remains as to whether LADA is a discrete subtype, a milder form of type 1 diabetes, or a mixture of some patients with type 1 diabetes and others with type 2 diabetes. The uncertainty is increased by variation in the diagnostic criteria, with initial treatment based upon physician preference as well as the patient’s presentation [30]. In addition, among those with GAD autoantibodies, the phenotype varies with different autoantibody levels [31].
Subcategories of common forms of diabetes
The subcategorisation of type 1 or type 2 diabetes may not always be the optimal approach for precision diabetes diagnosis or therapy. Nevertheless, the ability to delineate type 1 or type 2 diabetes using non-traditional data and approaches may lead to improvements in prevention or treatment of the disease, including diabetes subclassifications beyond type 1 or type 2 diabetes.
Subcategories in type 1 diabetes
Subcategories in type 1 diabetes The age at which the initial islet autoantibody appears and the type of autoantibody (e.g. which of the four primary antibodies among islet cell autoantigen 512/islet antigen 2 [ICA512/IA-2], insulin, GAD, zinc transporter 8 [ZnT8]) may be important in defining aetiological subtypes of type 1 diabetes [32]. Data supporting this potential subcategory are based upon those diagnosed in the first 10 years of life and in predominantly white European populations. The relevance to other ethnic groups and those diagnosed later in life is uncertain.
The genetic variants accounting for the majority of risk of type 1 diabetes are now known, and the sensitivity and specificity of type 1 diabetes genetic risk scores (T1D-GRS) both exceed 80% [5, 33,34,35]; however, a high T1D-GRS will have low positive predictive value in populations with a typically low prevalence. A T1D-GRS may prove most useful when integrated with clinical features and islet autoantibodies [3, 4]. There is variation in the genetic susceptibility with age at diagnosis but, at present, genetics is not suggested as an approach for defining subtypes of type 1 diabetes.
There is strong evidence for enrichment of immune cell types that are associated with genetic risk of type 1 diabetes, particularly T cells (CD4+ and CD8+) and B cells (CD19+). However, at present, there is no immune-based test sufficiently reproducible and robust that it can be used diagnostically for type 1 diabetes.
Persistent endogenous beta cell function in type 1 diabetes is associated with greater potential for improved glycaemic control and reduced complications [36]. A stimulated C-peptide measurement represents a candidate for defining subcategories of type 1 diabetes with different treatment aims. C-peptide levels exponentially fall in the ‘honeymoon period’ after type 1 diabetes diagnosis [37] but have been shown to be stable 7 years after diagnosis [38]. Persistent C-peptide is associated with a later age at diagnosis, although there are few data to predict those likely to maintain high levels of C-peptide.
Subcategories in type 2 diabetes
Subcategories in type 2 diabetes Family history of type 2 diabetes, as a surrogate for precise genetic evaluation, fails to meet many of the criteria of a robust test, as any assessment changes over time and depends on the relatives selected for reporting the ‘family’. The value of a family history may be greatest in monogenic diabetes, in which a pedigree will often demonstrate a pattern of inheritance consistent with a single gene disorder and a consistent phenotype.
Type 2 diabetes treatment response and disease progression can be predicted from continuous clinical features with specific models. These models appear to perform better than dividing into cluster-based subgroups [7]. An advantage of using clinical features is that they are widely available and easily obtained (e.g. sex, BMI, HbA1c). However, they are limited by the fact that clinical features may vary over time and with the natural history of the disease. Incorporation of longitudinal change with treatment response could be a strength as the model’s prediction would change in concert with changes in the phenotype of the patient.
Recent research has attempted to define subcategories of type 2 diabetes (and type 1 diabetes) based on cluster analysis at diagnosis to provide insights into likely progression, risk of complications, and treatment response [39, 40]. Barriers facing this and other approaches include collection of data that are not routinely obtained (e.g. a fasting C-peptide at the time of diagnosis, with considerable variation in results between laboratories [41]) and the change in biomarkers over time that are dependent on disease course or its treatment. Genetic data have been used to define type 2 diabetes subcategories by clustering genetic variants that associate with physiological traits and which are correlated with clinical outcomes [6]. At this time, the available genetic data for type 2 diabetes and the clustering does not have sufficient predictive accuracy to replace existing delineative approaches. None of the methods described above are established for subclassification of type 2 diabetes in clinical practice; nevertheless, it is true that in a minority of patients, their specific type of diabetes may be adequately characterised using genetic clustering [42, 43].
Precision therapeutics
Accurate diagnosis is necessary for successful precision therapy, whether for prevention or treatment (Fig. 3). This is true where subgroup(s) of the population must be defined to determine which targeted interventions will be applied, as well as for determination of treatment outcome. In monogenic diabetes, there are no currently known options for prevention. In type 1 diabetes, precision prevention currently involves mainly the optimisation of monitoring methods (Text box 3), thereby facilitating timely early detection, preventing early complications and allowing appropriate treatment. In contrast, type 2 diabetes has many avenues for prevention; thus, the possibilities for precision approaches, possibly through tailoring of lifestyle (e.g. diet), are broad in type 2 diabetes.
Precision prevention in diabetes (Text box 3)
Type 1 diabetes
Type 1 diabetes is characterised by damage, impairment and eventual destruction of the insulin-producing pancreatic beta cells, thought to be the result of an autoimmune process. Type 1 diabetes progression has been grouped into discrete ‘stages’ [44]: Stage 1 is defined by the presence of ≥2 islet autoantibodies, with normal blood glucose; Stage 2 is defined by the presence of ≥2 islet autoantibodies with elevation of blood glucose, signalling the functional impairment of the beta cells; and Stage 3 is characterised by symptoms of dysglycaemia, such as polyuria or diabetic ketoacidosis, although not all symptoms need be present. A clinical diagnosis of type 1 diabetes typically is not given until Stage 3. Type 1 diabetes is nearly inevitable once ≥2 islet autoantibodies appear, particularly in those of younger age, with a lifetime diabetes risk approaching 100% [45, 46]. Approximately half of the risk of type 1 diabetes is due to genetic factors, with over 30% of the genetic risk attributable to genes of the HLA complex, but also including more than 50 non-HLA loci [35]. Unknown environmental factors are thought to trigger the autoimmune process that results in initial beta cell damage and progression toward symptomatic type 1 diabetes [47].
Primary prevention trials in genetically susceptible individuals who have not yet developed autoantibodies (i.e. pre-stage 1) and secondary prevention trials in children with stages 1 and 2 have been conducted [48] using dietary interventions and immune-targeting approaches. Dietary manipulation studies have been largely unsuccessful in reducing islet autoimmunity [49,50,51] or type 1 diabetes [52]. Previous intervention studies among individuals at stage 1 or stage 2 have been unable to slow, halt or reverse the destruction of insulin-producing beta cells. Of nine completed secondary prevention trials [53,54,55,56,57,58,59,60], only one (using an anti-CD3 antibody) has shown a slight delay in progression to type 1 diabetes [61].
Most prevention trials in type 1 diabetes have not been effective, partially because the unique type 1 diabetes genetic risk profile of the individual and their unique response to the preventive agent (immune therapy or dietary intervention) have not been considered. For example, the inflammatory response to infection with enteroviruses implicated in the onset of type 1 diabetes has been shown to be genetically mediated [62] and diet has had different effects on development of autoimmunity and progression to type 1 diabetes [63] dependent on genetic risk. Several studies have suggested that susceptibility to islet autoimmunity and progression to type 1 diabetes may be related to the ability to adequately use vitamin D, as higher cord blood 25-hydroxyvitamin D was associated with a decreased risk of type 1 diabetes, but only in children who were homozygous for a vitamin D receptor gene (VDR) variant [64]. Risk of islet autoimmunity was observed with reduced dietary intake of the n-3 fatty acid α-linolenic acid, but only in those with a specific genotype in the fatty acid desaturase gene (FADS) cluster [65]. Thus, without considering the unique genetic profiles of children, dietary supplementation may not be successful, arguing for an appropriately validated precision approach.
