Abstract
Introduction
This study evaluated the cost effectiveness of adjuvant olaparib versus watch and wait (WaW) in patients with germline breast cancer susceptibility gene 1/2 (gBRCA1/2)-mutated, high-risk, human epidermal growth factor receptor 2 (HER2)-negative early breast cancer (eBC), previously treated with neoadjuvant or adjuvant chemotherapy, from a Swedish healthcare perspective.
Methods
A five-state (invasive disease-free survival [IDFS], non-metastatic breast cancer [non-mBC], early-onset mBC, late-onset mBC, death) semi-Markov state transition model with a lifetime horizon was developed. Transition probabilities were informed by data from the Phase III OlympiA trial, supplemented with data from additional studies in BRCA-mutated, HER2-negative mBC. Health state utilities were derived via mapping of OlympiA data and supplemented by literature estimates. Treatment, adverse events and other medical costs were extracted from publicly available Swedish sources. Incremental cost per life-year (LY) and quality-adjusted life-year (QALY) gained were estimated. Costs and outcomes were discounted at 3% annually. One-way deterministic and probabilistic sensitivity analyses (PSA) were conducted.
Results
Over a lifetime horizon, adjuvant olaparib was associated with an additional 1.50 LYs and 1.22 QALYs, and incremental cost of 471,156 Swedish krona (SEK) versus WaW (discounted). The resulting ICER was 385,183SEK per QALY gained for olaparib versus WaW. ICERs remained below 1,000,000SEK across a range of scenarios, and were consistent across subgroups (hormone receptor [HR]-positive/HER2-negative and triple-negative breast cancer [TNBC]). In PSA, the probability of olaparib being cost effective at 1,000,000SEK per QALY was 99.8%.
Conclusions
At list price, adjuvant olaparib is a cost-effective alternative to WaW in patients with gBRCA1/2-mutated, high-risk, HER2-negative eBC in Sweden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The results of this cost-effectiveness analysis suggest that 1 year of adjuvant olaparib is a cost-effective option compared with watch and wait (WaW) as a treatment for patients who have germline breast cancer susceptibility gene 1/2 (gBRCA1/2)-mutated, high-risk, human epidermal growth factor receptor 2 (HER2)-negative early breast cancer (eBC) previously treated with neoadjuvant or adjuvant chemotherapy in Sweden. |
Olaparib is expected to provide significant clinical and patient benefits evidenced by meaningful increases in life-years and QALYs gained versus WaW in this population. |
Olaparib is a cost-effective treatment option in both hormone receptor-positive/HER2-negative and triple-negative eBC. |
1 Introduction
Breast cancer is the most common form of cancer in females in Sweden, and is the fifth leading cause of cancer deaths worldwide [1, 2]. In Sweden in 2021, breast cancer accounted for approximately 30% of all newly diagnosed cancers in women; contrastingly, breast cancer is relatively uncommon in men (<1% of new cancer diagnoses) [3]. In 2022, there were 9491 new breast cancer diagnoses in Sweden [4]. The majority of breast cancers are diagnosed at an early stage (American Joint Committee on Cancer [AJCC] Tumor Node Metastasis [TNM] criteria [8th edition] stage I–III) before metastatic disease progression [5,6,7], in part due to the coordinated efforts of breast cancer screening programs [7].
Germline mutations in the breast cancer susceptibility gene 1 (BRCA1) and BRCA2 genes confer an increased risk for breast cancer [8]. Women with germline mutations in BRCA1/2 have a 45–72% lifetime risk of developing breast cancer, compared with 12% in the general female population [9]. Individuals with germline BRCA (gBRCA) mutations and a breast cancer diagnosis are often younger, have more aggressive disease and experience a higher rate of recurrence compared with individuals with a breast cancer diagnosis but without such mutations [10,11,12]. In patients with primary breast cancer in Sweden, the prevalence of gBRCA mutations has been estimated at 2.4–7.0% [1, 13, 14].
Classification of breast cancer into different histological subgroups, based on human epidermal growth factor receptor 2 (HER2) and hormone receptor (HR) status, is used to govern treatment decisions in breast cancer [15]. For breast cancer patients with high-risk early HER2-negative, either HR-positive (HR-positive/HER2-negative) or HR-negative (triple-negative breast cancer [TNBC]) disease, initial treatment typically consists of surgery, neoadjuvant chemotherapy, adjuvant radiotherapy and adjuvant chemotherapy; HR-positive/HER2-negative patients also receive endocrine therapy [15]. In Sweden in 2022, 10.1% of patients were diagnosed with TNBC, 11.4% were diagnosed with HER2-positive breast cancer and 78.4% were diagnosed with HR-positive/HER2-negative breast cancer [4].
Despite the curative intent of this treatment, and the relatively high long-term survival for patients with early breast cancer (eBC) [6, 16], patients with high-risk disease have an increased risk of disease recurrence and a poor prognosis [10, 11, 17]. High-risk disease is variably defined, but assessments of risk typically consider biomarker status (i.e., mutation status, e.g., gBRCA1/2-mutation; or HER2-negative, particularly TNBC), lymph node involvement at diagnosis and the presence of residual disease or positive pathologically confirmed lymph nodes following surgery and chemotherapy [18, 19].
Whilst TNBC and HR-positive/HER2-negative patients with high-risk disease have a similarly poor prognosis, the risk of recurrence over time differs for these two populations [17]. TNBC patients face the highest risk of recurrence during the first 5 years after diagnosis, with a significant decrease and plateauing of the recurrence rate over subsequent decades [20,21,22]. Contrastingly, patients with HR-positive/HER2-negative disease remain at a constant risk of recurrence for at least 20 years after diagnosis [22, 23]. Despite these differences in long-term outcomes, patients with gBRCA1/2-mutated, high-risk, HER2-negative eBC enrolled in OlympiA had a similar short-term risk of disease recurrence, regardless of HR status [19].
