Abstract
Background
Atopic dermatitis (AD) affects both adults and children, impacting their quality of life and productivity; however, traditional systemic treatments such as cyclosporine have limitations. Emerging novel systemic interventions, including monoclonal antibodies and Janus kinase (JAK) inhibitors, have been shown to improve patient outcomes.
Objective
This study evaluates the cost-effectiveness of novel systemic interventions for moderate-to-severe AD in adults compared with the best supportive care (BSC) in Singapore.
Methods
The economic evaluation used a hybrid model consisting of a decision tree and Markov model. Treatment responses at 16 weeks were based on a network meta-analysis that was developed specifically for this study. Long-term response, discontinuation rates, episodes of flares and treatment-emergent adverse events were obtained from key dupilumab, abrocitinib, baricitinib and upadacitinib trials. The study had a 5-year time horizon and considered the healthcare payer's perspective. Sensitivity and scenario analyses were performed as well.
Results
Baricitinib 4 mg and 2 mg have the lowest incremental cost-effectiveness ratios, at Singapore dollars (S$) 60,730/quality-adjusted life-year (QALY) and S$66,842/QALY, respectively. Upadacitinib 30 mg offers the highest incremental QALY gain, while baricitinib 2 mg offers the least. The cost of the intervention drugs accounted for the highest proportion of the overall expenses (68–93%) for those in the maintenance state. Other influential factors within the model included (1) the incremental utility derived from intervention response; (2) the probability of achieving Eczema Area and Severity Index 75 (EASI-75) with BSC; and (3) the relative risk of achieving EASI-75 with the interventions. In a scenario where the cost of all drugs is matched to the lowest-priced drug, the top three cost-effectiveness interventions are dupilumab, upadacitinib 30 mg and abrocitinib 200 mg, respectively.
Conclusion
The interventions are found to be cost-effective at their existing prices when compared with BSC. Ideally, a composite score of treatment success and quality-of-life scores ought to be included, but such data were unavailable. Future research should consider conditional discontinuation data and long-term outcomes when such data become accessible.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
While several novel interventions for atopic dermatitis, such as dupilumab, abrocitinib, baricitinib and upadacitinib, may improve the quality of life for patients, they may not be cost-effective due to the cost of these interventions, which is a key driver of cost-effectiveness. |
Price negotiations and value-based pricing may help ensure these interventions become cost-effective and accessible to those who need them. |
Ideally, a composite score of treatment success and quality-of-life scores ought to be included when such data also become available. |
1 Introduction
Atopic dermatitis (AD) is a common and chronic skin condition, characterised by dry, itchy and inflamed skin [1], that affects about 11.1% of adults [2] and 20.8% of children and adolescents [3] in Singapore. It may arise for various reasons, including immune system activation, genetics, environmental triggers, and stress [1]. The disease course is chronic but intermittent, and when active, the intense pruritus and rash can be debilitating [4]. The skin appearance may cause social embarrassment and isolation, which consequentially leads to work impairment and productivity loss, school absenteeism, as well as an overall decrease in quality of life [2].
For patients with moderate-to-severe AD, cyclosporine (CsA) has been a mainstay among patients with inadequate response to topical therapy, such as topical corticosteroids (TCS). Nevertheless, the administration of CsA should be discontinued after 1 year due to safety concerns of possible nephrotoxicity risks [5]. After discontinuation of CsA, some patients may experience inadequate disease management.
Novel systemic interventions, including monoclonal antibodies and Janus kinase (JAK) inhibitors, have emerged as alternative treatments for patients with moderate-to-severe AD. Dupilumab [6, 7], a human monoclonal immunoglobulin (Ig) G4 antibody, disrupts interleukin (IL)-4 and IL-13 signalling by binding to IL-4Rα subunits. Conversely, abrocitinib [8, 9], baricitinib [10,11,12], and upadacitinib [13, 14] are reversible, selective inhibitors of JAKs—vital cytoplasmic protein tyrosine kinases essential for cell nucleus signal transduction. A network meta-analysis (NMA) by Drucker et al. [15] encompassing monotherapy and combination trials of these four intervention drugs demonstrated their notable impact on Eczema Area and Severity Index (EASI) scores versus placebo. Additionally, these interventions yielded enhanced patient quality of life, evidenced by changes in Patient-Oriented Eczema Measure (POEM) and Dermatology Life Quality Index (DLQI) scores. Abrocitinib [16], baricitinib [17], dupilumab [18], and upadacitinib [19] have subsequently secured Health Science Authority (HSA) approval for treating moderate-to-severe AD in adults in Singapore.
In light of the emerging therapeutic options, this study aims to comprehensively assess the cost-effectiveness of these interventions for the local population of patients with moderate-to-severe AD, specifically from the perspective of the decision makers within the Singapore healthcare system. Understanding the cost-effectiveness of these interventions is crucial for informing healthcare policies and resource allocation decisions in Singapore. In this paper, we present a detailed methodology encompassing data sources, model structure, and analytical techniques to achieve this.
