Abstract
Objective
To estimate the costs and cost-effectiveness of introducing highly active antiretroviral therapy (HAART) in Denmark based on real-world evidence for the three treatment eras pre-HAART (1985–1995), early HAART (1996–2005), and late HAART (2006–2017).
Methods
We performed a cohort study using Danish clinical and administrative registries to estimate costs, quality-adjusted life-years (QALYs), and life-years (LY) gained per person living with human immunodeficiency virus (PLHIV) in three treatment eras. The study utilized Markov modeling for a health economic evaluation, which summarized inputs from real-world evidence and estimated the cost-effectiveness in 2017 prices of the introduction of HAART in Denmark. We performed deterministic and probabilistic sensitivity analyses to assess the robustness of the results.
Results
The total annual costs per PLHIV increased with the introduction of HAART for the index year but decreased in the incremental years and the last year of life. The total lifetime discounted (and undiscounted) cost for an average PLHIV was €91,010 (€128,981) in pre-HAART, €103,130 (€199,062) in early HAART, and €126,317 (€254,964) in late HAART. The estimated incremental cost-effectiveness ratios showed that early HAART was cost-effective compared with pre-HAART with an incremental cost-effectiveness ratio (ICER) of €1378 per QALY, and that late HAART was cost-effective compared with early HAART with an ICER of €7385 per QALY. Sensitivity analyses confirmed cost-effectiveness in all scenarios.
Conclusions
The introduction and implementation of HAART in Danish healthcare was cost-effective, and in some scenarios, even disruptive, i.e., led to both cheaper and more effective care of PLHIV.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
The introduction of highly active antiretroviral therapy (HAART) was found to be cost-effective in Danish healthcare. Despite the increase in annual costs per patient from pre-HAART to the latter periods, the number of hospital days per patient decreased due to effective treatment. |
The study emphasizes the necessity of retrospective economic evaluations for informed decision-making by societies, policymakers, and clinicians. |
To bridge the current and prevailing life expectancy gap for people living with human immunodeficiency virus (HIV) and minimize side effects, further advancements in HAART are imperative. |
1 Introduction
Globally, human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) has caused over 39 million HIV/AIDS-related deaths to date and more than 36 million people are currently living with HIV [1,2,3].
The first breakthrough HIV drug, the anti-retroviral therapy (ART) azidothymidine marketed in 1987 under the brand name Retrovir, provided temporal improvements in the excess mortality caused by HIV [3, 4]. Being the most expensive drug in the world at the time, however, its use was limited to only symptomatic patients experiencing opportunistic infections [5, 6]. In 1995, protease inhibitors were introduced, and a year later came non-nucleoside reverse transcriptase inhibitors (NNRTI) [7, 8], paving the way for highly active antiretroviral therapy (HAART) [7, 11]. Drug prices remained among the world’s highest, however, and the early HAART period was marked by international debate about patent laws and drug prices, questioning whether the international community accepted too high prices of vital medicine [12, 13].
In this period, countries did not have national health technology assessment (HTA) organizations like today doing appraisals of new pharmaceuticals and judging whether pharmaceutical prices are ‘fair’ or not [14]. The methodology of health economic evaluation with quality-adjusted life-years (QALY) and national threshold values, which is used to perform such appraisals today, was not developed at the time [15]. Instead, there was an expressed need for standardization of economic methods to reduce variation in methods and estimates of costs per patient and cost-effectiveness [16].
Despite existing health economic studies [5, 14, 17,18,19,20,21,22,23,24], the cost-effectiveness of the introduction of HAART remain unclear. To our knowledge, none of the previous studies has conducted an ex-post cost-utility analysis according to international guidelines for health economic evaluation in the same way as analyzes are presented to decision-makers in national HTA bodies today.
Today, more than 30 HIV antiretroviral drugs are available. Modern-day health economic evaluations of the costs and effects of various HAART combinations have demonstrated value for money of many newer improvements in HIV treatment strategies [19, 21, 22]. Nonetheless, these analyses are prospective and aim to determine whether new treatment combinations as incremental improvements compared to already implemented treatments would be cost-effective and financially advantageous in the future. Ex-post (historic) economic evaluations take place after the implementation of the initiatives and are retrospective in format. They can be used to measure impact, policy learning, accountability, and provide feedback on future ex-ante evaluations [25, 26]. Ex-post evaluation allows you to assess the economic consequences of past actions and evaluate the actual costs and the effects on the patients [25]. Thereby we can assess whether the introduction of HAART has been worth the money as we understand cost-effectiveness today.