Olaparib, a poly-ADP ribose polymerase (PARP) inhibitor, has received approval from the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the adjuvant treatment of adult patients with gBRCA1/2 mutations who have high-risk, HER2-negative eBC previously treated with neoadjuvant or adjuvant chemotherapy [24, 25]. The approvals were based on the results of the phase III OlympiA clinical trial, the only clinical trial to date which assesses the use of adjuvant olaparib in eBC [19]. In OlympiA, patients were randomized to receive olaparib or placebo, and were stratified according to HR status, receipt of prior neoadjuvant or adjuvant chemotherapy, and prior platinum therapy [19].
OlympiA demonstrated that treatment with olaparib for up to 1 year is associated with significantly longer invasive disease-free survival (IDFS) or distant disease-free survival (DDFS) compared with placebo [19]. At the primary IDFS analysis, with a median follow-up of 2.5 years, 3-year IDFS was 85.9% in the olaparib arm versus 77.1% in the placebo arm (hazard ratio [HR] 0.58; 99.5% confidence interval [CI] 0.41–0.82; p < 0.001) [19]. At the second interim analysis of overall survival (OS), after a median follow-up of 3.5 years, olaparib demonstrated a significant improvement in OS relative to the placebo group (HR 0.68; 98.5% CI 0.47–0.97; p = 0.009) [26]. Furthermore, olaparib demonstrated a continued improvement in IDFS and DDFS. Specifically, 4-year IDFS was 82.7% in the olaparib arm versus 75.4% in the placebo arm (HR 0.63; 95% CI 0.50–0.78); 4-year DDFS was 86.5% in the olaparib arm versus 79.1% in the placebo arm (HR 0.61; 95% CI 0.48–0.77) [26]. The efficacy of olaparib observed in the overall trial population was consistent across stratification and pre-specified subgroups, including HR status (HR-positive/HER2-negative versus TNBC) [26].
To support its introduction into Swedish clinical practice, this study sought to evaluate the cost effectiveness, from a Swedish healthcare perspective, of olaparib as an adjuvant treatment compared with ‘watch and wait’ (WaW) in adult patients with gBRCA1/2 mutations who have high-risk, HER2-negative eBC previously treated with neoadjuvant or adjuvant chemotherapy.
2 Methods
2.1 Patient Population, Intervention and Comparators
The target population for this analysis was adult patients with gBRCA1/2 mutations who have high-risk, HER2-negative eBC previously treated with neoadjuvant or adjuvant chemotherapy. The baseline characteristics for the patient cohort in this cost-effectiveness analysis were based on the patient population in OlympiA and are presented in Table 1 [27].
As long-term recurrence patterns and post-recurrence treatment options differ based on histological subtype, the total costs and outcomes of treatment were first estimated separately for the TNBC and HR-positive/HER2-negative subgroups of OlympiA. The subgroup results were then combined to calculate the incremental cost-effectiveness ratio (ICER) for olaparib in the HER2-negative (‘intention-to-treat’ [ITT]) population. Following the method reported by Murphy et al. [28], the pooled ITT ICER was estimated as the weighted average of the incremental costs divided by the weighted average of the incremental quality-adjusted life years (QALYs) for olaparib versus WaW in each subgroup. The weights used in the base-case analysis were based on the prevalence of HR-positive/HER2-negative (17.7%) and TNBC (82.3%) in OlympiA [27]. Alternate estimates of prevalence were tested in scenario analyses.
Prior to the initiation of OlympiA, no maintenance treatments were available following neoadjuvant or adjuvant chemotherapy for patients with gBRCA1/2-mutated, high-risk, HER2-negative eBC. Furthermore, there were no treatments available that specifically targeted BRCA1/2 mutations [15]. Therefore, the intervention of interest is olaparib and the comparator used was the placebo arm of OlympiA as a proxy for WaW. Patients with HR-positive/HER2-negative disease could also receive endocrine therapy alongside olaparib or WaW.
2.2 Model Structure, Time Horizon and Discounting
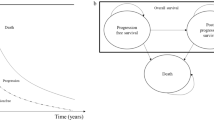
A Microsoft Excel®-based, five-state semi-Markov state transition model was developed following approaches accepted in previous health technology assessment (HTA) submissions in eBC [29,30,31,32,33]. The five health states used in the model were IDFS (starting state), non-metastatic breast cancer (non-mBC), early-onset mBC, late-onset mBC, and death (Fig. 1); the definitions of metastatic and non-metastatic recurrence to inform health state occupancy were based on the Standardized Definitions for Efficacy End Points (STEEP) criteria, as per the primary and secondary endpoints of IDFS and DDFS, respectively, in OlympiA [19]. Tunnel states were included to model time-varying transition risks from entry to the non-metastatic or mBC states.
Inclusion of two mBC health states enables the model to capture differences in risk of death based on the timing of recurrence; patients with early recurrence, defined as occurring within the first 2 years of the time horizon, typically have more aggressive disease that is less responsive to subsequent treatment than patients who experience late recurrence, occurring after 2 years [34,35,36]. Alternative definitions of early versus late recurrence were tested in scenario analyses.
Costs, life years (LYs) and QALYs were modelled over a lifetime horizon of 57 years, calculated based on a mean age in OlympiA of 43 years [27]. The cycle length of the model was 1 month (30.4 days). A life-table approach to half-cycle correction was adopted for costs and outcomes accrued over each model cycle. Exceptions include treatment costs for adjuvant olaparib, which were applied at the start of each model cycle to account for wastage by assuming a full monthly pack price was accrued by patients who received treatment at the start of the month but discontinued before the end of the cycle, and one-off costs and QALY adjustments. In line with Tandvårds- och läkemedelsförmånsverket (TLV) guidelines, costs and health outcomes were discounted at an annualized rate of 3% [37].
2.3 Clinical Effectiveness
The primary data source used to model transition probabilities (TPs) was the OlympiA trial (TP1, TP2 and TP4–6), derived from the analysis of patient-level data at the second interim analysis for OS [26].
