Abstract
Background and Objectives
Ulcerative colitis is highly prevalent in Canada and cost-effective ulcerative colitis therapies are warranted. Vedolizumab subcutaneous (SC) formulation was recently approved for ulcerative colitis maintenance therapy. We assessed vedolizumab SC cost effectiveness vs conventional and advanced therapeutics in patients with moderately to severely active ulcerative colitis from a Canadian public healthcare payer perspective.
Methods
A hybrid decision tree/Markov model was developed to evaluate vedolizumab SC costs, quality-adjusted life-years, and cost effectiveness vs conventional therapy, adalimumab SC, infliximab intravenous, golimumab SC, tofacitinib, ustekinumab SC, and vedolizumab intravenous. This model predicts the number of patients achieving clinical response and remission after treatment induction, and sustained benefit during maintenance treatment. To account for statistical uncertainties, the base-case analysis was conducted in a probabilistic manner. Scenario analyses examined the impact of previous treatment with anti-tumor necrosis factor agents, dose escalation, loss of efficacy, and treatment adherence.
Results
In the base-case analysis, conventional therapy was the most cost-effective therapeutic option in the overall population. Vedolizumab SC was cost effective and dominant compared with other advanced therapies (adalimumab, golimumab, infliximab, tofacitinib 5 mg, ustekinumab, and vedolizumab intravenous). The annual vedolizumab SC cost per patient was reduced vs ustekinumab SC, tofacitinib 5 mg, vedolizumab intravenous, and golimumab SC by $47,024, $3251, $2120, and $2004 (Canadian dollars), respectively, and exceeded that of infliximab, adalimumab, and conventional therapy by $582, $3293, and $41,024, respectively. Among the treatments, vedolizumab SC generated the highest quality-adjusted life-years overall (14.21), which translated into the best incremental cost per quality-adjusted life-years gained over conventional therapy in the overall population ($109,374) and in anti-tumor necrosis factor-naïve and anti-tumor necrosis factor-experienced patients ($41,658/$114,287).
Conclusions
Conventional therapy offered the most cost-effective therapeutic option followed by vedolizumab SC. Based on a $50,000/quality-adjusted life-year threshold, vedolizumab was cost effective in anti-tumor necrosis factor-naïve patients but not the overall population also when compared to conventional therapy.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
This health economic evaluation demonstrates that in patients with moderately to severely active ulcerative colitis in Canada, conventional therapy offers the most cost-effective therapeutic option in the overall population followed by vedolizumab subcutaneous (SC), which is cost effective compared with advanced therapies (adalimumab SC, infliximab intravenous, golimumab SC, low-dose tofacitinib, ustekinumab SC, and vedolizumab intravenous), showing either dominance or a very low incremental cost-effectiveness ratio. |
Vedolizumab SC is cost effective compared with conventional therapy based on a threshold of $50,000/quality-adjusted life-years in the anti-tumor necrosis factor-naïve patients. |
1 Introduction
Ulcerative colitis (UC) is an inflammatory bowel disease (IBD) characterized by bloody diarrhea, weight loss, fatigue, urgency or tenesmus, and abdominal pain [1, 2]. Uncontrolled immune activity in UC can lead to chronic inflammation and tissue damage that affects the patient for life [3].
Inflammatory bowel disease is most common in Western societies [4], and Canada has amongst the highest rates of IBD in the world. In 2018, approximately 120,000 Canadian residents had UC, with regional incidence rates that ranged from 8.4 cases per 100,000 people in Alberta to 21.4 cases per 100,000 in Nova Scotia [2].
Ulcerative colitis is associated with a high patient burden, as symptoms, psychological distress, and impact on social functioning reduce health-related quality of life at some point for all patients, and persistently for many [2, 4]. Moreover, as a lifelong condition with a 70–80% risk of relapse within 10 years and nearly 50% of patients requiring at least one disease-related hospitalization, UC is also associated with a high economic burden [3]. As of 2018, the estimated annual overall costs for IBD in Canada were 2.6 billion Canadian dollars, nearly half of which were estimated as direct care costs ($1.28 billion; $4731/patient), and $1.29 billion in annual indirect costs, mainly from productivity losses and out-of-pocket expenses [5].
Treatment for UC is intended to help patients safely achieve complete remission, improve quality of life, and avoid hospitalization and surgery. The 2015 Toronto Ulcerative Colitis Consensus Group statement recommended a stepwise treatment approach beginning with oral and/or rectal administration of 5-aminosalicylic acid or corticosteroids, depending on disease severity, followed by immunomodulators and biologic agents such as tumor necrosis factor (TNF) inhibitors (anti-TNFs). Included among these is vedolizumab, an integrin inhibitor with a gut-selective mechanism of action [6]. Treatment selection must consider patient-specific factors such as disease severity, disease activity (active vs remission), and responsiveness to treatment, in addition to the long-term risks associated with corticosteroids and immunomodulators.
