Abstract
Introduction
This study aimed to update cost-effectiveness and public health impact estimates of the two-dose recombinant zoster vaccine (RZV) compared with no vaccination against herpes zoster (HZ) in the Japanese population aged 65 years. List price of the vaccine and latest RZV efficacy and waning estimates were incorporated.
Methods
A multicohort static Markov model with a cycle length of 1 year was used to follow a hypothetical cohort of one million people aged 65 years over their remaining lifetime (base case). Age-stratified vaccine efficacy and waning rates were updated on the basis of the latest clinical trial data (interim ZOE-LTFU; NCT02723773). First-dose coverage was assumed at 40%, and second-dose compliance was assumed at 95%. Costs and outcomes were discounted at 2% annually, and the incremental cost-effectiveness ratio (ICER) was calculated from payer and societal perspectives. The societal perspective considered productivity loss due to suffering HZ, or due to suffering HZ and time required for vaccination. Sensitivity analyses explored the overall uncertainties in the model. Scenario analyses for Japanese adults aged 50, 60, 70, 80, ≥ 50, and ≥ 65 years (main scenario) were conducted. An ICER below ¥5–6 million/quality-adjusted life-year (QALY) was considered cost-effective.
Results
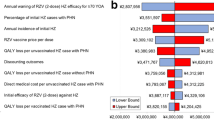
RZV was estimated to prevent 71,423 HZ cases and 15,858 post-herpetic neuralgia (PHN) cases per million people aged 65 years compared with no vaccine in Japan. The ICER was ¥4,205,515 from a payer perspective and was most sensitive to assumptions regarding vaccine efficacy waning, proportion of patients with HZ developing PHN, and HZ incidence. From societal perspectives, ICERs were ¥3,854,192 (productivity loss from suffering HZ only) and ¥4,622,212 (productivity loss from suffering HZ and time required for vaccination). Overall, the results were considered robust under extensive sensitivity and scenario analyses.
Conclusion
Vaccination against HZ with RZV is cost-effective compared with no vaccination in Japanese adults aged 65 years.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Herpes zoster (HZ), commonly known as shingles, is caused by the reactivation of the varicella-zoster virus (chickenpox virus) and typically affects older individuals. |
HZ and its complications such as post-herpetic neuralgia (PHN) can be prevented through vaccination. |
Previous studies, based on vaccine efficacy data collected up to 4 years post-vaccination from global studies and on assumed prices of the recombinant zoster vaccine (RZV) in Japan, estimated the number of shingles cases prevented and the potential cost-effectiveness of RZV in Japan. |
Here, we revised the mathematical model with the most up-to-date information to update previous analyses in the Japanese population. In particular, longer-term vaccine efficacy data and the list price of the vaccine were utilized. |
What was learned from the study? |
On the basis of a hypothetical cohort of one million Japanese adults aged 65 years, RZV vaccination was estimated to reduce the disease burden by 71,423 HZ and 15,858 PHN cases avoided compared with no vaccination in Japan. Moreover, an incremental cost-effectiveness ratio (ICER) of approximately ¥4.2 million per QALY gained (payer perspective) was observed, which is below the generally accepted threshold in Japan. |
Of the ages evaluated, the optimal age(s) at vaccination predicted by this model was 65 or ≥ 65 years, which can be taken into consideration for vaccination strategy policies. |
Digital Features
This article is published with digital features, including a graphical plain language summary, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.19711894.
Introduction
Herpes zoster (HZ), also known as shingles, is caused by the reactivation of the varicella-zoster virus (VZV) [1, 2]. HZ typically presents as a painful rash which resolves without intervention, although early antiviral therapy can shorten the length and severity of the illness [2]. However, some patients experience HZ-related complications, the most common being post-herpetic neuralgia (PHN) [1, 2]. PHN is pain that persists for at least 3 months after rash onset and is a condition that is challenging to manage clinically [1, 2]. HZ and PHN have substantial impact on the quality of life of patients and have been shown to incur a substantial economic burden [3, 4]. For example, the annual economic burden of HZ in Japan has been approximated at ¥20 billion (¥18.8 billion from a payer perspective and ¥24.5 billion from a societal perspective), consisting of direct medical costs predominantly and societal costs arising from working hours lost for patients and their caregivers [4].
Advancing age is a major risk factor of HZ and PHN due to age-related decline in immunity [1]. With increasing age, there is a natural decline in VZV-specific T-cell-mediated immunity and hence increased susceptibility to VZV reactivation, which likely explains the increase in HZ incidence with age [5]. Therefore, in countries with aging populations, such as Japan, the absolute number of HZ cases is predicted to increase in the next decade [6, 7]. The prevention of HZ and its complications is an ongoing public health goal, facilitated by the development of HZ vaccines [1].
In Japan, the one-dose live-attenuated zoster vaccine live (ZVL; Zostavax, Merck), which is used in several countries to prevent HZ, is not currently available. However, a varicella vaccine that contains the same attenuated live VZV (Oka strain) and a similar VZV titer as the ZVL is available (BIKEN, manufactured by the Research Foundation for Microbial Diseases of Osaka University). The BIKEN/Oka vaccine, previously approved for the prevention of chickenpox, was approved for an additional indication of HZ prevention in immunocompetent adults aged ≥ 50 years in March 2016 in Japan [8, 9]. More recently, a two-dose adjuvanted recombinant zoster vaccine (RZV; Shingrix, GSK) was made available in Japan.
