Abstract
Background
Today, there are many treatment options available for the management of ulcerative colitis, creating challenges in selecting the most efficacious and cost-effective treatment sequences. Treatments in the anti-tumor necrosis factor alpha (TNFα) therapeutic class, as well as vedolizumab, are widely used and endorsed as first-line options according to treatment guidelines. The aim of this study was to compare treatment sequences involving vedolizumab and the anti-TNFα treatment adalimumab in terms of cost-effectiveness in the treatment of moderately to severely active ulcerative colitis in Italy.
Methods
A cost-effectiveness model comparing treatment sequences within the Italian National Health Service in terms of costs and quality-adjusted life years (QALYs) with a lifetime horizon was developed. The analysis focused on the relative positioning of vedolizumab and adalimumab, leveraging the results of the landmark head-to-head VARSITY clinical trial as key inputs. The robustness of the results was investigated through a range of sensitivity and scenario analyses.
Results
The strategy of vedolizumab as a first-line advanced therapy followed by adalimumab was associated with higher costs and health benefits compared with first-line adalimumab followed by vedolizumab. The incremental cost-effectiveness ratio was €16,146/QALY, which was found to be robust to changes to inputs associated with areas of high uncertainty.
Conclusion
This economic evaluation estimated a 94% probability that vedolizumab as a first-line advanced therapy is cost-effective at a threshold of €33,004/QALY when compared with first-line adalimumab sequences. Using clinical trial evidence to inform the efficacy of second-line treatments estimated that the effectiveness of anti-TNFα treatments is not substantially reduced by vedolizumab exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Our model suggests that treatment with vedolizumab as a first-line advanced therapy is cost effective when compared with adalimumab as a first-line biologic in patients with ulcerative colitis in Italy. |
Even with a significant discount on adalimumab’s price, vedolizumab remains a cost-effective option compared with adalimumab. |
The outcomes of the model could provide justification for the earlier administration of vedolizumab within the treatment pathway in clinical practice. |
1 Introduction
Ulcerative colitis (UC) is a chronic, inflammatory, relapsing–remitting bowel disease with an increasing global incidence [1]. The condition negatively affects patients’ quality of life by burdening their well-being and physical, emotional, and social functioning [2], as well as being costly to manage [3]. There are a range of treatments available to manage UC with varying mechanisms of action, modes of administration, and safety and efficacy profiles. Typically, < 20% of patients achieve clinical remission following treatment induction, and a treatment response may not be maintained [4]. It is, therefore, common for patients to sequence through multiple treatment options to attempt to achieve and maintain a clinical response or remission [5].
Should first-line conventional therapies, such as 5-aminosalicylates and immunosuppressants, fail to produce an adequate response, patients can attempt advanced therapies, including biologic (e.g., vedolizumab) and small-molecule treatments (e.g., tofacitinib). Given the number of advanced therapies available for use in UC, as well as the scarcity of direct head-to-head treatment comparisons, it is challenging to identify the most efficacious and cost-effective sequence [4]. Clinical guidelines, including those from the European Crohn’s and Colitis Organisation and American Gastroenterological Association, contain conflicting recommendations regarding the choice of first-line advanced therapy [6, 7]. In Italy, clinical guidelines produced by the Italian Group for the Study of Inflammatory Bowel Disease also do not specify a preferred first-line advanced therapy [8].
Vedolizumab, an anti-lymphocyte trafficking agent, and treatments in the anti-TNFα therapeutic class, such as adalimumab, are well-established options endorsed as either first-line or second-line advanced therapies in published clinical guidelines [6, 7]. The first treatment in the anti-TNFα class was infliximab, which was approved for use in Italy in 2000; adalimumab was approved in 2003. Intravenous vedolizumab was approved for use in Italy in 2014 and has been reimbursed since 2016 [9]. A retrospective analysis of administrative healthcare records for Tuscany, Italy, between 2015 and 2019, showed that—at that time—vedolizumab was a common second-line advanced therapy following the more established treatments in the anti-TNFα class [10].
Few head-to-head clinical trials of advanced therapies for UC have been completed to date; however, a comparison between vedolizumab and adalimumab was provided by the VARSITY clinical trial [11]. This was a phase 3b, double-blind, double-dummy trial in which patients were randomized 1:1 to receive either intravenous (IV) vedolizumab at a dose of 300 mg every 8 weeks or subcutaneous (SC) adalimumab at a dose of 40 mg every 2 weeks, after standard induction dosing. Vedolizumab was found to be superior to adalimumab with respect to achievement of clinical remission and endoscopic improvement but not corticosteroid-free clinical remission. The exposure-adjusted incidence rates of infection were lower in the vedolizumab group than in the adalimumab group. Both adalimumab and vedolizumab were less effective in patients with prior exposure to an anti-TNFα treatment, in terms of clinical response or remission rates at week 52 [11].
Given that either vedolizumab or anti-TNFαs may be used as first-line advanced therapies, decision-making may be informed by estimates of the cost-effectiveness of alternative treatment sequences. The aim of this study was to assess the cost-effectiveness of vedolizumab as a first-line advanced therapy versus first-line adalimumab, making use of the head-to-head comparison provided by the VARSITY clinical trial. A de novo cost-effectiveness model was developed to capture the long-term health and cost impacts of treatment sequences within the National Health Service in Italy. Competing sequences reflect clinical practice in which all patients are eligible for both treatments but where a decision is made regarding whether they will be used as first-line or second-line advanced therapies. The uncertainties of key inputs and assumptions were also investigated through a series of scenario analyses.