The Danish public healthcare sector constitutes a useful setting for ex-post health economic evaluations. Since 1968, a unique personal identifier has been assigned to all residents at birth or upon immigration by the Civil Registration System (CRS) [27]. This identifier allows unambiguous data linkage at an individual level and tracks changes in vital status and migration for the entire population with daily updates. In an international comparison, Denmark has a relatively large number of high-quality data files containing information about individuals’ health and healthcare consumption, and a long research tradition due to the possibilities available from linking such information [28]. With a modern public healthcare system providing healthcare free of charge to all citizens, register-based analyses have been shown to provide unbiased cost estimates [28, 29].
The objective of this study was to estimate the costs and cost-effectiveness of introducing HAART in Denmark based on real-world evidence for the three treatment eras pre-HAART (1985–1995), early HAART (1996–2005), and late HAART (2006–2017). The primary measure of health effectiveness was QALY, and secondary outcome was survival in years after diagnosis.
2 Methods
We conducted a cohort study using Danish clinical and administrative registries and methods described in Jespersen et al (2021) and Ehlers et al (2022) [30, 31]. The results from the cohort study were used as input in the cost-effectiveness analysis in accordance with international guidelines for health economic evaluations [15, 32, 33]. In the health economic evaluation, we compare the costs, mortality, and QALYs of the average patient diagnosed and treated in each of the three eras using real-world evidence. The results are presented as the incremental cost-effectiveness ratio (ICER) from pre-HAART to early HAART and from early HAART to late HAART.
2.1 Study Sample and Setting
In Denmark, the National Health Service is tax-funded, offering all citizens free access to hospitals and outpatient specialty clinics as well as free hospital-dispensed medicines for all citizens [29]. HIV medicine is given to patients in need of care without any co-payment.
We used real-world evidence (RWE) from a study sample identical to the population in a previous study by Jespersen et al. [30]. The InfCare HIV database, the Danish National Patient Registry (DNPR), and the Danish HIV Cohort Study (DHCS) were used to identify PLHIV in the Central Denmark Region above the age of 18 with a confirmed first diagnosis of HIV during the years 1985–2017. The population is described in detail elsewhere [30]. In brief, 1043 PLHIV were included (Table 1). Overall, the median age at diagnosis was 36.6 years (IQR 29.3–45.5) differing from 33 years in pre-HAART to 39.5 years in late HAART. A higher proportion of PLHIV in late HAART was born in Western Europe compared to pre- and early HAART and, overall, 71.6% were of the male gender.
2.2 Estimating Survival Using Real-World Evidence
Utilizing the CRS, DNPR, DHCS, and the InfCare HIV database, cumulative survival in the first 9 years following diagnosis was calculated for each treatment era similar to Jespersen et al. [30]. Lifetime parametric extrapolation of survival probabilities was conducted in Stata (version SE17.0) using published extrapolation techniques [34,35,36] and calibrated to match long-term survival for PLHIV from the three eras based on published literature [10, 37,38,39,40,41,42] and estimations from a clinical expert (Supplementary Material S1). Choice of distribution was made by statistical verification using the Akaike information criterion (AIC), Bayesian information criterion (BIC), published literature [9, 10, 37, 39, 41], and visual inspection by Danish clinical experts. Ultimately, the exponential distribution was chosen for pre-HAART and the Weibull distribution for early and late HAART. Furthermore, the survival probabilities were adjusted using the relative survival method to account for the improvement over the whole period from 1985 to 2017 in life expectancy in the gender-matched Danish population [43]. The base case includes adjusted life expectancy but results without adjustment are also shown as well as a short time horizon reflecting only real-world evidence (RWE) without lifetime extrapolation.
2.3 Estimating Costs Using Real-World Evidence
The cost data and methods were previously reported by Ehlers et al. (2022) for late HAART [31] but extended to include the whole period from 1985 to 2017 with a healthcare sector perspective. In brief, the patient’s personal identifier (the CPR number) was used to link patients with information on healthcare consumption from the Danish National Patient Registry (DNPR), the Danish National Prescription Registry, and the National Health Insurance Service Register. Annual cost per patient was estimated for each of the three eras for the index year, incremental years, and the last year of life. Costs were adjusted to 2017 and converted from Danish Kroner (DKK) to Euro (€) following Statistics Denmark and the consumer price index [44]. Discounting of 3.5% for the first 35 years and 2.5% for the following years were applied in the base case according to Danish guidelines [45, 46], and analysis without discounting was also conducted.