The RZV was approved for HZ prevention in adults aged ≥ 50 years in March 2018 in Japan, following results of two global phase 3 clinical trials that assessed the efficacy of RZV [10, 11]. The ZOE-50 (NCT01165177) and ZOE-70 (NCT01165229) studies presented efficacy estimated up to 4 years post-vaccination for patients aged ≥ 50 years and ≥ 70 years, respectively. Using these data, the cost-effectiveness of RZV in Japan compared with no vaccination from a payer perspective was found to range from ¥4,316,457 to ¥8,952,500/quality-adjusted life-year (QALY) gained at a target vaccination age of 60 years and older [12,13,14]. Specifically, the incremental cost-effectiveness ratios (ICERs) were ¥4,316,457 per QALY gained for the population aged ≥ 65 years [12], ¥6,278,557 per QALY gained for those aged 65–84 years [13], and ¥8,952,550 per QALY gained for those aged 60 years [14]. However, these analyses were published before the launch of RZV in Japan (January 2020 [15]) and assumed RZV prices of ¥12,000–13,000 per dose [12,13,14], comparable to RZV prices used in the cost-effectiveness analyses for the USA [16] and Germany [17]. The current list price of RZV in Japan is ¥16,500 per dose [18].
Additionally, the previous cost-effectiveness analysis of RZV in Japanese adults does not reflect the most up-to-date parameters of RZV efficacy and waning rate. Interim results from the ongoing long-term follow-up (LTFU) of the ZOE-50 and ZOE-70 studies (ZOE-LTFU; NCT02723773) have shown that RZV efficacy remained high for up to 8 years post-vaccination [19]. When the updated vaccine efficacy results were incorporated into a Markov model, RZV was found to improve public health and cost-effectiveness results compared with previous analyses in the German population aged ≥ 50 years [20], although the effects in the Japanese population have not been explored.
It is imperative for cost-effectiveness models to be updated when new input data become available, so that cost-effectiveness estimates remain relevant to appropriately inform vaccination recommendations [21]. This study therefore aimed to provide supportive evidence for policymaking by updating a previously published cost-effectiveness model undertaken in the Japanese setting [12] with the latest long-term RZV efficacy data [19], as well as up-to-date parameters and analysis settings such as vaccine price. The primary objective of the present analysis was to reevaluate the cost-effectiveness of RZV vaccination from a payer’s perspective in preventing HZ and its complications compared with no vaccination in Japanese adults aged 65 years. The cost-effectiveness of RZV was also explored in other age cohorts and from a societal perspective.
Methods
Model Overview
The ZOster ecoNomic Analysis (ZONA) model is a static multicohort Markov model developed in Microsoft Excel to assess the public health impact and cost-effectiveness of RZV [16, 22]. The current study used a ZONA model previously adapted for the Japanese context by Shiragami et al. [12], with updates made to the estimates on vaccine efficacy and waning, vaccine price, and other parameters (including population size, annual all-cause mortality rates, proportion of mortality due to HZ, and costs) where necessary. In the current study, the model calculated a number of outputs, including the number of HZ cases, PHN cases, other HZ-related complications, deaths due to HZ, discounted life-years, discounted QALYs, and discounted costs accumulated for the cohort. In addition, the number needed to vaccinate with the RZV to avoid one case of HZ and PHN, and the number of visits and hospitalizations due to HZ, were each also estimated per one million cohort. Both the payer’s perspective and societal perspective were considered (details in the following sections).
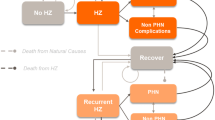
The model considers hypothetical cohorts split into five age groups (ages 50–59, 60–64, 65–69, 70–79, and ≥ 80 years). When considering vaccination scenarios, for example, directed at ages ≥ 65 years, the model combines the results of the age groups of 65–69, 70–79, and ≥ 80 years, assuming that all individuals in these age groups are vaccinated as in a “catch-up” campaign. Further details regarding the model structure, including health states, have been published elsewhere by Curran et al. [22]. In the current study, the probability of an individual moving between health states uses an annual time step. These age-specific transitional probabilities were derived from Japanese data, where available, and are detailed in Tables 1 and 2 and Supplementary Table 1.
In accordance with the Guideline for Economic Evaluation of Prophylactic Vaccines in Japan [23], lifetime health outcomes and associated costs were examined to capture the full effects of RZV vaccination through reduced morbidity and mortality. Annual discount rates of 2% on costs and life-years/QALYs were applied as the cohorts were evaluated over a lifetime and rates ranging between 0% and 4% were considered in a sensitivity analysis [23].
A deterministic sensitivity analysis (DSA), probabilistic sensitivity analysis (PSA), and threshold analysis for the base-case and main scenario (details in the following sections) were further carried out to explore the overall uncertainties in the model.
Base-Case Analysis
In the base-case analysis, vaccination age was set to 65 years, in line with the recommended routine vaccination age for the pneumococcal polysaccharide vaccine (23-PPV) in the national immunization program for older adults in Japan [24]. The analysis followed one million hypothetical individuals over their remaining lifetimes from the year of vaccination with annual cycle lengths. This hypothetical cohort size was chosen to allow for comparison with existing literature, especially from the public health impact perspective [12, 16, 20]. The two-dose RZV vaccination strategy (the second dose assumed to be given 2 months after the first dose) was compared with no vaccination, as there is no nationally recommended vaccine for HZ prevention in Japan [23].