2 Materials and Methods
2.1 Decision Problem
The treatment pathway for patients with moderately to severely active UC in Italy consists of first-line use of conventional therapies, such as 5-aminosalicylates or corticosteroids [6]. Where a response is inadequate, where response is lost, or where conventional therapies are not well tolerated by patients, then advanced therapies are used, which may include an anti-TNFα treatment (infliximab, adalimumab, and golimumab), vedolizumab (a α4β7 integrin antagonist), ustekinumab (an IL-12/23 inhibitor), or tofacitinib [a Janus kinase (JAK) inhibitor] [8]. If a response to an advanced therapy is inadequate, if response is lost, or if patients discontinue treatment because of adverse events, then patients are expected to cycle through all available advanced therapies until a response is achieved.
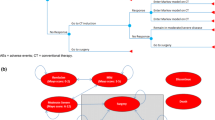
Where clinical guidelines provide statements regarding the choice of first-line advanced therapy, those guidelines suggest either infliximab (an anti-TNFα) or vedolizumab [7, 8]. This study compared sequences involving vedolizumab and adalimumab because of the availability of the direct comparison provided by the VARSITY clinical trial. The model was used to assess the impact of different levels of drug pricing and efficacy, such as may be observed for other therapies in the anti-TNFα class (e.g., infliximab). A comparison of the treatment sequences, in which vedolizumab and adalimumab appear as either first-line or second-line advanced therapies, is shown in Fig. 1. All advanced therapies beyond second-line treatments are represented as a single composite because of uncertainty regarding the number and ordering of subsequent treatments. Finally, patients may abandon advanced therapies and return to conventional therapies or surgery.
2.2 Model Structure
The cost-effectiveness model used a cohort state-transition structure (Markov model) throughout, with the treatment induction phase modeled as a series of tunnel states. The model used a cycle length of 2 weeks, chosen to capture the variable duration of treatment induction phases. The structure was closely related to the approach described in a recent submission to the National Institute for Health and Care Excellence (NICE) in the United Kingdom [12], and it was implemented in Microsoft Excel for Microsoft 365 (2023).
The health state structure of the cost-effectiveness model is shown in Fig. 2.
Health state structure of the cost-effectiveness model. Pale purple indicates Markov model health states where patients may remain for more than one cycle. Dark purple indicates tunnel states where patients can remain for one model cycle only. Grey background indicates on-treatment health states where patients receive advanced therapy. UC ulcerative colitis
The starting cohort consists of patients in the active UC health state, having failed to achieve an adequate response on conventional therapies. These patients immediately begin induction on a form of first-line advanced therapy. Patients may discontinue treatment during any cycle of the induction phase and do discontinue at the end of induction if a clinical response or remission is not achieved. Transitions following discontinuation depend on the availability of subsequent lines of advanced therapy. Where these are available, patients immediately begin the induction phase of the subsequent treatment or, if unavailable, move to the active UC health state.
After completion of the induction phase, a proportion of patients enter the clinical response (without remission) or clinical remission health states. The definitions of clinical remission and clinical response are based on the Mayo and partial Mayo scores, respectively, and are aligned with criteria used in the VARSITY clinical trial [11]. Patients remain in these states during the treatment maintenance phase unless discontinuation occurs because of a loss of response or an adverse event. In the base case, patients cannot transition between response and remission states; a scenario analysis relaxes this assumption (Table 1, scenario 3).
Patients entering the active UC health state are subsequently at risk of death or surgical intervention. The latter is the final treatment option for patients whose UC cannot be managed with pharmacotherapy; there is no option for spontaneous response or remission. The quality of life and healthcare effects of surgery were assumed to last for 6 months, in line with previous economic models [12], and they were modeled via tunnel states spanning 13 model cycles or approximately 6 months. After undergoing an initial surgical intervention, patients entered the post-surgery remission state or experienced post-surgery complications. However, patients with post-surgery remission were at risk of experiencing complications in subsequent cycles. Post-surgery complications may lead to a corrective surgical procedure, following which patients enter the post-second surgery remission state.
2.3 Model Inputs and Data Sources
2.3.1 Patient Population
The target patient population are those with moderately to severely active UC who had an inadequate response to conventional therapies. The baseline characteristics of these patients was assumed to be represented by the cohort enrolled in the VARSITY clinical trial [11]. The mean age of these patients was 40.7 years, 42% were female, and their mean body weight was 72.7 kg. The proportion of enrolled patients who were naïve to treatment with an anti-TNFα therapy was 79%.
2.3.2 Perspective, Time Horizon, and Discounting
The cost-effectiveness model was developed to capture the effects of alternative treatment strategies from the perspective of the National Health Service in Italy. The time horizon adopted for this decision problem was the lifetime of the typical patient, which was limited to an age of 100 years. Shorter time horizons of 5 and 10 years are tested in scenario analysis as per relevant guidelines for Italy [18]. Also aligned with Italian guidelines, costs and health benefits were discounted at a rate of 3% per annum [18].
