Abstract
Introduction
Limited data exist on real-world treatment patterns and the effectiveness of cyclin-dependent kinase (CDK) 4/6 inhibitors in germline BRCA (gBRCA)-mutated breast cancer.
Methods
Adults with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) metastatic breast cancer (mBC) treated with CDK4/6 inhibitor therapy between 2013 and 2018 were retrospectively selected from the Flatiron Health database. Patients with known gBRCA status were classified as mutated (gBRCAm) or wild type (gBRCAwt). Time-to-first subsequent therapy or death (TFST) and overall survival (OS) were calculated from the earliest line of therapy with a CDK4/6 inhibitor.
Results
Of 2968 patients with HR+/HER2− mBC receiving a CDK4/6 inhibitor, 859 (28.9%) had known gBRCA status, of whom 9.9% were gBRCAm and 90.1% gBRCAwt. Median (95% confidence interval [CI]) TFST was 10 (7–11) months in the gBRCAm group, 10 (9–11) months in the gBRCAwt group, and 11 (10–12) months in the combined gBRCAwt and unknown gBRCA group; median (95% CI) OS was 26 (21–not estimated), 37 (31–51), and 33 (31–35) months, respectively. Cox models indicated the gBRCAm group had shorter TFST (stratified hazard ratio [sHR] 1.24; 95% CI 0.96–1.59) and OS (sHR 1.50; 95% CI 1.06–2.14) than the gBRCAwt group. The gBRCAm group had shorter TFST (sHR 1.38; 95% CI 1.08–1.75) and OS (sHR 1.22; 95% CI 0.88–1.71) than the combined group.
Conclusion
The results of this real-world study suggest that treatment outcomes with CDK4/6 inhibitors may be worse in patients with gBRCAm mBC than in their counterparts with gBRCAwt and unknown gBRCA status, suggesting potential differences in tumor biology. This result highlights the unmet need in patients with gBRCAm requiring optimized treatment selection and sequencing. Future exploration in larger samples of patients who have had biomarker testing is warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Limited data exist on the real-world treatment patterns and effectiveness of cyclin-dependent kinase (CDK) 4/6 inhibitors in patients with germline BRCA (gBRCA)-mutated breast cancer. |
The current study addressed key evidence gaps surrounding the real-world outcomes of CDK4/6 inhibitor use in hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer (mBC) by gBRCA status. |
What was learned from the study? |
The results of this real-world study suggest that treatment outcomes with CDK4/6 inhibitors may be worse in patients with mutated gBRCA (gBRCAm) mBC than in those with wild-type gBRCA mBC and unknown gBRCA status, suggesting potential differences in tumor biology. |
The results highlight the unmet need in patients with gBRCAm requiring optimized treatment selection and sequencing; future exploration in larger samples of patients who have had biomarker testing is warranted. |
Introduction
Over the past two decades, great strides have been made in the understanding and classification of different types of breast cancer (BC) [1]. BC is segmented by distinct molecular subtypes based on the presence of hormone receptors (HR) (including estrogen receptors [ER] and progesterone receptors) and on human epidermal growth factor receptor type 2 (HER2) status [2,3,4]. These subtypes respond to different types of treatment [5], which have improved patient outcomes [6,7,8].
Germline deleterious breast cancer susceptibility gene (gBRCA) mutations are associated with a well-known increase in the risk of developing BC [9]. Approximately 2–8% of patients [10, 11] with HR-positive (HR+) BC are also positive for the gBRCA mutation (gBRCAm); this is even higher, about 40%, in those with low ER-positive (ER+) BC [12], which is defined as ≤ 10% positivity [13]. Patients with gBRCAm HR+ disease tend to be younger at diagnosis than patients with sporadic HR+, with an average age of < 45 years [14,15,16,17,18]. These patients have more aggressive disease, with higher levels of nodal involvement and Ki67 expression compared to patients with non-gBRCAm BC [18,19,20]. gBRCAm HR+ disease is also associated with higher recurrence scores compared to sporadic HR+ disease, with > 80% of patients classified as having intermediate- or high-risk disease [21,22,23].