Contacts with Danish hospitals are registered in the National Patient Register with information on the length of stay as well as unique linkages to individual-level information of the diagnosis-related group (DRG) and relevant Danish reimbursement tariffs designed to equal the average cost per Danish patient [28, 47]. Danish DRG tariffs include the costs of free hospital-dispensed medicines such as ART, but prices of hospital medicine are confidential, thus, cannot be estimated individually. The Danish National Prescription Registry was used to estimate expenditures of prescription medicine for selected noncommunicable diseases at the individual patient level using the pharmacy selling prices, however, only a subsample of the most relevant prescription drugs was included. All costs associated with primary care services including general practitioners and physiotherapy were obtained through the National Health Service Register.
For pre-HAART, Danish registers did not contain any information on unit costs, but only information on hospital days and visits. To estimate unit costs we assumed the same relative change in unit costs from pre-HAART to early HAART as reported by Beck for UK [19].
2.4 Quality of Life Estimates
Quality of life was estimated on the basis of the World Health Organization (WHO) Global Burden of Disease (GBD) study [48] using disability-adjusted life-years (DALY) disability weights as estimates of disutility. For each of the health states in the model, index year, incremental years, and last year of life, Danish clinical experts were consulted to help choose the relevant disutility values for PLHIV in each of the three periods, pre-HAART, early HAART, and late HAART. Disutility values were subtracted from a baseline utility from the Danish population norms to reflect the quality of life for Danish PLHIV [49]. QALYs were discounted similarly to costs according to Danish guidelines.
2.5 Comparing Cost, Survival, and QALY
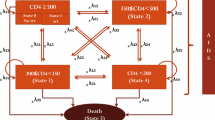
We constructed a simple decision-analytic Markov model to summarize inputs from the cohort study and estimate the total cost per PLHIV in the three treatment eras and assess the value of medical innovation in HAART. The Markov model simulated the impact of the HIV pandemic if it were introduced today reflecting three scenarios: pre-HAART without treatment, early HAART with the first generation of treatments, and late HAART with highly effective treatments (Supplementary Material S3). The model was constructed in TreeAge Pro (Healthcare, version 2022) and allowed for disease prediction in two mutually exclusive health states: HIV and death. All patients began in the HIV health state at the age of 36, corresponding to the time of HIV diagnosis (Table 1). The model simulated a monthly cycle length with half-cycle correction over a 65-year time horizon during which all patients were expected to die. The outputs of the model were used to calculate pairwise incremental cost-effectiveness ratios (ICERs), specifically comparing early HAART to pre-HAART and late HAART to early HAART. The British National Institute for Health and Care Excellence (NICE) threshold of £20,000–30,000 approximately equal to €23,000–34,000 was used for reference (conversion rate 1.13, 13 February 2023) due to the absence of an official willingness-to-pay (WTP) threshold of cost per incremental QALY in Denmark.
2.6 Statistical Analyses
All statistical analyses on costs and survival from Danish registries were performed in the SAS statistical software package v.9.4. Lifetime extrapolation of survival data was performed in Stata (version SE/17.0) and comparisons of costs, LY, and QALY were estimated in TreeAge Pro Healthcare (version 2022, R2.0).
Deterministic one-way sensitivity analyses were conducted with a range of ±20% of the mean input value to reflect uncertainty (Supplementary Material S4) and a probabilistic sensitivity analysis (PSA) with 10,000 second-order Monte Carlo iterations to assess the uncertainty around the cost-effectiveness estimate (Supplementary Material S5). Uncertainty was included using a beta distribution based on the standard deviation (SD) assigned to utility values and a gamma distribution reflecting standard error (SE) to costs calculated directly from RWE (Table 2). Furthermore, a cost-effectiveness acceptability curve was generated (Supplementary Material S6).
3 Results
3.1 Costs
The total annual costs per person living with HIV (PLHIV) increased with the introduction of HAART for the index year but decreased in the incremental years and the last year of life (Table 3).
3.2 Comparing Cost, Survival, and QALY
The total lifetime discounted (undiscounted) cost for an average PLHIV was €91,010 (€128,981) in pre-HAART, €103,130 (€199,062) in early HAART, and €126,317 (€254,964) in late HAART (Table 4). The lifetime projection of QALY (and LY) showed an average QALY per PLHIV of 4.56 (8.1), 13.35 (17.9), and 16.49 (20.4) for pre-, early, and late HAART respectively. Undiscounted QALY (and LY) were 6.44 (11.6) for pre-HAART, 24.98 (34.0) for early HAART, and 32.08 (40.4) for late HAART. Discounted (undiscounted) life expectancy for the Danish general population in 2006–2017, corresponding to late HAART, was 22.3 (44.9) LYs (Supplementary Material S2).
