Abstract
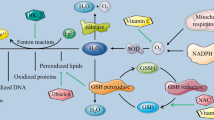
Among the several unresolved issues, profound/total loss of low-frequency residual hearing after cochlear implant fixation is the most frequent event. Even after several attempts such as modifications in the design of electrodes, improvement in the surgical procedures and use of protective drugs to minimize the trauma and its after-effects, residual hearing has been retained in less than 50% patients only. Surgical procedure and mechanical trauma at the electrode insertion site are thought to be responsible for excess generation of reactive oxygen species (ROS) following initiation of inflammatory cytokines resulting into loss of residual hearing due to programmed cell death of essential inner ear structures. Though very recent studies have reported the use of conventional antioxidants to preserve the residual hearing, they have their own limitations. With the emerging need of better and effective antioxidants, nanoceria has spurred immense research interest on utilizing its unique catalytic characteristics for ROS-associated diseases. Nanoceria has shown effective protection against several ROS-induced damages compared to conventional antioxidants such as vitamin C and vitamin E. The objective of the present work is to develop an understanding about the underlying mechanism of loss of residual hearing and propose a novel method based on delivery of nanoceria to minimize it. The first part of the article highlights the failure of cochlear implants, nature of failures and revised surgeries due to loss of residual hearing. Subsequently, the article explores the relation among surgical/mechanical trauma, excess generation of ROS at electrode insertion site, progressive death of hair cells and loss of residual hearing. Finally, effectiveness of radical scavenging characteristics of nanoceria along with controlling parameters and involved mechanisms has been reviewed. The present work also focuses on the limitations and challenges of nanoceria in clinical applications. Based on the literature review, it is hypothesized that the residual hearing loss is associated with excess generation of ROS and it is proposed that the delivery of effective antioxidants/radical scavengers having a good longevity and regenerative ability is expected to reduce the excess level of ROS and retain the residual hearing.




(Reprinted by permission of American Scientist, magazine of Sigma Xi, The Scientific Research Honor Society).




(Reprinted from Biomaterials, 28/10, M. Das, S. Patil, N. Bhargava, J. F. Kang, L. M. Riedel, S. Seal, and J. J. Hickman, Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons, Pages No. 1918-1925, Copyright (2019), with permission from Elsevier).

Similar content being viewed by others
References
Gantz BJ, Turner CW (2003) Combining acoustic and electrical hearing. Laryngoscope. 113(10):1726–1730. https://doi.org/10.1097/00005537-200310000-00012
Gantz BJ, Hansen MR, Turner CW, Oleson JJ, Reiss LA, Parkinson AJ (2009) Hybrid 10 clinical trial: preliminary results. Audiol Neurotol. 14(SUPPL. 1):32–38. https://doi.org/10.1159/000206493
Lenarz T, Stöver T, Buechner A, Lesinski-Schiedat A, Patrick J, Pesch J (2009) Hearing conservation surgery using the hybrid-L electrode: results from the first clinical trial at the Medical University of Hannover. Audiol Neurotol. https://doi.org/10.1159/000206492
Woodson EA, Reiss LAJ, Turner CW, Gfeller K, Gantz BJ (2009) The hybrid cochlear implant: a review. Cochlear Implant Hear Preserv 67:125–134. https://doi.org/10.1159/000262604
Barbara M, Mattioni A, Monini S et al (2003) Delayed loss of residual hearing in Clarion® cochlear implant users. J Laryngol Otol 117(11):850–853. https://doi.org/10.1258/002221503322542836