For IDFS (TP1 and TP2), TNBC-specific IDFS data were used for the analysis of the TNBC subgroup because they were sufficiently mature and provided the most robust dataset for this analysis. Alternatively, ITT data were used as a proxy for the HR-positive/HER2-negative analysis due to the limited number of IDFS events observed in this subgroup (25 and 34 invasive disease events occurred in the olaparib and placebo arms, respectively, after a median follow-up of 3.5 years) [26]. In OlympiA, the observed hazard rate for IDFS in the placebo arm was greater than or approximately equal to olaparib throughout follow-up (electronic supplementary material [ESM] Fig. S3). Therefore, the modelled hazard rates for olaparib were assumed to be the same as for placebo from the point at which the rates converged across arms, thereby assuming no further treatment benefit. The transitions from IDFS to non-metastatic (TP1) or metastatic (TP2) breast cancer were modelled using a constant probability of an IDFS event being non-metastatic (23.8%) or metastatic (76.2%). For TNBC, the risks of non-mBC or mBC were assumed to decline to zero at 5 years, in line with clinical expectations and the literature [20, 21, 38]. For the HR-positive/HER2-negative population, breast cancer events could occur throughout the lifetime horizon [23].
Event numbers in OlympiA were too low to inform the transition from IDFS to death (TP3), therefore Swedish lifetables were used to estimate the probability of this transition in the model [39]. Background mortality risk was age- and gender-matched to the characteristics of the OlympiA population and further adjusted for the excess mortality associated with gBRCA1/2 mutations [40]. Transition probabilities for the non-metastatic and metastatic recurrence (TP4–6) were informed by data from the OlympiA ITT population. ITT data were preferred over subgroup data to maximize the sample size, given the limited event numbers and considering the assumption that the risk of an event occurring was not meaningfully impacted by HR status. Limited crossover occurred during OlympiA and therefore this would not be expected to impact the transition probabilities. Data from additional studies in BRCA-mutated, HER2-negative mBC were used to inform survival modelling from the late-onset mBC state (TP7) [41,42,43,44,45], as data from the OlympiA trial were not sufficiently mature; OS data from OlympiA were used to validate the model predictions.
Standard parametric models (including exponential, lognormal, Weibull, loglogistic, Generalized Gamma, Gompertz and Gamma) were fit to the clinical data informing each transition probability in order to extrapolate clinical outcomes beyond the trial period. The choice of preferred model focused on statistical fit, clinical plausibility and external validation of the extrapolations.
To ensure clinical plausibility of the long-term model extrapolations, all-cause mortality data (life tables from Statistics Sweden) were used to constrain the risk of death from any state in the model to be greater than or equal to the background risk of death as determined by age [39].
A summary of each transition is provided in Table S8 (see ESM) and long-term model projections of IDFS and OS for the HER2-negative/HR-positive and TNBC populations are presented in Fig. 2. Further information on the modelling of clinical effectiveness is provided in the ESM (Sect. 1.1).
Model long-term prediction of IDFS (top) and OS (bottom) for TNBC (left) and HR-positive/HER2-negative (right) populations. For the TNBC population, the KM curves reflect the TNBC subgroup; for the HR-positive population, the KM curves reflect the ITT population. HER2 human epidermal growth factor receptor 2, HR hormone receptor, IDFS invasive disease-free survival, ITT intention-to-treat, KM Kaplan–Meier, OS overall survival, TNBC triple-negative breast cancer
2.4 Adverse Events
The model considered the QALY losses and costs associated with Grade ≥3 treatment-related adverse events that occurred in ≥3% of patients in either treatment arm of OlympiA. These outcomes were accrued at the start of the model time horizon.
2.5 Health-Related Quality of Life
Utility values were assumed to vary by health state only, given the absence of a significant or meaningful difference in health-related quality of life (HRQoL) between the arms of OlympiA (Table 2) [19, 46]. Utility values for the IDFS and non-metastatic health states were derived by mapping EORTC QLQ-C30 data from OlympiA to the EQ-5D-3L, using the Crott and Briggs algorithm, as it was derived from a cohort with similar characteristics to OlympiA (i.e., locally advanced breast cancer) [47]. An alternative mapping algorithm was applied in a scenario analysis (ESM Table S9).
The utility value for non-mBC was assumed to be the same as IDFS, as supported by the literature and assumed by previous models [48]. There were limited data from OlympiA to inform post-recurrence utilities because EORTC QLQ-C30 assessments were limited to 2 years and therefore relatively little data were collected after recurrence in OlympiA [19]. Consequently, utility values for the early and late-onset mBC health states were informed by published literature [48]. The health state utility values were age-adjusted using the general population health state utility norm equation from Ara and Brazier [49]. This was undertaken to prevent health state utility values in the model exceeding those of the general population, and to incorporate the effects of increasing comorbidities with age on HRQoL. In the absence of Swedish utility values, the health state utilities for all states were based on values derived from the UK 3L value set [50]. Disutility due to adverse events in the adjuvant setting was included in the analysis. For each arm of the model, the total QALY impact of adverse events was estimated based on the incidence, duration, and disutility of each event, and this combined QALY impact was applied to the first cycle in the model as a one-off adjustment (ESM Table S10). The impact of adverse events experienced by patients receiving subsequent treatment was not considered on the assumption that post-recurrence adverse events would impact both arms of the model, and therefore have a minimal influence on the incremental results.
2.6 Costs and Resource Use
The following costs and healthcare resource use were modelled: adjuvant therapy costs, treatment costs for recurrence of disease (drug, surgical and radiotherapy treatment costs), disease management and monitoring costs, adverse event costs and end-of-life costs. Unit cost data for the base case were extracted from a range of Swedish sources (2022 SEK) [51,52,53,54,55,56,57,58,59]. Resource use data (2022) were obtained from an interview with a Swedish clinical expert, who was involved in the OlympiA trial and was chosen because of their extensive experience in the treatment of breast cancer. Cost and resource use inputs are summarized in the following sections, with full details of the key model inputs and assumptions provided in the ESM (Sects. 1.3 and 1.4).
