Abstract
Objectives
The aim of this study was to update previously estimated public health impact and cost effectiveness of recombinant zoster vaccine (RZV) for the prevention of herpes zoster (HZ) in Canadians aged ≥50 years using longer-term RZV efficacy and waning data and real-world coverage and completion.
Methods
A multicohort Markov model was used to conduct a cost-utility analysis comparing RZV with no HZ vaccination among Canadians aged ≥50 years. Real-world data were used for first-dose coverage (17.5%) and second-dose completion (65%). Vaccine efficacy and waning data were applied from up to 8-year follow-up from the ZOE-50 and ZOE-70 clinical trials. Incremental costs and benefits were calculated using a lifetime horizon from the healthcare payer (base case) and societal perspectives. A discount rate of 1.5% was applied to costs and quality-adjusted life-years (QALYs).
Results
The model estimated that RZV would prevent 303,835 HZ cases, 83,256 post-herpetic neuralgia (PHN) cases, 39,653 other complications, and 99 HZ-related deaths compared with no HZ vaccination. Incremental cost-effectiveness ratios (ICERs) were estimated to be $27,486 and $22,097 per QALY (2022 Canadian dollars [CAN$]) from the healthcare payer and societal perspectives, respectively. The base-case ICER was most sensitive to a lower percentage of initial HZ cases with PHN. Almost all probabilistic sensitivity analysis simulations (98.1%) resulted in ICERs <CAN$50,000 per QALY.
Conclusions
RZV is expected to remain a cost-effective option for Canadian adults aged ≥50 years when using longer-term RZV efficacy and waning estimates, although the estimated public health impact was smaller than in the previous analysis (due to lower coverage/completion estimates).
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
A 2017 analysis predicted that recombinant zoster vaccine (RZV) would be cost effective for the prevention of herpes zoster (HZ) among adults aged ≥50 years in Canada. However, longer-term vaccine efficacy and waning data were not available and first-dose coverage and second-dose compliance had to be assumed since RZV was not available on the Canadian market. |
The current analysis used longer-term RZV efficacy and waning data (based on up to 8 years of follow-up) and real-world Canadian first-dose coverage and second-dose compliance. |
In the current study, RZV was predicted to be slightly more cost effective than in the 2017 study. However, coverage and compliance rates would need to be improved to obtain a similar predicted public health impact as the 2017 study. |
1 Introduction
Most Canadian adults will have been infected with varicella-zoster virus (VZV) during childhood, leaving them at risk for developing herpes zoster (HZ) later in life, following reactivation of VZV due to a decline in immune system function from older age or compromised immune systems due to disease or immunosuppressive therapy [1]. The incidence of HZ increases with age, from approximately 5 per 1000 people aged 50–54 years to around 11 per 1000 people aged ≥85 years [2]. The incidence of HZ also increases among immunocompromised patients, e.g. 12 per 1000 people with cancer or rheumatoid arthritis and 43 per 1000 people who have undergone bone marrow or stem cell transplantation [3]. The overall lifetime risk of HZ in Canada is nearly one in three [4]. As the incidence of HZ appears to be increasing over time due to an aging Canadian population, the burden of HZ is likely to increase [5].
HZ is characterized by a painful dermatomal rash lasting up to 4 weeks [1]. Approximately 20% of patients with HZ go on to develop post-herpetic neuralgia (PHN) [6], defined as pain that lasts for >3 months after the HZ rash has healed [1] but that can last for considerably longer. Around 10–20% of patients with HZ will develop HZ ophthalmicus [7], which can result in vision loss without prompt treatment [8]. HZ and its complications can therefore have a profound effect on the quality of life of affected patients [9, 10].
Canada’s National Advisory Committee on Immunization (NACI) first recommended vaccination with zoster vaccine live (ZVL) in 2010 for the prevention of HZ and its complications in people aged ≥60 years without contraindications [11]. NACI’s recommendations were updated in 2018: recombinant zoster vaccine (RZV; Shingrix, GSK) should be offered to people aged ≥50 years without contraindications, while ZVL may be considered for immunocompetent people aged ≥50 years without contraindications when RZV is contraindicated or not available/accessible [12]; however, ZVL has since been discontinued in Canada (in 2022) [13].
RZV for Canadian adults aged ≥60 years was estimated by McGirr et al. [14] to be a cost-effective intervention versus no HZ vaccination, with an incremental cost-effectiveness ratio (ICER) of 28,360 Canadian dollars (CAN$) per quality-adjusted life-year (QALY) gained, and for adults aged ≥50 years with an ICER of CAN$30,402 per QALY gained (2016). However, at the time of the McGirr et al. study, RZV efficacy data were only available up to 4 years post-vaccination and assumptions for first-dose RZV coverage and second-dose completion rates were required as RZV was not yet available in Canada. The publication of an interim analysis [15] of follow-up for up to 8 years since initial vaccination from Zoster Efficacy Study in Adults 50 Years of Age or Older (ZOE-50) and Zoster Efficacy Study in Adults 70 Years of Age or Older (ZOE-70) RZV pivotal clinical trial populations [16, 17] has provided longer-term vaccine efficacy (VE) and waning data. Moreover, the publication of real-world evidence for several data inputs for RZV has prompted an updated analysis. In order to ensure that cost-effectiveness models are relevant for policy decision making, it is important that they are updated with new inputs when these become available. The primary objective of the analysis was therefore to apply the new VE and waning data and other updated data inputs that have become available since the McGirr et al. [14] study in the ZOster ecoNomic Analysis (ZONA) 50+ model to update the cost effectiveness and public health impact estimates for Canada.