The model calculated the public health impact and costs accumulated for a hypothetical cohort of one million people in the observed population over the entire time horizon under each intervention strategy (RZV or no vaccination). The outcomes were then compared to calculate incremental differences; the key outcome measure for all analyses was the ICER. Japan does not have an explicit ICER threshold. While the suggested willingness-to-pay (WTP) value per QALY gained in Japan is ¥5 million/QALY gained [25], an ICER range rather than a uniform price threshold has been proposed to be more appropriate in policy settings in Japan [26]. Hence, in the current study, an ICER below the range of ¥5–6 million/QALY gained was considered as cost-effective in Japan, consistent with the generally accepted cost-effectiveness threshold previously reported for the cost-effectiveness analysis of pneumococcal vaccination [27].
The base-case analysis considered a payer perspective, considering only vaccination and direct medical costs, based on the Guideline for Economic Evaluation of Prophylactic Vaccines in Japan [23].
Scenario Analysis
Multiple sets of scenario analyses were conducted. Six scenarios for single or combined age cohorts over 50 years were investigated: 50, 60, 70, 80, ≥ 50, and ≥ 65 years. The age cohort of ≥ 65 years was evaluated as the main scenario analysis to inform a potential catch-up vaccination program in adults above 65 years of age.
Estimates from a societal perspective, where indirect costs were added alongside vaccination and direct medical costs, were examined for the cohorts of age 65 years (base case) and age ≥ 65 years (main scenario). Two scenario analyses from the societal perspective were performed: (1) considering productivity loss due to suffering HZ only, and (2) considering productivity loss due to suffering HZ and time required for vaccination.
In addition, a scenario focusing on HZ morbidity only (i.e., excluding HZ mortality) was examined since deaths due to HZ could be relatively uncommon. A scenario considering the incidence of recurrent HZ to be different from that of the initial occurrence was also performed (details in the following section).
Model Inputs
Model inputs were divided into five categories: demographics, epidemiology, vaccine-specific parameters, costs, and utilities. A targeted literature review was conducted to assess for additional or new information of each parameter since the previous analysis by Shiragami et al. [12]. Additional data or insight were critically assessed and model inputs for the current analysis were updated where appropriate, as detailed below. All other model inputs have been described previously by Shiragami et al. [12] and are included in Supplementary Table 1.
Demographics and Epidemiological Data
Age-stratified population figures and annual all-cause mortality rates were updated with data from the portal site of Official Statistics in Japan (e-STAT), specifically the Estimates of the Population 2019 and Abridged Life Tables 2019, respectively [28].
The proportion of mortality due to HZ was derived by dividing the number of deaths due to HZ (obtained from e-Stat Vital Statistics in Japan 2019 [28]) with the estimated number of patients with HZ (calculated by HZ incidence; Supplementary Table 1 [12, 29] and e-Stat Estimation of Population Size in Japan 2019 [28]).
A new report for the incidence of recurrent HZ in Japan was identified, in which Shiraki et al. observed lower recurrent HZ incidence than initial HZ incidence (1.7 versus 4.8 per 1,000 person-years) in the population of Miyazaki Prefecture, Japan [30]. However, this was inconsistent with other global studies, where recurrent HZ incidence was comparable to [31] or higher than [32] initial HZ incidence. In view of the conflicting evidence, the current analysis adopted the approach taken by Shiragami et al. [12], which assumed that recurrent HZ incidence was the same as initial HZ incidence. This assumption was further tested using a scenario analysis, where a lower recurrent HZ incidence, estimated by applying the ratio to the incidence of initial HZ (i.e., 1.7/4.8 from Shiraki et al. [30]), was examined as a conservative scenario.
All other epidemiological model inputs, including HZ incidence rates, the proportion of HZ cases with PHN or other complications, and healthcare resource utilization per HZ case, have been described previously by Shiragami et al. [12] and are included in Supplementary Table 1.
Vaccine Efficacy and Waning
Vaccine efficacy and waning rate estimates for two doses of RZV against HZ were updated on the basis of data from interim ZOE-LTFU [19] (Table 1). The initial vaccine efficacy was estimated at 98.9% and 95.4% for adults aged 50–69 years and ≥ 70 years, respectively, as detailed in Curran et al. [20]. The waning rate was estimated at 1.5% and 2.3% annually for adults aged 50–69 years and ≥ 70 years, respectively [20]. The vaccine efficacy and its waning rate estimates for one dose of RZV against HZ were not changed from the Shiragami et al. analysis [12], and are included in Table 1.
The first-dose coverage was assumed at 40% on the basis of the 23-PPV vaccination coverage for older adults in Japan [24], as used by Shiragami et al. [12]. The second-dose compliance of RZV was assumed at 95% on the basis of clinical trial data and the second-dose compliance of pediatric vaccines in Japan [10, 11, 33], as used by Shiragami et al. [12]. The interval between the first and second doses was set at 2 months, in alignment with the standard interval recommended for RZV dosage in Japan [15].
Costs
Vaccination costs, direct medical costs, and indirect costs (for analysis from the societal perspective only) were considered to comprehensively capture the costs associated with HZ and HZ vaccination.
Vaccination costs consisted of vaccine price, administration costs, and adverse event (AE) costs due to vaccination. The RZV price per dose was updated from the previous analysis to ¥16,500 on the basis of the list price of the vaccine [18] (Table 2). The administration cost per dose (inclusive of the cost of initial visit, biologics, and injection) followed the same assumptions reported in Shiragami et al. [12] and were calculated on the basis of a Medical Fee Point Scheme 2020 [34] (Table 2). The costs associated with AEs similarly followed the same assumptions reported in Shiragami et al. [12], and updated cost data were obtained from a Medical Fee Point Scheme 2020 [34], Drug Price 2020 [35], or Diagnosis Procedure Combination (DPC) Point Scheme 2020 [34] (Table 2).