The ICERs using discounted costs and QALYs showed that early HAART was cost-effective compared with pre-HAART at €1378 per QALY, and late HAART was cost-effective compared with early HAART at €7385 per QALY. The results were not sensitive to alterations in the time horizon, methods for estimating life expectancy, or discounting (Table 4).
The PSA showed a >99,9% likelihood of late HAART being cost-effective at €23,000 per QALY, i.e., the lower range of the threshold value. Early HAART had the highest probability of being cost-effective at a threshold range of approximately €1400–€7300 per QALY.
4 Discussion
In this first study to perform an ex-post cost-effectiveness analysis of HAART using individual-level register data we showed that the introduction of HAART was cost-effective in Danish healthcare and, in some scenarios, even seemed to be a truly disruptive innovation, i.e., HAART being both cheaper and more effective as in the scenario with a 9-year time horizon. The result showed that annual costs per patient with HIV increased from pre-HAART to early HAART and late HAART, however, because of the effective treatment the number of hospital days per patient also decreased.
Several limitations and challenges arise when conducting retrospective cost-effectiveness analyses, especially when dealing with RWE going several decades back in time, in the present study back to 1985 [50]. Due to limitations in data availability, we only had access to DRG tariffs for individual hospital costs from the year 2004. However, we were able to use data on the number of hospital days per patient for the entire period to estimate average costs per patient with HIV in the pre-HAART period. This provided a reasonable estimate of average costs as hospital budgets and personnel were linked to the number of hospital beds during that time [51].
Observational analyses may also be susceptible to bias when comparing patient populations in different time periods, which can lead to inaccuracies in the estimations of the impact of the intervention, i.e., the treatment effects of early and late HAART. The challenges of controlling for all relevant developmental factors were handled by adjusting for the increase in life expectancy of the general population. We did not correct for potential changes in socioeconomic status, ethnicity, the presence of comorbidities, and improved living conditions in the general population, which may influence the outcomes being compared. Other limitations include that we did not investigate the impact of CD4 cell count on the cost-effectiveness [28, 29]. Furthermore, there is a large uncertainty in the estimation of QALY gain using the WHO GBD disability values for PLHIV as estimates of disutility [15]. Estimating the gain in utility over a period of 30 years is difficult as preferences for health and instruments for measuring health-related quality of life have changed significantly over the last 30 years [15, 49]. We believe our assumptions about disutility are conservative in the sense that getting the HIV diagnosis in the pre-HAART and early HAART days may have felt like a “death sentence,” and our estimates mainly capture the difference in utility due to the state of the HIV disease and the availability of antiretroviral therapy [48]. As assumed in our study, published meta-analyses and results from the SMART trial present utility data showing an increase in quality-of-life for PLHIV over the three periods [52,53,54,55,56,57].
Furthermore, comparing treatment strategies over different time periods using RWE is contingent upon the fact that the healthcare system in general is changing over time. Thus, the increased effectiveness of HIV treatment over time cannot be solely related to specific breakthrough drugs or even competencies in HIV departments, rather it relies on the entire healthcare sector and society’s knowledge and competencies that have evolved. As such, ART was undoubtedly a central part of the disruptive innovation, but the increased effectiveness also relates to the way drugs were used as well as the healthcare system’s ways of dealing with HIV. This is the strength of ex-post health economic evaluation; it captures cost and effectiveness of treatments in the way they have been used in clinical practice. Furthermore, advances in other therapeutic areas during the whole period from pre- to late HAART have also affected mortality and have therefore contributed to the findings [30].
The findings from our study are generally in accordance with other health economic research, and it seems to ‘sum up’ the existing economic evidence in a coherent conclusion about cost-effectiveness.
For the pre-HAART period, the lack of a standardized methodology for health economic analysis gave a large variation in estimates of cost per patient with HIV (from 2000 to 70,000 US dollars per patient per year) [16]. Most of these earlier cost estimates showed patient costs that were higher than our estimates, presumably because they represented selected patient groups receiving treatment, rather than a population-based average like our study. We found no indication that our figures from the pre-HAART period were higher than other European healthcare systems, but like other studies from that era, we confirmed that the last year of life was very expensive [58].
Other health economic analyses from the early HAART period also showed the apparent paradox, that despite the increasing cost of pharmaceuticals, the total cost per patient with HIV did stabilize or even drop after the introduction of triple therapy [19, 22, 59]. In the late HAART, several cost-effectiveness analyses were published, all pointing to the further implementation of cost-effective new treatment possibilities for HIV. Summing up late HAART CUAs, it does not seem strange that our overall picture of the entire period also turns out to be cost-effective. At the same time, the same trend continues during late HAART with a continuous substitution of bed days with more effective medicine; however, in this period also at a slightly increasing annual cost per patient with HIV. Again, our study is population-based showing the average for all PLHIV, where only a few have received the most expensive treatments. Further, most PLHIV have not received specialized HIV drugs immediately after diagnosis until the result of the START INSIGHT study, demonstrating that early initiation of ART is beneficial for nearly all HIV-infected patients, regardless of the CD4+ count, were implemented in Danish clinical practice at the end of the late HAART period [60].