2 Methods
2.1 Model Overview
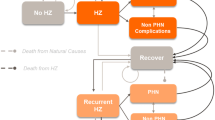
The structure of the ZONA model (Online Resource Fig. 1) has already been described [14, 18,19,20,21,22,23,24,25,26] and the ZONA model used by McGirr et al. underwent external, technical validation [14]. The ZONA model is a static multicohort Markov model developed in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) and considers five age cohorts (50‒59, 60‒64, 65‒69, 70‒79, and ≥80 years). The analysis was conducted over the remaining lifetimes of the cohorts from the year of vaccination, with annual cycles. States in the model were no HZ, HZ, PHN, other complications, recovery, recurrent HZ, death from HZ, and death from other causes. Two strategies were considered: vaccination with two doses of RZV, or no HZ vaccination. Comparisons with ZVL were not conducted because ZVL has been discontinued in Canada [13].
2.2 Input Parameters
Updated population estimates from 2021 [27] and annual all-cause mortality rates from 2020 [28] from Statistics Canada were used (Online Resource Table 1). Inputs from McGirr et al. [14] were used for the annual probability of HZ [29]; the risk of developing PHN [30] and ocular, neurological, cutaneous, and other non-pain complications [6]; and the case fatality rates for HZ [31] as no new data were identified.
First-dose RZV coverage was updated from the 80% assumed by McGirr et al. [14] (which was based on influenza vaccine uptake rates) to 17.5%, based on first-dose real-world coverage data up to 2022 [32] (Table 1). Second-dose completion was updated from an assumed 75% [14] to 65%, based on a Canadian retrospective database study [33]. McGirr et al. [14] used VE and waning data for two doses of RZV from two randomized controlled trials (ZOE-50 and ZOE-70) with mean follow-up durations of 3.2 and 3.7 years, respectively [16, 17]. VE and waning rates in the current analysis were updated with longer-term follow-up data from these two studies, for up to 8 years since initial vaccination (Table 1). VE and waning rates for one dose of RZV [18] were not changed from the McGirr et al. analysis [14].
The costing methods for HZ cases with and without PHN were consistent with those used by McGirr et al. [14], but used the most recent available data for resource utilization and unit costs for general practitioner (GP) visits, hospitalizations, and prescription drugs [34,35,36,37], which resulted in slightly lower costs (Online Resource Table 2). The price per dose of RZV was updated to CAN$130.14, which reflects the IMS Canada Price List on 18 April 2022. Vaccine administration costs were assumed to be CAN$4.95 for the first dose of RZV and CAN$10.55 for the second dose (including a CAN$5.60 premium for an immunization-only visit) [36]. RZV adverse event (AE) costs were estimated based on the proportions of patients in the ZOE studies [16, 17] who required a GP visit, emergency room (ER) visit, or hospitalization (as per McGirr et al. [14]) and the latest unit costs [35, 36], which were inflated to 2022 CAN$ when necessary [38, 39].
Utility values at baseline (i.e. without HZ) were updated from the McGirr et al. analysis [14] using data obtained from Szende et al. [40], who reported EuroQol 5 Dimensions (EQ-5D) index values for Canada, which were weighted for each age group based on Statistics Canada 2021 population estimates [27] (Online Resource Table 3). QALY losses per HZ-only case and per HZ with PHN case were updated using input data from a modeling study by Drolet et al. [41], who estimated QALY losses using data from a Canadian study of patients aged ≥50 years with HZ or PHN. QALY losses for RZV AEs [42] were not updated as no new data were identified since the analysis by McGirr et al. [14].
2.3 Base-Case Analysis
The base-case analysis used the updated values presented in Table 1 and Online Resource Tables 1–4 and an annual discount rate of 1.5% for costs and QALYs, in line with recent guidelines from Canada’s Drug and Heath Technology Agency (CADTH) [43]. To incorporate potential uncertainty and assess the sensitivity of results to changes in the discount rate, additional analyses using rates of 0% and 3% were conducted.
2.4 One-Way and Probabilistic Sensitivity Analyses
One-way sensitivity analysis, where inputs were varied one at a time across defined ranges, was conducted to examine the sensitivity of the ICER to the value of each input. The first-dose coverage was varied by ±20% of the base-case value. The range for second-dose completion was 55–75%, with the upper bound consistent with the percentage of adults who received a second vaccine dose in the ‘12-month cohort’ of McGirr et al. [33]. The ranges for the baseline utility values obtained from Szende et al. [40] were assumed to be ±20% of the base-case values. The ranges for QALY losses were equal to the limits published in Drolet et al. [41]. The vaccine price was not varied in sensitivity analyses because vaccine prices are fixed in Canada. Further details on the ranges for the one-way sensitivity analysis are presented in Table 1 and Online Resource Tables 2 and 3.