Intravenous (IV) vedolizumab was approved for the treatment of UC in 2014 after clinical trials demonstrated that vedolizumab every 4 or 8 weeks for 52 weeks led to significant improvements vs placebo in clinical and histological endpoints, which were maintained based on evidence from long-term extension and registration studies [7,8,9]. A subcutaneous (SC) formulation of vedolizumab was recently developed to provide a maintenance treatment option for patients who require SC administration in a community or at-home setting. In the phase III VISIBLE 1 trial in patients with moderately to severely active UC, patients who responded to induction treatment with vedolizumab IV were subsequently treated in maintenance phase with vedolizumab SC (n = 106), vedolizumab IV (n = 54), or placebo (n = 56). The trial met its primary endpoint with clinical remission at week 52 achieved by 46.2%, 42.6%, and 14.3% of patients in the vedolizumab SC, vedolizumab IV, and placebo groups, respectively. The SC formulations showed comparable efficacy to the IV formulation across all endpoints. The safety profile of vedolizumab SC was similar to that of the vedolizumab IV maintenance therapy reference arm in the trial [10]. Vedolizumab SC (108 mg every 2 weeks) is currently approved as maintenance therapy for both UC and Crohn’s disease in Europe, Canada, and Australia [11,12,13]. This study aimed to assess the cost effectiveness of vedolizumab SC (following vedolizumab IV induction) vs existing treatments, including biologics and biosimilars, for the management of patients with moderately to severely active UC from a Canadian public healthcare payer perspective.
2 Materials and Methods
2.1 Model Concept and Structure
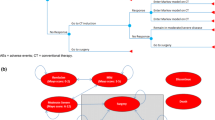
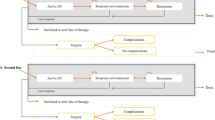
An economic model was developed in Microsoft Excel to conduct a cost-effectiveness analysis of vedolizumab SC (following vedolizumab IV induction) vs existing treatments for patients with moderately to severely active UC from a Canadian public healthcare payer perspective over a lifetime horizon. The economic model used a decision tree structure to model the treatment induction phase, and a Markov cohort approach to model the treatment maintenance phase (Fig. 1). The model structure was adapted from a previously published vedolizumab IV model in UC used for health technology assessment [14,15,16], and was developed to address feedback from the Canadian Agency for Drug and Technologies in Health to the previous vedolizumab IV model, which applied a calibration technique to derive transition probabilities [17]. The current model does not use calibration to estimate transition probabilities and instead uses transition probabilities that were informed directly by network meta-analysis (NMA) data.
a Diagram of the model concept with key model parameters, outcomes, and their interrelationships, b model structure for the induction phase, and c model structure for the maintenance phase. $ and U represent associated costs and health utilities, respectively. AE adverse event, CT conventional therapy, UC ulcerative colitis. aAnti-tumor necrosis factor-naïve patients can switch to a second advanced therapy under the “Treatment Sequences” model option. bApplies only to patients who had advanced therapy at the onset
2.2 Model Inputs and Data Sources
2.2.1 Patient Population and Baseline Characteristics
The model cohort described a patient population with moderately to severely active UC (Mayo score ≥ 6), of which 60.2% were male, with a mean age of 39.3 years, and a mean body weight of 76.0 kg [10, 18]. The average cohort age was updated on each cycle. Patients were required to have evidence of an inadequate response to, loss of response to, or intolerance to at least one other conventional treatment.
2.2.2 Perspective, Time Horizon, and Discounting
The model was developed from a public healthcare payer’s perspective in Canada over a lifetime horizon, defined as the time when the mean age of the cohort reaches 110 years, which was deemed sufficient to assume all individuals would be deceased by the last cycle of the model. The cycle length was 1 year. Costs and health outcomes were discounted annually at the rate of 1.5% per year in the base case according to the Canadian guidelines for cost-effectiveness evaluations [19].
2.2.3 Treatment Comparators
Treatment comparators comprised (1) biologics (originals and biosimilars) that were available in Canada in 2019, including the monoclonal antibodies adalimumab SC, infliximab IV, golimumab SC, ustekinumab SC, and vedolizumab IV; (2) small-molecule inhibitor treatments, such as low-dose and high-dose tofacitinib (5 mg and 10 mg, respectively); and (3) conventional therapy using mesalazine (5-aminosalicylic acid), azathioprine, or prednisone. Details of these comparators are summarized in Table 1 in the Electronic Supplementary Material (ESM).
2.2.4 Treatment Efficacy
A systematic literature review and a NMA of clinical trials was conducted to allow indirect comparisons of advanced therapies (biologics and the small molecule, tofacitinib) used in the treatment of moderately to severely active UC, and networks of evidence were developed separately for the overall combined anti-TNF-naïve and anti-TNF-experienced population, and each subgroup separately [20]. Because of differences in trial design, population, and reported results among the clinical trials of advanced therapies, a connected network of clinical trials to compare all the treatments of interest for the outcomes of interest in the NMA was not available for all patient subgroups (e.g., anti-TNF-α-naïve, exposed, and mixed) in the model. Results from the NMA were extrapolated to determine efficacy for all patient subgroups across all treatments.
Clinical response and remission, as defined in the randomized controlled trials (RCTs), where remission is a subset of the broader category of response, were considered ordered categorical data with three mutually exclusive states: no response, response but no remission, and remission. Data of this nature were analyzed with a multinomial probit model. The multinomial model assumed that the treatment effects were the same for the three categories (no response, response but no remission, and remission).
Normal non-informative prior distributions were used for all parameters obtained from the NMA (mean 0; variance of 10,000). Relative treatment effects were expressed as odds ratios (ORs) with 95% credible intervals, which reflect a 95% probability that the estimate is contained within the specified range. For the induction phase, Bayesian NMA models were used to simultaneously synthesize the results of the included trials for each outcome of interest to obtain the relative treatment effects. Relative risk (RR) values were generated from ORs for each agent of interest using placebo as a common comparator, and transition probabilities between health states were adjusted by converting ORs to RRs and associated 95% credible intervals using the following formula, where p is the probability of the outcome of interest observed for the placebo:
In the models, placebo was assumed to be the equivalent of conventional therapy because clinical trials would typically have continued background conventional therapy in the placebo arm. An NMA of clinical trials for the maintenance phase was also conducted to derive RRs between treatments during maintenance therapy. The clinical studies included in the NMA had two distinct trial designs in the maintenance phase, some of the trials re-randomized induction treatment responders at weeks 6–10, while other trials used a treat-through design, in which all patients received the same treatment for 52 weeks during induction and maintenance. To account for the differences in the maintenance phase in this NMA, data from treat-through study designs were converted and re-randomized to estimate sustained response (RR for maintenance response on advanced therapy vs conventional therapy, conditional on the induction response for advanced therapy) and remission (RR for maintenance remission on advanced therapy vs conventional therapy, conditional on the induction response to advanced therapy) [20]. The treatment efficacy values produced for the model are summarized in Table 1.
