Abstract
The study investigated the emissions of volatile organic compounds (VOCs) from a mining soil amended with sewage sludge and irrigated with wastewater with or without tomato plants. The aim is to find out whether amendment and irrigation change VOC emissions from the soil and whether tomato changes emissions compared to uncultivated soil. Soil and plant experiments were done in assembled pots. All pots were placed inside a closed glass chamber inside an isolated and windowless room. Experiments with soil without plants were done independently from experiments with soil and plants. An aspirating pump coupled with Tenax adsorbent tubes was used for sampling of VOCs emitted from pots. Volatile organic compounds trapped in the tubes were quantified by gas chromatography–mass spectrometry detection. The study detected a total of nine VOCs emitted from the polluted soil: benzene, toluene, ethylbenzene, p-xylene, m-xylene, o-xylene, styrene, benzene-1,2,4-trimethyl and tetrachloroethylene, among which the most abundant were toluene, m-xylene and styrene. Differences between pots with or without amendments (C and A-pots) showed a general tendency to a decline of VOCs emissions in the mining soil amended with sewage sludge. Plants contributed to increase significantly the emissions of all VOCs in both A and C-pots. The soil amended with sewage sludge reduced the emission of VOCs: styrene in pots without plants and benzene and xylenes in pots with plants. Tomato plants contributed to increase significantly the emissions of all VOCs except styrene in both amended and non-amended soils.
Article Highlights
-
The addition of sewage sludge to a contaminated soil reduces atmospheric emissions of VOCs.
-
Growing tomato plants in soil increases emissions of soil pollutants.
-
Repeated irrigation of soils with wastewater is a higher source of soil contamination by VOCs than sludge.
-
The application of tomato plants is useful for soil remediation, but is not recommended for food use.
-
The plants contribute to significant increases in benzene, toluene and ethylbenzene emissions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Volatile organic compounds (VOCs) are emitted to the atmosphere from soils and vegetation, although VOCs emitted by vegetation are often 1–2 orders of magnitude higher than those emitted by soils. In soils, VOCs are produced by all living organisms (Leff and Fierer 2008) such as microbes or worms, and by non-living components, including dissolved OM, particulate OM pools (Mc Bride et al. 2020) and landfill wastes (Randazzo et al. 2020).
The patterns of VOC emission by a soil can change depending on soil conditions, including nutrient availability (Levesque et al. 2018), soil fertilization (Giagnoni et al. 2020), soil microbial abundance and composition (Trowbridge et al. 2020) or soil pollution (Franco et al. 2014; Faubert et al. 2017). VOCs are emitted into the atmosphere in large quantities from a variety of different natural (Guenther 2013) and anthropogenic sources (Qiu et al. 2014). The addition of organic matter to the soil leads to an increase or a reduction of gas emissions in the atmosphere, depending on the type of amendment (Levesque et al. 2018). Regarding natural compounds, Giagnoni et al. (2020) reported a shift from the production of acetate towards the production of acetone and acetaldehyde after liming the soil and an increase in methanol release as a consequence of tillage. With respect to anthropogenic compounds, the main VOCs studied in soils amended with sewage sludge are benzene, toluene, ethylbenzene, xylenes and styrene (Urionabarrenetxea et al. 2021). The emission reduction of VOCs after addition of biochar has been attributed to increased adsorption of VOCs by the soil, such as benzene, toluene, ethylbenzene, and xylenes (BTEXs) (Vikrant et al. 2020). This adsorption is highly dependent on the biochar source and pyrolysis conditions and on the physicochemical properties of the volatiles, being lower for benzene and higher for the rest of non-polar and hydrophobic compounds (Saiz-Rubio et al. 2019).
BTEXs are hydrocarbons of utmost concern in contamination events, because of their greater solubility in water and mobility in soils, being also enlisted among the top 100 priority pollutants by the US-EPA (Feng et al. 2021). Usually, when metal-contaminated soils are amended with sewage sludge or irrigated with wastewater, they receive volatile organic aromatic compounds of the BTEXs type from these inputs (Feng et al. 2021). In petroleum contaminated soils, BTEXs and PAHs are usually used as toxic indicators to assess their hazardousness (Niu and Lin 2021). The highly mutagenic and carcinogenic effects of BTEXs require effective remediation (Akmirza et al. 2017).
Reclaiming a contaminated soil with plants is a practice that intends to improve the quality of both the soil and the plant cover, which may mitigate the harmful effects of pollutants, by reducing their mobility and bioavailability or by improving their degradation (Wyszkowska et al. 2019; Xie and Van Zyl 2020). Furthermore, the use of industrial or urban organic wastes as soil conditioners is considered one of the most effective and inexpensive operations to improve biochemical properties of soils contaminated by trace metals (Burgos et al. 2010; Asemaninejad et al. 2021). Therefore, knowing whether the application of both ameliorating strategies produces an increase of VOCs emissions to the atmosphere is essential to avoid possible environmental problems in the future. Although VOCs’ emission from soil amended with organic waste products has been previously studied (Seewald et al. 2010; Abis et al. 2018), literature on VOC emission from soil/vegetation of mining areas is scarce (Faubert et al. 2017; Giagnoni et al. 2020).