2 Method
2.1 Model Validation
A scoping workshop was convened initially to delineate the relevance of the model to local clinical practice and patient needs. This process encompassed several key components, including understanding the heterogeneity within patient populations, selecting appropriate surrogate outcome measures, describing the significance of various treatment-emergent adverse events (TEAEs), and elucidating how the anticipated clinical benefits manifest within the local context. Additionally, efforts were made to identify suitable comparators and comprehend the clinical pathways associated with the administration of intervention and comparator drugs.
Throughout this process, the medical experts engaged in comprehensive discussions regarding the model schematic, relative efficacy of interventions, and resource utilisation considerations. Subsequently, a follow-up consultation workshop was conducted, during which the outcomes of the NMA and proposed parameterisation for the model were presented. Moreover, simplifying assumptions and strategies for addressing missing data were established and collectively endorsed. Following the completion of the cost-effectiveness analysis, the base-case results and sensitivity analyses were also presented to these experts.
After extensive discussions and consideration of diverse viewpoints, consensus among the medical experts was reached through transparent deliberation. We iteratively weighed evidence, evaluated implications for local practice, and adhered to established guidelines. Any remaining disagreements were addressed through further exploration until all members agreed on the final decisions.
The medical experts in these workshops included heads of departments from dermatology departments across various hospitals within distinct healthcare clusters in Singapore. Additionally, clinicians from the KK Women's and Children’s Hospital were invited to contribute their insights, particularly concerning the treatment pathways involving these intervention drugs in the adolescent population, despite the eventual exclusion of this demographic from the model. The involvement of pharmacists and nurses further enriched the discussions and ensured a comprehensive exploration of relevant clinical perspectives.
2.2 Patients Population
The population of interest is adults (aged 18 years and older) with moderate-to-severe AD who achieve inadequate response to, cannot tolerate, or are contraindicated to CsA. The model considers patients with characteristics similar to those in the BREEZE AD-4 trial [10]. Referencing the placebo + TCS arm, patients have a mean age of 38.7 years, and 62.4% have prior CsA use. All patients taking any intervention drugs are treated in combination with TCS and topical calcineurin inhibitors (TCI), also known as combination therapy.
2.3 Model Structure
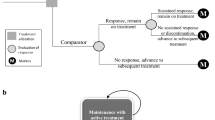
A hybrid model with a decision tree and Markov model structure (Fig. 1) was designed on TreeAge Healthcare Pro to capture short-term (first year) and long-term (second to fifth year) effects of treatment. The interventions taken in combination with TCS include (1) dupilumab, taken once every 2 weeks; (2) abrocitinib 100 mg, taken daily; (3) abrocitinib 200 mg, taken daily; (4) baricitinib 2 mg, taken daily; (5) baricitinib 4 mg, taken daily; (6) upadacitinib 15 mg, taken daily; and (7) upadacitinib 30 mg, taken daily. The choice of interventions is justified by their status as novel systemic therapeutics increasingly prescribed within the local healthcare system, with the potential to replace current standard of care if proven cost-effective. The interventions are compared with the best supportive care (BSC), which involves treatment with TCS and TCI only. The model is available as electronic supplementary material (ESM).
The short-term parameters for the model include the probability of responding to treatment at week 16 and the probability of unsustained response to treatment between weeks 17 and 52, whereas the long-term parameters include the discontinuation from treatment and the survival of patients. Outcomes in the Markov model were modelled over a 4-year time horizon, with each cycle representing 1 month (48 cycles in total). The 4-year time horizon in the Markov model was selected due to the absence of long-term treatment data to inform the longer-term disease course of AD. An annual discount rate of 3% is applied to all costs and QALYs in the Markov model [20]. In this study, we conducted the cost-effectiveness analysis from the healthcare payer’s perspective.
Our analysis adheres to the standards of Health Technology Assessment (HTA) practices in Singapore, focusing solely on direct healthcare costs from the perspective of the healthcare payer. While the Drug Advisory Committee does not use a precise incremental cost-effectiveness ratio (ICER) threshold, we consider historical evidence, which suggests that positive subsidies for drugs in Singapore typically do not exceed Singapore dollars (S$) 45,000 per quality-adjusted life-year (QALY) gained or S$75,000/QALY depending on the drug being evaluated [21].
2.4 Treatment Response, Discontinuation and Mortality
Treatment response at week 16 is defined by a 75% decrease in EASI (EASI-75) score after the patient has received treatment. The selection of EASI-75 as the primary outcome was based on consensus among key stakeholders, following the guidelines of the global Harmonising Outcome Measures for Eczema (HOME) initiative, affirming EASI as the preferred core instrument for assessing clinical signs in AD trials [22]. The probability of achieving EASI-75 in the BSC arm is informed by the proportion of patients achieving EASI-75 in the placebo + TCS arm in the BREEZE AD-4 trial [10]. We conducted an NMA to inform the relative risk ratios of the treatment effect between the interventions of interest and BSC (see ESM 1). The probabilities of achieving EASI-75 in the intervention arms are thus obtained by applying the respective relative risk ratios to the proportion of patients achieving EASI-75 in the BSC arm.