A probabilistic sensitivity analysis (PSA) was conducted to examine the impact of uncertainty about the values of model inputs on the ICER estimate. For each PSA, ICERs were estimated from 5000 Monte Carlo simulations in which input values were simultaneously sampled from appropriate probability distributions. Costs and vaccine coverage rates were varied using gamma and uniform distributions, respectively. All other inputs were varied using beta distributions. The base-case values and standard errors (SEs) were used to estimate beta and gamma distribution parameters, while ranges were used to define upper and lower bound values for the uniform distributions. All inputs with SEs and/or ranges (as detailed in Table 1 and Online Resource Tables 2 and 3) were varied in the PSA. Age-dependent parameters that varied across age groups were assumed to be correlated.
Inputs that were not updated were varied in the one-way sensitivity analysis and the PSA as per the study by McGirr et al. [14].
2.5 Scenario Analyses
First-dose coverage values of 40%, 60%, and 80% were tested in three scenario analyses. A fourth scenario analysis estimated the cost effectiveness of RZV versus no HZ vaccination from the societal perspective. Indirect costs of HZ were estimated from absenteeism and presenteeism losses for HZ cases from Drolet et al. [44]. These were multiplied by age-specific employment rates as of February 2022 [45] and total compensation per hour worked in 2020 [46] inflated to 2022 CAN$ using the consumer price index for all items [38, 39] (Online Resource Table 4). Indirect costs for vaccination administration were estimated similarly, based on an assumed 1 h lost. Indirect costs for AEs were based on AE incidences in the ZOE-50 and ZOE-70 studies [16, 17], employment rates and compensation as above, and 4, 12, and 40 h of lost productivity for an AE resulting in a GP visit, ER visit, or hospitalization, respectively. Hours of lost productivity for vaccine administration and AEs were based on the opinion of clinical experts.
2.6 Outcomes
Health and economic outcomes included cases of HZ, PHN, and other complications (ocular, neurological, cutaneous, and other non-pain) avoided; HZ-related deaths avoided; life-years and QALYs gained; direct costs (vaccination, HZ costs, and total); and ICERs. For most analyses, these were from the perspective of the publicly funded healthcare system, and the willingness-to-pay threshold was assumed to be CAN$50,000 per QALY gained, as is commonly used in Canada [47]. All costs are in 2022 CAN$.
3 Results
3.1 Base-Case
In the base case, the model estimated that RZV at a first-dose coverage of 17.5% versus no HZ vaccination would prevent 303,835 cases of HZ, 83,256 cases of PHN, 39,653 complications, and 99 HZ-related deaths, and save 751 life-years and 16,814 QALYs (Table 2). Vaccination costs were estimated to be approximately CAN$606 million, with a direct cost saving of around CAN$144 million, for a total incremental direct cost of CAN$462 million. These outcomes equate to an ICER of CAN$27,486 per QALY gained. Discount rates of 0% and 3% resulted in ICERs of CAN$21,519 and CAN$33,816 per QALY gained, respectively. The number needed to vaccinate (NNV) to prevent one HZ case was 9 and the NNV to prevent one PHN case was 32.
3.2 One-Way and Probabilistic Sensitivity Analyses
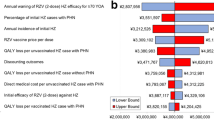
In the one-way sensitivity analysis, the ICER was most sensitive to the percentage of initial HZ cases with PHN, the QALY loss per unvaccinated HZ case with PHN, the annual waning of RZV second-dose VE against HZ, and the annual incidence of initial HZ (Fig. 1). The highest ICER was observed when the percentage of initial HZ cases with PHN was at its lower bound (CAN$44,724 per QALY gained).
One-way sensitivity analysis resultsa for the ICERs of RZV vs. no HZ vaccination for Canadian adults aged ≥50 years from a payer’s perspective (base-case ICER: CAN$27,486). aCosts are in 2022 CAN$. The 10 most influential inputs are shown. CAN$ Canadian dollars, HZ herpes zoster, ICERs incremental cost-effectiveness ratios, PHN post-herpetic neuralgia, QALY quality-adjusted life-year, RZV recombinant zoster vaccine
Stochastic results from 5000 Monte Carlo simulations estimated total median incremental direct healthcare costs of CAN$456 million and an incremental gain of 16,800 QALYs for RZV versus no HZ vaccination (Fig. 2). The runs had a median ICER of CAN$27,174 per QALY gained. Almost all simulations (98.1%) resulted in ICERs below the assumed willingness-to-pay threshold of CAN$50,000 per QALY gained (Fig. 3).
Incremental costsa vs. incremental QALYs from 5000 PSA simulations of RZV vs. no HZ vaccination for Canadian adults aged ≥50 years from a payer’s perspective. aCosts are in 2022 CAN$. CAN$ Canadian dollars, HZ herpes zoster, PSA probabilistic sensitivity analysis, QALYs quality-adjusted life-years, RZV recombinant zoster vaccine
Cost-effectiveness acceptability curve from PSA for RZV vs. no HZ vaccination for Canadian adults aged ≥50 yearsa from a payer’s perspective. aCosts are in 2022 CAN$. CAN$ Canadian dollars, HZ herpes zoster, ICER incremental cost-effectiveness ratio, PSA probabilistic sensitivity analysis, QALY quality-adjusted life-year, RZV recombinant zoster vaccine, WTP willingness-to-pay
3.3 Scenario Analyses
As first-dose RZV coverage was increased to 40%, 60%, and 80%, the estimated numbers of HZ cases prevented also increased, to 694,481, 1,041,721, and 1,388,962, respectively (Table 3). The estimated numbers of PHN cases, other complications, and HZ-related deaths prevented at various first-dose coverage rates increased similarly (Table 3), as did life-years and QALYs gained, vaccination costs, HZ costs averted, and total direct costs. However, as increasing coverage increased vaccination costs and HZ cases averted to the same extent, the ICER remained the same in all scenarios.