There is a paucity of long-term data for patients with gBRCAm HR+ disease; as such, there is no adequate evidence to conclude that patients with gBRCAm HR+ disease have different long-term outcomes than patients with sporadic HR+ disease [19]. A meta-analysis of ten studies comparing the safety of breast-conserving surgery in patients with gBRCAm versus controls found a significantly higher risk of ipsilateral BC recurrence in studies with a median follow-up period of ≥ 7 years [24]. The study also identified a higher risk of contralateral breast cancer (CBC) in those with gBRCAm disease [24], which was reinforced in another study that found CBC to be more likely in those with gBRCA1m disease than in those with sporadic BC [25].
The treatment landscape for gBRCAm HR+ BC is evolving. However, data on the use of tamoxifen for early gBRCAm BC is contradictory, with one study showing a negative impact on survival [26] and another suggesting a significant reduction in the incidence of CBC [27]. Recent evidence shows benefits of treatment with poly (ADP ribose) polymerase (PARP) inhibitors for gBRCAm metastatic BC (mBC), compared to chemotherapy. In the phase III OlympiAD clinical trial, patients with HER2-negative (HER2−) gBRCAm mBC treated with olaparib saw an improvement in median progression-free survival (PFS) (7.0 vs. 4.2 months for patients treated with olaparib and standard therapy, respectively; hazard ratio [HR] 0.58; 95% confidence interval [CI] 0.43–0.80) [28]. The phase III OlympiA clinical trial that included patients with HER2− gBRCAm early BC found a 3-year invasive disease-free survival of 85.9% after adjuvant olaparib, compared to 77.1% in the group receiving placebo (HR 0.58; 95% CI 0.41–0.82) [29]. In the EMBRACA trial, median PFS was significantly longer for patients with mBC and gBRCAm who received talazoparib monotherapy, compared to those who received standard chemotherapy (8.6 vs.5.6 months; HR 0.54; 95% CI 0.41–0.71) [30].
Endocrine therapy for ER+/HER2− mBC may include combinations with palbociclib, abemaciclib, or ribociclib, oral agents that inhibit cyclin-dependent kinase 4/6 (CDK4/6). A pooled analysis of three randomized trials investigating the addition of CDK4/6 inhibitors to endocrine therapy in patients with HR+, HER2− advanced or mBC found a substantial benefit in all subgroups of interest, but did not examine gBRCA status [31]. Preclinical data suggest that certain gBRCA1m cell lines may not be sensitive to CDK4/6 inhibitors; however, more investigation is warranted [32,33,34]. The current study addressed key evidence gaps surrounding the real-world outcomes of CDK4/6 inhibitor use in patients with HR+/HER2− mBC by gBRCA status.
Methods
Data Source
The database used for this study was Flatiron Health, a nationwide, longitudinal, and demographically and geographically diverse database derived from de-identified electronic health record (EHR) data. This database includes data from > 265 primarily community-based cancer clinics (approximately 800 sites of care) available for analysis, representing > 2 million US cancer patients. De-identified patient-level data include structured and unstructured data, curated via technology-enabled abstraction [35].
This retrospective study included 2968 patients diagnosed with HR+/HER2− mBC between 1 January 2013 and 31 January 2018. Patients were followed longitudinally until death or last visit prior to data cutoff (31 July 2018; data censoring). Demographic information, tumor status, cancer treatment, medical history/comorbidities, disease characteristics, biomarker testing rates, and results were utilized. Flatiron Health contains oncologist-defined, rule-based lines of therapy in the metastatic setting. The rules are objective and are created through indication-specific algorithms developed by a team of oncologists, engineers, and biostatisticians. They are based on literature review, guidelines, and deep clinical experience and are applied to the treatments documented as actually received by the patient, without relying on order sets or care plans [36]. All drugs given within 28 days of an initial therapy were considered part of the same regimen. The addition of a new therapy after 28 days was considered a switch and the start of a subsequent regimen. Mortality data were used to estimate the overall survival (OS) of patients. Tumor progression data were not available; therefore, the time-to-first subsequent therapy (TFST) or death was examined in place of progression.
All study data were fully compliant with US patient confidentiality requirements, including the Health Insurance Portability and Accountability Act (HIPAA) of 1996. The study used only de-identified patient records and, therefore, was exempted from Institutional Review Board approval. Informed consent was not required as this was not an interventional study, and routinely collected, anonymized data were used.