The probabilities of unsustained response between week 17 and week 52 are informed by the unconditional discontinuation risk due to lack of efficacy from long-term trials [6, 10, 23]. The unconditional discontinuation risk due to all causes informed the monthly probabilities of treatment discontinuation in the Markov model. No trial data beyond 16 weeks were available for abrocitinib, therefore the probabilities of treatment discontinuation for patients taking abrocitinib are assumed to be equal to patients taking upadacitinib, as supported by medical experts. To derive probabilities across various time durations, such as for the purposes of extrapolation, adjustments are made using the formula \(-\text{ln}(1-p)/t\) to obtain the instantaneous rate. Subsequently, the period-appropriate proportion and probabilities are obtained using \(1-{e}^{rt}\), after adjusting for time. By using this equation, we assume the hazard to be constant over time. Table 1 presents the transition probabilities used in the model.
The daily dosage of TCS and TCI for patients is determined using the average fingertip unit (FTU) method. This method is calculated as follows: \(total FTU*\% BSA*0.5 g/FTU\). For the body, excluding the face and neck, the total FTU is approximately 38, allocated for TCS application. For the face and neck, the total FTU is approximately 2.5, designated for TCI application.
For patients continuing or achieving sustained response to the intervention drugs, we assume a 50% reduction of resource use for TCS and TCI compared with non-responders or discontinuers. Notably, patients receiving BSC who are non-responders do not technically discontinue treatment. These patients will continue on BSC (i.e., BSC Failure health state) but achieve lower utility levels due to treatment failure. As treatment for AD is not expected to affect mortality, the general population mortality rate informed the transition to the death state.
2.5 Flares
Patients may experience acute exacerbations of symptoms, called flares, during the treatment for moderate-to-severe AD. The risk of flares may vary depending on the treatment received by a patient. The risk of flares was not an endpoint in the trials; thus, receipt of rescue medication is considered a proxy for flares [6, 10, 23]. Rescue medication was not permitted in the abrocitinib trials [8]. Hence, as supported by medical experts, we assume patients taking abrocitinib would have an equal risk of flare as patients taking upadacitinib. Patients with an episode of flare are prescribed high-potency TCS for 14 days, with the daily dosage of high-potency TCS determined using the same method as that for low-to-mid potency TCS. However, we also assume that such patients continue to use low-to-mid potency TCS at the same time. Of these patients, 50% are assumed to require additional second-line treatment with prednisolone. This stepwise regimen was recommended by medical experts from several public health institutions. Flares are included in the model as an additional cost. The proportion of flares for each strategy are available in ESM 2.
2.6 Treatment-Emergent Adverse Events
Allergic conjunctivitis, infectious conjunctivitis, herpes zoster infection and herpes simplex infection were selected by medical experts as key TEAEs. In trials where conjunctivitis was unspecified, we assumed that the number of allergic and infectious conjunctivitis cases is 50% of the total conjunctivitis cases, based on epidemiological evidence indicating that allergic and infectious conjunctivitis are among the most common forms of conjunctivitis encountered in clinical practice. The number of cases of herpes simplex infections was not reported in the upadacitinib trial; hence, we assume patients taking upadacitinib will have an equal risk of herpes simplex infection as patients taking abrocitinib, as supported by medical experts. Patients with allergic conjunctivitis are treated with sodium cromoglycate eyedrops, while patients with infectious conjunctivitis are treated with gentamycin eyedrops. Patients with infectious conjunctivitis are assumed to have a 50% chance of being referred to the ophthalmologist. The regimen for treating herpes zoster and herpes simplex infection involves taking acyclovir 800 mg and 400 mg, respectively. The proportion of TEAEs for each strategy is available in ESM 2.
2.7 Cost and Resource Use
The average costs of drugs and services were derived from three public healthcare institutions, each from one of the three public healthcare clusters in Singapore. These costs include drug acquisition costs, drug administration costs (for patients taking dupilumab, an injectable biologic drug), concomitant medication costs (e.g. TCS), healthcare resource use costs, cost of managing flares, and cost of managing TEAEs. The listed prices of the drugs and services were obtained from the pricing databases of each institution. These prices were provided by the heads of department from the respective institutions, ensuring access to accurate and up-to-date pricing information. The average cost was subsequently calculated by aggregating the listed drugs and services from the three institutions. All costs are reported in Singapore dollars and were based on 2022 price levels (S$1 = US$0.7255 = €0.6896).
The cost of acquiring the intervention drugs is determined based on dosage. Upadacitinib 30 mg was unavailable locally at the evaluation stage, therefore its average cost was unknown. Given the similar pricing of high and low doses of abrocitinib and baricitinib, we extrapolated a similar cost structure to both upadacitinib doses. A scenario analysis was performed to address the uncertainty surrounding the pricing of upadacitinib 30 mg, assuming a price 1.5 times that of upadacitinib 15 mg. Drug administration expenses are factored in solely for patients prescribed dupilumab.