2.2.5 Treatment-Related Adverse Events
The adverse event (AE) rates from the NMA were not fit for purpose for the model as they did not provide the granular level required to capture the costs and utility due to AEs; therefore, AE rates of at least 10% in the manufacturer’s submission to the Canadian Agency for Drug and Technologies in Health for tofacitinib were used. Adverse events considered in the model were serious infection, upper respiratory tract infection, tuberculosis, malignancies, acute infusion-related AE, injection-site reaction, and herpes zoster infection (Table 2 in the ESM) [21]. When the rate of AEs was 0 (e.g., for serious infections), a value of 0 (i.e., no cost) was included in the model. The reported 8-week rates were converted to 10-week probabilities for the induction phase and 52-week probabilities for the maintenance phase. Myocardial infarction and stroke were added because of a black-box warning from the US Food and Drug Administration about an increased risk of blood clots and death with the higher dose of tofacitinib in patients with rheumatoid arthritis [22], which has been extended to patients with UC [23]. The myocardial infarction and stroke event rates were extracted from published pooled data [24]. The probabilities of AEs were assumed to be similar across all moderate-to-severe, anti-TNF-based, UC subgroups in both the induction and the maintenance phases.
2.2.6 Treatment Discontinuation Because of AEs
Incidence rates of discontinuation because of AEs reported in the induction and maintenance phases were obtained from the NMA and calculated as a base-case 10-week probability for the induction phase and annual probability for the maintenance phase (Table 3 in the ESM) [20]. Not all reported AEs led to treatment discontinuation. The derived discontinuation probabilities were assumed to apply to the overall population, whereas coefficients of 0.9 (reduction) and 1.1 (increase) were applied to patients who were naïve and experienced, respectively, to anti-TNF therapy, to more accurately align with real-world discontinuation rates in these patients, as recommended by consulting expert clinicians.
2.2.7 Surgery
The UC-related surgery rate in Canada was derived from the University of Manitoba IBD Epidemiology Database [25]. The 20-year risk of 14.8% was converted to 10-week probabilities to estimate the proportion of non-responders undergoing surgery during the induction phase, and to annual probabilities during the maintenance phase using a standard logarithmic approach [26]. Surgery risk was adjusted using a 0.4226 correction factor to account for the probability of a lower colectomy risk, as patients are more recently diagnosed [21, 25].
2.2.8 Mortality
Calculations conducted to determine the impact of UC-related death revealed that UC population death rates were similar to the general population [27] and the impact was deemed to be negligible; thus, disease-specific mortality was excluded from the model.
2.2.9 Utilities
Utility values were sourced from a systematic review by the National Institute of Health and Care Excellence and applied by the Assessment Group in their de novo model-based utilities reported by Woehl et al. [28] and Archer et al. [14] (Table 2). All patients entered the model with a baseline utility equivalent to that of the “Active UC” health state. Health-state utilities were assumed to be independent of time and were applied in each cycle to patients in each health state over the model time horizon. Utility decrements associated with AEs were obtained from the National Institute of Health and Care Excellence submission for vedolizumab [29], and utilities of myocardial infraction and stroke were added for tofacitinib on the basis of non-UC specific utilities [30], as these events are not common in UC (Table 2).
2.2.10 Costs
Direct costs in 2019 Canadian dollars included treatment-related costs (i.e., drug acquisition and administration), disease management costs by health state, and AE management costs (Table 3). Drug costs were obtained from the Ontario Drug Benefit Formulary drug list for 2019 [31]. Acquisition costs during the induction and maintenance phases were calculated based on the advanced therapy regimen used in each phase. For infliximab IV, the biosimilar cost was used. Costs associated with the management of UC were applied to patients in the induction phase based on the final health state at the end of the 10-week induction phase, and to patients in the maintenance phase based on their health state in each cycle. Disease management costs included medical fees associated with UC-related resource utilization, and drug costs of conventional therapy, surgical procedure costs, and the costs of surgical complications [32]. The frequency of UC-related resource utilization was informed by the clinical opinions of two medical experts. The cost of surgery was based on the sum of fees related to hospitalization for UC surgery, including the costs for resource use and procedures. The costs of the “Post-surgery Complications” health state included a 14-day medical management cost of pouchitis, in addition to medical resource use incurred in this health state. Costs for AEs were derived using the Ontario Case Costing Initiative Costing Analysis Tool [33, 34].
2.3 Model Assumptions
Because of a paucity of data, model assumptions listed in Table 4 in the ESM were applied and validated by Canadian clinicians involved with the treatment and management of patients with UC.
2.4 Model Outcomes
Overall costs and cost by category were derived. Cost categories included treatment (acquisition and administration), disease management by health states (remission, response, active UC, surgery, post-surgery remission, and post-surgery complications), and AEs. Health outcomes included years on advanced therapy, years in response (no remission), years in remission, number of AEs experienced (at cohort level), surgeries required (at cohort level), life-years, and quality-adjusted life-years (QALYs), which were distributed by health state. The model reported an incremental cost-effectiveness ratio (ICER) in terms of incremental cost per QALY gained.