To our knowledge, only a single study has attempted to analyze with similar objectives as ours, but using modeling of cost instead of RWE data on cost. Perez-Elias et al. (2021) analyzed survival data from a cohort of PLHIV who started ART between 1985 and 2016 in Spain and compared their health outcomes and projected healthcare costs to those of a similar group of PLHIV who did not receive ART. The study found that ART has been a highly effective intervention for people living with HIV in Spain, with significant clinical and economic benefits. ART was associated with significant improvements in life expectancy and quality of life and a significant reduction in the incidence of AIDS-related illnesses. The study concluded that ART was cost-effective over the long term, as the healthcare costs associated with HIV care were lower for people receiving ART compared with those who did not [61].
Our findings suggest that societies, policymakers, and clinicians on an overall level have made cost-effective decisions when investing in the uptake of new treatment modalities for HIV. While breakthrough HIV drugs might have been among the most expensive in Danish healthcare, this analysis indicates that they have nevertheless been used cost-effectively. Historically, HIV has gradually changed in nature from a complicated, expensive disease, to a simpler, more manageable disease with a significantly improved prognosis [62, 63]. The introduction of ART led to a shift in the way that HIV was managed in health care, with a greater emphasis on prevention, early detection, and treatment, and not with a focus on managing symptoms of the disease [19, 64, 65]. Our analysis only includes innovation until 2017, and further improvements in the treatment of HIV have been implemented since then [66, 67].
This ex-post evaluation of the treatment of HIV highlights the importance of historical analyses. Future research should investigate the limitations of the single health technology evaluation approach usually applied to support decision-making in healthcare as the historical analysis points to the existence of important synergies between new technologies. Over the whole period, more than 30 effective HIV medicines emerged, and the treatments of noncommunicable diseases significantly contributed to the improved survival and quality of life in PLHIV. This development was not foreseen in earlier economic evaluations of antiretroviral therapies [5, 14, 17,18,19,20,21,22,23,24]. Future research should consider developing new methods for incorporating synergies and future innovation in single ex ante health economic evaluations.
5 Conclusion
We conclude that the introduction and implementation of HAART in Danish healthcare were cost-effective. Ex-post economic evaluations provide opportunities for measuring impact, policy learning, and feedback on future ex ante evaluations. Current and prevailing challenges in the treatment of HIV require further development of HAART to continue to close the gap in life expectancy and quality of life for PLHIV compared with the general population while minimizing costs.
References
Pandey A, Galvani AP. The global burden of HIV and prospects for control. Lancet HIV. 2019;6(12):e809–11.
World Health Organisation. HIV. Available from: https://www.who.int/data/gho/data/themes/hiv-aids
Richman DD. Susceptibility to nucleoside analogues of zidovudine-resistant isolates of human immunodeficiency virus. Am J Med. 1990;88(5B):8S-10S.
Fischl MA, Richman DD, Hansen N, Collier AC, Carey JT, Para MF, et al. The safety and efficacy of zidovudine (AZT) in the treatment of subjects with mildly symptomatic human immunodeficiency virus type 1 (HIV) infection. A double-blind, placebo-controlled trial. The AIDS Clinical Trials Group. Ann Intern Med. 1990;112(10):727–37.
Pinkerton SD, Holtgrave DR. A method for evaluating the economic efficiency of HIV behavioral risk reduction interventions. AIDS Behav. 1998;2(3):189–201.
Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, McMahon D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337(11):734–9.
Bartlett JA, Fath MJ, DeMasi R, Hermes A, Quinn J, Mondou E, et al. An updated systematic overview of triple combination therapy in antiretroviral-naive HIV-infected adults. AIDS. 2006;20(16):2051–64.
Yeni PG, Hammer SM, Hirsch MS, Saag MS, Schechter M, Carpenter CCJ, et al. Treatment for adult HIV infection: 2004 recommendations of the international AIDS society-USA panel. JAMA. 2004;292(2):251.
Gueler A, Moser A, Calmy A, Günthard HF, Bernasconi E, Furrer H, et al. Life expectancy in HIV-positive persons in Switzerland: matched comparison with general population. AIDS. 2017;31(3):427–36.
Lohse N, Obel N. Update of survival for persons with HIV infection in Denmark. Ann Intern Med. 2016;165(10):749.