Type 2 diabetes
The emergence of type 2 diabetes as a global public health crisis during recent decades has motivated numerous large randomised controlled trials assessing the efficacy of pharmacological or lifestyle interventions for prevention. An emphasis has been placed on intervening in people with ‘prediabetes,’ defined as a person with levels of fasting blood glucose, 2 h blood glucose or HbA1c that are chronically elevated but below the diagnostic thresholds for diabetes. Although prediabetes is a major risk factor for type 2 diabetes and other diseases [66], intervening in everyone with prediabetes may not be cost-effective [67]. Aggressive precision prevention in those with relevant risk factors is discussed in the current ADA SOC [68]. Youth with prediabetes should be the focus of preventive interventions, especially those with overweight or obesity and who have one or more additional risk factors (e.g. maternal history or exposure to gestational diabetes mellitus [GDM], a positive family history of diabetes in first- or second-degree relatives, signs of insulin resistance or specific high-risk ancestry).
Multiple interventions in adults with type 2 diabetes have been evaluated for risk reduction and prevention, both in the short and the long term. A recent systematic review [69] reported that after active interventions lasting from 6 months to >6 years, relative risk reduction achieved from lifestyle interventions (39%) was similar to that attained from use of drugs (36%); however, only lifestyle interventions had a sustained reduction in risk once the intervention period had ended. Analysis of the post-intervention follow-up period (~7 years) revealed a risk reduction of 28% with lifestyle modification compared with a non-significant risk reduction of 5% from drug interventions.
Most lifestyle intervention programmes use standardised approaches designed to change diet and exercise habits for reducing body weight. The Diabetes Prevention Program (DPP) evaluated the efficacy of lifestyle intervention and metformin therapy, compared with standard of care and placebo (control), for delay or prevention of diabetes in those with impaired glucose regulation at baseline. Although the reductions in diabetes risk from lifestyle (58% reduction) and metformin (31% reduction) compared with the control intervention were impressive [70], there was considerable variation across the study population [71], with many participants developing type 2 diabetes during the active intervention period (the first 2.8 years of the trial). Thus, the DPP lifestyle intervention did not truly ‘prevent’ diabetes. Indeed, in the decade after randomisation, during which participants were offered lifestyle reinforcement semi-annually, the average duration before disease onset was ~3 years [72]. Those participants in the DPP who progressed most rapidly were those who lost the least weight in the early stages of the intervention [73], with genetic variants representing significant predictors of peak weight loss and weight loss maintenance [74]. Results from the DPP and other large prevention trials suggest that a ‘one-size-fits-all’ lifestyle intervention strategy will not be efficacious for everyone, particularly if it cannot be sustained, strengthening the case for precision lifestyle interventions in type 2 diabetes prevention.
Although precision diabetes medicine is much more than genetics, the majority of relevant research has focused on evaluating the role of genetic variants in precision prevention. Large epidemiological studies [75] and intervention trials [76, 77] strongly suggest that standard approaches for lifestyle modification are equally efficacious in preventing diabetes regardless of the underlying genetic risk. This contrasts the extensive epidemiological evidence suggesting that the relationship of lifestyle with obesity is dependent on genetic risk [78,79,80,81]; however, with few exceptions (e.g., [74]), analyses in large randomised controlled trials have failed to show that these same genetic variants modify weight loss in response to lifestyle intervention [82]. It is also important to recognise that knowledge of increased genetic risk for diabetes may not motivate improvements in lifestyle behaviours. Indeed, knowledge of increased genetic risk for diabetes may decrease motivation to modify behaviour in genetic fatalists [83].
Diet recommendations optimised to the individual have been shown to reduce postprandial glycaemic excursions to a greater extent than standard approaches in healthy individuals [84]. Meal compositions that induce the most favourable glycaemic profiles have been guided by models derived from an individual’s biological data (e.g. microbiome, genome, and metabolome), information on lifestyle factors (e.g. sleep and exercise) and postprandial glycaemia following the consumption of a series of standardised meals. Although these studies indicate that personalised diet plans may help to minimise postprandial glycaemic excursions, no studies have reported the long-term impact of adhering to personalised diets on glycaemic control.
Of the 12 approved classes of diabetes drugs, many having been assessed for efficacy in prevention. Overall, drugs that enhance insulin action have proven more effective in diabetes prevention than those that increase insulin secretion. Some of the variability in the diabetes-reducing effect of metformin in the DPP has been associated with variation in the SLC47A1 gene that encodes the multidrug and toxin extrusion 1 (MATE1) transporter protein [85]. In the DPP Outcomes Study, the effects of lifestyle, metformin and placebo interventions on weight reduction during the 6–15 years that followed the end of the randomised intervention phase were assessed [86]. As a percentage of baseline weight, those assigned to metformin maintained an average weight loss of 6.2% compared with the lifestyle intervention group, which maintained a weight loss of 3.7%, and the placebo group, which maintained a weight loss of 2.8%. In the subgroup of DPP participants who lost <5% baseline weight at 1 year post randomisation (poor responders), body weight during the following 14 years remained essentially unchanged, whether receiving metformin or placebo interventions. In contrast, those participants in the lifestyle intervention group who lost <5% baseline weight gained and sustained ~2 kg excess body weight in the years that followed. These findings reveal a subgroup of DPP participants in whom lifestyle intervention led to weight gain, which presents a potential avenue for stratified intervention, where individuals who are unlikely to respond well to lifestyle modification might be better served by other therapeutic approaches.
Precision treatment (Text box 4)
Once diabetes develops, a variety of therapeutic steps may be clinically indicated to improve disease management. These steps include:
-
glucose monitoring
-
patient education and lifestyle intervention [87]
-
surgery
-
drug treatments to lower HbA1c
-
drug treatments to lower cardiovascular risk (e.g. statins, anti-hypertensives)
-
drug treatments targeting specific complications (e.g. ACE inhibitors/angiotensin II receptor blockers [ARBs] and sodium–glucose cotransporter 2 [SGLT2] inhibitors for proteinuric kidney disease, fibrates for retinopathy, atypical analgesics for painful neuropathy, and statins and antihypertensives for cardiovascular disease).
For each of these treatments, there will be patients who respond well and those who respond less well, in addition to those who have adverse outcomes from the therapy. Thus, precision treatment can be considered as using patient characteristics to guide the choice of an efficacious therapy to achieve the desired therapeutic goal or outcome while reducing unnecessary side effects (Fig. 3). Given the broad scope of precision treatment, pharmacological therapy in type 2 diabetes has the best evidence-base for precision therapeutics at present.
Subcategories and drug outcomes
Traditionally, trials of therapeutic interventions do not recognise variation in aetiological processes that lead to the development of type 2 diabetes. The MASTERMIND consortium recently re-analysed data from the A Diabetes Outcome Progression Trial (ADOPT) and Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes (RECORD) studies in order to highlight how clinical phenotype can be used to help guide treatment intervention. In ADOPT, on average, men without obesity showed a greater HbA1c reduction over 5 years with sulfonylureas than they did with thiazolidinediones; however, women with obesity treated with thiazolidinediones had sustained HbA1c lowering over the 5 years compared with sulfonylureas [88]. When considering the clinical and physiological variables used to subgroup individuals with diabetes [39], the insulin-resistant cluster defined in ADOPT and RECORD responded better to thiazolidinediones while the older patient cluster responded better to sulfonylureas [7].
Similar studies have been undertaken to investigate how simple clinical variables can be used to predict glycaemic response to dipeptidyl peptidase 4 inhibitors (DPP4i). In studies undertaken using prospective (Predicting Response to Incretin Based Agents in Type 2 Diabetes study [PRIBA]) and primary care data in the UK (Clinical Practice Research Datalink [CPRD]), an insulin-resistant phenotype of obesity and high triacylglycerols was associated with reduced initial response to DPP4i, and more rapid failure of therapy [89].