2.3.3 Treatment Efficacy
Inputs relating to the safety and efficacy of adalimumab and vedolizumab were taken from the VARSITY clinical trial [11]. This was a multicenter, phase 3, double-blind, double-dummy, randomized, active-controlled trial. It is one of the few trials that provides a head-to-head comparison between two advanced therapies, and it is the only study directly comparing vedolizumab and an anti-TNFα treatment. The trial had a treat-through design, and patients were assessed for clinical response and remission at weeks 14 and 52.
The proportions of patients achieving a clinical response at week 14, with or without clinical remission, are presented in Table 2. Because the standard durations of the induction phase for both treatments are < 14 weeks (8 and 4 weeks for vedolizumab and adalimumab, respectively), these data were adjusted to reflect estimates of the proportions immediately post-induction. The adjustment method used the ratio between the result at week 14 and the result at the end of the induction period based on partial Mayo scores [19]. The trial results, based on partial Mayo scores and the data in Table 2 (after adjustment), are provided in the Supplementary Appendix (Table S1).
The probability that a patient, having progressed to the maintenance phase, would subsequently discontinue treatment during each cycle was estimated based on data on durable response and remission from the VARSITY trial [11, 20] (Table 2). Durable clinical remission was defined as clinical remission at week 52 among subjects who were in clinical remission at week 14; durable clinical response was defined as a response among subjects with a response at week 6.
The transition probabilities from remission and from response (without remission) were derived by assuming that there would be no movement between response states during the maintenance phase. Therefore, the probability of transitioning from the remission to the active UC (\({P}_{\text{R},\text{UC}}\)) state during the maintenance phase was the complement of the probability of durable remission. The probability of transitioning from response (without remission) to active UC (\({P}_{\text{r},\text{UC}}\)) is calculated based on the probability of loss of the overall response (\({P}_{\text{Or},\text{UC}}\)), loss of clinical remission (\({P}_{\text{R},\text{UC}}\)), and the proportions of patients in the remission and response (without remission) states at the end of induction (\({\text{Ind}}_{\text{R}}\) and \({\text{Ind}}_{\text{r}}\) respectively). These probabilities are calculated as shown in Eq. (1). A scenario analysis was performed that allowed for transitions between response states during the maintenance phase.
2.3.4 Treatment Safety
The VARSITY study reported the incidence of adverse events occurring before the final safety follow-up at week 68 [11]. The only adverse events captured in these analyses were those due to serious infections and infestations because of their potential to have substantial cost and health implications; this is consistent with the approach of previous economic evaluations [12, 21]. The probability of each type of infection during each 2-week model cycle is given in Table 3. Because the incidence of adverse events is not reported for any other follow-up period, the risk of adverse events is assumed to be constant, and the probabilities in Table 3 are applied during every model cycle.
2.3.5 Third-Line Treatment Inputs
Should a second-line advanced therapy fail to produce an adequate response, or if a patient’s response is lost, it is expected that additional lines of advanced therapy would be attempted. There is uncertainty regarding the number and sequencing of advanced therapies that may be attempted as third-line treatments and beyond. In this cost-effectiveness model, the aim was to avoid making explicit assumptions regarding the sequencing of later lines of advanced therapy. Therefore, all third-line treatments and beyond were represented as part of a composite advanced therapy phase. The effects on the model results of omitting this composite treatment phase, and of modifying the relevant inputs, were assessed in the scenario analyses.
In the base case, the composite treatment is constructed based on the safety and efficacy of a 50:50 composition of ustekinumab and tofacitinib, two available options from different therapeutic classes. The model base case already evaluates the anti-TNFα adalimumab as either a first-line or second-line treatment, but also varies the treatment-related inputs in scenarios that aim to assess the impact of using other therapies in the anti-TNFα class. Therefore, use of an anti-TNFα therapy in the composite was not considered. The required clinical inputs for ustekinumab and tofacitinib were odds ratios, versus 8-weekly doses of 300 mg of IV vedolizumab, sourced from a published network meta-analysis [20], and the cost inputs are presented in Table 4.
The post-induction response and remission proportions for the composite treatment were estimated as the mean of ustekinumab and tofacitinib. The transit time for a patient through a two-treatment sequence will be greater than the transit time through any individual component, so the rate of discontinuation from the composite will be lower than the rate for any individual component. In the base case, the transitions in the maintenance phase (outside of the response states) used the minimum values for either ustekinumab or tofacitinib. The rate of discontinuation from the third-line composite is highly uncertain and was investigated in a scenario analysis (Table 1, scenario 2).
2.3.6 Surgery-Related Inputs
The final treatment option for patients whose UC cannot be managed with pharmacotherapy is to undergo surgery. Due to lack of Italian-specific guidance regarding surgical interventions, the health states related to surgery were based on previously developed models, in particular, the recent NICE submission for ozanimod [12]. The relevance of the surgical health states to the Italian patient pathway was confirmed during conversations with an Italian clinical expert who was external to the investigators and to Takeda [22].
The annual probability of surgery was estimated to be 1% based on Dai et al. [23]. The probability that surgery leads to complications was assumed to be 33.5% based on a 2023 UK clinical audit of inpatient care for adults with UC [24]. Following a successful colectomy, without complication, patients are at risk of developing complications at a later date; this probability was assumed to be 3.35% based on Segal et al. [25]. Patients whose surgery has led to complications are at risk of requiring a corrective procedure, with an annual probability of 5%; this was based on the opinion of the external Italian clinical expert [22].