Barry M, Mulcahy F, Back DJ. Antiretroviral therapy for patients with HIV disease. Br J Clin Pharmacol. 1998;45(3):221–8.
Halbert D. Moralized discourses: South Africa’s intellectual property fight for access to AIDS Drugs. Seattle J Soc Just. 2002;1(2):1–40.
There’s a Precedent for Overriding Patents on Vital Medications. Bloomberg.com. 2021; Available from: https://www.bloomberg.com/news/articles/2021-05-11/aids-drugs-in-south-africa-shows-precedent-for-overriding-patents-on-medications
Chancellor JV, Hill AM, Sabin CA, Simpson KN, Youle M. Modelling the cost effectiveness of lamivudine/zidovudine combination therapy in HIV infection. Pharmacoeconomics. 1997;12(1):54–66.
Drummond M. Methods for the economic evaluation of health care programmes. Fourth edition. Oxford, United Kingdom ; New York, NY, USA: Oxford University Press; 2015. 445 p.
Tolley K, Gyldmark M. The treatment and care costs of people with HIV infection or AIDS: development of a standardised cost framework for Europe. Health Policy. 1993;24(1):55–70.
Tran BX, Nguyen LH, Turner HC, Nghiem S, Vu GT, Nguyen CT, et al. Economic evaluation studies in the field of HIV/AIDS: bibliometric analysis on research development and scopes (GAPRESEARCH). BMC Health Serv Res. 2019;19(1):834.
Jacobsen MM, Walensky RP. Modeling and cost-effectiveness in HIV prevention. Curr HIV/AIDS Rep. 2016;13(1):64–75.
Beck EJ, Mandalia S, Sangha R, Sharott P, Youle M, Baily G, et al. The cost-effectiveness of early access to HIV services and starting cART in the UK 1996–2008. PLoS One. 2011;6(12): e27830.
Brogan A, Talbird S, Davis A, Wild L, Flanagan D. Is increased screening and early antiretroviral treatment for HIV-1 worth the investment? An analysis of the public health and economic impact of improvement in the UK. HIV Med. 2019;20(10):668–80.
Marcellusi A, Viti R, Russo S, Andreoni M, Antinori A, Mennini FS. Early treatment in HIV patients: a cost–utility analysis from the Italian perspective. Clin Drug Investig. 2016;36(5):377–87.
Miners A, Sabin C, Trueman P, Youle M, Mocroft A, Johnson M, et al. Assessing the cost-effectiveness of HAART for adults with HIV in England: the cost-effectiveness of HAART. HIV Med. 2001;2(1):52–8.
Oddone EZ, Cowper P, Hamilton JD, Matchar DB, Hartigan P, Samsa G, et al. Cost effectiveness analysis of early zidovudine treatment of HIV infected patients. BMJ. 1993;307(6915):1322–5.
Schackman BR, Goldie SJ, Weinstein MC, Losina E, Zhang H, Freedberg KA. Cost-effectiveness of earlier initiation of antiretroviral therapy for uninsured HIV-infected adults. Am J Public Health. 2001;91(9):1456–63.
Sculpher M, Drummond M, Buxton M. The iterative use of economic evaluation as part of the process of health technology assessment. J Health Serv Res Policy. 1997;2(1):26–30.
Boardman AE. Cost-benefit analysis: concepts and practice. Fifth edition. Cambridge, United Kingdom ; New York, NY: Cambridge University Press; 2018. 594 p.
Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39(7 Suppl):22–5.
Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. CLEP. 2015. https://doi.org/10.2147/CLEP.S91125.
Schmidt M, Schmidt SAJ, Adelborg K, Sundbøll J, Laugesen K, Ehrenstein V, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563–91.
Jespersen N, Axelsen F, Dollerup J, Nørgaard M, Larsen C. The burden of non-communicable diseases and mortality in people living with HIV (PLHIV) in the pre-, early- and late-HAART era. HIV Med. 2021;22(6):478–90.
Ehlers LH, Axelsen F, Bøjer Rasmussen T, Dollerup J, Jespersen NA, Larsen CS, et al. Cost of non-communicable diseases in people living with HIV in the Central Denmark Region. HIV Med. 2023;24(4):453–61.
Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 explanation and elaboration: a report of the ISPOR CHEERS II Good Practices Task Force. Value Health. 2022;25(1):10–31.
Motheral B, Brooks J, Clark MA, Crown WH, Davey P, Hutchins D, et al. A Checklist for retrospective database studies—report of the ISPOR task force on retrospective databases. Value in Health. 2003;6(2):90–7.