Gstoettner WK, Heibig S, Maier N, Kiefer J, Radeloff A, Adunka OF (2006) Ipsilateral electric acoustic stimulation of the auditory system: results of long-term hearing preservation. Audiol Neurotol. 11(SUPPL. 1):49–56. https://doi.org/10.1159/000095614
Bas E, Dinh CT, Garnham C, Polak M, Water TR (2012) Conservation of hearing and protection of hair cells in cochlear implant patients’ with residual hearing. Anat Rec 295(11):1909–1927. https://doi.org/10.1002/ar.22574
O’Leary SJ, Monksfield P, Kel G et al (2013) Relations between cochlear histopathology and hearing loss in experimental cochlear implantation. Hear Res 298:27–35. https://doi.org/10.1016/j.heares.2013.01.012
Bas E, Bohorquez J, Goncalves S et al (2016) Electrode array-eluted dexamethasone protects against electrode insertion trauma induced hearing and hair cell losses, damage to neural elements, increases in impedance and fibrosis: a dose response study. Hear Res 337:12–24. https://doi.org/10.1016/j.heares.2016.02.003
Eastwood H, Pinder D, James D et al (2010) Permanent and transient effects of locally delivered n-acetyl cysteine in a guinea pig model of cochlear implantation. Hear Res 259(1–2):24–30. https://doi.org/10.1016/j.heares.2009.08.010
Xu C, Qu X (2014) Cerium oxide nanoparticle: a remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 6(3):e90-16. https://doi.org/10.1038/am.2013.88
Kim H-W, Mozafari M, Hamzehlou S et al (2018) Biomedical applications of nanoceria: new roles for an old player. Nanomedicine. 13(23):3051–3069. https://doi.org/10.2217/nnm-2018-0189
Dobie RA, Van Hemel S (2004) Hearing loss: determining eligibility for social security benefits. National Academies Press (US). ISBN: 0-309-54514-5. https://doi.org/10.17226/11099
Gatehouse S, Naylor G, Elberling C (2006) Linear and non-linear hearing aid fittings—2. Patterns of candidature. Int J Audiol 45(3):153–171. https://doi.org/10.1080/14992020500429484
Hopkins K (2015) Deafness in cochlear and auditory nerve disorders, vol 129, 1st edn. Elsevier, Amsterdam. https://doi.org/10.1016/b978-0-444-62630-1.00027-5
Hochmair I, Nopp P, Jolly C, Schmidt M, Schösser H, Garnham C, Anderson I (2006) MED-EL cochlear implants: state of the art and a glimpse into the future. Trends Amplif 10(4):201–219. https://doi.org/10.1177/1084713806296720
Zeng F, Member S, Rebscher S, Harrison W, Sun X, Feng H (2008) Cochlear implants: system design, integration, and evaluation. IEEE Rev Biomed Eng 1(dc):115–142
Dorman MF, Wilson BS (2004) The design and function of cochlear implants. Am Sci. https://doi.org/10.1511/2004.49.942
Dhanasingh A, Jolly C (2017) An overview of cochlear implant electrode array designs. Hear Res 356:93–103. https://doi.org/10.1016/j.heares.2017.10.005
Timo STL (2009) Biomaterials in cochlear implants. GMS Curr Top Otorhinolaryngol Head Neck Surg 8(Ci):1–22
Eshraghi AA, Ocak E (2017) Cochlear implant electrode choice in challenging surgical cases : malformation, residual hearing, ossification, or reimplantation. Curr Otorhinolaryngol Rep. https://doi.org/10.1007/s40136-017-0171-3
Stefanescu EH, Poenaru M, Balica NC, Tudor A, Marinescu A, Georgescu M, Radulescu L, Cozma S, Necula V, Cosgarea M (2016) Reliability of Med-El cochlear implants in children: the Romania experience. Int J Eng Res Appl 6(7):25–30
Wang JT, Wang AY, Psarros C, Da Cruz M (2014) Rates of revision and device failure in cochlear implant surgery: a 30-year experience. Laryngoscope 124(10):2393–2399. https://doi.org/10.1002/lary.24649
Adunka O, Kiefer J (2006) Impact of electrode insertion depth on intracochlear trauma. Otolaryngol Head Neck Surg. 135(3):374–382. https://doi.org/10.1016/j.otohns.2006.05.002
Fang Y-Z, Yang S, Wu G (2002) Free radicals, antioxidants, and nutrition. Nutrition. 18(10):872–879. https://doi.org/10.1016/S0899-9007(02)00916-4