Direct medical costs included healthcare utilization due to HZ, PHN, and other HZ-related complications, and followed the data sources used in Shiragami et al. [12]. For the current analysis, direct medical cost data were adjusted by multiplying a revision rate of medical fees accumulated from year 2014 to year 2020 (-2.5%) [36] (Table 2).
Indirect costs were considered for scenario analyses from a societal perspective (for ages 65 and ≥ 65 only) and included costs arising from working hours lost for those vaccinated, as well as for patients with HZ and their caregivers. To estimate the productivity loss for individuals, the current study followed the data sources used in Shiragami et al. [12]. The age-specific wage and the employment rates were updated from the Basic Survey of Wage Structure 2019 and Survey of Labour Force 2019, respectively, included in e-Stat by age groups over 50 years in Japan [28]. The calculated hourly wages were ¥966 for ages 50–59, ¥692 for ages 60–69, ¥300 for ages 70–79, and ¥66 for ≥ 80 years, respectively. Productivity loss due to suffering HZ considered productivity loss of both patients and their caregivers and was calculated using the hourly wage described above (Table 2). The time required for vaccination was assumed to be 4 h per inoculation [37], and productivity loss was calculated by using the same hourly wage described above (Table 2).
Utilities
All baseline utility and QALY loss per HZ with or without PHN inputs have been described previously by Shiragami et al. [12], and are included in Supplementary Table 1.
Sensitivity Analysis
DSA was conducted to validate the robustness of the base-case (age group 65 years) and main scenario (age group ≥ 65 years) analysis results. The DSA was conducted by varying each of the model’s inputs one at a time across ranges. The ranges were informed by published confidence intervals or other variance data, where available. If appropriate data were not available, the ranges were determined on the basis of ±20% of the base-case estimates with some exceptions.
The examined range for the proportion of HZ cases with PHN was set at −50% to +20% of the base-case estimates. The lower bound of −50% was set on the basis of the total PHN proportion observed in two studies (9.2% in Sato et al. [38] and 19.7% in Takao et al. [29]), and the upper bound was assumed at +20% of the base-case estimates. The examined range for vaccination costs, including the vaccine price and administration cost, was set at ±10% of their point estimates owing to the limited uncertainty associated with the parameters. The examined range for AE costs per vaccination, as weighted AE costs based on the incidence of four AEs, was set at −50% to +100% of the base-case estimates, as used in Shiragami et al. [12]. Finally, the second-dose compliance of RZV was varied, with a range of 70–100%. The lower bound was assumed to be similar to the compliance rate within 6 months post-initial dose reported in the USA [39], while the upper bound was set to the maximum value. In Japan, the second RZV dose is recommended to be administered 2 months after the first dose, or within 6 months if the recommended interval has lapsed [15].
PSA was conducted to observe the variation of the cost-effectiveness in the base-case (age group 65 years) and main scenario (age group ≥ 65 years) analysis results. ICERs were estimated from 5000 Monte-Carlo simulations, in which input values were simultaneously sampled from probabilistic distributions. As done in Shiragami et al. [12], cost parameters and all other parameters except for the second-dose compliance were sampled across gamma and beta distributions, respectively. A uniform distribution was applied to the second-dose compliance for RZV. Age-specific incidence parameters that varied across age groups were assumed to be correlated using a correlation of 0.5.
A threshold analysis was conducted to investigate the values that selected key inputs could hold, while still maintaining an ICER of RZV vaccination versus no vaccination below various hypothetical WTP thresholds in the base-case analysis. Six sets of inputs, including the top sensitive parameters identified in the DSA, were examined: HZ incidence (initial and recurrent), percentage of HZ cases with PHN (initial and recurrent), initial efficacy of RZV (two dose), waning of RZV (one and two dose), vaccine price of RZV, and QALY loss per HZ case (HZ and PHN).
Compliance with Ethics Guidelines
This article is based on mathematical modeling with inputs informed primarily by previously conducted studies, and does not contain any studies with human participants or animals performed by any of the authors.
Results
Base-Case Population (Aged 65 Years)
On the basis of a fixed hypothetical cohort of one million Japanese adults aged 65 years, RZV was estimated to prevent 71,423 HZ cases, 15,858 PHN cases, and 6603 other HZ-related complications compared with no vaccination over the remaining lifetimes of one million individuals included in the model (Table 3). This corresponded to 2807 QALYs gained.
The HZ cases prevented allowed for direct medical cost savings of approximately ¥3.67 billion, with a vaccination cost of approximately ¥15.47 billion. These outcomes equated to an ICER of ¥4,205,515 per QALY gained from a payer perspective (Table 3). From a societal perspective, the approximate incremental costs were approximately ¥10.8 billion (considering productivity loss due to suffering HZ only) and ¥13.0 billion (considering productivity loss due to suffering HZ and time required for vaccination). The resulting ICERs were ¥3,854,192 per QALY gained and ¥4,622,212 per QALY gained, respectively (Table 4).
The number needed to vaccinate with RZV to prevent one case of HZ and PHN was 6 and 26, respectively (Table 3). Vaccination with RZV was associated with a reduction of 2720 hospitalizations and 428,960 outpatient visits due to HZ (Table 3).
Main Scenario Analysis (Aged ≥ 65 Years)
For the aged ≥ 65 years main scenario analysis cohort, RZV was estimated to prevent 56,272 HZ cases, 14,490 PHN cases, and 5730 other HZ-related complications compared with no vaccination over the remaining lifetimes (Table 5). This corresponded to 2706 QALYs gained.
For adults aged ≥ 65 years, similar to the results for adults aged 65 years, the ICER was ¥4,533,853 per QALY gained from a payer perspective, ¥4,244,476 per QALY gained for considering productivity loss due to suffering HZ only, and ¥4,614,515 per QALY gained for considering productivity loss due to suffering HZ and time required for vaccination.