Diaby V, Adunlin G, Montero AJ. Survival modeling for the estimation of transition probabilities in model-based economic evaluations in the absence of individual patient data: a tutorial. Pharmacoeconomics. 2014;32(2):101–8.
Wei Y, Royston P. Reconstructing time-to-event data from published Kaplan–Meier curves. 2018;
Guyot P, Ades A, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12(1):9.
Collaboration ATC. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. The Lancet. 2008;372(9635):293–9.
King JT, Justice AC, Roberts MS, Chang CCH, Fusco JS. Long-term HIV/AIDS survival estimation in the highly active antiretroviral therapy era. Med Decis Making. 2003;23(1):9–20.
Lohse N, Hansen ABE, Pedersen G, Kronborg G, Gerstoft J, Sørensen HT, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med. 2007;146(2):87.
McManus H, O’Connor CC, Boyd M, Broom J, Russell D, Watson K, et al. Long-Term survival in HIV positive patients with up to 15 years of antiretroviral therapy. Polis MA, editor. PLoS One. 2012;7(11): e48839.
the CASCADE Collaboration. Survival after introduction of HAART in people with known duration of HIV-1 infection. Lancet. 2000;355(9210):1158–9.
Trickey A, Zhang L, Sabin CA, Sterne JAC. Life expectancy of people with HIV on long-term antiretroviral therapy in Europe and North America: a cohort study. Lancet Healthy Longevity. 2022;3:S2.
Bower H, Andersson TML, Crowther MJ, Dickman PW, Lambe M, Lambert PC. Adjusting expected mortality rates using information from a control population: an example using socioeconomic status. Am J Epidemiol. 2018;187(4):828–36.
Statistics Denmark. Forbrugerprisindeks. Available from: https://www.dst.dk/da/Statistik/emner/oekonomi/prisindeks/forbrugerprisindeks
Medicinrådet. Metodevejledning for omkostningsanalyser af nye lægemidler og indikationer i hospitalssektoren. Medicinrådet; 2020. Available from: https://medicinraadet.dk/media/jgbiri0j/metodevejledning-for-omkostningsanalyser-af-nye-l%C3%A6gemidler-og-indikationer-i-hospitalssektoren-vers-1-6_adlegacy.pdf
Danish Ministry of Finance. Dokumentationsnotat - den samfundsøkonomiske diskonteringsrente. 2021. Available from: https://fm.dk/media/18371/dokumentationsnotat-for-den-samfundsoekonomiske-diskonteringsrente_7-januar-2021.pdf
Landspatientregisteret (LPR) - Sundhedsdatastyrelsen. [cited 2021 Jun 8]. Available from: https://sundhedsdatastyrelsen.dk/da/registre-og-services/om-de-nationale-sundhedsregistre/sygedomme-laegemidler-og-behandlinger/landspatientregisteret
Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019—The Lancet. Available from: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30925-9/fulltext#seccestitle280
Sørensen J, Davidsen M, Gudex C, Pedersen KM, Brønnum-Hansen H. Danish EQ-5D population norms. Scand J Public Health. 2009;37(5):467–74.
Sculpher MJ, Claxton K, Drummond M, McCabe C. Whither trial-based economic evaluation for health care decision making? Health Econ. 2006;15(7):677–87.
Borchmann O, Omland LH, Gerstoft J, Larsen CS, Johansen IS, Lunding S, et al. Length of stay in Denmark before HIV diagnosis and linkage to care: a population-based study of migrants living with HIV, Denmark, 1995 to 2020. Eurosurveillance. 2022. https://doi.org/10.2807/1560-7917.ES.2022.27.30.2100809.
Tengs TO, Lin TH. A meta-analysis of utility estimates for HIV/AIDS. Med Decis Making. 2002;22(6):475–81.
Holtgrave DR, Pinkerton SD. Updates of cost of illness and quality of life estimates for use in economic evaluations of HIV prevention programs. J Acquir Immun Defic Syndr Hum Retrovirol. 1997;16(1):54–62.
Freedberg KA, Scharfstein JA, Seage GR, Losina E, Weinstein MC, Craven DE, et al. The cost-effectiveness of preventing AIDS-related opportunistic infections. JAMA. 1998;279(2):130–6.
Schackman BR, Goldie SJ, Freedberg KA, Losina E, Brazier J, Weinstein MC. Comparison of health state utilities using community and patient preference weights derived from a survey of patients with HIV/AIDS. Med Decis Making. 2002;22(1):27–38.
Honiden S, Sundaram V, Nease RF, Holodniy M, Lazzeroni LC, Zolopa A, et al. The effect of diagnosis with HIV infection on health-related quality of Life. Qual Life Res. 2006;15(1):69–82.