As outlined under ‘Precision diagnostics’ and elsewhere, the most current examples of how genetics impacts precision treatment can be seen in monogenic diabetes, for which single gene mutations are causal for the development of diabetes and for which targeted treatments can, in effect, bypass the aetiological defect (e.g. sulfonylurea sensitivity in HNF1A-MODY [MODY3] [20] and insulin independence with high-dose sulfonylureas in neonatal diabetes due to KATP channel defects [14]). In some instances, precision treatment may result in cessation of unnecessary medication, as is the case in people with GCK-MODY (MODY2), where blood glucose remains somewhat elevated, but stable, over time.
Unlike monogenic forms of diabetes, type 2 diabetes is a common complex disease characterised by thousands of aetiological gene variants. It is uncertain whether individual genetic variants will be highly predictive of drug outcomes. Similar to the underlying genetic architecture of type 2 diabetes, it is possible that drug response in type 2 diabetes will be influenced by many genetic variants of small to modest effect. Genetic studies of drug response in type 2 diabetes have largely been based on candidate genes of known aetiological processes or drug pathways. These studies have been limited in their success. For example, some studies have shown that the KCNJ11/ABCC8 E23K/S119A risk variant increases glycaemic response to sulfonylureas [90,91,92]; in contrast, the TCF7L2 diabetes risk variant reduces glycaemic response to sulfonylureas [93,94,95]. The PPARG Pro12Ala diabetes risk variant has been associated with reduced glycaemic response to thiazolidinediones [96,97,98].
Genome-wide association studies (GWAS) have the potential to provide novel insights as they make no assumptions about drug mechanism or disease process, in contrast to candidate gene/pathway studies. Only GWAS of metformin have been reported to date [99, 100], identifying that variants at the ATM/NPAT and SLC2A2 loci are associated with an altered glycaemic response. In SLC2A2, the non-coding rs8192675 variant C allele is associated with greater response to metformin and is associated with reduced expression of the SLC2A2 transporter in liver, intestines and kidneys. In individuals with obesity, those with two copies of the C allele had an absolute HbA1c reduction of ~1.55% (compared with a reduction of ~1.1% in those without the C allele). While this may appear to be a small difference, the SLC2A2 genotype effect is the equivalent of a difference in metformin dose of 550 mg, or about half the average effect of starting a DPP4i.
When considering aetiological variation, recent work partitioning diabetes-associated genetic variants by their presumed aetiological process (partitioned polygenic scores) [6, 42, 101] may define genetically driven dominant processes. These processes, such as beta cell dysfunction, lipodystrophy or obesity could respond differently to drugs that act on these pathways, such as sulfonylureas, glucagon-like peptide 1 receptor agonist (GLP-1RA), DPP4i and thiazolidinediones.
Genetic variation can not only capture aetiological variation but also variation in drug pharmacokinetics (absorption, distribution, metabolism, excretion [ADME]) and in drug action (pharmacodynamics). Studies of ADME genes have revealed some variants with a moderate to large effect. For example, the 8% of the white population who carry two loss-of-function variants in CYP2C9 are 3.4 times more likely to achieve HbA1c target than those with normal function cytochrome P450 family 2 subfamily C member 9 (CYP2C9), due to reduced metabolism of sulfonylureas and increased serum concentrations [102]. SLCO1B1 and CYP2C8 genotypes that alter liver uptake and metabolism of rosiglitazone can alter glycaemic response (HbA1c) by as much as 0.7% [103]. While these studies have promoted pharmacogenetic approaches in precision diabetes therapeutics, some studies have been surprisingly negative. For example, loss-of-function variants in the SLC22A1 gene, encoding the organic cation transporter 1 (OCT1), which transports metformin into the liver [104, 105], do not reduce the glucose-lowering efficacy of metformin in patients with type 2 diabetes [106, 107]. Thus, there is genetic evidence that metformin does not work to lower glucose solely via hepatic mechanisms.
The diabetes phenotype is markedly different across ethnic groups; thus, it is likely that drug outcomes will differ between populations. The current and growing burden of diabetes is growing rapidly in all populations, particularly in South and East Asians, yet, these populations are under-represented in clinical and drug outcomes trials. A lack of systematic reviews and meta-analyses from these high-prevalence regions still points to differences in drug response. For example, the DPP4i response is greater in Asian than white people [108], a result supported by a subgroup analysis of the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) showing a greater HbA1c reduction to sitagliptin in East Asians compared with white individuals [109]. Glycaemic response to metformin has also been reported to differ by ethnic group, with African-American individuals having a greater response than European Americans [110].
At this time, it is evident that we have the potential to use simple clinical (e.g. BMI, sex, ethnicity), physiological and genetic variables to predict who is more or less likely to benefit from a treatment. The reducing costs of genotyping panels mean that genotype information could potentially be available at the point of prescribing, when the modest effect sizes described may start to have clinical utility. There is a need to develop implementation and evaluation strategies to assess the effectiveness and cost-effectiveness of such approaches compared with conventional treatment approaches.
Precision approaches to diabetes in pregnancy
In women, being affected by GDM is a major risk factor for type 2 diabetes. The risk of developing type 2 diabetes in women with prior GDM approaches 70% after the index pregnancy [111], climbing to an 84% risk of developing type 2 diabetes in women of East Indian ancestry [112]. Currently, genetic studies of GDM have identified those variants known to increase risk of type 2 diabetes [113]; however, other variants have been shown to influence glycaemic traits specifically in pregnancy [114]. Furthermore, like type 2 diabetes, GDM is a heterogeneous condition linked to primary defects in either insulin secretion or sensitivity [115, 116]. GDM can also result from monogenic forms of diabetes, as numerous studies have shown. Models that attempt to predict pregnancy complications [117] or subsequent type 2 diabetes [118] in GDM using clinical characteristics, biomarkers and/or genetic variants have yet to be adopted, even though both lifestyle interventions and metformin use have demonstrated benefits in reducing the risk of type 2 diabetes in women with prior GDM [119].
The target for all patients with type 1 or type 2 diabetes in pregnancy is to achieve as near normal glucose as possible, particularly around the time of conception (to reduce developmental anomalies) and in the third trimester (to reduce the risk of macrosomia) [120]. In pregnancy, the only clear exception so far is for mothers with GCK-MODY (MODY2) as fetal growth is determined predominantly by fetal genotype [121]. In mothers whose fetus inherits the mother’s GCK-MODY mutation, fetal growth is normal despite the maternal hyperglycaemia; thus, treatment of the maternal hyperglycaemia is not recommended [121, 122]. Establishing whether the fetus is likely to be affected is usually determined by ultrasound scan. In the future, the use of non-invasive cell-free DNA methods in maternal blood will likely establish fetal risk [123]. In GDM, whether maternal hyperglycaemia is closely monitored and treated in the third trimester is based on the degree of hyperglycaemia determined by an oral glucose tolerance test at 24–28 weeks’ gestation [10]. In the future, this decision could be modified by non-glycaemic factors that impact fetal growth.
Patient-centred mental health and quality-of-life outcomes
Precision diabetes medicine holds the promise of reducing uncertainty by providing therapies that are more effective, less burdensome and with fewer adverse outcomes, which ultimately improve quality of life and reduce premature death (see Text box 5). Highly relevant in this context is mental health (e.g. risk of distress and depression), yet little has been done to investigate how precision medicine might play a useful role in improving mental health outcomes.
Depression and anxiety are twice as common in people with diabetes than in the general population, occurring in up to 20% of adult patients [124]. Distress occurs in ~30% of people with diabetes [125], reflecting the emotional and psychological burden that comes with diabetes and its complications, the life adjustments it requires, and anxiety about hypoglycaemia or the impact on the fetus for GDM. Distress has been reported as being more common in patients in secondary, rather than primary, care and in populations with non-European ancestry. Depression is more common in lower- and middle-income countries, where ~75% of people with type 2 diabetes reside [125]. Both depression and distress in diabetes are more common in those who progress from oral agents to insulin therapy [126]. The onset of complications with the initiation of a more complex pattern of treatment is associated with increased rates of depression [126].
There are key points in the life course of a person with diabetes when both rational and irrational fears are often elevated, typically coinciding with ‘events’, including:
-
increased medication dose
-
transition to insulin or other injectables or devices
-
emergence of complications or worsening of complications
-
following a severe hypoglycaemic event
-
change in diabetes care provider.