The number needed to vaccinate with RZV to prevent one case of HZ and PHN was 8 and 28, respectively. Vaccination with RZV was associated with a reduction of 2374 hospitalizations and 353,312 outpatient visits due to HZ.
Other Ages Scenario Analyses
The ICER for age cohorts 50, 60, 70, 80, and ≥ 50 years ranged from ¥4,290,994 to ¥5,212,264 per QALY gained, from a payer perspective (Table 6).
Other Scenario Analyses
In the scenario focusing on HZ morbidity, where HZ mortality was excluded, the ICER for RZV versus no vaccination in Japanese adults aged 65 years was ¥4,277,936 per QALY gained, from a payer perspective (Table 7).
In the scenario considering a lower incidence of recurrent HZ than incidence of initial HZ occurrence, the ICER for RZV versus no vaccination in Japanese adults aged 65 years was ¥4,837,023, from a payer perspective (Table 7).
Deterministic Sensitivity Analysis
For the age 65 years cohort (base case), the ICER was the most sensitive to annual waning of RZV (second dose) HZ efficacy after age 70 years, followed by the percentage of initial HZ cases with PHN, annual incidence of initial HZ, discount rate for outcomes, and QALY loss per unvaccinated HZ case with PHN, in this order. The highest ICER achieved through the DSA was ¥5,995,155 per QALY gained, which remained below the generally accepted threshold range (Fig. 1).
Deterministic sensitivity analysis results for the ICER of RZV versus no vaccine for Japanese adults aged 65 years from a payer perspective. An ICER below the threshold range of ¥5–6 million/QALY gained was considered cost-effective in Japan. HZ herpes zoster, ICER incremental cost-effectiveness ratio, PHN post-herpetic neuralgia, QALY quality-adjusted life-year, RZV recombinant zoster vaccine, YOA years of age
DSA results for the age ≥ 65 years cohort were comparable to that of the age 65 years cohort. However, varying the two most sensitive parameters [annual waning of RZV (second dose) HZ efficacy after age 70 years and percentage of initial HZ cases with PHN] increased the maximum ICER to ¥6,246,366 and ¥6,658,248 per QALY gained, respectively, which were slightly over the generally accepted threshold range (Supplementary Fig. 1).
Probabilistic Sensitivity Analysis
For the age 65 years cohort (base case), approximately 76% and 92% of simulations resulted in a cost per QALY gained below the lower (¥5 million) and upper (¥6 million) bounds of the accepted WTP threshold range, respectively (Fig. 2).
Probabilistic sensitivity analysis results of RZV versus no vaccine for Japanese adults aged 65 years from a payer perspective, based on 5000 Monte-Carlo simulations, presented in a (a) scatterplot (lower-bound threshold was used) and (b) cost-effectiveness acceptability curve. Among the simulations, 75.6% and 91.7% resulted in an ICER (cost per QALY gained) below the lower and upper bounds of the generally accepted WTP threshold range, respectively (green). ICER incremental cost-effectiveness ratio, PSA probabilistic sensitivity analysis, QALY quality-adjusted life-years, RZV recombinant zoster vaccine, WTP willingness-to-pay
For the age ≥ 65 years cohort, approximately 64% and 88% of simulations resulted in a cost per QALY gained below the lower (¥5 million) and upper (¥6 million) bounds of the accepted WTP threshold range, respectively (Supplementary Fig. 2).
Threshold Analysis
Threshold analyses showed that increasing RZV price or waning by approximately 20% or 30%, respectively, would result in an ICER over the lower bound of the acceptable threshold (¥5 million). Decreasing the efficacy of RZV (two doses) or HZ incidence by approximately 10%, or decreasing the proportion of HZ with PHN or the QALY loss by approximately 20%, also resulted in an ICER over the lower bound of the acceptable threshold (Fig. 3).
Threshold analysis results for RZV versus no vaccine for Japanese adults aged 65 years from a payer perspective. An ICER below the threshold range of ¥5–6 million/QALY gained was considered cost-effective in Japan. Green dotted line denotes the lower-bound threshold (¥5 million/QALY gained). HZ herpes zoster, ICER incremental cost-effectiveness ratio, PHN post-herpetic neuralgia, QALY quality-adjusted life-year, RZV recombinant zoster vaccine
Discussion
The current study shows that with updated model input parameters, RZV had a substantial public health impact and remained cost-effective in Japanese adults aged 65 years in the base-case setting. Despite the increased vaccine price, sustained vaccine efficacy led to an estimated ICER of ¥4,205,515 per QALY gained from a payer perspective, which is below the ICER range of ¥5–6 million considered to be cost-effective in Japan. When examined from societal perspectives (scenario analysis), the estimated ICERs for those aged 65 years were approximately ¥3.9–4.6 million per QALY gained, which remained below the generally accepted ICER threshold in Japan. The ICERs were similarly below the generally accepted threshold in the scenarios focusing on HZ morbidity only (i.e., excluding HZ mortality) and considering a lower incidence of recurrent HZ.