Sample Selection
The study population consisted of patients aged ≥ 18 years with histologically or cytologically documented BC, evidence of metastatic disease, and biomarker test results indicating HR+/HER2− disease. Included patients received at least one line of therapy containing a CDK4/6 inhibitor (i.e., palbociclib, abemaciclib, or ribociclib) starting on or after the first diagnosis of mBC and no later than 31 January 2018; the index date was defined as the start date of the first line of treatment containing a CDK4/6 inhibitor.
Measures
The main characteristic of interest was gBRCAm status (gBRCAm vs. gBRCA wild type [wt] vs. a combined group of patients with gBRCAwt and unknown gBRCA status). Patients with unknown gBRCA status were those who were untested or had invalid test results. Treatment patterns prior to and during the first line of treatment containing a CDK4/6 inhibitor were described. Other variables examined included demographic and clinical characteristics. The primary outcomes were TFST and OS from the index date. TFST was calculated as the time from the index date to the start of the next line of therapy or death, whichever was earlier. OS was defined as the time from the index date to the date of death.
Analysis
Time-to-first subsequent therapy and OS were censored at the last activity date for patients without the outcome. Kaplan–Meier medians and associated 95% CIs were estimated for TFST and OS. These outcomes were compared using Cox models by gBRCA status, stratified by line of therapy and adjusting for demographic and clinical characteristics that modified HRs for gBRCA status by > 10%.
Results
Of 2968 patients with HR+/HER2− mBC receiving a CDK4/6 inhibitor (Fig. 1), 929 (31.3%) were tested for gBRCA status. Of these 929 patients, the status was known for 859 patients (28.9%), and 70 (2.4%) had uncertain or equivocal mutation results. Of those with known gBRCA status, 85 (9.9%) were gBRCAm. Of those with gBRCAm, 17.6% were gBRCA1m, 78.8% were gBRCA2m, and 3.5% had both gBRCA1m and gBRCA2m.
Study attrition flowchart for the CDK4/6-inhibitor-treated HR+/HER2− mBC cohort. Each box shows the number (n) of patients with percentage (in parentheses) of total number (N). Asterisk indicaes the index date, which is the start date of the first treatment line containing the CDK4/6 inhibitor. CDK4/6 Cyclin-dependent kinases 4 and 6, ER estrogen receptor, HER2 human epidermal growth factor receptor 2, HR+ hormone receptor positive, mBC metastatic breast cancer, PR progesterone receptor
Based on age at the index date, patients with gBRCAm were younger than those with gBRCAwt or unknown gBRCA status (Table 1). Mean (standard deviation) age was 52.7 (13.4), 58.1 (12.0), and 64.3 (11.6) years for those with gBRCAm, gBRCAwt, and gBRCAwt/unknown gBRCA status, respectively. There were small differences based on stage at initial BC diagnosis, with a higher proportion of the patients with unknown gBRCA status being stage IV versus more of those with gBRCAm and gBRCAwt being stage II. The other demographic and clinical characteristics assessed were largely similar between the groups.
For most patients (42.4, 37.9, and 40.2% of those with gBRCAm, gBRCAwt, and gBRCAwt/unknown gBRCA status, respectively), the earliest use of CDK4/6 therapy occurred in the first-line metastatic setting (Table 2). Patients who received their first CDK4/6 therapy in the second-line setting had most often been previously treated with an aromatase inhibitor or fulvestrant. A majority of those who had their CDK4/6 treatment in the third line or higher had been treated with an aromatase inhibitor in the prior line (Table 2).
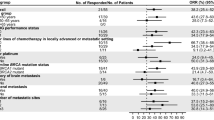
Median (95% CI) TFST from the start of the index line of therapy (all lines combined) was 10 (7–11) months in the gBRCAm group, 10 (9–11) months in the gBRCAwt group, and 11 (10–12) months in the combined group of those with gBRCAwt and unknown gBRCA status. Median (95% CI) OS from the start of the index line of therapy was 26 (21–not estimated) months in the gBRCAm group, 37 (31–51) months in the gBRCAwt group, and 33 (31–35) months in the combined group. These trends were consistent when looking at OS and TFST by individual line of CDK4/6 therapy (Figs. 2, 3).