From a societal perspective (but otherwise, as per the base case), overall vaccination costs were estimated to increase to approximately CAN$695 million, but there were estimated savings of around CAN$179 million in indirect cost savings of HZ cases prevented (Table 4). These outcomes equate to an ICER of CAN$22,097 per QALY gained, which is more favorable than in the base-case analysis from the healthcare system perspective (CAN$27,486). Discount rates of 0% and 3% resulted in ICERs of CAN$16,061 and CAN$28,594 per QALY gained, respectively.
4 Discussion
This study provides updated results compared with a previous cost-effectiveness analysis for RZV in Canada by McGirr et al. [14], which are shown side-by-side in Online Resource Table 5. When compared with no HZ vaccination, the current analysis estimated that vaccination of adults aged ≥50 years with RZV at recent coverage (17.5%) and completion (65%) rates for an age-appropriate population approaching 15 million would prevent 303,835 cases of HZ, 83,256 cases of PHN, and 99 deaths, and save 16,814 QALYs. Although the direct vaccination costs would be approximately CAN$606 million, the prevention of HZ cases would save approximately CAN$144 million in direct medical costs due to HZ, resulting in an incremental direct cost of CAN$462 million and an ICER of CAN$27,486 per QALY saved. When societal costs relating to indirect costs of HZ were estimated from absenteeism and presenteeism losses, the ICER was reduced to CAN$22,097.
The most notable data input changes for this analysis were values for first-dose RZV coverage, RZV VE waning, QALY losses per case of HZ alone and HZ with PHN, and RZV AE costs (see Online Resource Table 6 for a side-by-side comparison of all updated input parameters). We also updated the age of the vaccinated population in the base case from ≥60 to ≥50 years because at the time of the analysis by McGirr et al. [14], HZ vaccination (with ZVL) was only recommended for people aged ≥60 years in Canada [11, 48], but this was updated by NACI in 2018 to recommended vaccination (with RZV) for Canadians aged ≥50 years [12].
In the analysis by McGirr et al. [14], 80% first-dose RZV coverage was assumed, along with 75% second-dose completion. Assumptions were used because RZV had not yet been introduced in the Canadian market and were assumed to be higher than influenza vaccine coverage rates. The updated analysis used real-world data for the Canadian population and applied 17.5% first-dose RZV coverage (up to 2022) [32], and, among first-dose recipients, 65% second-dose completion within the 2- to 6-month recommended time period [33]. The lower first-dose coverage resulted in lower estimated reductions in HZ in comparison with results in a ≥50-year-old population in the 2017 analysis (303,835 vs. 1,024,771 [14]) and PHN (83,256 vs. 264,605 [14]). Scenario analyses demonstrated that reductions in HZ (1,388,962) and PHN (380,598) cases were estimated to be greater in the current analysis than in the analysis by McGirr et al. [14] when first-dose RZV coverage was assumed to be the same as in the analysis by McGirr et al. (80%). This opportunity for an increased public health impact for RZV relative to the estimates by McGirr et al. [14] is driven by less waning of RZV VE and aging of the Canadian population.
RZV VE and waning estimates were updated in the current analysis based on up to 8 years of follow-up of the ZOE clinical trial populations [15], compared with up to 4 years of follow-up [16, 17] used in the analysis by McGirr et al. [14]. Among people aged 50–69 years, waning was reduced from 1% for the first 4 years and 2.3% thereafter to 1.5% overall, while among people aged ≥70 years, waning was reduced from 3.6 to 2.3%, as estimated by Curran et al. [24]. The changes also impacted the NNV to prevent one HZ case, which was reduced from 10 [14] to 9, and to prevent one PHN case, which reduced from 37 [14] to 32.
QALY losses per HZ case were reduced from 0.036 in the analysis by McGirr et al. [14] to 0.009 (age 50–59 years) or 0.01 (≥60 years), and were updated per PHN case from 0.1357 [14] to 0.041 (age 50–59 years), 0.192 (60–69 years), and 0.234 (≥70 years) based on data published in 2019 by Drolet et al. [41]. This would have reduced the numbers of QALYs saved due to HZ cases but increased the numbers of QALYs saved due to PHN cases, resulting in similar overall QALYs saved per HZ case avoided (55,361/1,024,771 = 0.054 [14] and 16,814/303,835 = 0.055). As the same HZ and PHN probabilities were used in both analyses, these QALY loss changes had little impact on the overall outcomes.