The age cohort of ≥ 65 years was evaluated as the main scenario to inform a potential catch-up vaccination program, with reference to the ongoing 23-PPV routine and catch-up vaccination strategy in Japan [24]. In the current study, for the target population of adults aged ≥ 65 years, the ICER of RZV compared with no vaccination remained cost-effective at approximately ¥4,530,000 per QALY gained. ICERs were less favorable in scenario analyses where the starting age for introducing RZV vaccination was changed from age 65 years to 50, 60, 70, and 80 years, although these differences were small and the ICERs were still below the generally accepted cost-effectiveness threshold. The ICER for the age ≥ 50 years cohort was also below the generally accepted cost-effectiveness threshold but was slightly higher than that of the age ≥ 65 years cohort. Thus, solely based on ICER comparisons, RZV vaccination at ages 65 or ≥ 65 years may be considered optimal from a cost-effectiveness perspective compared with the other ages evaluated. Accordingly, the potential for HZ vaccination to adopt a similar strategy to pneumococcal vaccination in Japan—routine RZV vaccination at age 65 years and a catch-up program at age ≥ 65 years—may be positively considered.
The cost-effectiveness of RZV compared with no vaccination in Japan has been reported in previous studies with varying results [12,13,14]. These differences were driven mainly by differing assumptions on vaccine waning rates. The strength of our study is that the most up-to-date efficacy and waning data up to 8 years post-vaccination were incorporated into the model [19]. In contrast, initial data available on RZV efficacy were limited to a maximum of 4 years post-vaccination [10, 11]. Moreover, it should be noted that vaccine efficacy data, as opposed to effectiveness data, were used in the current study. Vaccine efficacy data are less prone to selection bias and confounders as they are accounted for in randomized control trials, and thus are considered higher-grade evidence than vaccine effectiveness data usually obtained from retrospective analyses of healthcare databases.
In addition, the current study incorporated the list price of RZV, whereas an assumed vaccine price was used in the previous analysis [12]. By incorporating the list price, which is higher than the previously assumed vaccine price, the current study provides an up-to-date estimation of the cost-effectiveness of RZV in Japan’s context.
Overall sensitivity analysis showed that the results were considered robust under both DSA and PSA. DSA suggested that the cost-effectiveness of RZV compared with no vaccine was sensitive to assumptions regarding the annual waning of two-dose RZV efficacy after age 70 years, percentage of HZ developing to PHN, annual incidence of initial HZ, discount rate for outcomes, and QALY loss per unvaccinated HZ case with PHN, in this order. The three most sensitive parameters were the same as those identified in the previous analysis by Shiragami et al. [12]. In addition, all five parameters had been identified in the sensitivity analyses of other cost-effectiveness studies examining RZV in Japan and elsewhere [13, 14, 16, 17, 40, 41], suggesting that these parameters are indeed important factors that affect the cost-effectiveness evaluation of RZV. The majority of these studies similarly observed that waning-related parameters and the annual incidence of initial HZ had a significant impact on the ICER [13, 16, 17, 40]. The magnitudes of impact on ICER caused by varying other parameters differed depending on the range examined. In the threshold analysis, we determined the range of parameters that would allow the ICER to remain cost-effective at the lower bound of the generally acceptable threshold (i.e., ¥5 million). These ranges may have been wider if the model used the upper bound of the ICER threshold, meaning that the ICER remains cost-effective even with a greater variation in the parameters.
Despite the strengths of our analysis, some limitations remain. There is a lack of data regarding the second-dose compliance for RZV in Japan and the study assumed a rate of 95% in the base-case analysis, on the basis of clinical trial data [10, 11]. Compliance rates of more than 95% for multiple-dose pediatric vaccines have been reported in the Japanese setting [33]. Even though these observations are not directly generalizable to other age groups, they suggest that high compliance rates are achievable in Japan. In the sensitivity analysis, setting the second-dose compliance rate to 70%, comparable to what has been observed in the USA [39], had little impact on the ICER in the current study. Second-dose compliance rate was not within the top ten sensitive parameters in the DSA. However, a lower compliance rate may reduce the number of HZ cases avoided [42].
In the current model, the direct medical costs used may not fully reflect the most up-to-date healthcare utilizations in Japan. The direct medical costs were based on a study conducted in 2013–2015, as used by Shiragami et al. [12]. Although the overall standard of care for HZ has not changed in Japan since then, there have been introductions of generics, new medications and diagnostics, as well as fluctuations in the fees of existing medical services and drugs [34, 35]. It was not feasible to fully incorporate these changes into the direct medical cost estimates in the current analysis. Instead, the direct medical cost estimates from 2014–2020 were adjusted according to the accumulated revision rate (−2.5%) for the base-case cost value and an extensive sensitivity analysis was conducted for this set of parameters. From the DSA, the ICER was not sensitive to the direct medical cost estimates, suggesting that this limitation has minimal impact on the conclusion of the current analysis.
Furthermore, a review of epidemiological studies suggested that more than 30% of patients with PHN experienced persistent pain for over a year [43]. In the present model, the impact of PHN on utility and costs was conservatively counted during one annual cycle. If the model allowed for PHN to last for more than a year, the resulting ICER would decrease.
Despite the limitations, this cost-effectiveness analysis model simulation incorporated the most up-to-date evidence and the robustness of our cost-effectiveness findings of RZV was supported by the sensitivity analyses.
Conclusions
Vaccination against HZ with RZV is cost-effective compared with no vaccination for Japanese adults aged 65 years at the up-to-date vaccine price, given the high and sustained efficacy of RZV observed from long-term data. Scenario analyses further suggest that RZV vaccination is also cost-effective when initiated in multiple age cohorts, which may represent the catch-up vaccination setting, particularly in those aged ≥ 65 years. RZV remained cost-effective under most of the alternative parameters and assumptions explored. The current analysis may aid local policymakers in their value assessment of RZV vaccination.
References
Gershon AA, Breuer J, Cohen JI, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1:15016. https://doi.org/10.1038/nrdp.2015.16.