Time-to-first subsequent therapy by gBRCA status from start of CDK4/6 as first-line therapy (a), from start of CDK4/6 as second-line therapy (b), and from start of CDK4/6 as third-line or higher therapy (c). 95% CIs were calculated using the Brookmeyer–Crowley method. CI Confidence interval, gBRCA germline BRCA, gBRCAm germline BRCA mutation, gBRCAwt germline BRCA wild type, TFST time-to-first subsequent therapy, unk unknown
Cox model results indicated that the gBRCAm group had a shorter TFST than individuals with gBRCAwt with a hazard ratio (stratified by index line [sHR]) of 1.24, although the 95% CI crossed the null value (95% CI 0.96–1.59) (Fig. 2). However, TFST was significantly shorter for patients with gBRCAm compared to the combined gBRCAwt/unknown gBRCA group, with an sHR of 1.38 (95% CI 1.08–1.75) (Fig. 2). OS was significantly shorter for patients with gBRCAm than for those with gBRCAwt (sHR 1.50; 95% CI 1.06–2.14); it was also shorter for patients with gBRCAm than for those with gBRCAwt/unknown gBRCA status but the difference did not meet statistical significance (sHR 1.22; 95% CI 0.88–1.71) (Fig. 3). None of the demographic or clinical characteristics met the criteria to be included in the final multivariate analysis models for TFST or OS.
Discussion
This study examined a retrospective cohort of patients with mBC under routine clinical practice in the Flatiron Health database. Of 2968 patients with HR+/HER2− mBC receiving a CDK4/6 inhibitor, gBRCA status was known for nearly 30%. Of those with a known status, about 10% were gBRCAm. Patients most commonly received letrozole + palbociclib or fulvestrant + palbociclib as their initial line of therapy containing a CDK4/6 inhibitor.
Median TFST was slightly shorter in those with gBRCAm compared to gBRCAwt and the combined group, especially when the first use of a CDK4/6 inhibitor was during first-line therapy. When examining all lines of therapy, combined median OS was > 10 months longer in those with gBRCAwt and about 7 months longer in those with gBRCAwt/unknown status compared to those with gBRCAm status. This effect was even more apparent when the initial line of therapy containing a CDK4/6 inhibitor was the first line, as median OS was approximately 2 years shorter for patients with gBRCAm compared to the group with gBRCAwt and the combined group.
The goal of the current study was to address the evidence gaps surrounding the real-world treatment patterns and clinical outcomes of CDK4/6 inhibitor use in gBRCAm and gBRCAwt HR+/HER2− mBC. To date, studies of clinical outcomes after CDK4/6 therapy have typically not been stratified by gBRCA status. In one retrospective study of 411 patients with HR+ mBC, median PFS was 8.9 months for those receiving letrozole + palbociclib and 10.3 months for those receiving fulvestrant + palbociclib [37]. A recent pooled analysis examining the efficacy of adding CDK4/6 inhibitors to endocrine therapy in patients with HR+/HER2− advanced BC or mBC found a substantial benefit. Across all seven pooled trials, the difference in median PFS was 8.8 months in favor of the combination of endocrine therapy with a CDK4/6 inhibitor (range 6.8–13.3 months across studies; HR 0.59; 95% CI 0.54–0.64; n = 2616 patients) compared to endocrine therapy alone [31]. However, while this study demonstrated the overall efficaciousness of CDK4/6 inhibitors, the results were not examined by gBRCA status.
A recent study utilizing Flatiron Health data [38] estimated the median OS after mBC diagnosis in patients with HR+/HER2− gBRCAm to be 38.0 months. The present study’s lower median OS in patients with gBRCAm (26.0 months) reflects survival only among patients treated with CDK4/6 inhibitors, with the patients followed from the start of that therapy, which in many cases was not the first line after mBC diagnosis. In addition, the prior study required at least one follow-up visit for patients to enter the outcome analyses, which may have biased survival upward by dropping patients who died after diagnosis without having another visit.