Overall, the updated data inputs between the analysis by McGirr et al. [14] and the current analysis resulted in an updated cost per QALY saved of CAN$27,486 (2022 costings) in comparison with CAN$30,402 (2016 costings) [14], indicating that RZV for adults aged ≥50 years in Canada remains a cost-effective option at a willingness-to-pay threshold of CAN$50,000 per QALY gained. We also evaluated the cost effectiveness of RZV from a societal perspective, which was not performed in the analysis by McGirr et al. [14]. The cost per QALY was lower in comparison with the healthcare payer perspective when indirect costs for vaccination and HZ were included (CAN$22,097). These findings are aligned with results from other ZONA model analyses that have predicted RZV to be cost effective versus no HZ vaccination in the United States (US) [19], Japan [22, 26], and Germany [24]. Three recent reviews have examined the cost effectiveness of RZV [49,50,51]. A 2022 meta-analysis of 12 studies (from the US, Asia, Canada, and Europe) that compared RZV versus no HZ vaccination concluded that RZV was cost effective for people aged 60–79 years from a societal perspective, and for those aged 60–69 years from a third-party payer perspective [49]. A 2023 review of 18 studies (from the US, Europe, Asia, and Canada) reported that RZV vaccination was predicted to be cost effective in 15 of the 18 included studies [50]. A 2022 systematic review of seven US studies concluded that RZV was a cost-effective strategy but that second-dose compliance is important [51]. Recent research has shown that barriers to the completion of RZV two-dose regimens include out-of-pocket costs, insurance coverage, accessibility, and patient forgetfulness, while healthcare provider encouragement, reminders, and self-motivation can improve completion [52]. Interventions addressing these factors could therefore help to improve the magnitude of the public health impact of RZV.
In the current one-way sensitivity analysis, the factors that increased the ICER to the largest values were a lower proportion of HZ cases developing PHN, lower HZ incidence, and higher RZV VE waning, but the ICER remained below the CAN$50,000 willingness-to-pay threshold. ZONA analyses from other countries also showed that higher waning [22, 24, 26], a lower proportion of HZ cases developing PHN [22, 24, 26], and a lower HZ incidence [24, 26] were key factors that increased ICER estimates. Given that the incidence of HZ is increasing with an aging Canadian population [5], a higher HZ incidence should result in lower ICERs.
Increasing first-dose coverage had a large impact on the potential public health impact of RZV. Research has shown that barriers to HZ vaccination include vaccine distrust [53], cost concerns [54, 55], and low perceived risk [55]. However, uptake and/or completion could potentially be improved by provider education [56, 57], physician’s recommendations [55], nurse/pharmacy administration [56], and use of prompts/reminders [56, 58]. Interventions such as these could help to improve uptake and/or completion, thus improving the public health impact of RZV in Canada.
4.1 Strengths and Limitations
The main strengths of the current analysis are the availability of Canada-specific first-dose coverage [32] and second-dose compliance data [33], and new RZV VE and waning estimates [15, 24]. The latter were based on the availability of up to 8 years of follow-up data [15], compared with up to 4 years of follow-up [16, 17] that were used in the McGirr et al. analysis [14]. However, estimating the QALYs lost for HZ and PHN is challenging, as evidenced by the rather different values used in the analysis by McGirr et al. [14] and the current study, which used newer values [41]. Moreover, the QALYs lost per case of HZ and PHN were assumed to be the same among vaccinated and unvaccinated individuals, but breakthrough HZ tends to be shorter [59]. Thus, the ICERs may have been overestimated. We also did not consider a larger indirect cost per case of HZ with PHN compared with HZ alone, which could have resulted in an underestimation of the value of RZV. Lastly, we assumed a willingness-to-pay threshold of CAN$50,000 per QALY, but this is not explicitly stated by Canadian health technology assessment bodies, such as CADTH, although there is some evidence to support this threshold [47].
5 Conclusions
RZV presents a cost-effective option, robust to a variety of sensitivity and scenario analyses, for vaccinating Canadian adults aged ≥50 years against HZ compared with no HZ vaccination (ICER CAN$27,486 per QALY gained from the healthcare payer perspective). Compared with the previous analysis [1], cost effectiveness was retained and the estimated public health impact was smaller (due to lower coverage/completion estimates). However, the potential public health impact was estimated to be greater when first-dose RZV coverage was assumed to be the same as the previous analysis driven by less waning of RZV VE and aging of the Canadian population.
Change history
17 July 2024
A Correction to this paper has been published: https://doi.org/10.1007/s41669-024-00502-w
References
Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84(3):274–80. https://doi.org/10.4065/84.3.274.
Curran D, Callegaro A, Fahrbach K, Neupane B, Vroling H, van Oorschot D, et al. Meta-regression of herpes zoster incidence worldwide. Infect Dis Ther. 2022;11(1):389–403. https://doi.org/10.1007/s40121-021-00567-8.
Chen SY, Suaya JA, Li Q, Galindo CM, Misurski D, Burstin S, et al. Incidence of herpes zoster in patients with altered immune function. Infection. 2014;42(2):325–34. https://doi.org/10.1007/s15010-013-0550-8.
Government of Canada. Herpes zoster (shingles) vaccine: Canadian Immunization Guide. 2018. Available at: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-8-herpes-zoster-(shingles)-vaccine.html. Accessed 18 Jan 2023.
Varghese L, Standaert B, Olivieri A, Curran D. The temporal impact of aging on the burden of herpes zoster. BMC Geriatr. 2017;17(1):30. https://doi.org/10.1186/s12877-017-0420-9.
Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82(11):1341–9. https://doi.org/10.4065/82.11.1341.
Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology. 2008;115(2 Suppl):S3–12. https://doi.org/10.1016/j.ophtha.2007.10.009.
Minor M, Payne E. Herpes zoster ophthalmicus. Treasure Island: StatPearls; 2022.