2.3.7 Mortality
There is no evidence that patients with UC have a lower life expectancy than the general population [26]. Therefore, the risk of death in all health states except surgery states was derived from life tables for the general population of Italy [27]. Patients with UC are at an increased risk of death following a surgical intervention, and a standardized mortality ratio of 1.3 was applied to all surgery tunnel states [28]. These assumptions are consistent with previous economic evaluations reviewed during the development of this cost-effectiveness model.
2.3.8 Cost Inputs
All costs are reported in 2022 euros. The unit costs for the acquisition of advanced therapies included in this evaluation were sourced from the Gazzetta Ufficiale. These prices, presented in Table 4 reflect net ex-manufacturer prices with mandatory 10% discounts applied. For adalimumab, costs for both the originator and biosimilar products are provided, and the market share used for the originator product is 11.6% [29]. The IV administration costs for those treatments using this modality were taken from Ravasio et al. [30]. Treatments administered as SC injections were assumed to require a one-off training visit, with a cost equal to that of an IV administration visit. Per-cycle costs were calculated using the doses and regimens for the advanced therapies, as presented in Table 4.
Patients receiving an advanced therapy may continue to receive concomitant corticosteroids. The mean doses of oral corticosteroids in use, at baseline and week 52, were sourced from the VARSITY study for vedolizumab and adalimumab [11]. Corticosteroids were costed as prednisone [34], and the volume of use for other advanced therapies was calculated using the data from the adalimumab arm of the VARSITY study. Following the failure of all advanced therapy options, patients are assumed to remain in the active UC state and to receive a mix of conventional therapies. The type and usage of conventional therapies were obtained from an Italian real-world evidence study [10], and unit costs were obtained from the Gazzetta Ufficiale [35].
The healthcare resource use associated with each health state was informed through discussions with the external Italian clinical expert [22]. The unit costs for each resource item were obtained from the Ministero della Salute [36], and the per-cycle health state costs are given in Table 5. The unit cost for the first surgery was €13,793, based on the cost of major interventions in the large and small intestines; the unit cost for the second surgery was €11,203, based on the cost of a rectal resection [36]. Finally, unit costs were sourced for the management of serious infection and infestation events [36]. Complete tables of these cost inputs are provided in the Supplementary Appendix (Table S2).
2.3.9 Health State Utilities
To align with many recent evaluations [12, 24, 37], the model used health state utilities from Woehl et al. [14] and Arseneau et al. [16] (Table 5) in the base case. Woehl gathered information on 329 UK-based patients with Crohn’s disease to estimate health state utility values. Utility values from Arseneau were generated using the time-trade off (TTO) method on responses from 48 American UC patients to a questionnaire about their health-related quality of life. On model entry, all patients are in the active UC state, which they remain in during treatment induction. The health state utilities were not adjusted as patients aged. The occurrence of serious infection or infestation events incurs a disutility of 0.156 [38]. Several alternative sets of health state utilities from other published sources were used in the scenario analyses.
2.4 Base-Case Analysis
The base-case analysis compared the relative positioning of adalimumab or vedolizumab as either first-line or second-line advanced therapies. When first-line advanced therapy is initiated, patients are thus naïve to any anti-TNFα treatments. Therefore, the treatment efficacy inputs used the results available for this subgroup from the VARSITY clinical trial. Following exposure to adalimumab, such as for second-line vedolizumab treatment, the treatment efficacy inputs were sourced for the anti-TNFα-exposed subgroup. For treatments given after the failure of vedolizumab therapy, no changes in treatment safety or efficacy were applied. This is line with evidence that the effectiveness of anti-TNFα therapy is not substantially affected by prior vedolizumab exposure [39, 40]. The inputs related to treatment safety, disaggregated by anti-TNFα exposure subgroups, were not available. A full list of assumptions that drive the base-case analysis can be found in the Supplementary Appendix (Table S3).
2.5 Model Outcomes
The economic model captures the cost outcomes according to subcategories including advanced therapy drug, conventional therapy drug, surgery, healthcare, and adverse event management costs. Health outcomes, both in terms of life years and QALYs, were captured according to the health state in which they were accrued. Treatment sequences were compared in terms of incremental costs, incremental QALYs, and incremental cost-effectiveness ratios (ICERs). Results are presented for both the model evaluated using inputs at estimated means (deterministic model), as well as averaged over the results of a probabilistic sensitivity analysis (PSA) obtained using 10,000 resampled sets of inputs (probabilistic model). The modeled ICERs were compared with the mean ICER for approved medicines in Italy of €33,004/QALY; this threshold was derived from submissions to the Italian Medicines Agency between October 2016 and January 2021 [41].
2.6 Scenario and Sensitivity Analyses
Scenario analyses were used to investigate the potential impact of key model limitations or areas of high uncertainty on the model results. Twelve separate scenarios were evaluated; they are numbered and described in Table 1. Scenarios 1 and 2 assessed alternative assumptions relating to later lines of advanced therapy after second-line treatment, which are considered to be areas of high uncertainty. Scenario 3 assessed the effect of allowing patients to move between the response and remission states during maintenance, with the lack of data to inform these transitions in the base case being an important limitation. Scenarios 4–6 used alternative sources for health state utilities. Scenarios 7 and 8 evaluated the impact of adalimumab being more effective and less costly, respectively. Scenarios 9 and 10 assessed the effect of varying the rate at which patients receive surgical intervention. Finally, scenarios 11 and 12 investigated alternative modelled time horizons.