Koshy E, Mengting L, Kumar H, Jianbo W. Epidemiology, treatment and prevention of herpes zoster: a comprehensive review. Indian J Dermatol Venereol Leprol. 2018;84(3):251–62.
Mizukami A, Sato K, Adachi K, et al. Impact of herpes zoster and post-herpetic neuralgia on health-related quality of life in Japanese adults aged 60 years or older: results from a prospective, observational cohort study. Clin Drug Investig. 2018;38(1):29–37.
Nakamura H, Mizukami A, Adachi K, et al. Economic burden of herpes zoster and post-herpetic neuralgia in adults 60 Years of age or older: results from a prospective, physician practice-based cohort study in Kushiro, Japan. Drugs Real World Outcomes. 2017;4(4):187–98.
Weinberg A, Lazar AA, Zerbe GO, et al. Influence of age and nature of primary infection on varicella-zoster virus-specific cell-mediated immune responses. J Infect Dis. 2010;201(7):1024–30.
Varghese L, Standaert B, Olivieri A, Curran D. The temporal impact of aging on the burden of herpes zoster. BMC Geriatr. 2017;17(1):30.
Cabinet Office Government of Japan. White paper on ageing society 2020. https://www8.cao.go.jp/kourei/whitepaper/w-2020/html/zenbun/index.html. Accessed Jan 2022.
Japan Pharmaceuticals and Medical Devices Agency. Package insert for varicella vaccine live attenuated "Biken". https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/630144_631340ED1022_1_24. Accessed Jan 2022.
Hoshi SL, Kondo M, Okubo I. Cost-effectiveness of varicella vaccine against herpes zoster and post-herpetic neuralgia for elderly in Japan. Vaccine. 2017;35(24):3264–71.
Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–96.
Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–32.
Shiragami M, Mizukami A, Kaise T, et al. Cost-effectiveness of the adjuvant recombinant zoster vaccine in Japanese adults aged 65 years and older. Dermatol Ther (Heidelb). 2019;9(2):281–97.
Hoshi SL, Seposo X, Shono A, Okubo I, Kondo M. Cost-effectiveness of recombinant zoster vaccine (RZV) and varicella vaccine live (VVL) against herpes zoster and post-herpetic neuralgia among adults aged 65 and over in Japan. Vaccine. 2019;37(27):3588–97.
Shibahara H. Cost-effectiveness analysis of herpes zoster vaccine for the elderly in Japan. In: The 16th annual conference of Japan Health Economics Association (JHEA); Virtual, 2019. Presentation M-5.
Japan Pharmaceuticals and Medical Devices Agency. Package insert for recombinant zoster vaccine Shingrix. https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/340278_631341BE1028_2_04. Accessed Jan 2022.
Curran D, Patterson B, Varghese L, et al. Cost-effectiveness of an adjuvanted recombinant zoster vaccine in older adults in the United States. Vaccine. 2018;36(33):5037–45.
Van Oorschot D, Anastassopoulou A, Poulsen Nautrup B, et al. Cost-effectiveness of the recombinant zoster vaccine in the German population aged ≥ 60 years old. Hum Vaccin Immunother. 2019;15(1):34–44.
Iwatsuki K. Herpes Zoster Vaccine. J Jpn Med Assoc. 2020;149(7):1226.
Boutry C, Hastie A, Diez-Domingo J, et al. The adjuvanted recombinant zoster vaccine confers long-term protection against herpes zoster: interim results of an extension study of the pivotal phase III clinical trials (ZOE-50 and ZOE-70). Clin Infect Dis. 2021;74(8):1459–67.
Curran D, Van Oorschot D, Matthews S, et al. Long-term efficacy data for the recombinant zoster vaccine: impact on public health and cost effectiveness in Germany. Hum Vaccin Immunother. 2021;17(12):5296–303.
Szucs TD, Pfeil AM. A systematic review of the cost effectiveness of herpes zoster vaccination. Pharmacoeconomics. 2013;31(2):125–36.
Curran D, Van Oorschot D, Varghese L, et al. Assessment of the potential public health impact of herpes zoster vaccination in Germany. Hum Vaccin Immunother. 2017;13(10):2213–21.
Ikeda S. Guideline for study on the economic evaluation of vaccination. MHLW grants study. https://www.mhlw.go.jp/file/05-Shingikai-10601000-Daijinkanboukouseikagakuka-Kouseikagakuka/0000184902_1.pdf. Accessed Jan 2022.
Ministry of Health, Labour and Welfare (MHLW). Implementation guideline for National Immunization Program. https://www.mhlw.go.jp/content/000757429.pdf. Accessed Jan 2022.
Shiroiwa T, Sung YK, Fukuda T, et al. International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ. 2010;19(4):422–37.
Igarashi A, Goto R, Yoneyama-Hirozane M. Willingness to pay for QALY: perspectives and contexts in Japan. J Med Econ. 2019;22(10):1041–6.
Igarashi A, Hirose E, Kobayashi Y, Yonemoto N, Lee B. Cost-effectiveness analysis for PCV13 in adults 60 years and over with underlying medical conditions which put them at an elevated risk of pneumococcal disease in Japan. Expert Rev Vaccines. 2021;20(9):1153–65.
Statistics Bureau (Ministry of Internal Affairs and Communications). E-Stat: Portal Site of Official Statistics of Japan. https://www.e-stat.go.jp/. Accessed Aug 2021.
Takao Y, Miyazaki Y, Okeda M, et al. Incidences of herpes zoster and postherpetic neuralgia in Japanese adults aged 50 years and older from a community-based prospective cohort study: the SHEZ study. J Epidemiol. 2015;25(10):617–25.