2.6.1 Adjuvant Therapy Costs
Drug acquisition costs for olaparib were calculated as a function of drug formulation, list price per pack and dosing schedule. The cost per model cycle (30.4 days) for olaparib treatment was 51,897.50SEK. Olaparib treatment was assumed to be administered until recurrence of disease, tolerability issues or adverse events, or the completion of 12 months of treatment; duration of adjuvant olaparib treatment was modelled on the proportion of patients remaining on treatment over time in OlympiA [26]. Following discontinuation or completion of olaparib treatment, patients were assumed to undergo WaW, alongside adjuvant endocrine therapy for HR-positive/HER2-negative patients, until disease recurrence. No administration costs were included in the model for olaparib as the treatment is given orally [24].
WaW comprises monitoring and surveillance for disease recurrence, therefore no drug acquisition or administration costs were assigned to this intervention; however, HR-positive/HER2-negative patients can receive, and incur costs for, adjuvant endocrine therapy. In the comparator arm of the model, patients underwent WaW from model entry to disease recurrence or death.
Drug acquisition costs for adjuvant endocrine therapy (in HR-positive/HER2-negative patients only) were modelled as a weighted average of the costs of tamoxifen, anastrozole and letrozole. Based on 72.5% of patients receiving adjuvant endocrine therapy (assumption based on clinical input), the weighted-average cost per model cycle (30.4 days) of endocrine therapy was 32.54SEK. Treatment with endocrine therapy was assumed to continue until disease recurrence, or for a maximum of 6.8 years; this value was based on clinical expert opinion and reflects the clinical estimate of 60% of patients receiving treatment for approximately 8 years, and 40% of patients receiving treatment for approximately 5 years in the base case. Healthcare resource use for the administration of endocrine therapies was assumed to be captured by the routine disease management costs assigned to the IDFS state.
2.6.2 Treatment Costs for Recurrent Disease
Patients entering the non-metastatic or metastatic disease states were assumed to receive further treatment, including surgery, radiotherapy and additional drugs. The share of each treatment depended on the health state (non-metastatic or metastatic), prior adjuvant treatment (olaparib or WaW), and the HR status (HR-positive/HER2-negative or TNBC) of patients.
Treatment costs for drugs, surgery and radiotherapy were modelled as a series of weighted average total treatment costs (drug, surgery, radiotherapy) that were applied as one-off costs to each patient entering the relevant health states. The application of one-off costs for post-recurrence treatment simplified the model calculation with minimal impact on results given that surgery and radiotherapy are expected to occur close to recurrence, and ongoing drug costs are expected to be limited due to the poor prognosis of patients after recurrence and the widespread use of low-cost chemotherapy.
2.6.3 Disease Monitoring and Management Costs
Disease monitoring and management costs were modelled using a series of health state costs that reflected resource use during routine care of breast cancer patients, including medical consultations and routine-tests. A time-in-state method was used to model the health state costs, which were independent of treatment arm.
To reflect the changing patterns of care over the course of follow-up, monthly health state costs for IDFS were calculated for three periods (Years 0–1, Years 1–5 and Year 5+). The patterns of care were expected to remain approximately constant over the first 5 years for the non-mBC health state and no further costs were assumed beyond Year 5, while for the mBC health states, the patterns of care were expected to be the same over time until the end of life.
2.6.4 Adverse Event Costs
A one-off cost was implemented to account for managing adverse events in the first cycle of the model.
2.6.5 End-of-Life Costs
Patients who transitioned to death were assumed to incur a one-off cost associated with terminal care. This was based on the end-of-life cost included in a previous economic analysis in HER2-positive breast cancer [55, 56].
2.7 Sensitivity and Scenario Analyses
One-way deterministic sensitivity analyses (DSAs) and scenario analyses were conducted to assess the robustness of the model results and the impact of individual parameters or assumptions on the model. The DSAs were performed on 309 model input parameters including the discount rate, clinical inputs, cost inputs and health state utility inputs. Whenever available, the standard error of selected comparators was obtained directly from the same data source that informed the mean value; otherwise, the standard error for each parameter was assumed to be 10% of the mean value.
A probabilistic sensitivity analysis (PSA) was conducted to estimate the probability of olaparib being cost effective relative to WaW, based on different willingness-to-pay thresholds. A Monte-Carlo simulation with 10,000 iterations was conducted. In each iteration, the model inputs were randomly drawn from the pre-specified distributions (ESM Table S13). The standard error of the selected distribution was obtained in the same way as the standard errors of parameters in the one-way DSAs.
3 Results
3.1 Base-Case Cost-Effectiveness Analysis Results
In the TNBC population, over a 57-year time horizon, total discounted costs were 666,012SEK for olaparib and 200,084SEK for WaW; total discounted QALYs were 15.66 and 14.40, respectively (Table 3). The resulting ICER was 371,522SEK per QALY gained for olaparib versus WaW.
In the HR-positive/HER2-negative population, total discounted costs were 773,103SEK for olaparib and 277,640SEK for WaW; total discounted QALYs were 13.30 and 12.22, respectively (Table 3). The resulting ICER was 458,975SEK per QALY gained for olaparib versus WaW.
Based on a weighted average of the results for the TNBC and HR-positive/HER2-negative populations, in the HER2-negative ITT population, adjuvant olaparib was associated with an incremental gain in LYs and QALYs (1.50 and 1.22, respectively) versus WaW, and incremental costs of 471,156SEK versus WaW (Table 3). The resulting ICER was 385,183SEK per QALY gained for olaparib versus WaW.
Differences in total costs across treatment arms, in both the TNBC and HR-positive/HER2-negative populations, were largely driven by the cost of olaparib, which accounted for ~90% of the difference in total costs across arms; breakdowns of the costs and QALYs by treatment arm are provided in the ESM (Sect. 1.5). Drug acquisition costs for early-onset mBC accounted for ~5–6%, whilst all other individual parameters contributed <1.2% each. The largest contributor to incremental QALYs was the health outcomes of IDFS, which accounted for ~87% of the total absolute incremental QALYs.
3.2 One-Way Deterministic Sensitivity and Scenario Analyses
In the TNBC population, the greatest changes in base-case results arose from variations in the discount rate for health benefits, the utility assigned to IDFS, the probability of non-distant metastasis and the excess mortality risk associated with gBRCA1/2 mutations. In the HR-positive/HER2-negative population, parameters with the greatest influence on the ICERs included the discount rate for health benefits, the probability of an IDFS event being a non-distant recurrence and the utility assigned to IDFS.