2.5 Base-Case Analysis
The base-case analysis compared vedolizumab IV as induction followed by vedolizumab SC as maintenance to different model comparators in the overall cohort of patients with moderately to severely active UC from a public healthcare payer perspective over the lifetime horizon. Up-dosing was assumed in the base-case analysis for tofacitinib, infliximab IV, and golimumab SC, but not for vedolizumab SC or IV and adalimumab SC, as up-dosing is not permitted according to product labeling in Canada. For ustekinumab SC, dose de-escalation from every 8 weeks to every 12 weeks was assumed [20]. A sequential analysis of cost effectiveness was conducted following standard rules for estimating ICERs [19]. The expected values of costs and outcomes were obtained through probabilistic analyses, whereby all uncertain parameters are defined probabilistically. To account for statistical uncertainties of multiple key parameters, the base-case analysis was conducted in a probabilistic manner by simultaneously varying the following parameters: (1) the relative risks of response and remission for the advanced therapies compared with conventional therapy, the weekly rate of each AE in the induction phase, and the annual rate of each AE in the maintenance phase—using a log-normal distribution; (2) the proportions of male patients and patients discontinuing treatment because of AEs in the induction and maintenance phases, health-state utilities, and AE disutilities—using a beta distribution; and (3) the AE management costs and disease management costs—using a gamma distribution. Where a standard error or confidence interval was not available for a selected parameter, 10% of the mean was assumed as the standard error. Analysis was performed taking a mixed cohort from patients assuming a public healthcare perspective and lifetime modeling horizon. Table 5 in the ESM provides a list of all parameters and mean and standard error values informed by the NMA and included in the probabilistic base-case analysis. The cohort size was assumed to be 1000 patients and the number of replications was set to 10,000 to reach convergence (Fig. 1 in the ESM). Treatment was considered cost effective at ICERs below a willingness-to-pay threshold of $50,000 [35].
The base-case analysis also included the efficiency frontier approach [36], whereby interventions were ranked in terms of costs (lowest to highest). If an intervention was less effective than the previous less costly intervention, it was considered strictly “dominated” and subsequently excluded from further analyses. Incremental cost-effectiveness ratios were then calculated for each intervention, compared with the next most expensive, non-dominated strategy. If the ICER for an intervention was higher than that of the next most effective intervention, it was ruled out by “extended dominance,” and the process was reiterated to determine the interventions that make up the efficiency frontier. An efficiency frontier was created by plotting each treatment’s costs (horizontal axis) and QALYs (vertical axis) and drawing a line linking treatments that were not dominated by any of the other treatments in consideration.
2.6 Scenario Analyses
In addition to the base-case analysis, several scenario analyses were performed. The scenarios selected were of most interest from a clinical perspective and represented the highest uncertainty around the model structure or parameters. The “other patient subgroups” scenario used anti-TNF-naïve and anti-TNF-experienced patients as reference groups. The “100% up-dosing” scenario set the rates of dose escalation and regain of response to 100% values of dose escalation. The “all comparators” scenario analysis tested the effect of adding high-dose tofacitinib, which is not commonly used in Canada for maintenance therapy. The “waning effect” scenario analysis tested the effect of a 10% deterioration of efficacy of all therapies compared with the prior cycle each year over the lifetime horizon. The “societal perspective” scenario tested the productivity loss costs on the base-case ICER. The “discount rate” scenario examined the impact of setting the discount rate for costs and effects to 0% and 3%. The “10-year time horizon” scenario was examined to address findings from the base-case analysis that patients spent the majority of the model in the active UC health state, which may or may not be clinically plausible. The “vedolizumab utilities” scenario assessed the impact of applying utilities from the vedolizumab IV clinical trial, GEMINI [8]. The “drug holiday” scenario determined the impact of a 10% reduction in patient adherence to advanced therapies. Treatment was considered cost effective at ICERs below a willingness-to-pay threshold of $50,000 [35].
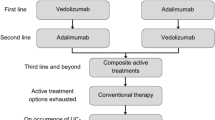
A separate treatment sequencing scenario compared the cost effectiveness of vedolizumab SC in the first-line vs second-line advanced therapy use, on the basis of a cohort size of 1000 patients and 10,000 iterations, and with all other model settings the same as for the base case (Table 6 in the ESM).
3 Results
3.1 Base-Case Analysis
Summaries of the base-case results for the full patient population are reported in Table 4, and the efficacy frontier is shown in Fig. 2. Overall, conventional therapy was most cost effective in the overall population. Vedolizumab SC was cost effective and dominant (i.e., more effective and less expensive) compared with other advanced therapies (adalimumab SC, golimumab SC, infliximab IV, tofacitinib 5 mg, ustekinumab SC, and vedolizumab IV). The annual cost of vedolizumab SC was lower than ustekinumab SC, tofacitinib 5 mg, vedolizumab IV, and golimumab SC by $47,024, $3251, $2120, and $2004, respectively, and exceeded that of infliximab IV (biosimilar), adalimumab SC, and conventional therapy by $582, $3293, and $41,024, respectively. Vedolizumab SC resulted in the highest total QALYs (14.21) and highest QALYs on advanced therapy (1.82), QALYs in treatment response (0.8), and QALYs in remission (0.75) vs all the other treatments.