The aim of this study was the assessment of VOCs emission from a mining soil irrigated with wastewater, (a) without amendments, (b) amended with sewage sludge and (c) planted with tomato plants (Lycopersicum esculentum L.). We studied whether amendment and irrigation change the VOCs emission by the soil and if tomato causes changes with respect to emissions from the only-soil.
Materials and Methods
Soil and Amendments
Soil samples were collected in the mining dump of Riotinto mining area (Nerva, province of Huelva, Spain). It is located in the Iberian Pyrite Belt (37º 42′ 4.5″ N–6º 33′ 35.1″ W), the largest deposit of pyrite (FeS2) and other metallic and polymetallic sulfides (Chopin and Alloway 2007). Random subsamples were collected from the site to a depth of 20 cm and pooled into a composite soil sample. The composite soil sample was homogenized in the laboratory with a cement mixer, air dried for 2 weeks and sieved through a 8-mm mesh for pot assays or through 2-mm mesh and ground to 50 μm for analysis following standard methodologies.
The soil was characterized as sandy-loam and, according to X-ray fluorescence analysis, SiO2 (46.9%), Fe2O3 (23.0%) and Al2O3 (12.7%) represent more than 80% of the soil mineralogical composition (Mingorance et al. 2014). It is very acid (pH 2.4) with low organic carbon content (OC 1.4%), and high electrical conductivity (EC 1.3 dS m−1, at 1:2.5 ratio), 24% water content at field capacity, humification index (HIX) 1.16 and specific UV absorbance 10.8 L g−1 cm−1. Soil ionic content (mg kg−1) is Na+ 26.1 ± 0.2, NH4+ 4.45 ± 0.07, K+ 26 ± 5, Ca2+ 118 ± 17, Mg2+ 25 ± 2, Cl− 4.90 ± 0.13, NO2− 0.180 ± 0.005, NO3− 65.1 ± 0.6, PO43− 0.42 ± 0.03 and SO42− 1334 ± 65 (Peña et al. 2015). The soil content of some potential hazardous elements (As, 3951; Cd, 13; Cu, 694; Pb, 3976, all in mg kg−1, determined by X-ray fluorescence (Mingorance et al. 2014)) is above the regional guidelines for agricultural soils (Aguilar et al. 1999).
Stabilized sewage sludge (SSL), obtained from the wastewater treatment plant (WTP) of ‘Carrión de los Céspedes’ (Sevilla, SW Spain), was used as amendment of the mining soil (MS). The main properties of SSL were: neutral pH 6.90 ± 0.01, HIX 0.43, EC (at 1:10 ratio, w/v) 2.8 ± 1.2 dS m−1 and high organic carbon (OC) content (%) 35.50 ± 2.94. Humic acid content was 1.6% and fulvic acid content 0.47%. The potential toxic metal load of SSL at the 2% dose used (approximately 40 Mg ha−1) was lower than that corresponding to European guidelines concerning external application to soil (European Commission 1986). Ionic content (mg kg−1) was higher than the soil: Na+ 55 ± 11, NH4+ 551 ± 29, K+ 20.7 ± 0.6, Ca2+ 1222 ± 28, Mg2+ 40 ± 8, Cl− 86.7 ± 0.4, NO2− 2.128 ± 0.002, NO3− 8.0 ± 0.4, PO43− 0.53 ± 0.06 and SO42− 3317 ± 184 (Peña et al. 2015). SSL was sieved through a 2-mm mesh.
Irrigation was performed with wastewater (wW) from the same WTP, whose main properties are: pH 8.7 ± 0.3, EC 1.063 ± 0.088 dS m−1 and high OC content.
Pot Assembling, Treatments and Experiments
Prior to pot assembling, the original MS was limed with Carbocal® (CAL), a liming amendment proceeding from the Azucarera Iberia, a sugar Spanish company. It is based on a sugar factory sub-product, rich in calcium carbonate (83.4%) and with an OC content of 5.1% (at an equivalent rate of 1.5% w/w). This calcium carbonate—0.75 g kg−1—raises soil to pH 7.3, obtaining a limed soil (MSL).
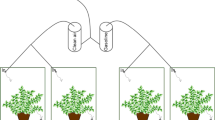
All pots were placed inside a closed glass chamber of 90 L, which was located in an isolated, windowless room during 5 days. Experiments with soil without plants were done separately from experiments with plants (Fig. 1a, b). In the glass chamber, indoor relative humidity and level of CO2 was increasing continuously during the incubation, mainly in pots with plants. After 5 days of incubation, volatile compounds emitted by each pot were collected by aspirating the air inside a conical glass hood, which covered completely the pots (Fig. 1c). The indoor air was aspirated by a suction pump located outside the chamber, which channelled it to an adsorbent tube where the VOCs were trapped.