In many cases, patient self-evaluations may be distorted at these times because the patient attributes blame for the disease to his/her self, the future feels uncertain and distress peaks. In the setting of precision diabetes medicine, providers should assess symptoms of diabetes distress, depression, anxiety, disordered eating and cognitive capacities using appropriate standardised and validated tools at the initial visit, at periodic intervals and when there is a change in disease, treatment or life circumstance [127], information that, when combined with other data, are likely to improve the precision of clinical decision making.
Psychological counselling can help patients understand and manage their emotional reactions to major events by developing a more optimistic outlook and more realistic, modulated and adaptive emotional reactions [128]. Precision medicine may be used in the future to help predict the frequency and extent of emotional crises. As a result, precision diabetes medicine may lessen the patient burden, help patients to objectivise their disease, and provide targets for behavioural and point-of-care interventions at critical moments in the clinical care cycle. Effective and tailored education and professional counselling will be necessary to mitigate the risk that a clearer prognosis may raise anxiety about the future for some patients.
Equity in precision diabetes medicine
The experience with monogenic diabetes has shown that there is a large degree of regional, national and international variation in how, and how often, these cases are diagnosed [1, 129, 130]. This variation is, in part, due to differences in access to general medical care and treatments, access to relevant healthcare professionals with the necessary education, training and experience, and access to laboratories with the necessary experience, assays and standards [131]. A precision approach to diabetes care will require that the relevant laboratory methods and assays are carefully standardised and comparable. Assessments that need to be standardised include:
-
type 1 diabetes-associated autoantibodies
-
C-peptide
-
clinical genetic/genomic risk scores
-
decision-support interpretation
One challenge to standardisation is that the frequency of various diabetes phenotypes and risk genotypes may vary by regions of the world and between ethnicities within a region. For example, type 2 diabetes often manifests very differently in Native Americans than in people of European ancestry, with Native Americans tending to develop diabetes at a much younger age and experience loss of beta cell function earlier in the life course of the disease [132]. Recent insights following the ADA Precision Diabetes Medicine meeting in Madrid (held in October 2019) confirm that case-based interactive learning is an excellent way to support this type of postgraduate education for clinicians at all levels of training.
The road to implementation
Advances in science allow for generation of large-scale biological and physiological data that can be harnessed for precision diagnostic (Fig. 2), therapeutic (Fig. 3) and prognostic (Fig. 4) purposes. Programmes are needed to train, foster and retain individuals with biological and data science expertise who will contribute to precision diabetes medicine efforts. Furthermore, clinicians, scientists and regulators must collaborate to develop standards and safeguards for protecting the accumulated ‘precise’ data, which in some instances may lead to unintended and sensitive revelations, on individuals in a secure manner across populations and across countries. Worldwide differences in prevalence of the forms of diabetes necessitates inclusion of currently understudied populations for the development of precision diagnostics and therapeutics. As a result, the precise subtype of diabetes a particular individual is diagnosed with may vary in different populations based on subtype frequency or genetic or dietary or lifestyle differences.
The communication strategy used by the interventionalist and the patient’s perception of risk may be important factors contributing to the successful implementation of precision diabetes medicine. Both personal and societal barriers may exist to the implementation of precision prevention across geographic regions and countries. Discussions with global and regional regulatory agencies will be needed to determine the level of evidence needed for approval and adoption of precision diagnostics and therapeutics. The development of tools and strategies to synthesise patient data and facilitate shared decision making will be needed to translate evidence for precision diabetes medicine into individualised diabetes care, accounting for patient preferences and behaviours, health literacy and socioeconomic considerations. Pragmatic studies of decision support systems utilising rich information in these healthcare systems, particularly those with biobank-linked electronic healthcare records, are needed to guide implementation of precision diabetes medicine into clinical practice and to generate the much-needed cost-efficacy data for broader adoption.
Building partnerships
Partnerships must be established between the scientific community, patients, healthcare systems, providers, payors, industry and regulatory bodies involved in the development, evaluation, approval, adoption and implementation of precision diagnostics, monitoring and therapeutics that are deemed acceptable for safe, efficacious and cost-effective use in precision diabetes care. Making the most of the opportunities offered by precision diabetes medicine will require many different stakeholders to form highly effective partnerships. Without networks of partnerships that span academic institutions, corporations, payors, regulators and medical and public interest groups with shared understanding and vision (Fig. 5), precision diabetes medicine is destined to fail. Partners in making precision diabetes medicine a reality include:
-
People with diabetes. People with diabetes are the most important stakeholders. In Western countries, between 1:10 and 1:20 people have diabetes, while in other parts of the world, diabetes is more prevalent (1:3 in some middle-eastern populations [133], and 1:2 in some Native American tribes [132]). The precision approach to diabetes will require effective patient-facing, bi-directional communication strategies that explain what precision medicine is and how it works. People with diabetes should be invited to contribute to research through advisory and advocacy positions, postgraduate educational programmes for clinicians, and play a central role in discussions with politicians, regulators and payors.
-
Regulatory agencies. The transition from current diabetes clinical practice to a precision medicine approach will have important implications for the development, prescription, and regulation of diagnostics and therapeutics. Involvement of regulators at the earliest stages of the precision diabetes medicine workflow will be critical to the successful implementation of the precision approach. Recognising these challenges, the US FDA and the European Medicines Agency (EMA) have initiated discussions relating to standards for evidence and the design of future clinical trials for precision diabetes medicine [134].
-
Payors. Payment for medical care related to diabetes varies greatly, including between regions within countries, with costs for diabetes often hidden in other areas of medical care. Fragmentation of sites of delivery for diabetes care and its costs directly impact payment policies. There is evidence in the case of monogenic diabetes that a precision medicine approach is cost-effective [135]. The delay, or prevention, of complications (the major contributor to diabetes costs) through precision diabetes medicine may be the strongest driver for adoption.
-
Product manufacturers. Diabetes technology, including the development of wearable devices for glucose monitoring and for regulating insulin infusions (i.e., the artificial pancreas), has developed rapidly and is an example of widespread personalised diabetes medicine. Technology and pharmaceutical implementation is currently at a pre-precision level, and treatment guidelines are quite generic. The European Federation of Pharmaceutical Industries and Associations (EFPIA) Diabetes Platform, in which six leading pharmaceutical companies are developing shared policy goals focused on improving diabetes clinical outcomes, has initiated multiple projects with strong precision diabetes medicine agendas, with other public–private partnerships focused on precision diabetes medicine underway [136].
-
Private and public supporters of research. Support for diabetes research funding has struggled as its priority has fallen among the general public and some political decision makers, where cancer and cardiovascular disease rank consistently higher than diabetes on the public agenda. For precision diabetes medicine to meaningfully improve the lives of patients, it will be necessary to build highly effective networks of key stakeholders, such that common agendas are agreed to and funding for research and implementation is made available. This in turn requires that the evidence justifying a precision diabetes medicine approach is clearly articulated to all major decision makers, including funders.
-
Clinicians and professional organisations. Medical care for the person with diabetes involves a wide-spectrum of healthcare providers, including tertiary and secondary specialists, general internists, primary care doctors, nurses, dietitians, podiatrists, pharmacists, and other paramedical professionals. Several organisations are engaged in the PMDI (ADA, EASD, NIDDK) and representatives of professional bodies in Asia, Africa and elsewhere are being engaged by the PMDI to ensure global impact. Tailoring educational modules and content to different professional and cultural settings is ideally suited to these partner organisations.
-
General public. The enormous burden that diabetes places on many healthcare systems is usually shouldered by the general public, owing to the high costs of treating the disease and loss of public revenue through decreased productivity. The effective implementation of precision prevention will require that the general public embraces the approach and that those in greatest need can access precision prevention programmes. Diabetes messaging for the general public can be modelled on precision oncology, for which public advocacy and engagement have been successful, effectively utilising social media as well as traditional media to communicate not only its strengths and weaknesses but also its benefits and risks.