3.2 Scenario Analyses
A summary of results for the modeled scenarios is provided in Table 5, and more detailed scenario results are provided in Tables 7–17 and Fig. 2 in the ESM. Notably, in the anti-TNF-naïve (Table 7 in the ESM) and anti-TNF-experienced (Table 8 in the ESM) subgroups, vedolizumab SC is cost effective compared with adalimumab SC, infliximab IV, golimumab SC, tofacitinib 5 mg and 10 mg, ustekinumab SC, and vedolizumab IV by showing either dominance or having a very low ICER. In a model comparing vedolizumab SC treatment sequencing, vedolizumab SC as first-line advanced therapy produced more patient QALYs at lower costs compared with vedolizumab SC as a second-line treatment (Table 6 in the ESM).
3.3 Cost-Effectiveness Acceptability Curve
In the probabilistic sensitivity analysis, the model was run for 10,000 replications to generate a cost-effectiveness plane and acceptability curve for the base-case analysis. The base-case cost-effectiveness plane and acceptability curve for the overall population are reported in Fig. 2. The acceptability curve displays how conventional therapy has the highest probability of being cost effective in the willingness-to-pay threshold of below $125,000, beyond which vedolizumab SC has the highest probability of being cost effective.
4 Discussion
To our knowledge, this is the first study to assess the cost effectiveness of vedolizumab IV induction followed by vedolizumab SC maintenance therapy in patients with moderately to severely active UC from Canada’s public healthcare payer perspective. The results revealed that in the overall population, conventional therapy is most cost effective followed by vedolizumab SC, which is cost effective compared with other advanced therapies (adalimumab SC, infliximab IV, golimumab SC, low-dose tofacitinib, ustekinumab SC, and vedolizumab IV), showing either dominance (more effective and less expensive) or having a very low ICER. Although conventional therapy was the most cost-effective option, this analysis focused on a population of patients considered for advanced therapy after failure of conventional treatment. In the overall population and anti-TNF-experienced subgroup, vedolizumab SC revealed ICERs of $109,374 and $114,287, respectively; therefore, it was not cost effective compared with conventional therapy for these patient groups based on a $50,000/QALY threshold. However, vedolizumab SC was cost effective vs conventional therapy in the anti-TNF-naïve subgroup based on a $50,000/QALY threshold. Furthermore, in treatment sequencing scenarios, the comparison of vedolizumab SC as first-line vs second-line advanced therapy demonstrated higher QALYs and lower costs for vedolizumab SC as first-line advanced therapy, suggesting a clinical and economic benefit for using vedolizumab SC immediately after conventional treatment.
Although tofacitinib was associated with higher remission and response rates, it generated fewer QALYs compared with vedolizumab, primarily owing to the higher rates of discontinuation because of AEs in patients treated with tofacitinib (5.3%) compared with those treated with vedolizumab (3.2%); secondarily, up-dosing was implemented in the model through loss of response and regain of response, and this led to only a proportion of patients receiving an up-dose regaining response. Up-dosing was implemented for tofacitinib in a proportion of the patients, while for vedolizumab SC or IV and adalimumab SC no up-dosing was assumed, as it was not permitted according to product labeling in Canada. As a result, patients treated with tofacitinib spent less time in response/remission and more time in active UC compared with patients treated with vedolizumab, and therefore accumulated slightly fewer QALYs.
Our model was informed by best practices in modeling for UC and was developed to overcome limitations associated with previous cost-effectiveness analyses of advanced therapies in the treatment of moderately to severely active UC [15, 16, 37]. Our study did not limit the modeled duration of advanced therapy, unlike previous models that assumed patients stopped advanced therapy after 1 year, regardless of whether they responded to treatment [16]. The latter assumption does not align with clinical practice in Canada or other settings in which a patient can experience benefit from advanced therapy for several years [38]. Additionally, to avoid bias toward any comparator advanced therapy and to follow standard practice, our model assumed that patients with treatment failure after a first-line advanced therapy were treated with conventional therapy. Furthermore, NMAs were used to assess the relative efficacy of multiple treatments in the absence of head-to-head RCTs, which is a common and accepted approach [39]. We extracted data via a systematic literature review and evidence from the VISIBLE 1 trial for the NMA [10, 20]. Despite the differences in RCT designs, outcome definitions for response and remission at 52, 54, and 60 weeks were comparable and consistent between re-randomized trials and treat-through studies.
Previous cost-effectiveness analyses characterized disease severity through three health states defined by complete Mayo scores, and used an 8-week cycle during maintenance and 8-week transition matrices derived from patient-level data in RCTs [15, 16, 40]. Limited availability of patient-level data and complete Mayo scores (obtained infrequently because of the endoscopy requirement) limits the ability to appropriately model transitions to remission or from moderately to severely active UC to mild UC [41]. Partial Mayo scores to derive 8-week transition matrices or complete Mayo scores based on only two timepoints require arbitrary assumptions and have been criticized by the National Institute of Health and Care Excellence, who recommend modeling observed transitions between moderately to severely active UC, response, and remission states [38]. Our model was designed to address these limitations by using a maintenance cycle length of 1 year, which is consistent with the maintenance phase in the VISIBLE 1 trial in which the complete Mayo score was only collected at the end of induction and the end of maintenance. At the beginning of each cycle, the number of patients in remission and response-only health states is known, and the probability of sustained response was used to calculate the percentage of patients remaining in these health states, and the probability of remission to determine the percentage of patients allocated to the remission health state at the end of the cycle. This allocation implies that some patients remain in remission, whereas others improve from response only to remission. The supplement of the probability of remission among those who experienced a sustained response is allocated to the response-only state, which implies that some patients remain in the response-only health state, whereas others worsen slightly from remission to response only. Patients who do not experience a sustained response are assumed to lose their remission or response-only status and transition to the active UC health state. With this approach, we can directly use the transitions observed during the maintenance phase for the placebo arm of the VISIBLE 1 trial and directly apply the comparative efficacy outcomes from the NMA (sustained response and remission) [20]. This can be done without having to optimize or calibrate transition probability matrices that are ultimately derived to match the transitions observed at the defined timepoints of the RCTs. This approach is consistent with that used in the cost-effectiveness analysis of vedolizumab in Spain [37].