Prior to the pot experiments, VOCs blanks were initially determined for each type of substance—solid/liquid—added to soil:
-
Ultrapure water (uW, reference of blanks).
-
The original mining soil (MS).
-
The liming product (CAL).
-
The stabilized sewage sludge (SSL).
-
The wastewater for irrigation (wW).
Glass Petri dishes were used as supports of these substances. An equal equivalent amount of each of the solid/liquid substances was added to each Petri dish (10 g or 10 mL), which was then aspirated through the conical glass hood into the adsorbent tube (Fig. 1d).
Two different treatments were carried out in pot experiments, each one with three replications, constituting a set of six pots (Fig. 1a, b): Control treatment (C) and amendment treatment (A). C-pots were prepared mixing the mining soil (250 g MS), glassy beads (40 g, 15% w/w) and liming amendment (5 g, 2% w/w CAL), giving a limed soil (MSL). A-pots were prepared adding stabilized sewage sludge (2% w/w SSL) to MSL, giving an amended soil (MSSL).
Two experiments were designed. The first pot experiment was done using C-pots and A-pots without plants (−P, Fig. 1a) and the second one was carried out using C-pots and A-pots planted with tomato plants (+P, Fig. 1b). Prior to sampling, prepared pots were irrigated with wastewater (45 mL, 80% field capacity), thus stabilizing them to reach a pH close to 7, at which the soil microbiota is activated. At this point, VOC emissions from the soil were sampled from each of the six pots (three C-pots and three A-pots).
Tomato plants were previously grown into a standard vegetal substrate until their size was high enough for transplanting them into the MS. One individual was transplanted into each pot. After 5 days, VOC emissions were sampled.
VOCs’ Sampling
Sampling of VOCs was performed using an aspirating pump coupled with adsorbent tubes. Glass tubes were manually assembled and conditioned. Each glass adsorbent tube (Perkin-Elmer) was filled with 60 mg of Tenax® TA 60/80 (Perkin-Elmer), which is the most commonly sorbent used (Balao et al. 2011). The sorbent was fixed to the centre of the tube with glass wool and stainless steel springs at both ends. Then, filled tubes were conditioned before sampling by oven heating at 250 °C for 2 h and quick nitrogen assisted cooling inside a desiccator. While awaiting sampling, the tubes were kept capped at both openings. CO2 concentration was measured and monitored with an air quality detection and data logging instrument (YesAir IAQ Monitor) designed for continuous indoor air quality monitoring of temperature, RH and gases such as CO2, CO and formaldehyde.
Blanks and added compounds were aspirated towards the adsorbent tube using an air sampling pump (Casella, Apex model) with the following conditions: air flow 1.6 mL min−1, air volume 72 L (45 min), temperature and relative humidity 22.4 ± 0.2 °C and 38.9 ± 0.6%, respectively, and CO2 rate 634 ± 33 ppm. After sampling, each tube was immediately capped and stored in the freezer (− 22 °C) until chromatographic analysis.
VOC Analysis
Volatile organic compounds in the air were quantified by gas chromatography–mass spectrometry detection (GC-MSD). VOCs trapped inside the tubes were desorbed and led to the chromatograph (Bruker 450-GC) using an automatic thermal desorption injector (Turbomatrix ATD 350, Perkin Elmer). Volatiles were desorbed using a thermal desorption program: 250 °C for 5 min at 30 mL min−1 of helium with a cold trap at 5 °C, and then heating the cold trap to 250 °C at 20 °C min−1. The VOCs were channelled to the chromatographic column (30 m length × 0.25 mm internal diameter × 0.25 µm film thickness). The oven temperature program for the separation of VOCs inside the DB-5 Agilent J&W column was: initial temperature of 40 °C for 3 min, 10 °C min−1 to 120 °C and 25 °C min−1 to 250 °C. Helium, the thermal desorber gas, was used as the carrier gas at a rate of 1 mL min−1. Gas chromatography separation was coupled with a quadrupole mass spectrometer (Bruker 300-MS). Detection of VOCs was carried out at full-scan mode (Q1 and Q3), for a mass range from 30 to 450 m/z, by electronic impact ionization. The identification was by comparison with the NIST spectral library (National Institute of Standards and Technology, Gaithersburg, MD, USA). The data obtained were collected using the Bruker software Chemical Analysis MS Workstation version 7.0 (Karlsruhe, Germany).