The path to precision diabetes medicine. HEA, health economic assessment. Adapted from [136] with the permission of American Diabetes Association. © 2018 American Diabetes Association
Summary and future perspectives
Precision diabetes medicine has found a firm foothold in the diagnosis and treatment of monogenic diabetes, while the application of precision medicine to other types of diabetes is at this time aspirational, rather than standard of care. The ability to integrate the diagnosis of monogenic diabetes into routine clinical care is one example where diagnostics are essential and meet many of the characteristics of the ideal test. Despite an excellent diagnostic paradigm, there are no known avenues for prevention in monogenic diabetes, although careful monitoring in presymptomatic variant carriers may lead to early detection of diabetes and rapid treatment.
Future precision diabetes medicine approaches are likely to include diagnostic algorithms for defining diabetes subtypes in order to decide the best interventional and therapeutic approaches. The scope and potential for precision treatment in diabetes is vast, yet deep understanding is lacking. It will be imperative to determine when and how the application of therapeutics in precision diabetes medicine improves outcomes in a cost-effective fashion.
There are many important stakeholders whose engagement will be necessary for the implementation of precision diabetes medicine to succeed (Fig. 5). Progress in translating advances in biology and technology will be governed by the identification, accurate measurement and scalable deployment of agents for diagnosis and therapy, so broad stakeholder engagement is essential. It is crucial that precision approaches are available to the full diversity of human populations and societal contexts, such that precision diabetes medicine does not widen health disparity but achieves the greatest benefits to all individuals and society as a whole. Highly functional partnerships with patient representatives and public organisations will be required to reap the benefits of precision diabetes medicine.
Abbreviations
- ADME:
-
Absorption, distribution, metabolism, excretion
- ADOPT:
-
A Diabetes Outcome Progression Trial
- DPP:
-
Diabetes Prevention Program
- DPP4i:
-
Dipeptidyl peptidase 4 inhibitor
- FDA:
-
Food and Drug Administration
- GDM:
-
Gestational diabetes mellitus
- GWAS:
-
Genome-wide association studies
- LADA:
-
Latent autoimmune diabetes in adults
- NIDDK:
-
National Institute of Diabetes and Digestive and Kidney Diseases
- PMDI:
-
Precision Medicine in Diabetes Initiative
- RECORD:
-
Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes
- T1D-GRS:
-
Type 1 diabetes genetic risk score
References
Hattersley AT, Patel KA (2017) Precision diabetes: learning from monogenic diabetes. Diabetologia 60:769–777
De Franco E, Flanagan SE, Houghton JA et al (2015) The effect of early, comprehensive genomic testing on clinical care in neonatal diabetes: an international cohort study. Lancet 386:957–963
Oram RA, Patel K, Hill A et al (2016) A type 1 diabetes genetic risk score can aid discrimination between type 1 and type 2 diabetes in young adults. Diabetes Care 39:337–344
Grubb AL, McDonald TJ, Rutters F et al (2019) A type 1 diabetes genetic risk score can identify patients with GAD65 autoantibody-positive type 2 diabetes who rapidly progress to insulin therapy. Diabetes Care 42:208–214
Sharp SA, Rich SS, Wood AR et al (2019) Development and standardization of an improved type 1 diabetes genetic risk score for use in newborn screening and incident diagnosis. Diabetes Care 42:200–207
Udler MS, McCarthy MI, Florez JC, Mahajan A (2019) Genetic risk scores for diabetes diagnosis and precision medicine. Endocr Rev 40:1500–1520
Dennis JM, Shields BM, Henley WE, Jones AG, Hattersley AT (2019) Disease progression and treatment response in data-driven subgroups of type 2 diabetes compared with models based on simple clinical features: an analysis using clinical trial data. Lancet Diabetes Endocrinol 7:442–451
Fleming GA, Petrie JR, Bergenstal RM, Holl RW, Peters AL, Heinemann L (2020) Diabetes digital app technology: benefits, challenges, and recommendations. A consensus report by the European Association for the Study of Diabetes (EASD) and the American Diabetes Association (ADA) Diabetes Technology Working Group. Diabetes Care 43:250–260
Fleming GA, Petrie JR, Bergenstal RM, Holl RW, Peters AL, Heinemann L (2020) Diabetes digital app technology: benefits, challenges, and recommendations. A consensus report by the European Association for the Study of Diabetes (EASD) and the American Diabetes Association (ADA) Diabetes Technology Working Group. Diabetologia 63:229–241
ADA (2020) 2. Classification and diagnosis of diabetes. In: Standards of medical care in diabetes–2020. Diabetes Care 43(Suppl 1):S14–S31
Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, Hattersley AT (2018) Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol 6:122–129
Thomas NJ, Lynam AL, Hill AV, Weedon MN, Shields BM, Oram RA et al (2019) Type 1 diabetes defined by severe insulin deficiency occurs after 30 years of age and is commonly treated as type 2 diabetes. Diabetologia 62:1167–1172
Gloyn AL, Pearson ER, Antcliff JF et al (2004) Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med 350:1838–1849
Pearson ER, Flechtner I, Njolstad PR et al (2006) Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med 355:467–477
Babenko AP, Polak M, Cave H et al (2006) Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med 355:456–466
Sagen JV, Raeder H, Hathout E et al (2004) Permanent neonatal diabetes due to mutations in KCNJ11 encoding Kir6.2: patient characteristics and initial response to sulfonylurea therapy. Diabetes 53:2713–2718
Bowman P, Sulen A, Barbetti F et al (2018) Effectiveness and safety of long-term treatment with sulfonylureas in patients with neonatal diabetes due to KCNJ11 mutations: an international cohort study. Lancet Diabetes Endocrinol 6:637–646
Steele AM, Shields BM, Wensley KJ, Colclough K, Ellard S, Hattersley AT (2014) Prevalence of vascular complications among patients with glucokinase mutations and prolonged, mild hyperglycemia. JAMA 311:279–286
Stride A, Shields B, Gill-Carey O, Chakera AJ, Colclough K, Ellard S et al (2014) Cross-sectional and longitudinal studies suggest pharmacological treatment used in patients with glucokinase mutations does not alter glycaemia. Diabetologia 57:54–56
Pearson ER, Starkey BJ, Powell RJ, Gribble FM, Clark PM, Hattersley AT (2003) Genetic cause of hyperglycaemia and response to treatment in diabetes. Lancet 362:1275–1281
Pearson ER, Pruhova S, Tack CJ et al (2005) Molecular genetics and phenotypic characteristics of MODY caused by hepatocyte nuclear factor 4α mutations in a large European collection. Diabetologia 48:878–885
Bowman P, Flanagan SE, Edghill EL et al (2012) Heterozygous ABCC8 mutations are a cause of MODY. Diabetologia 55:123–127
Shields BM, McDonald TJ, Ellard S, Campbell MJ, Hyde C, Hattersley AT (2012) The development and validation of a clinical prediction model to determine the probability of MODY in patients with young-onset diabetes. Diabetologia 55:1265–1272
Carlsson A, Shepherd M, Ellard S et al (2020) Absence of islet autoantibodies and modestly raised glucose values at diabetes diagnosis should lead to testing for MODY: lessons from a 5-year pediatric Swedish national cohort study. Diabetes Care 43:82–89
Ellard S, Colclough K, Patel KA, Hattersley AT (2020) Prediction algorithms: pitfalls in interpreting genetic variants of autosomal dominant monogenic diabetes. J Clin Invest 130:14–16