Patients also receive concomitant medications such as emollients, low-to-mid potency background TCS and TCI. Emollients are universally used among patients, irrespective of disease severity, thus their cost is omitted from the model as it would offset across the strategies. Given the diverse range of prescribed low-to-mid potency TCS formulations, the average cost across all variants is employed in the model. The quantity of TCS and TCI applied by each patient was estimated using the FTU guidelines of the National Eczema Association [24], assuming a 50% affected body surface area (BSA) for each patient.
Other healthcare resources include laboratory tests, doctor’s consultations, rescue therapy for flares and treatment medication for TEAEs. Patients receiving systemic therapies must undergo several laboratory tests before treatment initiation and as part of routine clinical monitoring while undergoing treatment. The type and frequency of each laboratory test for different treatments were modelled after the guidelines of a local public healthcare institution. We assume that no patient would discontinue treatment due to unsatisfactory laboratory results, and that compliance to treatment and consultation is 100%.
2.8 Utility Values
This study uses health state utilities that assume a common baseline utility for both interventions and comparator, common utility values for response to any interventions, and a separate utility to reflect response to BSC treatment. Treatment‐specific utilities added complexity to the model; hence health‐state utilities were preferred in alignment to the TA814 assumption [25].
The values used are derived from TA534 (dupilumab HTA appraisal) [26], which reported that the utility values were derived from pooled data from all patients in the LIBERTY AD CAFÉ trial [7] and a subset of patients from LIBERTY AD CHRONOS [6]. The report notes that the mean EASI and pruritus scores were slightly higher in the pooled populations, while the mean DLQI and EQ-5D scores were slightly lower. These findings may be attributed to these patients’ history of intolerance to, contraindication to, or inadequate response to systemic immunosuppressive therapies. Patients receiving BSC are assigned utility values based on their response to intervention, with responders given a higher value and non-responders given a lower value. Those who respond and maintain their response to the intervention are assigned a utility value corresponding to their response. In contrast, non-responders are assigned the same utility value as non-responders in BSC.
Disutilities associated with TEAEs and flares are already accounted for in the EQ-5D data from the trials, as specified in TA814 [25] and TA534 [26]. Hence, disutilities for TEAEs and flares are not considered to avoid double counting, given the use of trial-based utilities and data collection frequency.
Table 2 presents the cost and health utility values used in the model, while Table 3 presents the resource use frequency for each strategy.
2.9 Sensitivity Analyses
One-way sensitivity analyses (OWSA) are employed to assess the influence of feasible changes in individual input parameters on the model. Given the extensive parameter set, the OWSA primarily focuses on pivotal parameters, including the probability of achieving EASI-75 at week 16, the probability of unsustained response and discontinuation, and utility values. However, all parameters with uncertainty are varied in a probabilistic sensitivity analysis (PSA) comprising 10,000 Monte Carlo simulations.
For parameter distributions, the probabilities of achieving EASI-75, discontinuation, flares, TEAEs and utilities follow beta distributions, while the relative risks of patients achieving EASI-75 for intervention drugs adopt a log-normal distribution. Given the absence of standard errors for utility values in the trials, TA814 [25] and TA534 [26], we assume that the standard error equates to 5% of the mean utility values. The utilities derived from treatment success from BSC of the intervention are treated as incremental utilities. This is conducted to ensure that the utility values associated with all treatment responses consistently surpass the baseline utility from no response in the sensitivity analysis.
3 Results
3.1 Base-Case Analysis
The study presents its findings using ICERs and dominance analysis (Table 4). Baricitinib 4 mg shows the most favourable ICER at S$60,730/QALY, followed by baricitinib 2 mg at S$66,842/QALY, when compared with BSC. Conversely, all other interventions exceed S$100,000/QALY gained compared with BSC. Upadacitinib 30 mg demonstrates the highest incremental QALY gain, while baricitinib 2 mg exhibits the least. No interventions, when compared with BSC, are below the threshold of S$45,000/QALY.
Considering the mutually exclusive nature of the interventions, we applied the principle of extended dominance. A strategy is considered dominated when an alternative achieves greater QALYs at a lower cost. Abrocitinib 100 mg, abrocitinib 200 mg, and dupilumab were all dominated strategies. However, weak dominance occurs when a more costly strategy is both more effective and has a lower ICER than the comparator. Baricitinib 2 mg shows an ICER of approximately S$66,842/QALY gained compared with BSC (i.e., the least expensive alternative). Nevertheless, baricitinib 4 mg presents a more favourable ICER of approximately S$30,000/QALY gained compared with baricitinib 2 mg. Both upadacitinib 15 mg and upadacitinib 30 mg exhibit significantly higher ICERs compared with the next least expensive alternative, indicating them as less cost-effective options relative to other interventions.
However, it is essential to note that in the scenario where upadacitinib 30 mg is priced at 1.5 times that of upadacitinib 15 mg, the resultant ICER is S$162,536/QALY compared with BSC. When compared with the next least expensive alternative (i.e., abrocitinib 200 mg), the ICER is S$292,753/QALY (see ESM 3).