VOC–Mix 20 standard was used for the analytical calibration of the VOCs detected. The chromatographic signal of each VOC was relativized to the four internal standards pentafluoro-benzene, 1,4-difluoro-benzene, chloro-benzene-d5, 1,4-dichloro-benzene-d4 of the Mix 11–IS standard (both Mix standards were purchased from Dr. Ehrenstorfer). Calibration curves were prepared in the range 0.25–35 ng µL−1 (ppm). Afterwards, 10 µL of each diluted standard were added with a microsyringe into each glass adsorbent tube and then a continuous flow of nitrogen was passed through the tube. All tubes were handled inside a vertical laminar flow cabinet (equipped with an activated carbon filter). Each peak was identified by the absolute and the relative retention times, and by comparison with the mass spectral library of the instrument. Final concentrations in the air were expressed as µg m−3.
Statistical Analysis
Normality was tested by Shapiro–Wilk’s test and the homogeneity of variance was established with the Levene’s test (p > 0.05). One-way analysis of variance (ANOVA) was performed followed by a post hoc test (LSD) to analyse differences of VOC emissions between pot experiments. When data were non-normally distributed non-parametric tests were applied (Kruskal–Wallis and Mann–Whitney U-test). Data analyses were performed using StatSoft (Version 8).
Results
Nine volatile organic compounds were studied: benzene, toluene, m-xylene, p-xylene, o-xylene, ethylbenzene, styrene, tetrachloroethylene and benzene-1,2,4-trimethyl. The nine different VOCs identified represent the volatile compounds with the highest analytical signal obtained in the GC–MS (Table 1). The original mining soil was limed with CAL, amended with SSL and irrigated with wW. Before studying which VOC comes from the plant or from the soil it is essential to investigate if CAL, SSL or wW emit volatile compounds on their own. Results on VOCs emitted by the different substances are listed in Table 2, showing the contribution of each one to the total VOC emitted by each pot. Thus, we know their potential relative contribution at the start of the experiment (first day), prior to sampling after 5 days of incubation. As Table 2 shows, MS (45%) and wW (52%) would be the main contributors, with negligible contribution from ultrapure water or CAL (0.2%) and low supply from SSL (3%, except for benzene).
The mine soil showed an initial level of VOC similar (0.46 µg m−3) to that of wW (0.53 µg m−3); however, it should be noted that pots were irrigated with wW every day, so the wW relative contribution is expected to be higher at the moment of sampling (10th day). Besides, different volatile compounds have been reported in WTPs, being aromatic hydrocarbons and organosulfur compounds the most abundant chemical classes (Jiang et al. 2017). This would explain the relative high contribution of wW, taking into account its corresponding inputs to the pot experiment. Table 3 shows the relative proportion of the emitted volatiles in pot experiments. The first remarkable result was the high concentration of toluene emitted by pots in all experiments (77.4–86.2% of TVOCs), followed by m-xylene (2.8–5.6%) and styrene (1.2–5.1%). The less representative VOC emitted (0.42–0.55%) was benzene-1,2,4-trimethyl. Figure 2 shows the average amount of the nine volatile compounds measured in the experiments with or without plants in C and A-pots. Significant differences between pots with or without amendments (C and A-pots) were noticed pointing to a general tendency to a decline of VOCs emissions in the mining soil amended with sewage sludge. In pots without plants (−P), this reduction was remarkable but only significant for styrene (− 34%, p < 0.05), while for pots with plants (+ P), the reduction was significant for benzene, p, m, and o-xylene (− 46%) while for styrene, a significant increase was observed (+ 23%, p < 0.05). Plants contributed to increase significantly the emissions of all VOCs in both A and C-pots, except for styrene (p < 0.05). The presence of plants in pots increased significantly BTXs and BTEXs, while TVOCs emissions increased four times for non-amended-pots and three times for amended pots (Table 3, Fig. 3).
VOCs emitted in the experiment (mean ± standard deviation, n = 3). Different letters indicate significant differences (p < 0.05) between treatments. C-Pots/MSL-P mining soil without amendment and without plants, A-Pots/MSSL-P mining soil with amendment and without plants, C-Pots/MSL + P mining soil without amendment and with plants, A-Pots/MSSL + P mining soil with amendment and with plants
Discussion
In the composition of original pots (Table 2), the potential contribution was higher for benzene (39%) than for toluene (8%), although final benzene emission only reached 2.8–3.4% (Table 3), mainly originating from MS, wW and SSL. This decrease in the emitted VOCs also happened with the other studied VOCs (except m-xylene and styrene), in comparison with the initial pot conditions (at the first day). Toluene was presumably originated from the initially most concentrated compound in the pots, i.e., benzene, which had been also found in a limed degraded soil (Giagnoni et al. 2020). Other authors have reported that catechol, benzoate, phenol and toluene are the usual metabolites of benzene degradation (Oka et al. 2008). Ulrich et al. (2005) demonstrated that benzene was converted to toluene by benzene methylation and to phenol by benzene hydroxylation under anaerobic conditions. Under the current work conditions, the glass chamber was closed during 5 days, which would result in a continuous increase of CO2 level. Furthermore, MS and SSL have a high concentration of ions favoring anaerobic conditions (3300–4000 mg kg−1 SO4=, 551–1410 mg kg−1 NH4+, only 8–18 mg kg−1 NO3−, Peña et al. 2015). Therefore, it is to be expected that the increased anoxic conditions during incubation would have favoured the mechanism of benzene degradation by sulfate-reducing bacteria, allowing the formation of toluene by methylation. Similar processes may have occurred for ethylbenzene, p, o-xylenes and benzene-1,2,4-trimethyl. For instance, Pseudomonas putida has initial degradation rates higher for m- and p-xylene than for toluene (Duetz et al. 1998). In addition, according to Table 2, the major source of toluene was wW, whose contribution would increase until sampling, because the pots were incubated for 5 days. The emission of VOCs from a soil depends on oxygen availability and on the physiological status of the microorganisms (Insam and Seewald 2010), as well as on soil mineral composition and substrate quality (Abis et al. 2018).