In addition to the scenario analyses, one-way sensitivity analyses were performed to obtain model outputs when each input was varied between the upper and lower bounds. These upper and lower bounds either reflected the 95% confidence interval from the source data, where available, or the range obtained from varying the input mean by an arbitrary 25%. The PSA results were used to calculate—on the basis of parametric uncertainty—the probability of a treatment sequence being cost-effective versus a comparator sequence for a range of cost-effectiveness thresholds.
3 Results
3.1 Base-Case Results
The absolute and incremental modeled outcomes for treatment sequences initiated with either vedolizumab or adalimumab are presented in Table 6. Vedolizumab as a first-line advanced therapy leads to higher drug costs relative to first-line adalimumab, as it is initiated earlier in the treatment sequence, and is also more efficacious. Vedolizumab as a first-line advanced therapy leads to QALY gains because of its higher efficacy relative to adalimumab, as well as relative to second-line vedolizumab in patients with previous anti-TNFα exposure. For vedolizumab as a first-line advanced therapy versus first-line adalimumab, based on the PSA results, the mean incremental QALYs were 0.36 and the mean incremental costs were €5517, leading to an ICER of €15,397/QALY. This probabilistic ICER falls slightly below the result obtained using inputs set to their mean estimated values of €16,146/QALY. Both of these values are lower than the mean ICER for approved medicines in Italy of €33,004/QALY [41].
Further results are presented in the Supplementary Appendix, including disaggregated base-case results (Tables S4–7), results from the one-way sensitivity analysis (OWSA; Fig. S1; Table S8), and a diagram representing the ICER ranges at different price discounts for both vedolizumab and adalimumab (Fig. S2).
3.2 Sensitivity Analyses
The PSA results, obtained using 10,000 sets of resampled model input values, are presented in the cost-effectiveness plane in Fig. 3. The lilac points show the incremental costs and QALYs obtained in a single iteration. The figure also shows the cost-effectiveness threshold of €33,004/QALY, the deterministic model result, and the 95% multivariate normal confidence ellipses. Overall, there is a slightly greater uncertainty associated with the incremental QALYs, and relatively few of the PSA results lie above the cost-effectiveness threshold.
The probability that vedolizumab as a first-line advanced therapy is cost-effective versus first-line adalimumab over a range of cost-effectiveness thresholds is shown in Fig. 4. At a threshold of €33,004/QALY, vedolizumab as a first-line advanced therapy is cost-effective with a probability of 94%.
3.3 Scenario Analyses
The incremental cost and QALY results obtained for the scenario analyses given in Table 1 are presented on the cost-effectiveness plane in Fig. 3. The number labels assigned to the scenario results correspond to the first column of Table 1. More detailed model results for these scenarios can be found in the Supplementary Appendix (Table S9). Overall, few of the scenarios assessed led to a substantial change in the ICER for vedolizumab as a first-line advanced therapy versus first-line adalimumab; only scenario 9 resulted in an ICER that was above €33,004/QALY.
Scenarios 1 and 2 considered alternative assumptions regarding the advanced therapies used after second-line treatments. In scenario 1, further lines of advanced therapy were removed, so patients discontinuing second-line treatments move to the active UC state and receive conventional therapy. Despite this change leading to an increase in the rate at which patients become eligible for surgery, it only had a small effect on the incremental outputs. The impact of scenario 2, increasing the time spent on advanced therapy after second-line treatments, was to reduce the incremental QALYs and, therefore, increase the ICER.
The assumption that there is no movement between response (without remission) and remission states during the maintenance phase was relaxed in scenario 3. Allowing these transitions had the effect of prolonging the time spent undergoing treatment, thereby increasing the incremental costs and QALYs. The overall impact was to slightly reduce the ICER. Scenarios 4 to 6 used alternative sources for health state utilities. The ICERs for these scenarios ranged from €14,628 to €23,017/QALY.
Scenarios 7 and 8 assessed the effect of adalimumab being more effective following induction and of having a lower acquisition cost, respectively. Both of these scenarios had very little impact on either the incremental costs or incremental QALYs. This is to be expected because adalimumab is included in both competing treatment sequences as either a first-line or second-line therapy. The minor differences that were observed were caused by a combination of discounting and changes in the size of the treated cohort as a result of mortality.
The next two scenarios varied the rate at which a surgical intervention is performed for patients whose advanced therapies have failed to achieve a response. Scenario 9 increased the annual probability to 5%, leading to an increase in incremental costs and a decrease in incremental QALYs. The ICER increased to €35,709/QALY. Scenario 10 decreased the annual probability according to Misra et al. [17]—a published source cited in many previous economic evaluations. This led to small increases in incremental QALYs and decreases in incremental costs, thereby lowering the ICER.
The final two scenarios investigated the impact of shortening the time horizon from the base case lifetime perspective. This was to adhere to Italian guidelines that require shorter time horizons of 5 and 10 years to be assessed [18]. Both scenarios had a minimal impact on the ICER, suggesting that model results are moderately insensitive to the adopted time horizon.