Clissold RL, Hamilton AJ, Hattersley AT, Ellard S, Bingham C (2015) HNF1B-associated renal and extra-renal disease-an expanding clinical spectrum. Nat Rev Nephrol 2015 11:102–112
Tranebjaerg L, Barrett T, Rendtorff ND WFS1 Wolfram syndrome spectrum disorder. In: Adam MP, Ardinger HH, Pagon RA et al (eds) GeneReviews®. University of Washington, Seattle. Available from https://www.ncbi.nlm.nih.gov/pubmed/20301750. Accessed 13 May 2020
Murphy R, Turnbull DM, Walker M, Hattersley AT (2008) Clinical features, diagnosis and management of maternally inherited diabetes and deafness (MIDD) associated with the 3243A>G mitochondrial point mutation. Diabet Med 25:383–399
Tuomi T, Groop LC, Zimmet PZ, Rowley MJ, Knowles W, Mackay IR (1993) Antibodies to glutamic acid decarboxylase reveal latent autoimmune diabetes mellitus in adults with a non-insulin-dependent onset of disease. Diabetes 42:359–362
Brophy S, Yderstraede K, Mauricio D et al (2008) Time to insulin initiation cannot be used in defining latent autoimmune diabetes in adults. Diabetes Care 31:439–441
Hawa MI, Kolb H, Schloot N et al (2013) Adult-onset autoimmune diabetes in Europe is prevalent with a broad clinical phenotype: action LADA 7. Diabetes Care 36:908–913
Battaglia M, Ahmed S, Anderson MS et al (2020) Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care 43:5–12
Onengut-Gumuscu S, Chen WM, Robertson CC et al (2019) Type 1 diabetes risk in African-ancestry participants and utility of an ancestry-specific genetic risk score. Diabetes Care 42:406–415
Rich SS (2017) Genetics and its potential to improve type 1 diabetes care. Curr Opin Endocrinol Diabetes Obes 24:279–284
Onengut-Gumuscu S, Chen WM, Burren O et al (2015) Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nat Genet 47:381–386
Rickels MR, Evans-Molina C, Bahnson HT et al (2020) High residual C-peptide likely contributes to glycemic control in type 1 diabetes. J Clin Invest 130:1850–1862
Hao W, Gitelman S, DiMeglio LA, Boulware D, Greenbaum CJ (2016) Fall in C-peptide during first 4 years from diagnosis of type 1 diabetes: variable relation to age, HbA1c, and insulin dose. Diabetes Care 39:1664–1670
Shields BM, McDonald TJ, Oram R et al (2018) C-peptide decline in type 1 diabetes has two phases: an initial exponential fall and a subsequent stable phase. Diabetes Care 41:1486–1492
Ahlqvist E, Storm P, Karajamaki A et al (2018) Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol 6:361–369
Zaharia OP, Strassburger K, Strom A et al (2019) Risk of diabetes-associated diseases in subgroups of patients with recent-onset diabetes: a 5-year follow-up study. Lancet Diabetes Endocrinol 7:684–694
Little RR, Rohlfing CL, Tennill AL et al (2008) Standardization of C-peptide measurements. Clin Chem 54:1023–1026
Udler MS, Kim J, von Grotthuss M et al (2018) Type 2 diabetes genetic loci informed by multi-trait associations point to disease mechanisms and subtypes: a soft clustering analysis. PLoS Med 15:e1002654
Mahajan A, Wessel J, Willems SM et al (2018) Refining the accuracy of validated target identification through coding variant fine-mapping in type 2 diabetes. Nat Genet 50:559–571
Insel RA, Dunne JL, Atkinson MA et al (2015) Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 38:1964–1974
Krischer JP (2013) The use of intermediate endpoints in the design of type 1 diabetes prevention trials. Diabetologia 56:1919–1924
Ziegler AG, Rewers M, Simell O et al (2013) Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 309:2473–2479
Rewers M, Stene LC, Norris JM (2017) Risk factors for type 1 diabetes. In: Cowie CC, Caagrande SS, Menke A et al (eds) Diabetes in America, 3rd edn. NIH, Bethesda, pp 11.1–11.29
Skyler JS, Krischer JP, Becker D, Rewers M (2017) Prevention of type 1 diabetes. In: Cowie CC, Caagrande SS, Menke A et al (eds) Diabetes in America, 3rd edn. NIH, Bethesda, pp 37.1–37.21
Knip M, Akerblom HK, Becker D et al (2014) Hydrolyzed infant formula and early beta-cell autoimmunity: a randomized clinical trial. JAMA 311:2279–2287
Vaarala O, Ilonen J, Ruohtula T et al (2012) Removal of bovine insulin from cow’s milk formula and early initiation of beta-cell autoimmunity in the FINDIA pilot study. Arch Pediatr Adolesc Med 166:608–614
Hummel S, Pfluger M, Hummel M, Bonifacio E, Ziegler AG (2011) Primary dietary intervention study to reduce the risk of islet autoimmunity in children at increased risk for type 1 diabetes: the BABYDIET study. Diabetes Care 34:1301–1305
Knip M, Akerblom HK, Al Taji E et al (2018) Effect of hydrolyzed infant formula vs conventional formula on risk of type 1 diabetes: the TRIGR randomized clinical trial. JAMA 319:38–48
Nanto-Salonen K, Kupila A, Simell S et al (2008) Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet 372:1746–1755
Krischer JP, Schatz DA, Bundy B, Skyler JS, Greenbaum CJ (2017) Effect of oral insulin on prevention of diabetes in relatives of patients with type 1 diabetes: a randomized clinical trial. JAMA 318:1891–1902
Elding Larsson H, Lundgren M, Jonsdottir B, Cuthbertson D, Krischer J (2018) Safety and efficacy of autoantigen-specific therapy with 2 doses of alum-formulated glutamate decarboxylase in children with multiple islet autoantibodies and risk for type 1 diabetes: a randomized clinical trial. Pediatr Diabetes 19:410–419
Lampeter EF, Klinghammer A, Scherbaum WA et al (1998) The Deutsche Nicotinamide Intervention Study: an attempt to prevent type 1 diabetes. DENIS Group. Diabetes 47:980–984
Gale EA, Bingley PJ, Emmett CL, Collier T (2004) European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet 363:925–931
(2002) Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 346:1685–1691
Vehik K, Cuthbertson D, Ruhlig H, Schatz DA, Peakman M, Krischer JP (2011) Long-term outcome of individuals treated with oral insulin: diabetes prevention trial-type 1 (DPT-1) oral insulin trial. Diabetes Care 34:1585–1590
Vandemeulebroucke E, Gorus FK, Decochez K, Weets I, Keymeulen B, De Block C, Tits J, Pipeleers DG, Mathieu C (2009) Insulin treatment in IA-2A-positive relatives of type 1 diabetic patients. Diabetes Metab 35:319–327
Herold KC, Bundy BN, Long SA et al (2019) An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 381:603–613
Hober D, Alidjinou EK (2013) Enteroviral pathogenesis of type 1 diabetes: queries and answers. Curr Opin Infect Dis 26:263–269
Hakola L, Takkinen HM, Niinisto S et al (2018) Infant feeding in relation to the risk of advanced islet autoimmunity and type 1 diabetes in children with increased genetic susceptibility: a cohort study. Am J Epidemiol 187:34–44
Tapia G, Marild K, Dahl SR et al (2019) Maternal and newborn vitamin D-binding protein, vitamin D levels, vitamin D receptor genotype, and childhood type 1 diabetes. Diabetes Care 42:553–559
Norris JM, Kroehl M, Fingerlin TE et al (2014) Erythrocyte membrane docosapentaenoic acid levels are associated with islet autoimmunity: the Diabetes Autoimmunity Study in the Young. Diabetologia 57:295–304
DECODE Study Group, the European Diabetes Epidemiology Group (2001) Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med 161:397–405
Diabetes Prevention Program Research Group (2003) Within-trial cost-effectiveness of lifestyle intervention or metformin for the primary prevention of type 2 diabetes. Diabetes Care 26:2518–2523
ADA (2020) 12. Older adults. In: standards of medical care in diabetes–2020. Diabetes Care 43(Suppl 1):S152–S162
Haw JS, Galaviz KI, Straus AN et al (2017) Long-term sustainability of diabetes prevention approaches: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med 177:1808–1817
Knowler WC, Barrett-Connor E, Fowler SE et al (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403
Crandall J, Schade D, Ma Y et al (2006) The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol A Biol Sci Med Sci 61:1075–1081