3.2 One-Way Sensitivity Analyses
Comparing the various strategies against BSC, (1) the incremental utility derived from intervention response, (2) the probability of achieving EASI-75 with BSC, and (3) the relative risk of achieving EASI-75 with the interventions emerge as highly influential parameters across most interventions.
The parameter of incremental utility from intervention response has the most significant impact on the ICER values of dupilumab, abrocitinib (100 mg, 200 mg), and upadacitinib (15 mg, 30 mg). The parameter brought about substantial shifts in ICER, with absolute changes ranging from 22% to 24% among these interventions. Similarly, baricitinib (2 mg, 4 mg) experiences a notable ICER change of approximately 26–28% in response to this parameter. However, the incremental utility from intervention response is not among the major influential parameters for baricitinib (2 mg, 4 mg). In fact, the monthly discontinuation after 1 year results in a significantly larger shift in ICER by approximately 50–55% for baricitinib (2 mg, 4 mg), driven by the huge magnitude and uncertainty surrounding this parameter.
While the relative risk of achieving EASI-75 for interventions proves influential across most interventions, its impact is relatively less pronounced for dupilumab, owing to the lower uncertainty around the relative risk ratio associated with this intervention.
The results for the OSWA and the tornado diagrams stratified by intervention are available in ESM 4.
3.3 Scenario Analysis
A disaggregated costing analysis revealed that the cost of drugs is the biggest driver of cost in the model (see ESM 5); thus, two scenarios are considered where the cost of the intervention drugs are reduced. A 30% reduction in the price of all intervention drugs reduces the ICER significantly when compared with BSC. However, all ICERs are still above the implicit threshold of S$45,000/QALY. We also considered a scenario where the cost of the intervention drugs are matched to the lowest-priced drug (i.e. baricitinib), with the aim of exploring potential cost-saving implications in a budget-constrained environment. This resulted in a drastic change in ICER ranking, with the top three cost-effective interventions compared with BSC being dupilumab, upadacitinib 30 mg and abrocitinib 200 mg, respectively (see Table 5).
3.4 Probabilistic Sensitivity Analysis
The acceptability curve represents the probability of an intervention being cost-effective at different willingness-to-pay thresholds (Fig. 2). The graph indicates that at the implicit threshold of S$45,000/QALY, baricitinib 2 mg and baricitinib 4 mg have a 1% and 4% probability of being cost-effective, respectively, compared with BSC. For all other interventions, none of the iterations meet the criteria for cost-effectiveness at this threshold. When the threshold is increased to S$75,000/QALY, baricitinib 2 mg and baricitinib 4 mg have a 28% and 60% probability of being cost-effective, respectively, compared with BSC. Similarly, none of the iterations meet the criteria for cost-effectiveness at this threshold for all other interventions.
4 Discussion
This study assessed the cost-effectiveness of dupilumab, abrocitinib, baricitinib and upadacitinib individually in combination with TCS/TCI using BSC as a comparator for treating moderate-to-severe AD. Among the interventions, baricitinib 4 mg has the lowest ICER value of S$60,730/QALY gained, followed by baricitinib 2 mg with an ICER of S$66,842/QALY gained when compared with BSC. Meanwhile, the other interventions (dupilumab, abrocitinib 100 mg and 200 mg, upadacitinib 15 mg and 30 mg) exceed S$100,000/QALY gained. When compared with the majority of previously subsidised drugs in Singapore (with ICERs ranging from dominance to S$45,000/QALY gained), the current interventions, at their existing price points, may not be considered cost-effective due to their higher incremental costs and relatively modest incremental QALYs.
It is noteworthy that several of the interventions under consideration have received reimbursement recommendation within other jurisdictions. Dupilumab, for instance, has obtained approval for monotherapy use in both the UK [26] and Australia [27], while in Canada [28], it is endorsed for reimbursement in combination with TCS. In the UK and Canada, dupilumab is recommended for patients who have not responded to at least one other systemic therapy. Similarly, upadacitinib has secured reimbursement approval for monotherapy in the UK [25] and Australia [29], and for combination therapy with TCS in Canada [30]. Abrocitinib, currently approved for reimbursement exclusively in the UK [25], is indicated for both combination and monotherapy use.
In contrast, while baricitinib has been deemed cost-effective and has been approved for monotherapy in the UK [31], it is not recommended for use in Australia [32]. This decision stems from disparities in treatment response magnitude compared with dupilumab, coupled with concerns regarding the relatively inferior safety profile of baricitinib. In many instances, the ICER compared with BSC may appear relatively favourable; however, the lack of publicly available list prices and disaggregated cost breakdowns from such reports pose challenges in identifying the primary cost drivers in such analyses.
In our analysis, apart from the increment utility and relative efficacy of the interventions, the cost of the intervention drugs remains the most significant cost driver, accounting for approximately 68–93% of the total cost for patients on maintenance in the Markov model. In terms of the incremental cost compared with BSC, the intervention cost accounts for approximately 78–97% of the total cost for patients on maintenance in the Markov model. It is thus unsurprising that a 30% reduction in the cost of all intervention drugs reduced the ICER by 22–30%. Further benchmarking the cost of all intervention drugs to the lowest costing drug (i.e. baricitinib) reduced the ICER even further, allowing for efficacious drugs such as dupilumab, abrocitinib 200 mg and upadacitinib 30 mg to be cost-effective based on the implicit threshold of S$45,000/QALY.