With regard to differences between pots with or without amendments (C and A-pots), a general reduction of VOCs’ emissions in the mining soil amended with sewage sludge was noticed (Fig. 2). The observed reduction was especially high for the more hydrophobic compounds (higher log Kow values) and could be ascribed to enhanced adsorption by sewage sludge, as previously reported for biochars (Saiz-Rubio et al. 2019; Vikrant et al. 2020).
The retention of VOCs in soils can be stimulated by the addition of activated carbon (Bonaglia et al. 2020), although it is more usual to add organic amendments to soils, such as sewage sludges or biochars. Based on the data from Table 3, the retention percentage of pollutants in the soil due to the presence of SSL for the overall concentrations of BTXs and BTEXs was only -10%, and was not significant, neither for TVOCs (p > 0.05, − 13%), due mainly to the low reduction rates of benzene and toluene. Although TVOCs, BTXs and BTEXs did not significantly differ among treatments without plants (with or without amendments) (Table 3), the decrease of VOCs emissions from pots with plant was higher in the presence of SSL (− 22%) than without SSL (− 13%) (Fig. 2).
Plants played an important role in the increase of the emissions of all VOCs in both A and C-pots. The most marked effects by the tomato plant were observed for both benzene and toluene (× 4 times/C-pots, × 3 times/A-pots) and ethylbenzene (× 3 times/C-pots, × 4 times/A-pots). The increases of emissions were also notable for p,o-xylenes (× 3 times/C-pots, × 2 times/A-pots) and for benzene-1,2,4-trimethyl (× 3 times/C-pots, × 3 times/A-pots).
The presence of plants would enhance VOCs emission, because these compounds would be uptaken and translocated to the aerial parts. Hydrophobicity and molecular weight may influence the translocation processes of organic molecules in plants (Wang et al. 2021; Liu et al. 2021). Although neutral compounds with log Kow between − 1 and 5 are considered mobile in the transpiration stream (Briggs et al. 1982), according to Sicbaldi et al. (1997), the efficiency of translocation would be best achieved by compounds with log Kow values ranging from 2 to 3, decreasing for compounds of lower or higher polarity. Therefore, VOCs with these log Kow values, like benzene, toluene and ethylbenzene (Table 1), are the ones preferentially translocated by the tomato plant and released to the atmosphere.
On the other hand, addition of sewage sludge to the soil would increase adsorption and reduce the bioavailability of organic compounds for plant uptake, because the uptake potential of a compound is mainly dependent on the available concentration in soil solution. Amendment addition enhances soil OC, which is the main controlling factor of the adsorption of BTEX and other neutral hydrophobic pollutants (Zytner 1994; Takaki et al. 2014). For instance, an inhibition of greenhouse gas emissions, such as CO2, N2O, methane or ammonia, after sewage sludge amendment has been reported (Awasthi et al. 2016; Pandiyan et al. 2021). Besides, addition of sewage sludge does not only modify the soil porous micro-structure, which in turn promotes adsorption, but also provides the soil with a source of labile OM, boosting the activity of soil microbes, which contribute to degrade VOCs (Agegnehu et al. 2016; Awasthi et al. 2020).
In summary, for both amended and non-amended mining soil, the tomato causes a large increase in VOC emissions, which means both a positive aspect for soils remediation but also a negative aspect for air pollution. This agrees with Yi et al. (2013), who indicated that in agricultural fields VOC emissions are largely produced by the aboveground living crops.
Conclusions
The addition of sewage sludge to the mining soil reduces the emission from the soil of the more hydrophobic compounds from 23.5 to 35.8%, which is of great importance in the fate of volatiles emission to the atmosphere. Nevertheless, the cultivation of tomato plants in soil further increases VOCs emission, especially of the more polar VOCs, toluene and benzene, for which the plant induces an increase in the release of 295.7–334.0%. However, addition of sewage sludge partially compensates this increase, which demonstrates that plants are a sink and a source of volatile compounds. On the other hand, repeated irrigation with wastewater (under the current conditions) constitutes a source of VOC emission similar to that of mine soil and one order of magnitude higher than sludge. Therefore, as part of the conclusions and recommendations of the current work, the use of wastewater for soil irrigation is not recommended in remediation methods of contaminated soils, because of the increase in VOCs release. Further studies should be carried out on VOC emissions from different types of soils, soil amendments, soil irrigation solutions and plant species. The overall balance of the results indicates that mining soils emit hazardous VOCs into the atmosphere when these plant-based remediation techniques are applied.