4 Discussion
This study presents the results of a de novo cost-effectiveness model developed to compare treatment sequences involving adalimumab and vedolizumab for the treatment of moderately to severely active ulcerative colitis within the Italian National Health Service. The analysis compared alternative positioning of vedolizumab and adalimumab as either first-line or second-line advanced therapies. Vedolizumab as a first-line advanced therapy was cost-effective versus first-line adalimumab: it was associated with higher costs and health benefits that resulted in an estimated ICER of €16,146/QALY. A probabilistic sensitivity analysis indicated a probability of an ICER below €33,004/QALY to be > 90% [41]. This result was found to be robust to alternative inputs and assumptions when key uncertainties and model limitations were investigated.
The cost-effectiveness of vedolizumab as a first-line advanced therapy versus first-line adalimumab is due, in part, to the reduced efficacy of second-line vedolizumab in patients previously exposed to an anti-TNFα treatment. The efficacy of second-line adalimumab being unchanged is supported by evidence that the effectiveness of anti-TNFα therapy is not substantially affected by prior vedolizumab therapy [39, 40]. As far as the authors are aware, the mechanisms giving rise to this asymmetry are unknown. However, there is evidence that anti-TNFα exposure may lead to changes relevant to the pharmacokinetics of vedolizumab [40, 42, 43]. Such findings serve to highlight the challenges associated with determining the most efficacious and cost-effective use of the available treatments for UC.
Notably, the results of the scenario analyses found the ICER comparing vedolizumab as a first-line advanced therapy with first-line adalimumab not to be sensitive to the price or efficacy of adalimumab. Insensitivity to cost should not be surprising where both sequences include adalimumab; only through discounting effects or mortality can the costs incurred differ. The absolute treatment effects are not key drivers of incremental differences in this comparison; rather, it is the relative change, if any, in efficacy in post-exposure subgroups. This suggests that similar results would be obtained for comparisons involving a different anti-TNFα therapy, where an assumption that there would be no loss of efficacy following vedolizumab exposure may apply. The inputs to which the results were sensitive include the annual risk of surgery, largely because of the difference in the quality of life of patients after successful surgery compared with active UC.
There are conflicting results available from previous economic evaluations comparing treatment sequences involving vedolizumab and anti-TNFα therapies; however, these are often associated with limitations in the availability of efficacy data after first-line treatments. Shultz et al. [44] compared second-line sequences beginning with either vedolizumab or adalimumab in the USA, although neither of these treatments were subsequently available as second-line therapies. They found vedolizumab as a first-line advanced therapy to be dominant versus first-line adalimumab. Scott et al. [45] compared treatment sequences involving adalimumab, infliximab, and vedolizumab in the USA. Their study found vedolizumab as a first-line advanced therapy to be cost-effective versus first-line adalimumab but not versus first-line infliximab. However, the probability of a maintenance response for vedolizumab did not differ according to a patient’s anti-TNFα exposure.
Finally, Bouhnik et al. [46] compared sequences involving infliximab and vedolizumab, finding first-line infliximab sequences to be dominant compared with vedolizumab as a first-line advanced therapy in patients with UC. The efficacy inputs for infliximab in subsequent lines used data for patients exposed to anti-TNFα, which is not appropriate for modeling its use after vedolizumab. Furthermore, the efficacy of infliximab in subsequent lines was assumed to be equal to the least efficacious drug, an assumption that maximizes the relative benefits of first-line infliximab sequences.
This study, comparing vedolizumab and adalimumab treatment sequences, benefits from the landmark head-to-head VARSITY clinical trial as a source of key safety and efficacy inputs. This avoids the potential limitations associated with the indirect treatment comparisons often used to compare treatments for UC. Unlike many previous economic evaluations, such as that of Fenu et al. [37], this study used tunnel states rather than a decision tree to represent each treatment’s induction phase. This approach, used in previous health technology assessment submissions [12], allows for the straightforward modeling of treatment sequences where patients can move into, and out of, induction in any cycle. This study highlights the importance of comparing treatments in terms of their position in a treatment pathway, as would be the case in clinical practice.
Despite the advantages associated with basing this analysis on the VARSITY clinical trial, it only allows for a narrow scope of possible comparisons focusing on vedolizumab and adalimumab. Although there may be some scope to generalize these results to comparisons involving other treatments in the anti-TNFα class, it was not possible to present comparisons involving other advanced therapies, such as ustekinumab, ozanimod, or JAK inhibitors (such as tofacitinib and upadacitinib), without resorting to indirect treatment comparisons. Another substantial limitation of the study is that, where published inputs and information on patient pathway in Italy were unavailable, some inputs and assumptions were elicited or confirmed by the external Italian clinical expert. While published data are often preferred over expert opinion, this method of eliciting information is common in economic modelling, and the expert who was engaged was external to the investigators and manufacturer. In addition, the option for escalated dosing available for both vedolizumab and adalimumab was not included. This is not uncommon in economic evaluations because of the lack of evidence informing efficacy following escalation. Some studies focus only on accounting for cost implications [47]; however, it was decided that such an approach would not add value in this application. Further limitations include inconsistencies in the data available on durable response and remission (using different baselines), the fact that the model does not account for spontaneous remission in patients receiving conventional therapies, and that health state utility values based on the UK and the USA were used in lieu of a set of utility values generated with an Italian value set.
5 Conclusions
This study compared the alternative positioning of vedolizumab and adalimumab in treatment sequences in terms of their cost-effectiveness, finding vedolizumab as a first-line advanced therapy to be associated with increased QALYs and higher costs. This resulted in an ICER of €16,146/QALY, with a probability of > 90% that this ICER would be below a threshold of €33,004/QALY. This study highlights that the cost-effectiveness of alternative treatment sequences is sensitive to differences in treatment efficacy according to the location of a therapy within a sequence.