Johnson RW, Bouhassira D, Kassianos G, Leplege A, Schmader KE, Weinke T. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med. 2010;8(1):37. https://doi.org/10.1186/1741-7015-8-37.
Drolet M, Brisson M, Schmader KE, Levin MJ, Johnson R, Oxman MN, et al. The impact of herpes zoster and postherpetic neuralgia on health-related quality of life: a prospective study. CMAJ. 2010;182(16):1731–6. https://doi.org/10.1503/cmaj.091711.
National Advisory Committee on Immunization (NACI). Statement on the recommended use of herpes zoster vaccine. 2010. Available at: https://www.canada.ca/en/public-health/services/reports-publications/canada-communicable-disease-report-ccdr/monthly-issue/2010-36/canada-communicable-disease-report.html. Accessed 30 Jan 2023.
Warrington R, Ismail S, National Advisory Committee on Immunization (NACI). Summary of the NACI update on herpes zoster vaccines. Can Commun Dis Rep. 2018;44(9):220–5. https://doi.org/10.14745/ccdr.v44i09a06.
Drug Shortages Canada. Discontinuation report ZOSTAVAX II. Available at: https://www.drugshortagescanada.ca/discontinuance/159386. Accessed 1 May 2023.
McGirr A, Van Oorschot D, Widenmaier R, Stokes M, Ganz ML, Jung H, et al. Public health impact and cost-effectiveness of non-live adjuvanted recombinant zoster vaccine in Canadian adults. Appl Health Econ Health Policy. 2019;17(5):723–32. https://doi.org/10.1007/s40258-019-00491-6.
Boutry C, Hastie A, Diez-Domingo J, Tinoco JC, Yu CJ, Andrews C, et al. The adjuvanted recombinant zoster vaccine confers long-term protection against herpes zoster: interim results of an extension study of the pivotal phase 3 clinical trials ZOE-50 and ZOE-70. Clin Infect Dis. 2022;74(8):1459–67. https://doi.org/10.1093/cid/ciab629.
Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang SJ, Diez-Domingo J, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–32. https://doi.org/10.1056/NEJMoa1603800.
Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–96. https://doi.org/10.1056/NEJMoa1501184.
Curran D, Van Oorschot D, Varghese L, Oostvogels L, Mrkvan T, Colindres R, et al. Assessment of the potential public health impact of herpes zoster vaccination in Germany. Hum Vaccin Immunother. 2017;13(10):2213–21. https://doi.org/10.1080/21645515.2017.1345399.
Curran D, Patterson B, Varghese L, Van Oorschot D, Buck P, Carrico J, et al. Cost-effectiveness of an adjuvanted recombinant zoster vaccine in older adults in the United States. Vaccine. 2018;36(33):5037–45. https://doi.org/10.1016/j.vaccine.2018.07.005.
Watanabe D, Mizukami A, Holl K, Curran D, Van Oorschot D, Varghese L, et al. The potential public health impact of herpes zoster vaccination of people aged ≥ 50 years in Japan: results of a Markov model analysis. Dermatol Ther (Heidelb). 2018;8(2):269–84. https://doi.org/10.1007/s13555-018-0236-3.
van Oorschot DAM, Hunjan M, Bracke B, Lorenc S, Curran D, Starkie-Camejo H. Public health impact model estimating the impact of introducing an adjuvanted recombinant zoster vaccine into the UK universal mass vaccination programme. BMJ Open. 2019;9(5): e025553. https://doi.org/10.1136/bmjopen-2018-025553.
Shiragami M, Mizukami A, Kaise T, Curran D, Van Oorschot D, Bracke B, et al. Cost-effectiveness of the adjuvant recombinant zoster vaccine in Japanese adults aged 65 years and older. Dermatol Ther (Heidelb). 2019;9(2):281–97. https://doi.org/10.1007/s13555-019-0291-4.
Volpi A, Boccalini S, Dari S, Clarke C, Curran D, Loiacono I, et al. The potential public health impact of herpes zoster vaccination in the 65 years of age cohort in Italy. Hum Vaccin Immunother. 2020;16(2):327–34. https://doi.org/10.1080/21645515.2019.1657753.
Curran D, Van Oorschot D, Matthews S, Hain J, Salem AE, Schwarz M. Long-term efficacy data for the recombinant zoster vaccine: Impact on public health and cost effectiveness in Germany. Hum Vaccin Immunother. 2021;17(12):5296–303. https://doi.org/10.1080/21645515.2021.2002085.
Lee C, Jiang N, Tang H, Ye C, Yuan Y, Curran D. Potential public health impact of the adjuvanted recombinant zoster vaccine among people aged 50 years and older in Beijing. Hum Vaccin Immunother. 2021;17(10):3735–46. https://doi.org/10.1080/21645515.2021.1932216.
Teng L, Mizukami A, Ng C, Giannelos N, Curran D, Sato T, et al. Cost-effectiveness analysis update of the adjuvanted recombinant zoster vaccine in Japanese older adults. Dermatol Ther (Heidelb). 2022;12(6):1447–67. https://doi.org/10.1007/s13555-022-00744-8.
Statistics Canada. Population estimates on July 1st, by age and sex. 2022. Available at: https://doi.org/10.25318/1710000501-eng. Accessed 31 Mar 2022.