The analyses in the current study were carried out using data recorded in a collection of EHR systems. As expected with real-world data, some elements were underreported or missing. Progression information was not available; we used TFST as a proxy, which is not an accurate substitute for progression. Caution must be used in particular when comparing to PFS from clinical trials, where scans are done at consistent intervals across patients. TFST was censored at the last activity date for patients without the outcome; patients potentially could have moved to a different oncology clinic and received treatment that does not appear in the database. The mortality data may be incomplete, although a recent study examining the impact of missing death data on survival analyses by comparing data from the Flatiron Health database and the National Death Index (as a gold standard) showed high sensitivity (91%) in the Flatiron-derived cohort [39]. Information on surgery, radiation therapy, and other services received in hospitals was unavailable, as was pharmacy dispensing information. Lines of therapy were derived from information in the EHR using a rule-based algorithm, but this information may be inaccurate or incomplete as its accuracy depends on complete treatment documentation [36]. Information on treatment received prior to the metastatic setting was not available. Secondary tumors may have been erroneously assigned primary tumor codes, leading to an overestimation of a history of cancer other than BC prior to or on the index date. Comparisons between gBRCA groups were complicated by the fact that there are differences between the groups aside from just their gBRCA status. We attempted to adjust for this confounding by known factors that could be identified in the data, but unknown or residual confounding may exist.
In real-world clinical practice, a minority of patients with HR+ BC undergoes BRCA testing, likely in cases where a BRCA mutation is suspected. As only 31% of patients (929 of 2968) in the current study were tested, and gBRCAm is relatively rare, we expect that the group with an unknown status is more characteristic of patients with gBRCAwt. However, some differences were observed in the baseline characteristics (e.g., age and stage at initial BC diagnosis) between the patients with gBRCA of unknown status and those with gBRCAwt, likely reflective of testing guidelines. This raises the concern that the gBRCAwt population in the current study might be a biased sample of the larger gBRCAwt population. The group with unknown gBRCA status also likely includes some undiagnosed patients with gBRCAm, meaning that the gBRCA unknown group cannot be assumed to fully represent the gBRCAwt population. However, as gBRCAm is rare (approximately 2–8% [10, 11] of all HR+ BC), the impact of this will be minimal on the results. The outcomes for those with gBRCAwt and the group with unknown gBRCA status are largely similar, which provides evidence for the validity of the results for patients with gBRCAwt.
The Flatiron Health database represents a large convenience sample of outpatient oncology practices in the USA that use EHR systems. While this sample may not represent all oncology practice sites within the USA, these data are expected to be generalizable to US populations with mBC who meet the study selection criteria and who are treated in oncology clinics. Information in the Flatiron Health database on treatments received in the oncology clinic and on OS are considered to have reasonable accuracy, allowing a valid look at real-world treatment patterns and survival outcomes among US patients with mBC.
Conclusions
The results of this real-world study suggest that treatment outcomes with CDK4/6 inhibitors may be worse in patients with gBRCAm compared to those with gBRCAwt disease. Patients with gBRCAm on CDK4/6 therapy had a shorter TFST and OS time than those with gBRCAwt and unknown gBRCA status. These findings indicate a higher unmet need among patients with gBRCAm.
This study is one of the first to examine OS and TFST by gBRCA status following treatment with CDK4/6 inhibitors in a real-world setting. If BRCA testing increases in clinical practice, which may occur with the availability of treatments targeting this mutation, further real-world studies can be conducted using larger samples with improved generalizability to a broader population of CDK4/6-treated patients with and without the gBRCA mutation.
References
Russnes HG, Lingjaerde OC, Borresen-Dale AL, Caldas C. Breast cancer molecular stratification: from intrinsic subtypes to integrative clusters. Am J Pathol. 2017;187(10):2152–62.
Yip CH, Rhodes A. Estrogen and progesterone receptors in breast cancer. Future Oncol. 2014;10(14):2293–301.
Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5):dju055.
National Cancer Institute, Surveillance Epidemiology and End Results (SEER) Program. Cancer stat facts: female breast cancer subtypes. 2020. https://seer.cancer.gov/statfacts/html/breast-subtypes.html. Accessed 2020.
Dalmau E, Armengol-Alonso A, Munoz M, Segui-Palmer MA. Current status of hormone therapy in patients with hormone receptor positive (HR+) advanced breast cancer. Breast. 2014;23(6):710–20.