References
Ungaro R, et al. Ulcerative colitis. Lancet. 2017;389(10080):1756–70.
Yarlas A, et al. Burden of ulcerative colitis on functioning and well-being: a systematic literature review of the SF-36(R) health survey. J Crohns Colitis. 2018;12(5):600–9.
Cohen RD, et al. Systematic review: the costs of ulcerative colitis in Western countries. Aliment Pharmacol Ther. 2010;31(7):693–707.
Bressler B. Is there an optimal sequence of biologic therapies for inflammatory bowel disease? Ther Adv Gastroenterol. 2023;16:17562848231159452.
Singh H, et al. Systematic literature review of real-world evidence on dose escalation and treatment switching in ulcerative colitis. Clin Outcomes Res. 2023;15:125–38.
Raine T, et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohns Colitis. 2022;16(1):2–17.
Feuerstein JD, et al. AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020;158(5):1450–61.
Macaluso FS, et al. Use of biologics and small molecule drugs for the management of moderate to severe ulcerative colitis: IG-IBD clinical guidelines based on the GRADE methodology. Dig Liver Dis. 2022;54(4):440–51.
Agenzia Italiana del Farmaco. Rettifica della determina n. 459/2016 del 31 marzo 2016, relativa alla riclassificazione del medicinale per uso umano «Entyvio», ai sensi dell'articolo 8, comma 10, della legge 24 dicembre 1993, n. 537. Gazzetta Ufficiale, 2016. Available from: https://www.gazzettaufficiale.it/eli/gu/2016/07/22/170/sg/pdf. Accessed 31 Aug 2023.
Tuccori M, et al. Advanced treatment of ulcerative colitis using an Italian healthcare administrative database: drug utilization patterns, healthcare resource use and costs: the MICHELANGELO study (EUPAS40896; final report). European Network of Centres for Pharmacoepidemiology and Pharmacovigilance, 2021. Available from: https://www.encepp.eu/encepp/openAttachment/studyResult/42875. Accessed 1 Sept 2023.
Sands BE, et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med. 2019;381(13):1215–26.
National Institute for Health and Care Excellence. Ozanimod for treating moderately to severely active ulcerative colitis [TA828]. 2022. https://www.nice.org.uk/guidance/ta828. Accessed 8 Sept 2023.
Wilson MR, et al. Cost-effectiveness of vedolizumab compared with conventional therapy for ulcerative colitis patients in the UK. Clin Outcomes Res. 2017;9:641–52.
Woehl A, Hawthorne A, McEwan P. The relation between disease activity, quality of life and health utility in patients with ulcerative colitis. Gut. 2008;A153(Suppl. 1).
Swinburn P, et al. PTU-127. The impact of surgery on health related quality of life in ulcerative colitis. Gut. 2012;A237(Suppl 2).
Arseneau KO, et al. Do patient preferences influence decisions on treatment for patients with steroid-refractory ulcerative colitis? Clin Gastroenterol Hepatol. 2006;4(9):1135–42.
Misra R, et al. Colectomy rates for ulcerative colitis differ between ethnic groups: results from a 15-year nationwide cohort study. Can J Gastroenterol Hepatol. 2016;2016:8723949.
Agenzia Italiana del Farmaco. Linee guida per la compilazione del dossier a supporto della domanda di rimborsabilità e prezzo di un medicinale. 2021. https://www.aifa.gov.it/documents/20142/1283800/Linee_guida_dossier_domanda_rimborsabilita.pdf. Accessed 1 Sept 2023.
Lewis JD, et al. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14(12):1660–6.
Jairath V, et al. Integrating efficacy and safety of vedolizumab compared with other advanced therapies to assess net clinical benefit of ulcerative colitis treatments: a network meta-analysis. Expert Rev Gastroenterol Hepatol. 2021;15(6):711–22.
National Institute for Health and Care Excellence. Ustekinumab for treating moderately to severely active ulcerative colitis [TA633]. 2020. https://www.nice.org.uk/guidance/ta633. Accessed 8 Sept 2023.
Takeda. Internal communication with one external Italian clinical expert. 2023: Data on file.
Dai N, et al. Colectomy rates in ulcerative colitis: a systematic review and meta-analysis. Dig Liver Dis. 2023;55(1):13–20.
National Institute for Health and Care Excellence, Upadacitinib for treating moderately to severely active ulcerative colitis [TA856]. 2023. https://www.nice.org.uk/guidance/ta856. Accessed 8 Sept 2023.
Segal JP, et al. Incidence and long-term implications of prepouch ileitis: an observational study. Dis Colon Rectum. 2018;61(4):472–5.
Selinger CP, Leong RW. Mortality from inflammatory bowel diseases. Inflamm Bowel Dis. 2012;18(8):1566–72.
Italian National Institute of Statistics. Resident population, by age, sex and marital status on 1st January 2022. 2022. Available from: http://dati.istat.it/Index.aspx?QueryId=42869&lang=en. Accessed 1 Sept 2023.
Jess T, et al. Overall and cause-specific mortality in ulcerative colitis: meta-analysis of population-based inception cohort studies. Am J Gastroenterol. 2007;102(3):609–17.