Celardo I, Pedersen JZ, Traversa E, Ghibelli L (2011) Pharmacological potential of cerium oxide nanoparticles. Nanoscale. 3(4):1411. https://doi.org/10.1039/c0nr00875c
Nimse SB, Pal D (2015) Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 5(35):27986–28006. https://doi.org/10.1039/c4ra13315c
Weydert CJ, Cullen JJ (2010) Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc 5(1):51–66. https://doi.org/10.1038/nprot.2009.197.MEASUREMENT
Clark JR, Leon L, Warren FM, Abbott JJ (2012) Magnetic guidance of cochlear implants: proof-of-concept and initial feasibility study. J Med Dev 6(3):035002. https://doi.org/10.1115/1.4007099
Chen J, Patil S, Seal S, McGinnis JF (2006) Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat Nanotechnol 1(2):142–150. https://doi.org/10.1038/nnano.2006.91
Das M, Patil S, Bhargava N et al (2007) Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials 28(10):1918–1925. https://doi.org/10.1016/j.biomaterials.2006.11.036
Tsai Y-Y, Oca-Cossio J, Agering K et al (2007) Novel synthesis of cerium oxide nanoparticles for free radical scavenging. Nanomedicine 2(3):325–332. https://doi.org/10.2217/17435889.2.3.325
Colon J, Herrera L, Smith J et al (2009) Protection from radiation-induced pneumonitis using cerium oxide nanoparticles. Nanomed Nanotechnol Biol Med 5(2):225–231. https://doi.org/10.1016/j.nano.2008.10.003
Estevez AY, Pritchard S, Harper K et al (2011) Neuroprotective mechanisms of cerium oxide nanoparticles in a mouse hippocampal brain slice model of ischemia. Free Radic Biol Med 51(6):1155–1163. https://doi.org/10.1016/j.freeradbiomed.2011.06.006
Xue Y, Luan Q, Yang D, Yao X, Zhou K (2011) Direct evidence for hydroxyl radical scavenging activity of cerium oxide nanoparticles. J Phys Chem C 115:4433–4438. https://doi.org/10.1021/jp109819u
Lee SS, Song W, Cho M et al (2013) Antioxidant properties of cerium oxide nanocrystals as a function of nanocrystal diameter and surface coating. ACS Nano 7(11):9693–9703. https://doi.org/10.1021/nn4026806
Colon J, Hsieh N, Ferguson A et al (2010) Cerium oxide nanoparticles protect gastrointestinal epithelium from radiation-induced damage by reduction of reactive oxygen species and upregulation of superoxide dismutase 2. Nanomed Nanotechnol Biol Med 6(5):698–705. https://doi.org/10.1016/j.nano.2010.01.010
Zholobak NM, Sherbakov OB, Babenko LP et al (2014) The perspectives of biomedical application of the nanoceria. EPMA J. 5(Suppl 1):A136. https://doi.org/10.1186/1878-5085-5-S1-A136
Dillon C, Billings M, Hockey KS, DeLaGarza L, Rzigalinski BA (2011) Cerium oxide nanoparticles protect against MPTP-induced dopaminergic neurodegeneration in a mouse model for Parkinson’s disease. NSTI-Nanotechnology 3:451–454
Kim CK, Kim T, Choi IY et al (2012) Ceria nanoparticles that can protect against ischemic stroke. Angew Chemie Int Ed 51(44):11039–11043. https://doi.org/10.1002/anie.201203780
D’Angelo B, Santucci S, Benedetti E et al (2009) Cerium oxide nanoparticles trigger neuronal survival in a human alzheimer disease model by modulating BDNF pathway. Curr Nanosci 5(2):167–176. https://doi.org/10.2174/157341309788185523
DeCoteau W, Heckman KL, Estevez AY et al (2016) Cerium oxide nanoparticles with antioxidant properties ameliorate strength and prolong life in mouse model of amyotrophic lateral sclerosis. Nanomed Nanotechnol Biol Med 12(8):2311–2320. https://doi.org/10.1016/j.nano.2016.06.009
Das S, Dowding JM, Klump KE, McGinnis JF, Self W, Seal S (2013) Cerium oxide nanoparticles: applications and prospects in nanomedicine. Nanomedicine 8(9):1483–1508. https://doi.org/10.2217/nnm.13.133
Djuričić B, Pickering S (1999) Nanostructured cerium oxide: preparation and properties of weakly-agglomerated powders. J Eur Ceram Soc 19(11):1925–1934. https://doi.org/10.1016/S0955-2219(99)00006-0
Rajeshkumar S, Naik P (2017) Synthesis and biomedical applications of cerium oxide nanoparticles—a review. Biotechnol Rep 2018(17):1–5. https://doi.org/10.1016/j.btre.2017.11.008