Numerous published studies have similarly identified the price of the intervention as a key determinant of cost-effectiveness [33]. Interestingly, a majority of the studies focusing on dupilumab, abrocitinib, and baricitinib have reported them to be cost-effective compared with BSC [33]. However, upon closer examination of drug cost utilities across various studies, a notable trend emerges, i.e. drug costs in Singapore appear to be comparatively higher when compared with similar studies conducted in Spain [34], Japan [35], Italy [36], and the United States [37]. These disparities could include variations in healthcare system structures, pricing regulations, and negotiation strategies between pharmaceutical companies and healthcare payers across different countries. Thus, these observations underscore the importance of exploring alternative strategies to enhance cost-effectiveness, such as value-based pricing and other negotiation strategies with pharmaceutical manufacturers.
While all interventions are found to exceed the cost-effectiveness threshold of S$45,000/QALY, caution should be exercised when drawing conclusions due to the small incremental QALYs and the inherent limitations of our analysis. AD is a complex disease, with multiple instruments used to measure disease severity and treatment effectiveness. Although we used the EASI scale to determine treatment success in our model, it is not a catch-all outcome. For example, patients with localised moderate-to-severe lesions may have low EASI scores but high SCORing Atopic Dermatitis (SCORAD) scores, which are not accounted for in our analysis. Additionally, the severity of pruritus, which can significantly impact sleep and quality of life, was not captured in the EASI score. Ideally, a composite score such as RECAP (Recap of Atopic Eczema) [38] or ADCT (Atopic Dermatitis Control Tool) [39] combining EASI with other outcomes measuring quality of life, such as DLQI or POEM, would provide a more comprehensive assessment of the interventions’ benefits. However, such data were unavailable, limiting the scope of our analysis. Therefore, while the incremental cost and QALYs of the intervention drugs compared with BSC may be large and small, other clinical impacts beyond EASI should also be considered.
Moreover, our study is constrained by the data available in the published RCTs. The reliance on unconditional treatment discontinuation, due to the absence of conditional treatment discontinuation data, may lead to overestimating treatment failure and withdrawal rates. Consequently, this inflationary effect impacts the ICER, potentially portraying the interventions as not cost-effective compared with the comparator. Since the long-term discontinuation rate is derived from unconditional treatment discontinuation data between week 16 and week 52, the overestimation of treatment failure and withdrawal extends to the long-term model. As a result, the calculated ICER values might deviate from real-world dynamics. Presently, only long-term safety and effectiveness data are available for dupilumab. Future investigations should endeavour to incorporate conditional discontinuation information and evidence-based long-term outcomes wherever feasible. Additionally, the model does not account for treatment sequencing, as patients may try different treatment options if they do not respond to their initial treatment. While incorporating treatment sequencing may enhance the model’s ability to mirror real-world clinical practices, it introduces additional complexity to the model. It is also important to note that reliable data on treatment sequencing are currently unavailable for integration into the model.
Lastly, while the utility values utilised in our analysis were derived from UK NICE appraisals, specifically TA814 [25] and TA534 [26], it is important to recognise that utility values may vary across different populations, and thus may not fully capture the preferences and health-related quality of life of the Singaporean population. This limitation could introduce uncertainty into our analysis, particularly in terms of the generalisability to the local setting. We acknowledge this limitation and recommend cautious interpretation of the findings. Future studies incorporating locally derived utility values would be beneficial to enhance the robustness and applicability of cost-effectiveness analyses in our setting.
5 Conclusion
This study focused on assessing the cost-effectiveness of new treatments for moderate-to-severe AD in Singapore. We found that baricitinib 4 mg has the most favourable cost- effectiveness compared with BSC, but was however not cost-effective at the S$45,000/QALY threshold. Other treatment strategies such as abrocitinib, baricitinib 2 mg, dupilumab, and upadacitinib have even higher ICERs, indicating they may also not be cost-effective. Further analyses, including disaggregated cost analysis and sensitivity analysis, highlighted the significant impact of intervention drug prices on cost-effectiveness. Lowering the prices of these drugs could substantially improve their cost-effectiveness, especially for treatments demonstrating significantly greater efficacy than BSC.
Given the high cost of these drugs and the existing data limitations and uncertainties, we suggest exploring performance-based risk-sharing agreements between drug manufacturers and payers. Such agreements could help manage the financial risks associated with high drug costs, and uncertainty regarding long-term effectiveness and safety. By sharing these risks, manufacturers and payers can work together to ensure fair access to effective treatments while effectively managing healthcare costs. Moving forward, we encourage further research and collaborative efforts to address these limitations and uncertainties in modelling and healthcare policy. By doing so, we can strive to optimise patient outcomes and allocate healthcare resources more effectively in the management of AD.