Statistics Canada. Mortality rates, by age group. 2022. Available at: https://doi.org/10.25318/1310071001-eng. Accessed 31 Mar 2022.
Marra F, Chong M, Najafzadeh M. Increasing incidence associated with herpes zoster infection in British Columbia, Canada. BMC Infect Dis. 2016;16(1):589. https://doi.org/10.1186/s12879-016-1898-z.
Drolet M, Brisson M, Schmader K, Levin M, Johnson R, Oxman M, et al. Predictors of postherpetic neuralgia among patients with herpes zoster: a prospective study. J Pain. 2010;11(11):1211–21. https://doi.org/10.1016/j.jpain.2010.02.020.
Brisson M. Estimating the number needed to vaccinate to prevent herpes zoster-related disease, health care resource use and mortality. Can J Public Health. 2008;99(5):383–6. https://doi.org/10.1007/BF03405246.
IQVIA. Estimated Shingrix immunization rate from IQVIA CompuScript NRx (March 2022 DM) and IQVIA GPM by Payer (March 2022 DM), both for the Canadian Shingles market. All Rights Reserved; GSK Internal Sales Data (March 2022 DM) for the Canadian Shingles market, as well as population data published by Statistics Canada. The statements, findings, conclusions, views, and opinions contained and expressed in this report are based only in part on data obtained under license from IQVIA Solutions Canada Inc. (IQVIA), and therefore such statements, findings, conclusions, views, and opinions are not necessarily those of IQVIA or any of its affiliated or subsidiary entities. Durham, NC; IQVIA
McGirr A, Bourgoin T, Wortzman M, Millson B, McNeil SA. An early look at the second dose completion of the recombinant zoster vaccine in Canadian adults: a retrospective database study. Vaccine. 2021;39(25):3397–403. https://doi.org/10.1016/j.vaccine.2021.04.053.
Friesen KJ, Chateau D, Falk J, Alessi-Severini S, Bugden S. Cost of shingles: population based burden of disease analysis of herpes zoster and postherpetic neuralgia. BMC Infect Dis. 2017;17(1):69. https://doi.org/10.1186/s12879-017-2185-3.
Ontario Ministry of Health and Long-Term Care. Ontario Case Costing Initiative Costing Analysis Tool. Acute Inpatient FY 2017/2018 statistics by most responsible diagnosis ICD-10 Code. 2021. Available at: https://hsim.health.gov.on.ca/hdbportal/. Accessed 15 Jun 2021.
Ontario Ministry of Health. Schedule of benefits. Physician services under the Health Insurance Act. 2022. Available at: https://www.health.gov.on.ca/en/pro/programs/ohip/sob/physserv/sob_master_20221201.pdf. Accessed 1 Feb 2023.
Ontario Ministry of Health and Long-Term Care. Formulary search. Available at: https://www.formulary.health.gov.on.ca/formulary/. Accessed 1 Apr 2022.
Statistics Canada. Consumer Price Index, annual average, not seasonally adjusted. 2022. Available at: https://doi.org/10.25318/1810000501-eng. Accessed 31 Mar 2022.
Statistics Canada. Consumer Price Index, monthly, not seasonally adjusted. 2022. Availabel at: https://doi.org/10.25318/1810000401-eng. Accessed 1 Apr 2022.
Szende A, Janssen B, Cabases J. Self-reported population health: An international perspective based on EQ-5D. Dordrecht: Springer; 2014.
Drolet M, Zhou Z, Sauvageau C, DeWals P, Gilca V, Amini R, et al. Effectiveness and cost-effectiveness of vaccination against herpes zoster in Canada: a modelling study. CMAJ. 2019;191(34):E932–9. https://doi.org/10.1503/cmaj.190274.
Le P, Rothberg MB. Cost-effectiveness of herpes zoster vaccine for persons aged 50 years. Ann Intern Med. 2015;163(7):489–97. https://doi.org/10.7326/M15-0093.
Canada's Drug and Health Technology Agency (CADTH). Guidelines for the Economic Evaluation of Health Technologies: Canada — 4th Edition. Available at: https://www.cadth.ca/guidelines-economic-evaluation-health-technologies-canada-4th-edition. Accessed 18 Jan 2023.
Drolet M, Levin MJ, Schmader KE, Johnson R, Oxman MN, Patrick D, et al. Employment related productivity loss associated with herpes zoster and postherpetic neuralgia: a 6-month prospective study. Vaccine. 2012;30(12):2047–50. https://doi.org/10.1016/j.vaccine.2012.01.045.
Statistics Canada. Labour force characteristics, monthly, seasonally adjusted and trend-cycle, last 5 months. 2022. Available at: https://doi.org/10.25318/1410028701-eng. Accessed 5 Apr 2022.
Statistics Canada. Labour productivity and related measures by business sector industry and by non-commercial activity consistent with the industry accounts. 2022. Available at: https://doi.org/10.25318/3610048001-eng. Accessed 5 Apr 2022.
Griffiths EA, Vadlamundi NK. CADTH’s $50,000 cost-effectiveness threshold: Fact or fiction? [abstract PHP278]. Value Health. 2016;19:A488. https://doi.org/10.1016/j.jval.2016.09.821.