Availability of Data and Materials
Not applicable.
References
Abis L, Loubet B, Ciuraru R, Lafouge F, Dequiedt S, Houot S, Maron P-A, Bourgeteau-Sadet S (2018) Profiles of volatile organic compound emissions from soils amended with organic waste products. Sci Total Environ 636:1333–1343. https://doi.org/10.1016/j.scitotenv.2018.04.232
Agegnehu G, Bass AM, Nelson PN, Bird MI (2016) Benefits of biochar, compost and biochar–compost for soil quality, maize yield and greenhouse gas emissions in a tropical agricultural soil. Sci Total Environ 543:295–306. https://doi.org/10.1016/j.scitotenv.2015.11.054
Aguilar J, Dorronsoro C, Galán E, Gómez Ariza JL (1999) Los criterios y estándares para declarar un suelo como contaminado en Andalucía y la metodología y técnica de toma de muestras y análisis para su investigación. In Investigación y Desarrollo Medioambiental en Andalucía (eds) OTRI-University of Sevilla, pp 61–64
Akmirza I, Pascual C, Carvajal A, Pérez R, Muñoz R, Lebrero R (2017) Anoxic biodegradation of BTEX in a biotrickling filter. Sci Total Environ 587–588:457–465. https://doi.org/10.1016/j.scitotenv.2017.02.130
Asemaninejad A, Langley S, Mackinnon T, Spiers G, Beckett P, Mykytczuk N, Basiliko N (2021) Blended municipal compost and biosolids materials for mine reclamation: long-term field studies to explore metal mobility, soil fertility and microbial communities. Sci Total Environ 760:143393. https://doi.org/10.1016/j.scitotenv.2020.143393
Awasthi MK, Wang Q, Ren X, Zhao J, Huang H, Awasthi SK, Lahori AH, Li R, Zhou L, Zhang Z (2016) Role of biochar amendment in mitigation of nitrogen loss and greenhouse gas emission during sewage sludge composting. Bioresour Technol 219:270–280. https://doi.org/10.1016/j.biortech.2016.07.128
Awasthi MK, Duana Y, Awasthia SK, Liua T, Zhang Z (2020) Effect of biochar and bacterial inoculum additions on cow dung composting. Bioresour Technol 297:122407. https://doi.org/10.1016/j.biortech.2019.122407
Balao F, Herrera J, Talavera S, Dötterl S (2011) Spatial and temporal patterns of floral scent emission in Dianthus inoxianus and electroantennographic responses of its hawkmoth pollinator. Phytochemistry 72:601–609. https://doi.org/10.1016/j.phytochem.2011.02.001
Bonaglia S, Broman E, Brindefalk B, Hedlund E, Hjorth T, Rolff C, Nascimento FJA, Udekwu K, Gunnarsson JS (2020) Activated carbon stimulates microbial diversity and PAH biodegradation under anaerobic conditions in oil-polluted sediments. Chemosphere 248:126023. https://doi.org/10.1016/j.chemosphere.2020.126023
Briggs GG, Bromilow RH, Evans AA (1982) Relationships between lipophilicity and root uptake and translocation of non-ionised chemicals by barley. Pestic Sci 13:495–504. https://doi.org/10.1002/ps.2780130506
Burgos P, Madejón P, Cabrera F, Madejón E (2010) By-products as amendment to improve biochemical properties of trace element contaminated soils: effects in time. Int Biodeter Biodegr 64:481–488. https://doi.org/10.1016/j.ibiod.2010.05.009
Chopin EIB, Alloway BJ (2007) Trace elements partitioning and soil particle characterisation around mining and smelting areas at Tharsis, Riotinto and Huelva, SW Spain. Sci Total Environ 373:488–500. https://doi.org/10.1016/j.scitotenv.2006.11.037
Duetz WA, Wind B, van Andel JG, Barnes MR, Williams PA, Rutgers M (1998) Biodegradation kinetics of toluene, m-xylene, p-xylene and their intermediates through the upper TOL pathway in Pseudomonas putida (pwWO). Microbiology 144:1669–1675. https://doi.org/10.1099/00221287-144-6-1669
Faubert P, Durocher S, Bertrand N, Ouimet R, Rochette P, Tremblay P, Boucher JF, Villeneuve C (2017) Greenhouse gas emissions after application of landfilled paper mill sludge for land reclamation of a nonacidic mine tailings site. J Environ Qual 46:950–960. https://doi.org/10.2134/jeq2017.03.0119
Feng S, Gong L, Zhang Y, Tong Y, Zhang H, Zhu D, Huang X, Yang H (2021) Bioaugmentation potential evaluation of a bacterial consortium composed of isolated Pseudomonas and Rhodococcus for degrading benzene, toluene and styrene in sludge and sewage. Bioresour Technol 320:124329. https://doi.org/10.1016/j.biortech.2020.124329