Knowler WC, Fowler SE, Hamman RF et al (2009) 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 374:1677–1686
Delahanty LM, Pan Q, Jablonski KA et al (2014) Effects of weight loss, weight cycling, and weight loss maintenance on diabetes incidence and change in cardiometabolic traits in the Diabetes Prevention Program. Diabetes Care 37:2738–2745
Papandonatos GD, Pan Q, Pajewski NM et al (2015) Genetic predisposition to weight loss and regain with lifestyle intervention: analyses from the Diabetes Prevention Program and the Look AHEAD randomized controlled trials. Diabetes 64:4312–4321
Langenberg C, Sharp SJ, Franks PW et al (2014) Gene-lifestyle interaction and type 2 diabetes: the EPIC interact case-cohort study. PLoS Med 11:e1001647
Hivert MF, Christophi CA, Franks PW et al (2016) Lifestyle and metformin ameliorate insulin sensitivity independently of the genetic burden of established insulin resistance variants in Diabetes Prevention Program Participants. Diabetes 65:520–526
Hivert MF, Jablonski KA, Perreault L et al (2011) Updated genetic score based on 34 confirmed type 2 diabetes loci is associated with diabetes incidence and regression to normoglycemia in the diabetes prevention program. Diabetes 60:1340–1348
Kilpelainen TO, Qi L, Brage S et al (2011) Physical activity attenuates the influence of FTO variants on obesity risk: a meta-analysis of 218,166 adults and 19,268 children. PLoS Med 8:e1001116
Shungin D, Deng WQ, Varga TV et al (2017) Ranking and characterization of established BMI and lipid associated loci as candidates for gene-environment interactions. PLoS Genet 13:e1006812
Graff M, Scott RA, Justice AE et al (2017) Genome-wide physical activity interactions in adiposity – a meta-analysis of 200,452 adults. PLoS Genet 13:e1006528
Tyrrell J, Wood AR, Ames RM et al (2017) Gene-obesogenic environment interactions in the UK Biobank study. Int J Epidemiol 46:559–575
Livingstone KM, Celis-Morales C, Papandonatos GD et al (2016) FTO genotype and weight loss: systematic review and meta-analysis of 9563 individual participant data from eight randomised controlled trials. BMJ 354:i4707
Godino JG, van Sluijs EM, Marteau TM, Sutton S, Sharp SJ, Griffin SJ (2016) Lifestyle advice combined with personalized estimates of genetic or phenotypic risk of type 2 diabetes, and objectively measured physical activity: a randomized controlled trial. PLoS Med 13:e1002185
Zeevi D, Korem T, Zmora N et al (2015) Personalized nutrition by prediction of glycemic responses. Cell 163:1079–1094
Jablonski KA, McAteer JB, de Bakker PI et al (2010) Common variants in 40 genes assessed for diabetes incidence and response to metformin and lifestyle intervention in the diabetes prevention program. Diabetes 59:2672–2681
Apolzan JW, Venditti EM, Edelstein SL et al (2019) Long-term weight loss with metformin or lifestyle intervention in the Diabetes Prevention Program Outcomes Study. Ann Intern Med 170:682–690
Lean ME, Leslie WS, Barnes AC et al (2018) Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet 391:541–551
Dennis JM, Shields BM, Jones AG, Pearson ER, Hattersley AT, Henley WE (2018) Evaluating associations between the benefits and risks of drug therapy in type 2 diabetes: a joint modeling approach. Clin Epidemiol 10:1869–1877
Dennis JM, Shields BM, Hill AV et al (2018) Precision medicine in type 2 diabetes: clinical markers of insulin resistance are associated with altered short- and long-term glycemic response to DPP-4 inhibitor therapy. Diabetes Care 41:705–712
Feng Y, Mao G, Ren X (2008) Ser1369Ala variant in sulfonylurea receptor gene ABCC8 is associated with antidiabetic efficacy of gliclazide in Chinese type 2 diabetic patients. Diabetes Care 31:1939–1944
Zhang H, Liu X, Kuang H, Yi R, Xing H (2007) Association of sulfonylurea receptor 1 genotype with therapeutic response to gliclazide in type 2 diabetes. Diabetes Res Clin Pract 77:58–61
Javorsky M, Klimcakova L, Schroner Z et al (2012) KCNJ11 gene E23K variant and therapeutic response to sulfonylureas. Eur J Intern Med 23:245–249
Pearson ER, Donnelly LA, Kimber C et al (2007) Variation in TCF7L2 influences therapeutic response to sulfonylureas: a GoDARTs study. Diabetes 56:2178–2182
Schroner Z, Javorsky M, Tkacova R et al (2011) Effect of sulphonylurea treatment on glycaemic control is related to TCF7L2 genotype in patients with type 2 diabetes. Diabetes Obes Metab 13:89–91
Javorsky M, Babjakova E, Klimcakova L et al (2013) Association between TCF7L2 genotype and glycemic control in diabetic patients treated with gliclazide. Int J Endocrinol 2013:374858
Kang ES, Park SY, Kim HJ et al (2005) Effects of Pro12Ala polymorphism of peroxisome proliferator-activated receptor gamma2 gene on rosiglitazone response in type 2 diabetes. Clin Pharmacol Ther 78:202–208
Hsieh MC, Lin KD, Tien KJ et al (2010) Common polymorphisms of the peroxisome proliferator-activated receptor-gamma (Pro12Ala) and peroxisome proliferator-activated receptor-gamma coactivator-1 (Gly482Ser) and the response to pioglitazone in Chinese patients with type 2 diabetes mellitus. Metabolism 59:1139–1144
Pei Q, Huang Q, Yang GP et al (2013) PPAR-gamma2 and PTPRD gene polymorphisms influence type 2 diabetes patients’ response to pioglitazone in China. Acta Pharmacol Sin 34:255–261
Zhou K, Bellenguez C, Spencer CC et al (2011) Common variants near ATM are associated with glycemic response to metformin in type 2 diabetes. Nat Genet 43:117–120
Zhou K, Yee SW, Seiser EL et al (2016) Variation in the glucose transporter gene SLC2A2 is associated with glycemic response to metformin. Nat Genet 48:1055–1059
Mahajan A, Taliun D, Thurner M et al (2018) Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet 50:1505–1513
Zhou K, Donnelly L, Burch L et al (2010) Loss-of-function CYP2C9 variants improve therapeutic response to sulfonylureas in type 2 diabetes: a Go-DARTS study. Clin Pharmacol Ther 87:52–56
Dawed AY, Donnelly L, Tavendale R et al (2016) CYP2C8 and SLCO1B1 variants and therapeutic response to thiazolidinediones in patients with type 2 diabetes. Diabetes Care 39:1902–1908
Shu Y, Sheardown SA, Brown C et al (2007) Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest 117:1422–1431
Sundelin E, Gormsen LC, Jensen JB et al (2017) Genetic polymorphisms in organic cation transporter 1 attenuates hepatic metformin exposure in humans. Clin Pharmacol Ther 102:841–848
Zhou K, Donnelly LA, Kimber CH et al (2009) Reduced-function SLC22A1 polymorphisms encoding organic cation transporter 1 and glycemic response to metformin: a GoDARTS study. Diabetes 58:1434–1439
Dujic T, Zhou K, Yee SW et al (2017) Variants in pharmacokinetic transporters and glycemic response to metformin: a Metgen meta-analysis. Clin Pharmacol Ther 101:763–772
Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM (2013) Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia 56:696–708
Davis TME, Mulder H, Lokhnygina Y et al (2018) Effect of race on the glycaemic response to sitagliptin: insights from the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS). Diabetes Obes Metab 20:1427–1434
Williams LK, Padhukasahasram B, Ahmedani BK et al (2014) Differing effects of metformin on glycemic control by race-ethnicity. J Clin Endocrinol Metab 99:3160–3168
Kim C, Newton KM, Knopp RH (2002) Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 25:1862–1868
Das Gupta R, Gupta S, Das A, Biswas T, Haider MR, Sarker M (2018) Ethnic predisposition of diabetes mellitus in the patients with previous history of gestational diabetes mellitus: a review. Expert Rev Endocrinol Metab 13:149–158