National Advisory Committee on Immunization (NACI). Update on the use of herpes zoster vaccine. 2016. Available at: https://www.canada.ca/en/services/health/publications/healthy-living/updated-recommendations-use-herpes-zoster-vaccines.html. Accessed 30 Jan 2023.
Udayachalerm S, Renouard MG, Anothaisintawee T, Thakkinstian A, Veettil SK, Chaiyakunapruk N. Incremental net monetary benefit of herpes zoster vaccination: a systematic review and meta-analysis of cost-effectiveness evidence. J Med Econ. 2022;25(1):26–37. https://doi.org/10.1080/13696998.2021.2008195.
Giannelos N, Ng C, Curran D. Cost-effectiveness of the recombinant zoster vaccine (RZV) against herpes zoster: an updated critical review. Hum Vaccin Immunother. 2023. https://doi.org/10.1080/21645515.2023.2168952.
Meredith NR, Armstrong EP. Cost-effectiveness of herpes zoster vaccines in the U.S.: a systematic review. Prev Med Rep. 2022;29: 101923. https://doi.org/10.1016/j.pmedr.2022.101923.
George S, Awan A, O’Connor M, Foster A, Raymond K, Gorfinkel I, et al. Qualitative Interviews with Healthcare Providers and Adult Vaccine Recipients on the Attitudes, Barriers, and Facilitators to Completing the Recombinant Zoster Vaccine Regimen in Canada. Canadian Immunization Conference. 2023. Presented 26 Apr 2023.
Ricks T, Trent MJ, MacIntyre CR. Predictors of herpes zoster vaccination among Australian adults aged 65 and over. Vaccine. 2022;40(50):7182–6. https://doi.org/10.1016/j.vaccine.2022.10.064.
Hurley LP, O’Leary ST, Dooling K, Anderson TC, Crane LA, Cataldi JR, et al. Survey of physician practices, attitudes, and knowledge regarding recombinant zoster vaccine. J Gen Intern Med. 2023;38(4):986–93. https://doi.org/10.1007/s11606-022-07721-z.
Yang TU, Cheong HJ, Song JY, Noh JY, Kim WJ. Survey on public awareness, attitudes, and barriers for herpes zoster vaccination in South Korea. Hum Vaccin Immunother. 2015;11(3):719–26. https://doi.org/10.1080/21645515.2015.1008885.
Elkin ZP, Cohen EJ, Goldberg JD, Li X, Castano E, Gillespie C, et al. Improving adherence to national recommendations for zoster vaccination through simple interventions. Eye Contact Lens. 2014;40(4):225–31. https://doi.org/10.1097/ICL.0000000000000041.
O’Donnell M, Shurpin K, Janotha B. Improving herpes zoster vaccine rates: the impact of a targeted educational program. J Am Assoc Nurse Pract. 2018;30(8):435–40. https://doi.org/10.1097/JXX.0000000000000039.
Gatwood J, Brookhart A, Kinney O, Hagemann T, Chiu CY, Ramachandran S, et al. Clinical nudge impact on herpes zoster vaccine series completion in pharmacies. Am J Prev Med. 2022;63(4):582–91. https://doi.org/10.1016/j.amepre.2022.04.018.
Kim JH, Johnson R, Kovac M, Cunningham AL, Amakrane M, Sullivan KM, et al. Adjuvanted recombinant zoster vaccine decreases herpes zoster-associated pain and the use of pain medication across 3 randomized, placebo-controlled trials. Pain. 2023;164(4):741–8. https://doi.org/10.1097/j.pain.0000000000002760.
Acknowledgments
The authors thank Business & Decision Life Sciences Medical Communication Service Center for editorial assistance and manuscript coordination, on behalf of GSK. Jenny Lloyd (Compass Healthcare Communications Ltd., on behalf of GSK) provided medical writing support at all draft stages.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
GlaxoSmithKline Biologicals SA funded this study (GSK study identifier: VEO-000312) and all costs related to the development of the publications.
Conflicts Of Interest/Competing Interests
Sydney George is employed by GSK. Dessi Loukov, Cheryl Ng, Jessica Regan and Nikolaos Giannelos are employed by and hold shares in GSK. Justin Carrico and Katherine A. Hicks are employed by RTI Health Solutions and received consulting fees from GSK to perform the work disclosed in this publication. All authors declare no other financial and non-financial relationships and activities.
Author Contributions
SG, JC, KAH, DL, CN and NG were involved in the design of the study. SG, JC, and KAH collected and generated the data. All authors analyzed and/or interpreted the data and participated in the development of this manuscript. SG led the publication development and study design. All authors gave their final approval and are accountable for all aspects of the work.
Previous Congress Activities
CAPT, 17–18 October 2022, Toronto, ON, Canada.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
The model used during the current study is available from the corresponding author on reasonable request.
Clinical Trial Registration
Not applicable. GSK study identifier: VEO-000312.
Trademark Information
Shingrix is a trademark owned by or licensed to GSK.
Additional information
The original online version of this article was revise: The copyright holder for this article was incorrectly given as 'The Author(s)' but should have been 'GSK'.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
George, S., Carrico, J., Hicks, K.A. et al. Updated Public Health Impact and Cost Effectiveness of Recombinant Zoster Vaccine in Canadian Adults Aged 50 Years and Older. PharmacoEconomics Open 8, 481–492 (2024). https://doi.org/10.1007/s41669-024-00483-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-024-00483-w