In this model, better performance of vedolizumab SC as a first-line advanced therapy vs second-line treatment was observed (higher QALYs and lower costs). In a real-world setting, vedolizumab IV was effective in inducing and maintaining clinical response as a first-line advanced treatment in UC, and it also did not seem to impact the effectiveness of subsequent second-line anti-TNFs following discontinuation of first-line vedolizumab IV [42, 43]. Additionally, during the induction phase in the GEMINI 1 study, numerically greater efficacy of vedolizumab IV was observed in anti-TNF-naïve patients compared with patients in whom anti-TNFs previously failed [7]. Although these results are from vedolizumab IV studies, they might help to inform higher QALYs observed in this model in vedolizumab SC as a first-line advanced therapy.
This study had some limitations. First, some trials included in the NMA were international and not specific to the Canadian population, and health-state utility estimates were obtained from UK population studies [16, 20, 40] because of a lack of available quality-of-life data for Canadian patients. Second, the clinical trials used to inform efficacy and AE rates had an approximate follow-up duration of 1 year, whereas our base-case analysis assumed a constant treatment effect over the lifetime horizon if patients remained on treatment beyond 1 year. We included a waning treatment effect in the model to help address this. Third, our base-case analysis assumed 100% adherence to advanced therapies, possibly resulting in an overestimation of drug costs over the time horizon; however, this impacted all advanced therapies, potentially cancelling out any impact. Fourth, we did not model changes in Mayo scores over time. Even if available, the complete Mayo score was collected only at baseline, at the end of the induction phase, and at the end of the maintenance phase, providing insufficient data points to extrapolate changes over time. Fifth, there was a lack of comparative efficacy data for some of the first-line therapies given to anti-TNF-experienced patients and for subsequent lines of therapies. A linear extrapolation was conducted to fill in the missing data, which might have introduced a source of bias to the analysis. Sixth, there was uncertainty around healthcare resource utilization data, which was collected from two medical experts, and there may have been local variation in these estimates. Seventh, because not all treatments were present in all networks, the log-normal distribution was used to generate probabilistic sensitivity analysis realizations from the NMA instead of the CODA approach, which is generally preferrable. Finally, infliximab IV cost data were based on a biosimilar. If data for additional biosimilars become available, these comparators should be considered for future analyses.
5 Conclusions
The results of this health economic evaluation demonstrated that in the mixed population with moderately to severely active UC, in addition to anti-TNF-naïve and anti-TNF-experienced subgroups, vedolizumab SC is cost effective compared with adalimumab SC, infliximab IV, golimumab SC, tofacitinib 5 mg and 10 mg, ustekinumab SC, and vedolizumab IV, showing either dominance or a very low ICER. Vedolizumab SC offered the most cost-effective therapeutic option after conventional therapy. Based on a $50,000/QALY threshold, vedolizumab could be considered cost effective in the anti-TNF-naïve patients but not the overall population. These results demonstrate that after conventional therapy, vedolizumab SC is an optimal first-line advanced therapy in Canada for patients with UC.
References
Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365(18):1713–25. https://doi.org/10.1056/NEJMra1102942.
Kaplan GG, Bernstein CN, Coward S, Bitton A, Murthy SK, Nguyen GC, et al. The impact of inflammatory bowel disease in Canada 2018: epidemiology. J Can Assoc Gastroenterol. 2019;2(Suppl. 1):S6–16. https://doi.org/10.1093/jcag/gwy054.
Fumery M, Singh S, Dulai PS, Gower-Rousseau C, Peyrin-Biroulet L, Sandborn WJ. Natural history of adult ulcerative colitis in population-based cohorts: a systematic review. Clin Gastroenterol Hepatol. 2018;16(3):343–56.e3. https://doi.org/10.1016/j.cgh.2017.06.016.
Alatab S, Sepanlou SG, Ikuta K, Vahedi H, Bisignano C, Safiri S, et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5(1):17–30. https://doi.org/10.1016/s2468-1253(19)30333-4.
Benchimol EI, Bernstein CN, Bitton A, Murthy SK, Nguyen GC, Lee K, et al. The impact of inflammatory bowel disease in Canada 2018: a scientific report from the Canadian Gastro-Intestinal Epidemiology Consortium to Crohn’s and Colitis Canada. J Can Assoc Gastroenterol. 2019;2(Suppl. 1):S1–5. https://doi.org/10.1093/jcag/gwy052.
Bressler B, Marshall JK, Bernstein CN, Bitton A, Jones J, Leontiadis GI, Toronto Ulcerative Colitis Consensus Group, et al. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology. 2015;148(5):1035–58.e3. https://doi.org/10.1053/j.gastro.2015.03.001.
Feagan BG, Rubin DT, Danese S, Vermeire S, Abhyankar B, Sankoh S, et al. Efficacy of vedolizumab induction and maintenance therapy in patients with ulcerative colitis, regardless of prior exposure to tumor necrosis factor antagonists. Clin Gastroenterol Hepatol. 2017;15(2):229–39.e5. https://doi.org/10.1016/j.cgh.2016.08.044.
Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, GEMINI 1 Study Group, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699–710. https://doi.org/10.1056/NEJMoa1215734.