DSA plots can be found in the ESM (Figs. S9 and S10). Results of scenario analyses (ESM Table S20) suggest that the base case is robust to variations in several model choices, with the greatest change from the base case ICER resulting from use of alternative discount rates for health benefits (6.0%: +55.89%; 1.5%: −23.19%), a 30-year time horizon (+18.19%) and use of Lidgren et al. as the source of utility values for all model health states (+12.97%). The ICER remained below 1,000,000SEK across all scenarios tested.
3.3 Probabilistic Sensitivity Analysis
Across the 10,000 iterations of the PSA, the average incremental cost was 470,839SEK, and the average incremental QALY gain was 1.27 for olaparib versus WaW. The resulting probabilistic ICER was 371,651SEK per QALY gained for olaparib versus WaW. At a willingness-to-pay threshold of 1,000,000SEK per QALY gained, at which the probability of reimbursement for severe diseases is approximately 50% [60, 61], olaparib had a 99.8% probability of being cost effective versus WaW. Figures 3 and 4 display the cost-effectiveness plane and cost-effectiveness acceptability curve, respectively, for olaparib versus WaW.
4 Discussion
The treatment landscape in eBC is rapidly evolving. Prior to the initiation of OlympiA, standard of care included surgery, neoadjuvant or adjuvant chemotherapy and postoperative radiotherapy, while no maintenance treatments were available for patients with gBRCA1/2-mutated, high-risk, HER2-negative eBC with the aim to minimize disease recurrence and extend OS [15]. Furthermore, there were no treatments available that specifically target BRCA1/2 mutations [15], a disease biology which is associated with a higher recurrence rate and greater disease aggressiveness [10, 11]. However, since the initiation of OlympiA, abemaciclib has been granted reimbursement for the adjuvant treatment of high-risk, HR-positive/HER2-negative eBC in Sweden [62, 63]. Additionally, patients with TNBC can be offered pre- and postoperative pembrolizumab, and patients with remaining disease after neoadjuvant chemotherapy can be offered postoperative capecitabine [15, 64]. However, these therapies do not target the underlying tumor driver that results in the unique characteristics of the gBRCA1/2-mutated eBC patient population [62, 64, 65]. Furthermore, there is an evolving body of real-world evidence suggesting that the gBRCA1/2-mutated, HR-positive breast cancer population may not respond as well to cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors as the corresponding non-BRCA-mutated population [66,67,68]. Additionally, there are no data on the effect of postoperative capecitabine in a gBRCA1/2-mutated TNBC.
Recently, olaparib has been approved by the EMA as an adjuvant treatment for adult patients with gBRCA1/2 mutations who have high-risk, HER2-negative eBC previously treated with neoadjuvant or adjuvant chemotherapy [24]. To help payers and decision makers understand the economic value of olaparib in this indication, this five-state, semi-Markov model evaluated the cost effectiveness of olaparib versus WaW. Over a lifetime horizon, 1 year of adjuvant olaparib was expected to yield a substantial health benefit, with meaningful improvements in LYs and QALYs versus WaW, and an ICER of 385,183SEK per QALY gained versus WaW at list price in the ITT population. Results from the DSAs and PSA support the base-case findings; at a willingness-to-pay threshold of 1,000,000SEK per QALY gained, olaparib had a 99.8% probability of being cost effective versus WaW. As the willingness-to-pay threshold for severe diseases in Sweden is typically 1,000,000SEK [60], the current analysis suggests that 1 year of adjuvant olaparib, at list price, would likely be a cost effective option in Sweden compared with the current standard of care, regardless of HR status (deterministic ICER: 385,183SEK, 371,522SEK and 458,975SEK per QALY gained in the ITT, TNBC and HR-positive/HER2-negative populations, respectively).
The current analysis is considered to provide a robust estimate of the cost effectiveness of olaparib in the adjuvant setting. Firstly, it leverages data from the OlympiA clinical trial, a large, well-designed double-blinded randomized controlled trial that demonstrates a significantly reduced risk of disease recurrence and increased survival in patients who receive adjuvant olaparib versus WaW [26]. By separately assessing the cost effectiveness of olaparib versus WaW in TNBC and HR-positive/HER2-negative disease, the analysis was able to account for the long-term recurrence risk differences observed in published literature by histological subgroup [17]. Additionally, in the recent National Institute for Health and Care Excellence (NICE) appraisal of adjuvant olaparib in early breast cancer, clinical experts confirmed that the OlympiA trial was broadly generalisable to UK clinical practice [69]. Similarly, expert feedback received during the elicitation of Swedish resource use data confirmed that the trial design and inclusion criteria of OlympiA were applicable to Swedish practice. Further study of the real-world outcomes of adjuvant treatment in this population in Sweden is, however, warranted.
Despite the strengths of the analysis, the results should be considered in the context of several key limitations. At the time of analysis, the IDFS and interim OS data from OlympiA were relatively immature (14.5 to 22.6% and 8.1 to 11.9%, respectively) despite a median 3.5 years of follow-up [26]. The modelled cost-effectiveness analysis therefore relies on the extrapolation of these data to a lifetime horizon, leading to uncertainty in results. Evidence from the two available data cut-offs of OlympiA suggest that the benefit of 1-year olaparib is sustained with increasing follow-up, supporting the model predictions [19, 26]. Further follow-up of OlympiA will help address uncertainties.
Secondly, in OlympiA, there were insufficient data to model IDFS for the HR-positive/HER2-negative subgroup using data from the HR-positive/HER2-negative subgroup, which represented approximately 18% of the ITT cohort. The IDFS curve for the HR-positive/HER2-negative subgroup was modelled using data from the ITT population, under the assumptions of consistent treatment effects and short-term recurrence rates between high-risk gBRCA1/2-mutated patients with HR-positive/HER2-negative breast cancer and TNBC. This assumption was supported by data from OlympiA, and other PARP inhibitor studies in mBC, which have shown consistent efficacy by receptor status [26, 70].