Whitham HK, Hutchinson AB, Shrestha RK, Kuppermann M, Grund B, Shouse RL, et al. Health utility estimates and their application to HIV prevention in the United States: Implications for cost-effectiveness modeling and future research needs. MDM Policy Pract. 2020;5(2):238146832093621.
Hellinger FJ. Forecasting the medical care costs of the HIV epidemic: 1991–1994. Inquiry. 1991;28(3):213–25.
Krentz HB, Auld MC, Gill MJ, HIV Economic Study Group. The changing direct costs of medical care for patients with HIV/AIDS, 1995–2001. CMAJ. 2003;169(2):106–10.
The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795–807.
Pérez-Elías MJ, Podzamczer Palter D, Ventayol Bosch P, Jarrín I, Castro A, Rubio-Rodríguez D, et al. Clinical and economic benefit of 32 years of antiretroviral treatment for people living with HIV in Spain: Has it been an efficient intervention? Enfermedades infecciosas y microbiologia clinica (English ed). 2021;S2529993X21001313.
Disruptive Innovation In Health Care Delivery: A Framework For Business-Model Innovation - ProQuest. Available from: https://www.proquest.com/docview/204616768/fulltextPDF/F12D51BDF2824F34PQ/1?accountid=14468
Flows NR, Kocher R, Cutler DM. The future of medicine—where investors are putting their money. Forbes. Available from: https://www.forbes.com/sites/realspin/2015/05/01/the-future-of-medicine-where-investors-are-putting-their-money/
Fauci AS. 25 years of HIV. Nature. 2008;453(7193):289–90.
Joint United Nations Programme on HIV/AIDS. AIDS at 30: Nations at the Crossroads. Geneva: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2011.
Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. Available from: https://www.who.int/publications-detail-redirect/9789240031593
Hiv-1-infektion. Available from: https://medicinraadet.dk/anbefalinger-og-vejledninger/behandlingsvejledninger/hiv
Acknowledgements
We would like to thank the health economist Flemming Axelsen, NovoCure (former employee at Gilead Sciences Denmark), for his help in setting up the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The current analysis was partly funded by an unrestricted research grant from Gilead Sciences, Denmark to Nordic Institute of Health Economics and Aarhus University, respectively. The funding source approved the overall design of the study prior to initiating analyses. The funding source had no influence on the final study design and methods, data collection, analysis, interpretation of results, or decision to publish. The funding source was provided with the opportunity to comment on the manuscript, while the final version was solely based on the discretion of the authors.
Conflicts of Interest
M.S. received travel grants from AbbVie and is a current equity holder in Ambu. T.B.R. has no conflicts to declare. M.N. has no conflicts to declare. C.S.L. has received honoraria for lectures and participating in Advisory Boards from Gilead, GSK, and MSD. L.H.E. received consultancy fees or research funding from AbbVie, AstraZeneca, Boehringer Ingelheim, Gilead, GSK, Janssen, Merck, Pfizer, Radiometer, and Specsavers.
Availability of Data and Material
According to Danish legislation, our approvals to use the Danish data sources for the current study do not allow us to distribute or make patient data directly available to other parties. The full health economic model is available in a TreeAge viewer version at https://github.com/matildeslot/HIV_CUA. The health economic model can also be accessed at https://www.tpweb.treeage.com/modelShareId=da570xl5sxxi7wkplcu96ppl1. This repository allows for the user to conduct different analyses such as cost-effectiveness analyses, Tornado analyses, and Markov cohort analyses.
Ethics Approval
Studies based solely on data from the Danish national registers by Danish legislation do not require approval from the Danish Health Research Ethics committees, as study participants are never contacted, and consent is not required for the use of register information. The study was reported to the Danish Data Protection Agency through registration at Aarhus University (record number KEA-2017-36/605).
Code Availability
Please see 6.3 Availability of data and material.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Author Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) guidelines for authorship for this article. All authors contributed to the design and conceptualization of the analyses. M.S. was responsible for the first draft of the Markov model. T.B.R. was responsible for the cohort study. M.S. and L.H.E. wrote the first draft of the manuscript. C.S.L. and M.N. critically revised model inputs and assumptions and contributed to the manuscript for important intellectual content. All authors were responsible for data acquisition, analysis, and interpretation and approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Slot, M., Rasmussen, T.B., Nørgaard, M. et al. Evaluating Cost-Effectiveness of Antiretroviral Therapy over Time: A Cohort and Cost-Effectiveness Study. PharmacoEconomics Open (2024). https://doi.org/10.1007/s41669-024-00513-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s41669-024-00513-7