Mener AS, Aggarwal A. Advances in targeted therapy for breast cancer. Fed Pract. 2015;32(Suppl 4):46S–49S.
Swain SM, Miles D, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(4):519–30.
Edessa D, Sisay M. Recent advances of cyclin-dependent kinases as potential therapeutic targets in HR+/HER2- metastatic breast cancer: a focus on ribociclib. Breast Cancer (Dove Med Press). 2017;9:567–79.
King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302(5645):643–6.
Timms KM, Abkevich V, Hughes E, et al. Association of BRCA1/2 defects with genomic scores predictive of DNA damage repair deficiency among breast cancer subtypes. Breast Cancer Res. 2014;16(6):475.
Tung N, Lin NU, Kidd J, et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol. 2016;34(13):1460–8.
Sanford RA, Song J, Gutierrez-Barrera AM, et al. High incidence of germline BRCA mutation in patients with ER low-positive/PR low-positive/HER-2 neu negative tumors. Cancer. 2015;121(19):3422–7.
Allison KH, Hammond MEH, Dowsett M, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020;38(12):1346–66.
Peretz TY, Zick A, Kadouri L, et al. Abstract P3-03-02: ER dependent breast cancer phenotype in BRCA 1/2 carriers. In: 40th Annual San Antonio Breast Cancer Symposium (SABCS®); 5–9 December; San Antonio, TX, USA. 2017.
Pellegrino B, Bella M, Michiara M, Zanelli P, Naldi N, Porzio R, et al. Triple negative status and BRCA mutations in contralateral breast cancer: a population-based study. Acta Biomed. 2016;87(1):54–63.
Fountzilas E, Konstantopoulou I, Vagena A, et al. Pathology of BRCA1- and BRCA2-associated breast cancers: known and less known connections. Clin Breast Cancer. 2020;20(2):152–9.
Krammer J, Pinker-Domenig K, Robson ME, et al. Breast cancer detection and tumor characteristics in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat. 2017;163(3):565–71.
Mavaddat N, Barrowdale D, Andrulis IL, et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomark Prev. 2012;21(1):134–47.
Tredan O, de Talhouet S, Peron J, et al. Abstract P3-03-05: Association between BRCA1 and BRCA2 mutations and survival in breast cancer patients according to molecular subtype. In: 40th Annual San Antonio Breast Cancer Symposium (SABCS®); 5–9 December; San Antonio, TX, USA. 2017.
Aleskandarany M, Caracappa D, Nolan CC, et al. DNA damage response markers are differentially expressed in BRCA-mutated breast cancers. Breast Cancer Res Treat. 2015;150(1):81–90.
Shah PD, Patil S, Dickler MN, Offit K, Hudis CA, Robson ME. Twenty-one-gene recurrence score assay in BRCA-associated versus sporadic breast cancers: differences based on germline mutation status. Cancer. 2016;122(8):1178–84.
Halpern N, Sonnenblick A, Uziely B, et al. Oncotype Dx recurrence score among BRCA1/2 germline mutation carriers with hormone receptors positive breast cancer. Int J Cancer. 2017;140(9):2145–9.
Lewin R, Sulkes A, Shochat T, et al. Oncotype-DX recurrence score distribution in breast cancer patients with BRCA1/2 mutations. Breast Cancer Res Treat. 2016;157(3):511–6.
Valachis A, Nearchou AD, Lind P. Surgical management of breast cancer in BRCA-mutation carriers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014;144(3):443–55.
Kriege M, Jager A, Hooning MJ, et al. The efficacy of taxane chemotherapy for metastatic breast cancer in BRCA1 and BRCA2 mutation carriers. Cancer. 2012;118(4):899–907.
Goodwin PJ, Phillips KA, West DW, et al. Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: an International Prospective Breast Cancer Family Registry population-based cohort study. J Clin Oncol. 2012;30(1):19–26.
Phillips KA, Milne RL, Rookus MA, et al. Tamoxifen and risk of contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2013;31(25):3091–9.
Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523–33.
Tutt ANJ, Garber JE, Kaufman B, et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384:2394–405.
Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379(8):753–63.