In the absence of EQ-5D data from OlympiA, utility values were derived by mapping EORTC QLQ-C30 data from OlympiA to the EQ-5D-3L (UK value set), using the Crott and Briggs algorithm for the IDFS and non-mBC health states [47]. These data were combined with mBC utility estimates (EQ-5D-3L UK value set) from the published literature, which were based on breast cancer patients in Sweden. In the absence of Swedish utilities, all utility values were derived from the 3L UK value set. The resulting utility values are therefore subject to uncertainty because of the application of mapping to OlympiA, the use of multiple data sets, and the absence of utility values valued from a Swedish perspective. Nevertheless, results obtained in scenario analyses with an alternative mapping algorithm (that reported by Longworth et al. [71]) and alternative utility source (Lidgren et al. [48]) remained positive. Furthermore, although the utility value obtained for the IDFS health state was higher than values previously published in the literature for HER2-positive disease, recent studies have shown that disease-free eBC patients who remain disease free over time have a HRQoL comparable to that of the general population [72,73,74].
Expert validation of resource use data was obtained from a single clinical expert. Although the model results were relatively insensitive to assumptions surrounding healthcare resource use, as demonstrated in scenario and sensitivity analyses, validation from a singular clinical expert could potentially add uncertainty to the results, compared with gaining validation from a panel of experts or real-world studies of resource use.
Finally, this analysis does not consider the cost effectiveness of olaparib versus other treatment options that have received marketing authorization for use in the adjuvant setting since the initiation of OlympiA, namely abemaciclib (for HR-positive/HER2-negative disease only) and neoadjuvant/adjuvant pembrolizumab (for TNBC only), or those that are sometimes used off-label in clinical practice (adjuvant capecitabine in TNBC) [62,63,64, 75, 76]. However, there is no documented evidence for their efficacy in the gBRCA-mutated eBC patient population, precluding their inclusion in this analysis. If data become available for these treatments in the BRCA-mutated population, future research into the cost effectiveness of olaparib and other adjuvant treatments for eBC is warranted.
To the authors’ knowledge, this analysis is the first peer-reviewed published economic evaluation to establish the cost effectiveness of a treatment in gBRCA1/2-mutated eBC, and in early TNBC. However, a cost-effectiveness analysis of olaparib for BRCA-mutated eBC in the United States was presented at the American Society of Clinical Oncology Annual Meeting in 2022 and the conclusions were similar to those formed from this model. Specifically, the ICER was approximately 114,500USD per QALY gained for olaparib compared with placebo, and olaparib was deemed to be cost effective for women with BRCA-mutated eBC at a willingness-to-pay threshold of 150,000USD [77]. Additionally, NICE and the Canadian Agency for Drugs and Technologies in Health (CADTH) have recently published positive recommendations for the use of olaparib in the population under investigation in this analysis [69, 78]. These assessments had used the same model structure, which had been adapted to UK and Canadian clinical practice [69, 78]. The final analyses used in these appraisals adopted different assumptions on the utility values and extrapolation methods to those used in our base case [69, 78]. These alternative assumptions were considered in sensitivity analyses in this cost-effectiveness analysis and were shown to yield similar conclusions regarding the cost effectiveness of olaparib in the proposed indication.
5 Conclusions
The economic evaluation demonstrated that, at list price, 1 year of adjuvant olaparib treatment in patients with gBRCA1/2 mutations who have high-risk, HER2-negative eBC previously treated with neoadjuvant or adjuvant chemotherapy provides meaningful increases in LYs and QALYs, and is cost effective over a lifetime horizon compared with WaW. The outcomes of this analysis are generalizable to Swedish practice, but the limitations and uncertainties related to health economic modelling are acknowledged. However, in general, the ITT ICER was insensitive to most of the parameters tested in sensitivity and scenario analyses, which demonstrates the robustness of the analysis.
References
Socialstyrelsen och Cancerfonden. Cancer i siffror 2018:populärvetenskapliga fakta om cancer. 2018 29/11/2022].
Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today. Lyon: International agency for research on cancer; 2020.
Socialstyrelsen. Statistik om cancer: Bilaga—Tabeller—Statistik om nyupptäckta cancerfall 2021. 2021 29/11/2022].
National Quality Registry for Breast Cancer (NKBC). Antal bröstcancerfall Bland alla anmälda fall. Diagnosår: 2022. 2022 29/11/2022].
American Joint Committee on Cancer (AJCC). AJCC cancer staging manual. 8th ed. Cham: Springer; 2017.
National Cancer Institute. National Cancer Institute: surveillance, epidemiology and end results program. Cancer stat facts: female breast cancer subtypes. Delhi: National Cancer Institute; 2022.
Nationellt Kvalitetsregister för Bröstcancer. Årsrapport 2021 från. 2021.
Feng Y, Spezia M, Huang S, Yuan C, Zeng Z, Zhang L, et al. Breast cancer development and progression: risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes & Diseases. 2018;5(2):77–106.
Petrucelli N, Daly MB, Pal T. BRCA1- and BRCA2-associated hereditary breast and ovarian cancer. Gene Reviews. Washington: University of Washington; 1998. (Updated 2022 May 26 ed).
Fu X, Tan W, Song Q, Pei H, Li J. BRCA1 and Breast Cancer: molecular mechanisms and therapeutic strategies. Front Cell Dev Biol. 2022;10: 813457.
Baretta Z, Mocellin S, Goldin E, Olopade OI, Huo D. Effect of BRCA germline mutations on breast cancer prognosis: a systematic review and meta-analysis. Medicine (Baltimore). 2016;95(40): e4975.
Tilanus-Linthorst MMA, Lingsma HF, Evans DG, Thompson D, Kaas R, Manders P, et al. Optimal age to start preventive measures in women with BRCA1/2 mutations or high familial breast cancer risk. Int J Cancer. 2013;133(1):156–63.
Nilsson MP, Törngren T, Henriksson K, Kristoffersson U, Kvist A, Silfverberg B, et al. BRCAsearch: written pre-test information and BRCA1/2 germline mutation testing in unselected patients with newly diagnosed breast cancer. Breast Cancer Res Treat. 2018;168(1):117–26.