Loftus EV, Colombel JF, Feagan B, Vermeire S, Sandborn WJ, Sands BE, et al. Long-term effectiveness and safety of vedolizumab in patients with ulcerative colitis: 5-year cumulative exposure of GEMINI 2 completers rolling into the GEMINI open-label extension study. Presented at: Digestive Disease Week; 9 May 2017; Chicago (IL).
Sandborn WJ, Baert F, Danese S, Krznarić Z, Kobayashi T, Yao X, et al. Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. Gastroenterology. 2020;158(3):562–72.e12. https://doi.org/10.1053/j.gastro.2019.08.027.
Takeda Pharma A/S. Entyvio vedolizumab [summary of product characteristics]. Taastrup, Denmark: Takeda Pharma A/S; 2020.
Takeda Pharmaceuticals Australia Pty Ltd. Entyvio® vedolizumab [Australian product information]. Sydney (NSW): Takeda Pharmaceuticals Australia Pty Ltd; 2020.
Takeda Canada Inc. Entyvio® vedolizumab [product monograph]. Toronto (ON): Takeda Canada Inc.; 2020.
Archer R, Tappenden P, Ren S, Martyn-St James M, Harvey R, Basarir H, et al. Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional therapy (including a review of TA140 and TA262): clinical effectiveness systematic review and economic model. Health Technol Assess. 2016;20(39):1–326. https://doi.org/10.3310/hta20390.
Tsai HH, Punekar YS, Morris J, Fortun P. A model of the long-term cost effectiveness of scheduled maintenance treatment with infliximab for moderate-to-severe ulcerative colitis. Aliment Pharmacol Ther. 2008;28(10):1230–9. https://doi.org/10.1111/j.1365-2036.2008.03839.x.
Wilson MR, Bergman A, Chevrou-Severac H, Selby R, Smyth M, Kerrigan MC. Cost-effectiveness of vedolizumab compared with infliximab, adalimumab, and golimumab in patients with ulcerative colitis in the United Kingdom. Eur J Health Econ. 2018;19(2):229–40. https://doi.org/10.1007/s10198-017-0879-5.
Canadian Agency for Drugs and Technologies in Health. CADTH common drug review: pharmacoeconomic review report for Entyvio (vedolizumab). https://www.cadth.ca/sites/default/files/cdr/pharmacoeconomic/SR0421_Entyvio_PE_Report.pdf. Accessed 10 Nov 2021.
Institut National d'Excellence en Santé et Services Sociaux (INESS). Notice to manufacturers of medicines: conventions relating to average weight and average body surface area of patients. 2019. https://www.inesss.qc.ca/fileadmin/doc/INESSS/Inscription_medicaments/Avis_fabricants/20190806_Avis_fabricants_Analyses_economiques.pdf
Canadian Agency for Drugs and Technologies in Health. CADTH methods and guidelines: guidelines for the economic evaluation of health technologies: Canada. 4th ed. https://www.cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf. Accessed 30 Jun 2020.
Jairath V, Chan K, Lasch K, Keeping S, Agboton C, Blake A, et al. Integrating efficacy and safety of vedolizumab compared with other advanced therapies to assess net clinical benefit of ulcerative colitis treatments: a network meta-analysis. Expert Rev Gastroenterol Hepatol. 2021;15(6):711–22. https://doi.org/10.1080/17474124.2021.1880319.
Canadian Agency for Drugs and Technologies in Health. CADTH common drug review: pharmacoeconomic review report. Tofacitinib (Xeljanz). https://www.cadth.ca/sites/default/files/cdr/pharmacoeconomic/sr0572-xeljanz-pharmacoeconomic-report.pdf. Accessed 8 Feb 2021.
US Food and Drug Administration. FDA approves boxed warning about increased risk of blood clots and death with higher dose of arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR). https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-boxed-warning-about-increased-risk-blood-clots-and-death-higher-dose-arthritis-and. Accessed 30 Jun 2020.
US Food and Drug Administration. Initial safety trial results find increased risk of serious heart-related problems and cancer with arthritis and ulcerative colitis medicine Xeljanz, Xeljanz XR (tofacitinib). https://www.fda.gov/media/145590/download. Accessed 29 Jun 2021.
Charles-Schoeman C, DeMasi R, Valdez H, Soma K, Hwang LJ, Boy MG, et al. Risk factors for major adverse cardiovascular events in phase III and long-term extension studies of tofacitinib in patients with rheumatoid arthritis. Arthritis Rheumatol. 2019;71(9):1450–9. https://doi.org/10.1002/art.40911.
Targownik LE, Singh H, Nugent Z, Bernstein CN. The epidemiology of colectomy in ulcerative colitis: results from a population-based cohort. Am J Gastroenterol. 2012;107(8):1228–35. https://doi.org/10.1038/ajg.2012.127.
Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
Statistics Canada. Life expectancy and other elements of the life table, Canada, all provinces except Prince Edward Island. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310011401. Accessed 8 Feb 2021.
Woehl A, Hawthorne A, McEwan P. The relation between disease activity, quality of life and health utility in patients with ulcerative colitis (402). Gut. 2008;57(Suppl. 1):A153.
National Institute for Health and Care Excellence. NICE single technology appraisal. Vedolizumab (Entyvio®) for the treatment of adults with moderate to severe active ulcerative colitis. 2014. https://www.nice.org.uk/guidance/ta342/documents/ulcerative-colitis-moderate-to-severely-active-vedolizumab-id691-evaluation-report-part-22
Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26(4):410–20. https://doi.org/10.1177/0272989X06290495.