Gao JJ, Cheng J, Bloomquist E, et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US Food and Drug Administration pooled analysis. Lancet Oncol. 2020;21(2):250–60.
Dean JL, McClendon AK, Knudsen ES. Modification of the DNA damage response by therapeutic CDK4/6 inhibition. J Biol Chem. 2012;287(34):29075–87.
Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11(5):R77.
Tarasewicz E, Rivas L, Hamdan R, et al. Inhibition of CDK-mediated phosphorylation of Smad3 results in decreased oncogenesis in triple negative breast cancer cells. Cell Cycle. 2014;13(20):3191–201.
Flatiron Health. Real-world Evidence. New York: Flatiron Health; 2018. https://flatiron.com/real-world-evidence/. Accessed 2019.
Khozin S, Miksad RA, Adami J, et al. Real-world progression, treatment, and survival outcomes during rapid adoption of immunotherapy for advanced non-small cell lung cancer. Cancer. 2019;125(22):4019–32.
Eziokwu AS, Varella L, Kruse ML, et al. Real world clinical outcomes of palbociclib in hormone receptor positive (HR+) metastatic breast cancer (MBC) patients. J Clin Oncol. 2018;36(15_suppl):e13034-e.
Quek RGW, Mardekian J. Clinical outcomes, treatment patterns, and health resource utilization among metastatic breast cancer patients with germline BRCA1/2 mutation: a real-world retrospective study. Adv Ther. 2019;36(3):708–20.
Carrigan G, Whipple S, Taylor MD, et al. An evaluation of the impact of missing deaths on overall survival analyses of advanced non-small cell lung cancer patients conducted in an electronic health records database. Pharmacoepidemiol Drug Saf. 2019;28(5):572–81.
Acknowledgements
Funding
Evidera received funding from AstraZeneca (Gaithersburg, MD, USA/Cambridge, UK) and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., (Kenilworth, NJ, USA), who are co-developing olaparib. AstraZeneca and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., are also funding the journal’s Rapid Service and Open Access Fees.
Prior presentation
This work was accepted for a poster presentation at ASCO’s Annual Meeting, 31 May to 4 June 2019, Chicago, IL, USA.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship contributions
Jenna M. Collins, Beth L. Nordstrom, Kimmie K. McLaurin, Tapashi B. Dalvi, Susan C. McCutcheon, James C. Bennett, Brian R. Murphy, Puneet K. Singhal, Charles McCrea, and Josefa M. Briceno contributed to study design, data interpretation, as well as critical review of the results and manuscript. Reshma Shinde contributed to data interpretation and critical review of the results and manuscript. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures
Authors Kimmie K. McLaurin, Tapashi B. Dalvi, and Josefa M. Briceno are employees of and own stock for AstraZeneca Pharmaceuticals, LP (Gaithersburg, MD, USA). Susan C. McCutcheon and Charles McCrea are employees of and own stock for AstraZeneca (Cambridge, UK). James C. Bennett was a contractor for AstraZeneca during the time of the analysis (Cambridge, UK). Puneet K. Singhal and Reshma Shinde are employees of and own stock for Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Jenna M. Collins, Beth L. Nordstrom, and Brian R. Murphy are employees of Evidera, a healthcare research firm that provides consulting and other research services to pharmaceutical, device, government, and non-government organizations. In their salaried positions, they work with a variety of companies and are explicitly precluded from accepting any payment or honoraria directly from them for services rendered.
Compliance with Ethics Guidelines
All study data were fully compliant with US patient confidentiality requirements, including the Health Insurance Portability and Accountability Act (HIPAA) of 1996. The study used only de-identified patient records and, therefore, was exempted from Institutional Review Board approval. Informed consent was not required as this was not an interventional study, and routinely collected, anonymized data were used.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available as individual data cannot be shared, per HIPAA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Collins, J.M., Nordstrom, B.L., McLaurin, K.K. et al. A Real-World Evidence Study of CDK4/6 Inhibitor Treatment Patterns and Outcomes in Metastatic Breast Cancer by Germline BRCA Mutation Status. Oncol Ther 9, 575–589 (2021). https://doi.org/10.1007/s40487-021-00162-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40487-021-00162-4