Dhall A, Self W (2018) Cerium oxide nanoparticles : a brief review of their synthesis methods and biomedical applications. Antioxidants. https://doi.org/10.3390/antiox7080097
Thakur N, Manna P, Das J (2017) Synthesis and biomedical applications of nanoceria, a redox active nanoparticle. J Nanobiotechnol. https://doi.org/10.1186/s12951-019-0516-9
Zhang F, Wang P, Koberstein J, Khalid S, Chan SW (2004) Cerium oxidation state in ceria nanoparticles studied with X-ray photoelectron spectroscopy and absorption near edge spectroscopy. Surf Sci 563(1–3):74–82. https://doi.org/10.1016/j.susc.2004.05.138
Deshpande S, Patil S, Kuchibhatla SV, Seal S (2005) Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl Phys Lett 87(13):1–3. https://doi.org/10.1063/1.2061873
Korsvik C, Patil S, Seal S, Self WT (2007) Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem Commun 10:1056. https://doi.org/10.1039/b615134e
Pirmohamed T, Dowding JM, Singh S et al (2010) Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem Commun 46(16):2736. https://doi.org/10.1039/b922024k
Montini T, Melchionna M, Monai M, Fornasiero P (2016) Fundamentals and catalytic applications of CeO2-based materials. Chem Rev. https://doi.org/10.1021/acs.chemrev.5b00603
Andriopoulou C, Trimpalis A, Petallidou KC, Sgoura A, Efstathiou AM, Boghosian S (2017) Structural and redox properties of Ce1−xZrxO2−δ and Ce0.8Zr0.15RE0.05O2−δ (RE: La, Nd, Pr, Y) solids studied by high temperature in situ ram. J Phys Chem C 121(14):7931–7943. https://doi.org/10.1021/acs.jpcc.7b00515
Mamontov E, Egami T, Brezny R, Koranne M, Tyagi S (2000) Lattice defects and oxygen storage capacity of nanocrystalline ceria and ceria-zirconia. J Phys Chem B. 104(47):11110–11116. https://doi.org/10.1021/jp0023011
Madier Y, Descorme C, Le Govic AM, Duprez D (1999) Oxygen Mobility in CeO2 and CexZr(1−x)O2 compounds: study by CO transient oxidation and 18O/16O isotopic exchange. J Phys Chem B. 103(50):10999–11006. https://doi.org/10.1021/jp991270a
Reddy BM, Khan A, Lakshmanan P, Aouine M, Loridant S, Volta JC (2005) Structural characterization of nanosized CeO2–SiO2, CeO2–TiO2, and CeO2–ZrO2 catalysts by XRD, Raman, and HREM techniques. J Phys Chem B. 109(8):3355–3363. https://doi.org/10.1021/jp045193h
Priya NS, Somayaji C, Kanagaraj S (2013) Optimization of Ceria-Zirconia solid solution based on OSC measurement by cyclic heating process. Procedia Eng. 64:1235–1241. https://doi.org/10.1016/j.proeng.2013.09.203
Perez JM, Asati A, Nath S, Kaittanis C (2008) Synthesis of biocompatible dextran-coated nanoceria with pH-dependent antioxidant properties. Small 4(5):552–556. https://doi.org/10.1002/smll.200700824
Karakoti AS, Singh S, Kumar A et al (2009) PEGylated nanoceria as radical scavenger with tunable redox chemistry. J Am Chem Soc 131(40):14144–14145. https://doi.org/10.1021/ja9051087
Zhai Y, Zhou K, Xue Y, Qin F, Yang L, Yao X (2013) Synthesis of water-soluble chitosan-coated nanoceria with excellent antioxidant properties. RSC Adv. 3(19):6833. https://doi.org/10.1039/c3ra22251a
Caputo F, Mameli M, Sienkiewicz A et al (2017) A novel synthetic approach of cerium oxide nanoparticles with improved biomedical activity. Sci Rep. 7(1):1–13. https://doi.org/10.1038/s41598-017-04098-6
Acknowledgements
The authors acknowledge the kind financial support by Department of Biotechnology, (Sanction Number BT/PR16998/NER/95/449/2015), Government of India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rai, N., Raj, R. & Kanagaraj, S. Radical Scavenging of Nanoceria in Minimizing the Oxidative Stress-Induced Loss of Residual Hearing: A Review. J Indian Inst Sci 99, 529–545 (2019). https://doi.org/10.1007/s41745-019-00116-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41745-019-00116-w