Lowe WL Jr, Scholtens DM, Sandler V, Hayes MG (2016) Genetics of gestational diabetes mellitus and maternal metabolism. Curr Diab Rep 16:15
Hayes MG, Urbanek M, Hivert MF et al (2013) Identification of HKDC1 and BACE2 as genes influencing glycemic traits during pregnancy through genome-wide association studies. Diabetes 62:3282–3291
Powe CE, Allard C, Battista MC et al (2016) Heterogeneous contribution of insulin sensitivity and secretion defects to gestational diabetes mellitus. Diabetes Care 39:1052–1055
Benhalima K, Van Crombrugge P, Moyson C et al (2019) Characteristics and pregnancy outcomes across gestational diabetes mellitus subtypes based on insulin resistance. Diabetologia 62:2118–2128
Cooray SD, Boyle JA, Soldatos G, Wijeyaratne LA, Teede HJ (2019) Prognostic prediction models for pregnancy complications in women with gestational diabetes: a protocol for systematic review, critical appraisal and meta-analysis. Syst Rev 8:270
Tobias DK (2018) Prediction and prevention of type 2 diabetes in women with a history of GDM. Curr Diab Rep 18:78
Aroda VR, Christophi CA, Edelstein SL et al (2015) The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up. J Clin Endocrinol Metab 100:1646–1653
ADA (2020) 14. Management of diabetes in pregnancy. In: standards of medical care in diabetes–2020. Diabetes Care 43(Suppl 1):S183–S192
Spyer G, Macleod KM, Shepherd M, Ellard S, Hattersley AT (2009) Pregnancy outcome in patients with raised blood glucose due to a heterozygous glucokinase gene mutation. Diabet Med 26:14–18
Sanyoura M, Letourneau L, Knight Johnson AE et al (2019) GCK-MODY in the US Monogenic Diabetes Registry: description of 27 unpublished variants. Diabetes Res Clin Pract 151:231–236
De Franco E, Caswell R, Houghton JA, Iotova V, Hattersley AT, Ellard S (2017) Analysis of cell-free fetal DNA for non-invasive prenatal diagnosis in a family with neonatal diabetes. Diabet Med 34:582–585
Petrak F, Baumeister H, Skinner TC, Brown A, Holt RIG (2015) Depression and diabetes: treatment and health-care delivery. Lancet Diabetes Endocrinol 3:472–485
Snoek FJ, Bremmer MA, Hermanns N (2015) Constructs of depression and distress in diabetes: time for an appraisal. Lancet Diabetes Endocrinol 3:450–460
Moulton CD, Pickup JC, Ismail K (2015) The link between depression and diabetes: the search for shared mechanisms. Lancet Diabetes Endocrinol 3:461–471
ADA (2020) 5. Facilitating behavior change and well-being to improve health outcomes. In: Standards of medical care in diabetes–2020. Diabetes Care 43(Suppl 1):S48–S65
Fisher L, Polonsky WH, Hessler D (2019) Addressing diabetes distress in clinical care: a practical guide. Diabet Med 36:803–812
Shields BM, Hicks S, Shepherd MH, Colclough K, Hattersley AT, Ellard S (2010) Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia 53:2504–2508
Shepherd M, Colclough K, Ellard S, Hattersley AT (2014) Ten years of the national genetic diabetes nurse network: a model for the translation of genetic information into clinical care. Clin Med (Lond) 14:117–121
Owen KR (2018) Monogenic diabetes in adults: what are the new developments? Curr Opin Genet Dev 50:103–110
Poudel A, Zhou JY, Story D, Li L (2018) Diabetes and associated cardiovascular complications in American Indians/Alaskan Natives: a review of risks and prevention strategies. J Diabetes Res 2018:2742565
Al Busaidi N, Shanmugam P, Manoharan D (2019) Diabetes in the Middle East: government health care policies and strategies that address the growing diabetes prevalence in the Middle East. Curr Diab Rep 19:8
Meyer RJ (2016) Precision medicine, diabetes, and the U.S. Food and Drug Administration. Diabetes Care 39:1874–1878
Naylor R (2019) Economics of genetic testing for diabetes. Curr Diab Rep 19:23
Fitipaldi H, McCarthy MI, Florez JC, Franks PW (2018) A global overview of precision medicine in type 2 diabetes. Diabetes 67:1911–1922
O’Brien RL, Brinster RL, Storb U (1987) Somatic hypermutation of an immunoglobulin transgene in kappa transgenic mice. Nature 326:405–409
Acknowledgements
The authors thank P. Siming (Lund University) for editorial assistance, H. Fitipalidi (Lund University) for assistance with the design of the figures and H. Mulder (Lund University) for technical critique. The authors acknowledge the invited peer reviewers who provided comments on an earlier draft of this report: H. Colhoun (University of Edinburgh), B. Draznin (University of Colorado School of Medicine), T. Hansen (University of Copenhagen), P. Njølstad (University of Bergen) and M. C. Riddle (Oregon Health and Science University).
Authors’ relationships and activities
WKC is on the scientific advisory board of the Regeneron Genetics Center. JCF has received a speaking honorarium from Novo Nordisk and consulting fees from Janssen Pharmaceuticals. MIM has in the past 3 years served on advisory panels for Pfizer, Novo Nordisk A/S and Zoe Global Ltd, has received honoraria from Merck, Pfizer, Novo Nordisk A/S and Eli Lilly, and research funding from AbbVie, Astra Zeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk A/S, Pfizer, Roche, Sanofi Aventis, Servier and Takeda. As of June 2019, MIM is an employee of Genentech and a holder of Roche stock. ERP has received research funding from Boehringer Ingelheim, Eli Lilly, Janssen, Novo Nordisk A/S, Sanofi Aventis and Servier and honoraria from Eli Lilly. LP has received research funding from Janssen and Provention Bio. PWF has received research funding from Boehringer Ingelheim, Eli Lilly, Janssen, Novo Nordisk A/S, Sanofi Aventis and Servier, received consulting fees from Eli Lilly, Novo Nordisk and Zoe Global Ltd and has stock options in Zoe Global Ltd. The other authors on this Consensus Report declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Funding
Funding for the PMDI is from the American Diabetes Association. In-kind support has been provided by the academic institutions of each Task Force member. The ideas and opinions expressed in this report were derived in part from work undertaken by the coauthors, for which they report the following support: WKC (NIH: R01 DK52431, P30 DK26687, U54 TR001873, and U54DK118612); JMN (NIH: R01 DK104351, R21 AI142483); ATH (MASTERMIND Consortium sponsored by the Medical Research Council [MRC]: MR-K005707-1; a Wellcome Trust Senior Investigator award to ATH [and S. Ellard, University of Exeter Medical School, Exeter, UK]: WT098395/Z/12/Z; National Institute for Health Research [NIHR] Clinical Research Facility); M-FH (ADA 1-15-ACE-26, NIH 5R01HD94150-02); MIM (Wellcome Trust Senior Investigator and NIHR Senior Investigator: 203141 212259 098381; NIDDK: U01-DK105535); ERP (Wellcome Trust New Investigator award: 102820/Z/13/Z); LP (NIH: R01DK104942, P30 DK02059, U54DK118612); SSR (NIH: DP3 DK111906, R01 DK122586; University of Virginia Strategic Investment Fund SIF88); PWF (European Research Council: CoG-2015_681742_NASCENT; Swedish Research Council; Novo Nordisk Foundation; European Diabetes Research Foundation; Swedish Heart Lung Foundation; Innovative Medicines Initiative of the European Union: no. 115317 – DIRECT and no. 115881 – RHAPSODY; no. 875534 – SOPHIA).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is being simultaneously published in Diabetologia (https://doi.org/10.1007/s00125-020-05181-w) and Diabetes Care (https://doi.org/10.2337/dci20-0022) by the European Association for the Study of Diabetes and the American Diabetes Association.
Rights and permissions
About this article
Cite this article
Chung, W.K., Erion, K., Florez, J.C. et al. Precision medicine in diabetes: a Consensus Report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 63, 1671–1693 (2020). https://doi.org/10.1007/s00125-020-05181-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-020-05181-w