Franco MG, Corrêa SM, Marques M, Perez DV (2014) Emission of volatile organic compounds and greenhouse gases from the anaerobic bioremediation of soils contaminated with diesel. Water Air Soil Pollut 225:1879. https://doi.org/10.1007/s11270-014-1879-z
Giagnoni L, Taiti C, León P, Costa C, Menesatti P, Espejo R, Gómez-Paccard C, Hontoria C, Vázquez E, Benito M, Mancuso S, Renella G (2020) Volatile organic compound emission and biochemical properties of degraded Ultisols ameliorated by no tillage and liming. Pedosphere 30:597–606. https://doi.org/10.1016/S1002-0160(20)60024-8
Guenther A (2013) Biological and chemical diversity of biogenic volatile organic emissions into the atmosphere. ISRN Atmos Sci. https://doi.org/10.1155/2013/786290
Insam H, Seewald M (2010) Volatile organic compounds (VOCs) in soils. Biol Fertil Soils 46:199–213. https://doi.org/10.1007/s00374-010-0442-3
Jiang GM, Melder D, Keller J, Yuan ZG (2017) Odor emissions from domestic wastewater: a review. Crit Rev Environ Sci Technol 47:1581–1611. https://doi.org/10.1080/10643389.2017.1386952
Leff JW, Fierer N (2008) Volatile organic compound (VOC) emissions from soil and litter samples. Soil Biol Biochem 40:1629–1636
Levesque V, Rochette P, Ziadi N, Dorais M, Antoun H (2018) Mitigation of CO2, CH4 and N2O from a fertigated horticultural growing medium amended with biochars and a compost. Appl Soil Ecol 126:129–139. https://doi.org/10.1016/j.apsoil.2018.02.021
Lide DR (ed) (1996) Handbook of chemistry and physics (77th edn). CRC Press Inc, Boca Raton, Fl
Liu Q, Liu Y, Dong F, Sallach JB, Wu X, Liu X, Xu J, Zheng Y, Li Y (2021) Uptake kinetics and accumulation of pesticides in wheat (Triticum aestivum L.): impact of chemical and plant properties. Environ Pollut 275:116637
Mc Bride SG, Choudoir M, Fierer N, Strickland MS (2020) Volatile organic compounds from leaf litter decomposition alter soil microbial communities and carbon dynamics. Ecology 101:e03130. https://doi.org/10.1002/ecy.3130
Mingorance MD, Rossini S, Valdés B, Pina Gata FJ, Leidi EO, Guzmán I, Peña A (2014) Stabilized municipal sewage sludge addition to improve properties of an acid mine soil for plant growth. J Soils Sedim 14:703–712. https://doi.org/10.1007/s11368-013-0743-x
Niu A, Lin C (2021) Managing soils of environmental significance: a critical review. J Hazard Mater 417:125990. https://doi.org/10.1016/j.jhazmat.2021.125990
Oka AR, Phelps CD, McGuinness LM, Mumford A, Young LY, Kerkhof LJ (2008) Identification of critical members in a sulfidogenic benzene-degrading consortium by DNA stable isotope probing. Appl Environ Microbiol 74:6476–6480. https://doi.org/10.1128/AEM.01082-08
Pandiyan B, Mangottiri V, Narayanan N (2021) Carbon transformations of biochar based co-composting—a review. Mini-Rev Org Chem 18:1–14. https://doi.org/10.2174/1570193X17999200928221205)
Peña A, Mingorance MD, Guzmán-Carrizosa I, Fernández-Espinosa AJ (2015) Improving the mining soil quality for a vegetation cover after addition of sewage sludges: inorganic ions and low-molecular-weight organic acids in the soil solution. J Environ Manage 150:216–225. https://doi.org/10.1016/j.jenvman.2014.11.016
Qiu K, Yang L, Lin J, Wang P, Yang Y, Ye D, Wang L (2014) Historical industrial emissions of non-methane volatile organic compounds in China for the period of 1980–2010. Atmos Environ 86:102–112. https://doi.org/10.1016/j.atmosenv.2013.12.026
Randazzo A, Asensio-Ramos M, Melián GV, Venturi S, Padrón E, Hernández PA, Pérez NM, Tassi F (2020) Volatile organic compounds (VOCs) in solid waste landfill cover soil: chemical and isotopic composition vs. degradation processes. Sci Total Environ 726:138326. https://doi.org/10.1016/j.scitotenv.2020.138326
Saiz-Rubio R, Balseiro-Romero M, Antelo J, Díez E, Fiol S, Macías F (2019) Biochar as low-cost sorbent of volatile fuel organic compounds: potential application to water remediation. Environ Sci Pollut Res 26:11605–11617. https://doi.org/10.1007/s11356-018-3798-9