Ontario Ministry of Health. Ontario Health Insurance Plan (OHIP) schedule of benefits and fees. Available from: http://www.health.gov.on.ca/en/pro/programs/ohip/sob/. Accessed 24 Feb 2020.
Cohen RD, Yu AP, Wu EQ, Xie J, Mulani PM, Chao J. Systematic review: the costs of ulcerative colitis in Western countries. Aliment Pharmacol Ther. 2010;31(7):693–707. https://doi.org/10.1111/j.1365-2036.2010.04234.x.
Harbord M, Eliakim R, Bettenworth D, Karmiris K, Katsanos K, Kopylov U, European Crohn’s and Colitis Organisation [ECCO], et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11(7):769–84. https://doi.org/10.1093/ecco-jcc/jjx009.
McGirr A, Van Oorschot D, Widenmaier R, Stokes M, Ganz ML, Jung H, et al. Public health impact and cost-effectiveness of non-live adjuvanted recombinant zoster vaccine in Canadian adults. Appl Health Econ Health Policy. 2019;17(5):723–32. https://doi.org/10.1007/s40258-019-00491-6.
Griffiths EA, Vadlamudi NK. Cadth’s $50,000 cost-effectiveness threshold: fact or fiction? Value Health. 2016;19(7):A488–9. https://doi.org/10.1016/j.jval.2016.09.821.
Caro JJ, Nord E, Siebert U, McGuire A, McGregor M, Henry D, et al. The efficiency frontier approach to economic evaluation of health-care interventions. Health Econ. 2010;19(10):1117–27. https://doi.org/10.1002/hec.1629.
Trigo-Vicente C, Gimeno-Ballester V, Montoiro-Allué R, López-Del VA. Cost-effectiveness analysis of infliximab, adalimumab, golimumab and vedolizumab for moderate to severe ulcerative colitis in Spain. Expert Rev Pharmacoecon Outcomes Res. 2018;18(3):321–9. https://doi.org/10.1080/14737167.2018.1411193.
Essat M, Tappenden P, Ren S, Bessey A, Archer R, Wong R, et al. Vedolizumab for the treatment of adults with moderate-to-severe active ulcerative colitis: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2016;34(3):245–57. https://doi.org/10.1007/s40273-015-0334-3.
Jansen JP, Fleurence R, Devine B, Itzler R, Barrett A, Hawkins N, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health. 2011;14(4):417–28. https://doi.org/10.1016/j.jval.2011.04.002.
Wilson MR, Azzabi Zouraq I, Chevrou-Severac H, Selby R, Kerrigan MC. Cost-effectiveness of vedolizumab compared with conventional therapy for ulcerative colitis patients in the UK. Clinicoecon Outcomes Res. 2017;9:641–52. https://doi.org/10.2147/CEOR.S135609.
Hisabe T, Hirai F, Matsui T. Diagnosis of ulcerative colitis. Nippon Daicho Komonbyo Gakkai Zasshi. 2011;64(10):807–16. https://doi.org/10.3862/jcoloproctology.64.807.
Bressler B, Yarur A, Kopylov U, Bassel M, Brett N, Lissoos T, et al. Clinical effectiveness of first-line anti-TNF therapies and second-line anti-TNF therapy post-vedolizumab discontinuation in patients with ulcerative colitis or Crohn’s disease (P1091). United Eur Gastroenterol J. 2019;7(8 Suppl.):624.
Ritter TE, Mehta SA, Van Anglen LJ. Real world experience with vedolizumab as first-line therapy for the treatment of ulcerative colitis (S0850). Am J Gastroenterol. 2020;115:S439.
Acknowledgements
Medical writing support was provided by Chris Barnes, PhD, of ProEd Communications, and Milena Wagner, PhD, of Excel Medical Affairs, and was funded by Takeda.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by Takeda.
Conflict of interest
Elisabetta Fenu is an employee of and holds stock or stock options in Takeda. Vasily Lukyanov and Xenia Radu are employees of IQVIA, which received funding from Takeda. Annabel Acs is a former employee of IQVIA, which received funding from Takeda. Stephanie Stypa and Aren Fischer are employees of Takeda Canada, Inc. John K. Marshall has received honoraria for speaking and/or consulting from AbbVie, Allergan, Amgen, Bristol Myers Squibb, Ferring, Fresenius Kabi, Janssen, Lilly, Lupin, Merck, Novartis, Paladin, Pfizer, Pharmascience, Procter & Gamble, Roche, Shire, Takeda, and Teva. Mark Oppe is a former employee of Axentiva Solutions, which received funding from Takeda.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants data supporting the results reported in this article, will be made available within 3 months from initial request, to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization. Data are available upon request via application at https://search.vivli.org.
Code availability
Not applicable.
Authors’ contributions
Elisabetta Fenu, Aren Fischer, Vasily Lukyanov, Mark Oppe, Annabel Acs, and Xenia Radu were involved in the conception and design of the study. Elisabetta Fenu, Mark Oppe, and Aren Fischer analyzed and interpreted the data. All authors were involved in the acquisition of the data, drafted the article or critically revised it for intellectual content, and approved the final version for submission.
Additional information
Vasily Lukyanov and Annabel Acs are former employees of IQVIA.
Mark Oppe is a former employee of Axentiva Solutions.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Fenu, E., Lukyanov, V., Acs, A. et al. Cost Effectiveness of Subcutaneous Vedolizumab for Maintenance Treatment of Ulcerative Colitis in Canada. PharmacoEconomics Open 6, 519–537 (2022). https://doi.org/10.1007/s41669-022-00331-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-022-00331-9