Shiraki K, Toyama N, Daikoku T, Yajima M. Herpes zoster and recurrent herpes zoster. Open Forum Infect Dis. 2017;4(1):ofx007. https://doi.org/10.1093/ofid/ofx007.
Tseng HF, Bruxvoort K, Ackerson B, et al. The epidemiology of herpes zoster in immunocompetent, unvaccinated adults ≥ 50 years old: incidence, complications, hospitalization, mortality, and recurrence. J Infect Dis. 2020;222(5):798–806.
Kim YJ, Lee CN, Lee MS, et al. Recurrence rate of herpes zoster and its risk factors: a population-based cohort study. J Korean Med Sci. 2018;34(2): e1. https://doi.org/10.3346/jkms.2019.34.e1.
Ministry of Health, Labour and Welfare (MHLW). Vaccination information. http://www.mhlw.go.jp/topics/bcg/other/5.html. Accessed Jan 2022.
Ministry of Health, Labour and Welfare (MHLW). About medical fee revision in 2020. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000188411_00027.html. Accessed Jan 2022.
Ministry of Health, Labour and Welfare (MHLW). About listing in the National Health Insurance price list and generics in 2020. https://www.mhlw.go.jp/topics/2020/04/tp20200401-01.html. Accessed Jan 2022.
Ministry of Health, Labour and Welfare (MHLW). About medical fee revision. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000106602.html. Accessed Jan 2022.
Shiroiwa T. Examination of how to present the results of cost-effectiveness evaluation of vaccines-cost-effectiveness analysis of vaccination against rotavirus. MHLW Research Grant (Emerging/Reemerging Infectious Diseases and Vaccination Policy Promotion Research Project). 2016.
Sato K, Adachi K, Nakamura H, et al. Burden of herpes zoster and postherpetic neuralgia in Japanese adults 60 years of age or older: results from an observational, prospective, physician practice-based cohort study. J Dermatol. 2017;44(4):414–22.
Patterson BJ, Chen C-C, Mcguiness CB, et al. Early examination of real-world uptake and second-dose completion of recombinant zoster vaccine in the United States from October 2017 to September 2019. Hum Vaccin Immunother. 2021;17(8):2482–7.
Prosser LA, Harpaz R, Rose AM, et al. A cost-effectiveness analysis of vaccination for prevention of herpes zoster and related complications: input for national recommendations. Ann Intern Med. 2019;170(6):380–8.
Le P, Rothberg MB. Cost-effectiveness of the adjuvanted herpes zoster subunit vaccine in older adults. JAMA Intern Med. 2018;178(2):248–58.
Watanabe D, Mizukami A, Holl K, et al. The potential public health impact of herpes zoster vaccination of people aged ≥ 50 years in Japan: results of a Markov model analysis. Dermatol Ther (Heidelb). 2018;8(2):269–84.
Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6): e004833.
Honda M, Murata T, Ebata N, Fujii K, Ogawa S. Treatment patterns of postherpetic neuralgia patients before and after the launch of pregabalin and its effect on medical costs: analysis of Japanese claims data provided by Japan Medical Data Center. J Dermatol. 2017;44(7):767–73.
Acknowledgements
Funding
GlaxoSmithKline Biologicals SA was the funding source and was involved in all study activities and overall data management (collection, analysis, and interpretation) or this study (GSK identifier VEO-000184). GlaxoSmithKline Biologicals SA also funded all costs associated with the development and the publishing of the present manuscript. All authors had full access to all the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and/or Editorial Assistance
The authors thank Professor Ataru Igarashi, Yokohama City University School of Medicine, for critical review of the study. The authors also acknowledge Roeland Van Kerckhoven, PhD, GSK for publication management, Kyra Chan, PhD, from Costello Medical, Singapore, for medical writing and editorial assistance based on the authors’ input and direction, and Sharon Lee, PhD, from Costello Medical, Singapore, for publication coordination and editorial support.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception and design and were involved in the analyses or interpretation of the results. LT, AM, NG, CL, and TM participated in the collection or generation of the data. All authors were involved in drafting the article or revising it critically for important intellectual content. All authors read and approved the final manuscript.
Prior Presentation
This article includes data that have been presented at the 25th Annual Meeting of Japanese Society for Vaccinology (JSVAC), 3–5 December 2021, Karuizawa, Japan.
Disclosures
Lida Teng’s affiliation/department has received grants from CSL Behring Japan Inc., Fuji Film Inc., Otsuka Pharmaceutical Co. Ltd., Takeda Pharmaceutical Inc., and Terumo Corporation. Lida Teng has personally received supporting fees from the GSK group of companies during the conduct of the study. Akiko Mizukami, Cheryl Ng, and Nikolaos Giannelos are employees of the GSK group of companies. Desmond Curran, Tomohide Sato, and Taizo Matsuki are employees of and have stock ownership in the GSK group of companies. Christa Lee was an employee of and had stock ownership in the GSK group of companies at the time of the study, and is unaffiliated at the time of manuscript acceptance.
Compliance with Ethics Guidelines
This article is based on mathematical modeling with inputs informed primarily by previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article or as Supplementary Material.
Trademark Statement
Shingrix is a trademark of the GSK group of companies. Zostavax is a trademark of Merck Sharp & Dohme Corp.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Teng, L., Mizukami, A., Ng, C. et al. Cost-Effectiveness Analysis Update of the Adjuvanted Recombinant Zoster Vaccine in Japanese Older Adults. Dermatol Ther (Heidelb) 12, 1447–1467 (2022). https://doi.org/10.1007/s13555-022-00744-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-022-00744-8