Winter C, Nilsson MP, Olsson E, George AM, Chen Y, Kvist A, et al. Targeted sequencing of BRCA1 and BRCA2 across a large unselected breast cancer cohort suggests that one-third of mutations are somatic. Ann Oncol. 2016;27(8):1532–8.
Regionala Cancercentrum i Samverkan. Nationellt vårdprogram bröstcancer, Version 4.3, 2023-03-28, https://kunskapsbanken.cancercentrum.se/diagnoser/brostcancer/vardprogram/. 2023
Office for National Statistics. Cancer survival in England: adult, stage at diagnosis and childhood—patients followed up to 2018. 2019.
Hess KR, Esteva FJ. Effect of HER2 status on distant recurrence in early stage breast cancer. Breast Cancer Res Treat. 2013;137(2):449–55.
Ragaz J, Irving MA. High-risk breast cancer. 1st ed. Berlin: Springer; 1989.
Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P, et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384(25):2394–405.
Reddy SM, Barcenas CH, Sinha AK, Hsu L, Moulder SL, Tripathy D, et al. Long-term survival outcomes of triple-receptor negative breast cancer survivors who are disease free at 5 years and relationship with low hormone receptor positivity. Br J Cancer. 2018;118(1):17–23.
Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–34.
Sopik V, Sun P, Narod SA. Predictors of time to death after distant recurrence in breast cancer patients. Breast Cancer Res Treat Vol. 2019;173:465–74.
Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, et al. 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377(19):1836–46.
European Medicines Agency (EMA). Olaparib (Lynparza®) summary of product characteristics. 2022.
U.S. Food and Drug Administration (FDA). Lynparza® prescribing information. 2022
Geyer CEJ, Garber JE, Gelber RD, Yothers G, Taboada M, Ross L, et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann Oncol. 2022;33(12):1250–68.
European Medicines Agency (EMA). Lynparza-H-C-3726-II-0051-G: EPAR—assessment report—variation. https://www.ema.europa.eu/en/documents/variation-report/lynparza-h-c-3726-ii-0051-g-epar-assessment-report-variation_en.pdf. 2022.
Murphy P, Glynn D, Dias S, Hodgson R, Claxton L, Beresford L, et al. Modelling approaches for histology-independent cancer drugs to inform NICE appraisals: a systematic review and decision-framework. Health Technol Assess. 2021;25(76):1–228.
National Institute for Health and Care Excellence (NICE). TA632: Trastuzumab emtansine for adjuvant treatment of HER2-positive early breast cancer. 2020.
National Institute for Health and Care Excellence (NICE). TA612: Neratinib for extended adjuvant treatment of hormone receptor-positive, HER2-positive early stage breast cancer after adjuvant trastuzumab. 2019.
National Institute for Health and Care Excellence (NICE). TA569: Pertuzumab for adjuvant treatment of HER2-positive early stage breast cancer. 2019.
National Institute for Health and Care Excellence (NICE). TA501: Intrabeam radiotherapy system for adjuvant treatment of early breast cancer. 2018.
National Institute for Health and Care Excellence (NICE). TA424: Pertuxumab for the neoadjuvant treatment of HER2-positive breast cancer. 2016.
Dawood S, Broglio K, Ensor J, Hortobagyi GN, Giordano SH. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol. 2010;21(11):2169–74.
Lobbezoo DJ, van Kampen RJ, Voogd AC, Dercksen MW, van den Berkmortel F, Smilde TJ, et al. Prognosis of metastatic breast cancer: are there differences between patients with de novo and recurrent metastatic breast cancer? Br J Cancer. 2015;112(9):1445–51.
McKenzie HS, Maishman T, Simmonds P, Durcan L, Group PS, Eccles D, et al. Survival and disease characteristics of de novo versus recurrent metastatic breast cancer in a cohort of young patients. Br J Cancer. 2020;122(11):1618–29.
Tandvårds- och Läkemedelsförmånsverket (TLV). Ändring i Tandvårds- och läkemedelsförmånsverkets allmänna råd (TLVAR 2003:2) om ekonomiska utvärderingar. 2017.
Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–81.
Statistics Sweden. Population Statistics. 2022.
Mai PL, Chatterjee N, Hartge P, Tucker M, Brody L, Struewing JP, et al. Potential excess mortality in BRCA1/2 mutation carriers beyond breast, ovarian, prostate, and pancreatic cancers, and melanoma. PLoS ONE. 2009;4(3): e4812.
Robson M, Ruddy KJ, Im SA, Senkus E, Xu B, Domchek SM, et al. Patient-reported outcomes in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer receiving olaparib versus chemotherapy in the OlympiAD trial. Eur J Cancer. 2019;120:20–30.
Robson ME, Tung N, Conte P, Im SA, Senkus E, Xu B, et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019;30(4):558–66.
Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–21.
Collins JM, Nordstrom BL, McLaurin KK, Dalvi TB, McCutcheon SC, Bennett JC, et al. A real-world evidence study of CDK4/6 inhibitor treatment patterns and outcomes in metastatic breast cancer by germline BRCA mutation status. Oncol Therapy. 2021;9(2):575–89.
Emens LA, Molinero L, Loi S, Rugo HS, Schneeweiss A, Dieras V, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer: biomarker evaluation of the IMpassion130 study. J Natl Cancer Inst. 2021;113(8):1005–16.
Ganz PA, Bandos H, Spanic T, Friedman S, Müller V, Kümmel S, et al. Abstract GS4-09: Quality of life results from OlympiA: a phase III, multicenter, randomized, placebo-controlled trial of adjuvant olaparib after (neo)-adjuvant chemotherapy in patients with germline BRCA1/2 mutations and high-risk HER-2 negative early breast cancer. Can Res. 2022;82(4_Supplement): GS4-09-GS4-.
Crott R, Briggs A. Mapping the QLQ-C30 quality of life cancer questionnaire to EQ-5D patient preferences. Eur J Health Econ. 2010;11(4):427–34.
Lidgren M, Wilking N, Jönsson B, Rehnberg C. Health related quality of life in different states of breast cancer. Qual Life Res. 2007;16:1073–81.
Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health. 2010;13(5):509–18.
Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–108.
Sydöstra sjukvårdsregionen. Priser och ersättningar för Sydöstra sjukvårdsregionen. 2022.
Södra sjukvårdsregionen. Regionala priser och ersättningar för södra sjukvårdsregionen. 2022.
Region Örebro län. Prislista Röntgenkliniken 2021.
Region Skåne. Pris 2022—Region Skåne samt landstingen Blekinge, Södra Halland, Kronoberg—klinisk kemi. 2022.
Tandvårds- och Läkemedelsförmånsverket (TLV). Underlag för beslut i regionerna—Kadcyla (trastuzumab emtansin). 2019.
Statistics Sweden. Konsumentprisindex (1949=100). 2022.
Tandvårds- och Läkemedelsförmånsverket (TLV). Sök i databasen. 2022 March 2022]; https://www.tlv.se/beslut/sok-i-databasen.html.
Apoteket. apoteket.se. 2022. https://www.apoteket.se/.
Fass. Accofill—dosering. 2022. https://www.fass.se/LIF/product?userType=0&nplId=20140329000214.
Svensson M, Nilsson FOL, Arnberg K. Reimbursement decisions for pharmaceuticals in Sweden: the impact of disease severity and cost effectiveness. Pharmacoeconomics. 2015;33(11):1229–36.
Viollet J, O’Leary E, Camacho Gonzalez C, Lauppe R, Oldsberg L. HTA228 Willingness to pay for different severity levels in Sweden: an analysis of TLV decisions (2014–2022). Value in Health. 2022;25(12):S341.
European Medicines Agency (EMA). Summary of product characteristics abemaciclib. 2022.
Tandvårds- och Läkemedelsförmånsverket (TLV). Underlag för beslut om subvention - Omprövning. 2022.
European Medicines Agency (EMA). Summary of product characteristics pembrolizumab. 2022.
European Medicines Agency (EMA). Summary of product characteristics Xeloda. 2023.
Safonov A, Bandlamudi C, de Lara PT, Ferraro E, Derakhshan F, Will M, et al. Abstract GS4-08: comprehensive genomic profiling of patients with breast cancer identifies germline-somatic interactions mediating therapy resistance. Cancer Res. 2022;82(4_Supplement):GS4-08-GS4-.
Kim JY, Oh JM, Park YH, Ahn JS, Im YH. Which clinicopathologic parameters suggest primary resistance to palbociclib in combination with letrozole as the first-line treatment for hormone receptor-positive, HER2-negative advanced breast cancer? Front Oncol. 2021;11: 759150.
Bruno L, Ostinelli A, Waisberg F, Enrico D, Ponce C, Rivero S, et al. Cyclin-dependent kinase 4/6 inhibitor outcomes in patients with advanced breast cancer carrying germline pathogenic variants in DNA repair–related genes. JCO Precis Oncol. 2022;6: e2100140.
National Institute for Health and Care Excellence (NICE). TA886: Olaparib for adjuvant treatment of BRCA mutation-positive HER2-negative high-risk early breast cancer after chemotherapy. 2023.
Taylor AM, Chan DLH, Tio M, Patil SM, Traina TA, Robson ME, et al. PARP (Poly ADP-Ribose Polymerase) inhibitors for locally advanced or metastatic breast cancer. Cochrane Datab Syst Rev. 2021;4(4): CD011395.
Longworth L, Yang Y, Young T, Mulhern B, Hernandez Alava M, Mukuria C, et al. Use of generic and condition-specific measures of health-related quality of life in NICE decision-making: a systematic review, statistical modelling and survey. Health Technol Assess. 2014;18(9):1–224.
Criscitiello C, Spurden D, Piercy J, Rider A, Williams R, Mitra D, et al. Health-related quality of life among patients with HR+/HER2- early breast cancer. Clin Ther. 2021;43(7):1228-44.e4.
Hsu T, Ennis M, Hood N, Graham M, Goodwin PJ. Quality of life in long-term breast cancer survivors. J Clin Oncol. 2013;31(28):3540–8.
Sullivan J, Thornton Snider J, van Eijndhoven E, Okoro T, Batt K, DeLeire T. The well-being of long-term cancer survivors. Am J Manag Care. 2018;24(4):188–95.
Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147–59.
Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194–220.
Zettler C, Silva DD, Blinder VS, Robson ME, Elkin EB. Cost effectiveness of adjuvant olaparib for BRCA-mutated, early-stage breast cancer. J Clin Oncol. 2022;40(16_suppl):6593.
Canadian Agency for Drugs and Technologies in Health (CADTH). CADTH Reimbursement Recommendation: Olaparib (Lynparza). 2023.
Acknowledgements
Medical writing support, under the direction of the authors, was provided by Eleanor Atkinson, PhD, and Elysia Upton, MSc, from Costello Medical, UK, funded by AstraZeneca, Cambridge, UK, in accordance with Good Publication Practice (GPP2022) guidelines (https://www.ismpp.org/gpp-2022).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by AstraZeneca.
Conflicts of Interests/Competing Interests
MP, ME, AR, JJ, EK, EB and RH are employees and stockholders of AstraZeneca. BL has received honoraria for advisory boards and providing expert input on this economic analysis from AstraZeneca, as well as honoraria for various activities from Daiichi-Sankyo, Pfizer, Eli Lilly, Novartis, Gilead, Seagen and Merck.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Model inputs are described in the manuscript, electronic supplementary material, and references. No further data and material are available.
Code Availability
Not available.
Author Contributions
All authors contributed to the design of the study, were involved in drafting the outline, first, and second drafts, and approved the final version of the manuscript. RH is guarantor of this manuscript.
Data-sharing statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data-sharing policy, described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Polyzoi, M., Ekman, M., Reithmeier, A. et al. Cost-Effectiveness Analysis of Adjuvant Olaparib Versus Watch and Wait in the Treatment of Germline BRCA1/2-Mutated, High-Risk, HER2-Negative Early Breast Cancer in Sweden. PharmacoEconomics Open 8, 277–289 (2024). https://doi.org/10.1007/s41669-023-00457-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-023-00457-4