References
Tay YK, Chan YC, Chandran NS, Ho MS, Koh MJ, Lim YL, et al. Guidelines for the Management of Atopic Dermatitis in Singapore. Ann Acad Med Singap. 2016;45(10):439–50.
Cheok S, Yee F, Song Ma JY, Leow R, Ho MSL, Yew YW, et al. Prevalence and descriptive epidemiology of atopic dermatitis and its impact on quality of life in Singapore. Br J Dermatol. 2018;178(1):276–7.
Tay Y-K, Kong K-H, Khoo L, Goh C-L, Giam Y-C. The prevalence and descriptive epidemiology of atopic dermatitis in Singapore school children. Br J Dermatol. 2002;146(1):101–6.
Fishbein AB, Silverberg JI, Wilson EJ, Ong PY. Update on atopic dermatitis: diagnosis, severity assessment, and treatment selection. J Allergy Clin Immunol Pract. 2020;8(1):91–101.
(Singapore) HSA. Cyclosporine Full Prescribing Information. Register of Therapeutic Products; 2022.
Blauvelt A, de Bruin-Weller M, Gooderham M, Cather JC, Weisman J, Pariser D, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287–303.
de Bruin-Weller M, Thaçi D, Smith CH, Reich K, Cork MJ, Radin A, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ). Br J Dermatol. 2018;178(5):1083–101.
Bieber T, Simpson EL, Silverberg JI, Thaçi D, Paul C, Pink AE, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384(12):1101–12.
Reich K, Thyssen JP, Blauvelt A, Eyerich K, Soong W, Rice ZP, et al. Efficacy and safety of abrocitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis: a randomised, double-blind, multicentre phase 3 trial. Lancet. 2022;400(10348):273–82.
Bieber T, Reich K, Paul C, Tsunemi Y, Augustin M, Lacour JP, et al. Efficacy and safety of baricitinib in combination with topical corticosteroids in patients with moderate-to-severe atopic dermatitis with inadequate response, intolerance or contraindication to ciclosporin: results from a randomized, placebo-controlled, phase III clinical trial (BREEZE-AD4). Br J Dermatol. 2022;187(3):338–52.
Reich K, Kabashima K, Peris K, Silverberg JI, Eichenfield LF, Bieber T, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(12):1333–43.
Guttman-Yassky E, Silverberg JI, Nemoto O, Forman SB, Wilke A, Prescilla R, et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: a phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J Am Acad Dermatol. 2019;80(4):913-21.e9.
Katoh N, Kataoka Y, Saeki H, Hide M, Kabashima K, Etoh T, et al. Efficacy and safety of dupilumab in Japanese adults with moderate-to-severe atopic dermatitis: a subanalysis of three clinical trials. Br J Dermatol. 2020;183(1):39–51.
Reich K, Teixeira HD, de Bruin-Weller M, Bieber T, Soong W, Kabashima K, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397(10290):2169–81.
Drucker AM, Morra DE, Prieto-Merino D, Ellis AG, Yiu ZZN, Rochwerg B, et al. Systemic immunomodulatory treatments for atopic dermatitis: update of a living systematic review and network meta-analysis. JAMA Dermatol. 2022;158(5):523–32.
(Singapore) HSA. Abrocitinib Full Prescribing Information. ABRO-SIN-0922/PIL/2 ed. Register of Therapeutic Products; 2023.
(Singapore) HSA. Baricitinib Full Prescribing Information. Register of Therapeutic Products2022.
(Singapore) HSA. Dupilumab Full Prescribing Information. SG/DUP/0223/CCDS V13 - 16 and USPI Dec 2021 ed. Register of Therapeutic Products; 2021.
(Singapore) HSA. Upadacitinib Full Prescribing Information. CCDS05200722 ed. Register of Therapeutic Products; 2023.
Agency for Care Effectiveness. Procedures and guidelines for company submissions to the Agency for Care Effectiveness for funding consideration. Agency for Care Effectiveness; 2024.
Agency for Care Effectiveness. Drug and vaccine evaluation methods and process guide. Agency for Care Effectiveness; 2023.
Schmitt J, Spuls PI, Thomas KS, Simpson E, Furue M, Deckert S, et al. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol. 2014;134(4):800–7.
Silverberg JI, de Bruin-Weller M, Bieber T, Soong W, Kabashima K, Costanzo A, et al. Upadacitinib plus topical corticosteroids in atopic dermatitis: week 52 AD Up study results. J Allergy Clin Immunol. 2022;149(3):977-87.e14.
National Eczema Society. Topical steroids factsheet. National Eczema Society; 2023.
National Institute for Health and Care Excellence. Final appraisal document abrocitinib, upadacitinib, tralokinumab. National Institute for Health and Care Excellence; 2022.
National Institute for Health and Care Excellence. Final appraisal document: Dupilumab for treating moderate to severe atopic dermatitis. National Institute for Health and Care Excellence; 2018.
Pharmaceutical Benefits Advisory Committee. Public Summary Document – March 2020 PBAC Meeting (Dupilumab). Pharmaceutical Benefits Advisory Committee; 2020.