Seewald MSA, Singer W, Knapp BA, Franke-Whittle IH, Hansel A, Insam H (2010) Substrate-induced volatile organic compound emissions from compost-amended soils. Biol Fertil Soils 46:371–382. https://doi.org/10.1007/s00374-010-0445-0
Sicbaldi F, Sacchi GA, Trevisan M, Del Re AAM (1997) Root uptake and xylem translocation of pesticides from different chemical classes. Pestic Sci 50:111–119. https://doi.org/10.1002/(SICI)1096-9063(199706)50:2%3c111::AID-PS573%3e3.0.CO;2-3
Takaki K, Wade AJ, Collins CD (2014) Assessment of plant uptake models used in exposure assessment tools for soils contaminated with organic pollutants. Environ Sci Technol 48:12073–12082. https://doi.org/10.1021/es501086x
Trowbridge AM, Stoy PC, Phillips RP (2020) Soil biogenic volatile organic compound flux in a mixed hardwood forest: net uptake at warmer temperatures and the importance of mycorrhizal associations. J Geophys Res Biogeosci 125:e2019JG005479. https://doi.org/10.1029/2019JG005479
Ulrich AC, Beller HR, Edwards EA (2005) Metabolites detected during biodegradation of 13C6-benzene in nitrate-reducing and methanogenic enrichment cultures. Environ Sci Technol 39(17):6681–6691
Urionabarrenetxea E, Garcia-Velasco N, Anza M, Artetxe U, Lacalle R, Garbisu C, Becerril T, Soto M (2021) Application of in situ bioremediation strategies in soils amended with sewage sludges. Sci Total Environ 766:144099. https://doi.org/10.1016/j.scitotenv.2020.144099
Vikrant K, Kim KH, Peng WX, Ge SB, Ok YS (2020) Adsorption performance of standard biochar materials against volatile organic compounds in air: a case study using benzene and methyl ethyl ketone. Chem Eng J 387:123943. https://doi.org/10.1016/j.cej.2019.123943
Wang FY, Li X, Yu SM, He SH, Cao DT, Yao SJ, Fang H, Yu YL (2021) Chemical factors affecting uptake and translocation of six pesticides in soil by maize (Zea mays L.). J Hazard Mater 405:124269. https://doi.org/10.1016/j.jhazmat.2020.124269
Wyszkowska J, Borowik A, Kucharski J (2019) The resistance of Lolium perenne L. x hybridum, Poa pratensis, Festuca rubra, F. arundinacea, Phleum pratense and Dactylis glomerata to soil pollution by diesel oil and petroleum. Plant Soil Environ 65:307–312. https://doi.org/10.17221/42/2019-PSE
Xie LX, van Zyl D (2020) Distinguishing reclamation, revegetation and phytoremediation, and the importance of geochemical processes in the reclamation of sulfidic mine tailings: a review. Chemosphere 252:126446. https://doi.org/10.1016/j.chemosphere.2020.126446
Yi ZG, Zheng LL, Wu T, Wang XM (2013) Contribution of aboveground plants, the rhizosphere and root-free-soils to total COS and DMS fluxes at three key growth stages in rice paddies. Agric Ecosyst Environ 179:11–17. https://doi.org/10.1016/j.agee.2013.07.005
Zytner RG (1994) Sorption of benzene, toluene, ethylbenzene and xylenes to various media. J Hazard Mater 38:113–126. https://doi.org/10.1016/0304-3894(94)00027-1
Acknowledgements
Wastewater and sewage sludge were provided by the Andalusian Public Foundation ‘Centro de las Nuevas Tecnologías del Agua’ (CENTA) of the Junta de Andalucia Government. Carbocal was supplied by Juan Castellano Márquez from the Azucarera Iberia Company.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was supported by Proyecto de Excelencia-Junta de Andalucía (P10-RNM5814), co-financed by FEDER funds.
Author information
Authors and Affiliations
Contributions
The project conceptualization and design were mainly performed by Aránzazu Peña-Heras. Material preparation, analysis and data collection were mainly performed by Antonio José Fernández-Espinosa. Sabina Rossini-Oliva was mainly in charge of the preparation of the plant material. The investigation, review and editing of revised manuscript were performed by Antonio José Fernández-Espinosa, Sabina Rossini-Oliva and Aránzazu Peña-Heras.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fernández-Espinosa, A.J., Peña-Heras, A. & Rossini-Oliva, S. Atmospheric Emissions of Volatile Organic Compounds from a Mine Soil Treated with Sewage Sludge and Tomato Plants (Lycopersicum esculentum L.). Int J Environ Res 16, 47 (2022). https://doi.org/10.1007/s41742-022-00425-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41742-022-00425-6