Agenzia Italiana del Farmaco. Monitoraggio consumi e spesa biosimilari. April 2023. Biosimilari: Analisi della variabilità regionale. 2023. Available from: https://www.aifa.gov.it/en/monitoraggio-consumi-e-spesa-biosimilari. Accessed 8 Sept 2023.
Ravasio R, Viti R, Roscini AS. Cost per responder for vedolizumab and ustekinumab in Crohn’s disease patients after failure of TNF-α inhibitors in Italy. AboutOpen. 2023;10(1):13–21.
Gazzetta Ufficiale. Riclassificazione del medicinale per uso umano «Entyvio» ai sensi dell'art. 2023 [20 April 2023]. Available from: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2016-04-14&atto.codiceRedazionale=16A02888.
Gazzetta Ufficiale. Regime di rimborsabilita' e prezzo, a seguito di nuove indicazioni terapeutiche, del medicinale per uso umano «Xeljanz». [2023 20 April 2023]. Available from: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2022-04-05&atto.codiceRedazionale=22A02107&elenco30giorni=false.
Gazzetta Ufficiale. Regime di rimborsabilita' e prezzo a seguito di nuove indicazioni terapeutiche e rinegoziazione del medicinale per uso umano «Stelara». 2023 [20 April 2023]. Available from: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2021-12-03&atto.codiceRedazionale=21A07073&elenco30giorni=true.
Gazzetta Ufficiale. Gazzetta Ufficiale della Repubblica Italiana: 13 17-1-2020. 2023 [September 8, 2023]; Available from: https://www.gazzettaufficiale.it/eli/gu/2020/01/17/13/sg/pdf.
Gazzetta Ufficiale. Gazzetta Ufficiale della Repubblica Italiana. 2023. Available from: https://www.gazzettaufficiale.it/. Accessed 8 Sept 2023.
Ministero della Salute. Nomenclatore dell'assistenza specialistica ambulatoriale. 2017. Available from: https://www.salute.gov.it/portale/temi/p2_6.jsp?id=1767&area=programmazioneSanitariaLea&menu=lea. Accessed 1 Sept 2023.
Fenu E, et al. Cost effectiveness of subcutaneous vedolizumab for maintenance treatment of ulcerative colitis in Canada. Pharmacoeconomics. 2022;6(4):519–37.
Stevenson M, et al. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying antirheumatic drugs and after the failure of conventional disease-modifying antirheumatic drugs only: systematic review and economic evaluation. Health Technol Assess. 2016;20(35):1–610.
Bressler B, et al. Vedolizumab and anti-tumour necrosis factor alpha real-world outcomes in biologic-naive inflammatory bowel disease patients: results from the EVOLVE study. J Crohns Colitis. 2021;15(10):1694–706.
Pudipeddi A, et al. Vedolizumab has longer persistence than infliximab as a first-line biological agent but not as a second-line biological agent in moderate-to-severe ulcerative colitis: real-world registry data from the Persistence Australian National IBD Cohort (PANIC) study. Ther Adv Gastroenterol. 2022;15:17562848221080792.
Russo P, et al. Role of economic evaluations on pricing of medicines reimbursed by the Italian National Health Service. Pharmacoeconomics. 2023;41(1):107–17.
Biancheri P, et al. Effect of tumor necrosis factor-alpha blockade on mucosal addressin cell-adhesion molecule-1 in Crohn’s disease. Inflamm Bowel Dis. 2013;19(2):259–64.
Liefferinckx C, et al. Early vedolizumab trough levels at induction in inflammatory bowel disease patients with treatment failure during maintenance. Eur J Gastroenterol Hepatol. 2019;31(4):478–85.
Schultz BG, et al. Cost-effectiveness of intravenous vedolizumab vs subcutaneous adalimumab for moderately to severely active ulcerative colitis. J Manag Care Spec Pharm. 2021;27(11):1592–600.
Scott FI, et al. Identification of the most cost-effective position of vedolizumab among the available biologic drugs for the treatment of ulcerative colitis. J Crohns Colitis. 2020;14(5):575–87.
Bouhnik Y, et al. Cost-effectiveness analysis of subcutaneous infliximab for inflammatory bowel diseases in sequential biologic treatment. Inflamm Bowel Dis. 2023;29(6):898–913.
Sardesai A, et al. Cost-effectiveness of tofacitinib compared with infliximab, adalimumab, golimumab, vedolizumab and ustekinumab for the treatment of moderate to severe ulcerative colitis in Germany. J Med Econ. 2021;24(1):279–90.
Acknowledgements
The authors would like to thank Lucy Moffit (Putnam, UK) for her editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Takeda.
Conflict of interest
DHM, CH, and OO are employees of Putnam, UK, which received funding from Takeda to conduct this research. JS, RV, LS, ASR, and GDM are employees of Takeda and hold stock or stock options in Takeda.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All data used to support the findings of the study are publicly available.
Code availability
The code is not publicly available.
Author contributions
All authors were involved in the study design, analysis, and interpretation of the data; drafting; and revising the paper and approving the final version of the manuscript. All authors agree to be accountable for all aspects of the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Salcedo, J., Hill-McManus, D., Hardern, C. et al. Cost-Effectiveness of Vedolizumab as a First-Line Advanced Therapy Versus Adalimumab Treatment Sequences for Ulcerative Colitis in Italy. PharmacoEconomics Open (2024). https://doi.org/10.1007/s41669-024-00497-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s41669-024-00497-4