Canadian Agency for Drugs and Technologies in Health. CADTH Canadian Drug Expert Committee Recommendation (Dupilumab). Canadian Agency for Drugs and Technologies in Health; 2018.
Pharmaceutical Benefits Advisory Committee. Public Summary Document – July 2021 PBAC Meeting (Upadacitinib). Pharmaceutical Benefits Advisory Committee; 2021.
Canadian Agency for Drugs and Technologies in Health. CADTH Reimbursement Recommendation: Upadacitinib (Rinvoq). Canadian Agency for Drugs and Technologies in Health; 2021.
National Institute for Health and Care Excellence. Baricitinib for treating moderate to severe atopic dermatitis: Technology appraisal guidance. National Institute for Health and Care Excellence; 2021.
Pharmaceutical Benefits Advisory Committee. Public Summary Document – July 2021 PBAC Meeting (Baricitinib). Pharmaceutical Benefits Advisory Committee; 2021.
Heinz KC, Beaudart C, Willems D, Wiethoff I, Hiligsmann M. Cost-effectiveness of emerging treatments for atopic dermatitis: a systematic review. Pharmacoeconomics. 2023;41(11):1415–35.
Romero Jiménez RM, Herranz Pinto P, Campos Domínguez M, Aceituno Mata S, Bellmunt A, Prades M, et al. Cost-effectiveness analysis of abrocitinib compared with other systemic treatments for severe atopic dermatitis in Spain. Pharmacoecon Open. 2024;8(2):291–302.
Tanaka A, Yuasa A, Kamei K, Nagano M, Murofushi T, Bjerke A, et al. Cost-effectiveness analysis of abrocitinib compared with standard of care in adult moderate-to-severe atopic dermatitis in Japan. J Dermatol. 2024;51(6):759–71.
Costanzo A, Furneri G, Bitonti R, Pedone MP, Fanelli F, Di Turi R. Analisi costo-utilità di dupilumab per il trattamento della dermatite atopica grave negli adulti in Italia. Glob Reg Health Technol Assess. 2020;7:57–65.
Zimmermann M, Rind D, Chapman R, Kumar V, Kahn S, Carlson J. Economic evaluation of dupilumab for moderate-to-severe atopic dermatitis: a cost-utility analysis. J Drugs Dermatol. 2018;17(7):750–6.
Howells LM, Chalmers JR, Gran S, Ahmed A, Apfelbacher C, Burton T, et al. Development and initial testing of a new instrument to measure the experience of eczema control in adults and children: Recap of atopic eczema (RECAP). Br J Dermatol. 2020;183(3):524–36.
Staumont-Sallé D, Taieb C, Merhand S, Shourick J. The atopic dermatitis control tool: a high-performance tool for optimal support. Acta Derm Venereol. 2021;101(12):adv00618.
Acknowledgements
The authors thank the following for their involvement in our scoping workshop: Dr Charmaine Lim En-En, Ms Chen Mee Kuan, Mr Nicholas Teo and Ms Praveena Sures from the KK Women’s and Children's Hospital, Singapore; Ms Felicia Lim and Dr Jiang He from the National University Hospital, Singapore; and Dr Wang Aiwen from the Singapore General Hospital, Singapore.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study received no specific grants from any funding agencies.
Conflicts of interest
Clarence Ong, Jamaica Briones, Zhi Zhen Lim, Nisha Suyien Chandran, Haur Yueh Lee, Benny Kaihui Li, Yik Weng Yew, and Hwee-Lin Wee have no conflicts of interest to declare.
Data Availability
The disaggregated data that support the findings of this study are available from the corresponding author upon reasonable request; however, some data are also subject to the agreement of the respective public health institutions.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
The model is available as electronic supplementary material.
Author Contributions
Clarence Ong: Conceptualisation (equal), data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), project administration (equal), validation (equal), writing – original draft (equal), writing – review and editing (equal). Jamaica Briones: Conceptualisation (equal), data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), project administration (equal), supervision (equal), validation (equal), writing – review and editing (equal). Zhi Zhen Lim: Conceptualisation (equal), data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), project administration (equal), validation, writing – review and editing (equal). Nisha Suyien Chandran: Investigation (equal), methodology (equal), supervision (equal), validation (equal), writing – review and editing (equal). Haur Yueh Lee: Investigation (equal), methodology (equal), supervision (equal), validation (equal), writing – review and editing (equal). Benny Kaihui Li. Investigation (equal), methodology (equal), supervision (equal), validation (equal), writing – review and editing (equal). Yik Weng Yew: Investigation (equal), methodology (equal), supervision (equal), validation (equal), writing – review and editing (equal). Hwee-Lin Wee: Conceptualisation (equal), data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), project administration (equal), supervision (equal), validation, writing – review and editing (equal). All authors have read and approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ong, C., Briones, J., Lim, Z.Z. et al. Cost-Effectiveness of Dupilumab and Oral Janus Kinase Inhibitors for the Treatment of Moderate-to-Severe Atopic Dermatitis in Singapore. PharmacoEconomics Open (2024). https://doi.org/10.1007/s41669-024-00507-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s41669-024-00507-5