Abstract
Background
Chronic inflammatory demyelinating polyneuropathy (CIDP) is a rare, progressive autoimmune disease causing peripheral nervous system dysfunction. Guidelines recommend immunoglobulin (IG) therapy as an immunomodulatory agent in CIDP. Drawbacks and unmet needs with intravenous immunoglobulin (IVIG) include adverse effects and wear-off effects, along with the burden of administration based on site of care. Subcutaneous administration of Hizentra, a subcutaneous immunoglobulin (SCIG) reduces patient burden by allowing self-administration outside the hospital setting and has fewer adverse events (AEs).
Objective
We aimed to compare the expected cost of treatment and the budget impact of Hizentra compared with IVIG for maintenance treatment of CIDP in the United States.
Methods
A decision tree model was developed to estimate the expected budget impact of maintenance treatment with Hizentra for US stakeholders. The model adopts primarily a US integrated delivery network perspective and, secondarily, a commercial perspective over a 1-year time horizon. Pharmacy costs were based on a payment mix of average sales price (73%), wholesale acquisition cost (2%), and average wholesale price (25%). Costs in the model reflect 2022 US dollars. In accordance with the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) guidelines and recommendations for budget impact modeling, no discounting was performed. The PATH clinical study of Hizentra maintenance in CIDP was used to determine clinical inputs for relapse rates at initial assessment (24 weeks) and at 52 weeks for Hizentra. The ICE clinical study of Gamunex maintenance in CIDP was the basis of relapse rates for Gamunex (and other IVIGs). Literature-based estimates were obtained for infusion costs by site of care, costs of IVIG infusion-related complications, and significant IVIG AE rates. Hizentra AE rates from the US Hizentra prescribing information were assessed but were not included in the model as the AEs in CIDP were mild, easily treated, and self-limited. Sensitivity analyses and scenario analyses were conducted to evaluate variations from the base case.
Results
The model showed that a Hizentra starting dose of 0.2 g/kg is expected to result in annual cost savings of US$32,447 per patient compared with IVIG. For a hypothetical 25-million-member plan, the budget impact of a 10% market share shift from IVIG to Hizentra is expected to result in savings of US$2,296,235.
Conclusion
This analysis projects that Hizentra is likely associated with favorable economic benefit compared with IVIG in managing CIDP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hizentra is the first subcutaneous immunoglobulin (IG) for the treatment of chronic inflammatory demyelinating polyneuropathy (CIDP). As a subcutaneous treatment, Hizentra reduces patient burden by allowing for flexibly timed self-infusion at home compared with the burden of hospital or office administration of intravenous immunoglobulin (IVIG). |
The initial dose of Hizentra for the treatment of CIDP is expected to enable significant cost savings per patient compared with the corresponding higher dose of IVIG, even after accounting for upward titration of the initial Hizentra dose that may be needed for effective management of the minority of patients who relapse. |
1 Background
Chronic inflammatory demyelinating polyneuropathy (CIDP) is a rare, progressive autoimmune disease causing peripheral nervous system (PNS) dysfunction [1], with an estimated prevalence in an adult population of 8.9 per 100,000 persons [2]. CIDP is challenging to diagnose accurately and to distinguish from similar neuropathic or muscular conditions due to a heterogeneous patient population and varying clinical presentations across patients [3, 4]. The delayed recognition of CIDP symptoms and inaccurate diagnosis of true CIDP can lead to disease progression, resulting in axonal damage, increased loss of physical function, and permanent disability [5,6,7,8]. Furthermore, ineffective treatment of CIDP patients can result in relapse among stable patients, resulting in disability and additional management costs [9,10,11,12].
Corticosteroids are recommended as short-term induction therapy for patients with disabling symptoms of CIDP [13]. While short-term use of relatively inexpensive corticosteroids is arguably both clinically and financially attractive for decision makers, the long-term use of corticosteroids is known to be associated with substantial adverse consequences [13, 14]. Furthermore, corticosteroids are contraindicated in CIDP patients with pure motor-type CIDP or those with diabetes [15, 16]. Other immunosuppressives or immunomodulatory drugs are considered as corticosteroid-sparing agents, especially for patients refractory to first-line treatment [17,18,19]. Some of these treatments include azathioprine, cyclophosphamide, methotrexate, cyclosporine, mycophenolate mofetil, and rituximab and immunomodulatory agents such as interferon (IFN)-α and IFN-β. Plasmapheresis is an additional option for short-term treatment but can be associated with significant device-related costs, including the need for hospitalization, highly trained staff, and uncertain outcomes including possible serious adverse effects in long-term use [20, 21]. Key guidelines and consensus statements recommend the use of corticosteroids or immunoglobulin (IG) therapy as an immunomodulatory agent in CIDP for maintenance treatment [22,23,24,25]. In patients with CIDP, IG therapy has documented benefits in terms of preventing relapse of muscle weakness and numbness of the limbs [12, 26], improved and/or maintained functional performance of the arms and legs [26, 27], and improved and/or maintained quality of life in both physical function and mental health domains [27].
While the efficacy and safety of intravenous immunoglobulin (IVIG) treatment for CIDP has been established [28], originally via the ICE study, there are still drawbacks and unmet medical needs associated with this treatment option. These include adverse effects such as thromboembolic events [29], hemolysis (although this risk has been shown to be reduced recently with improved immunoaffinity chromatography production methods in the case of Privigen) [26, 30], aseptic meningitis [31], and venous access-related complications [32]. Furthermore, due to the relatively staggered dosing frequency, the pharmacodynamic profile of IVIGs suggests wear-off effects [33, 34], as measured by intra-cycle fluctuations in daily grip strength with IVIG treatment in CIDP [35].
In addition to issues germane to IVIGs in general noted above, there are others of particular relevance by site of IVIG infusions. Overall, patients across various conditions where IG therapy is indicated in the United States (US) predominantly receive IVIG therapy away from home [36]. Administering IVIG at standalone infusion or hospital outpatient centers is costlier than administering at home [37, 38]. Furthermore, patients administered IVIG at infusion or hospital outpatient centers report lower quality of life than those administered IVIG at home [38].
Patients with CIDP, once stabilized on IVIG, should be able to transition to subcutaneous Hizentra (immune globulin subcutaneous [Human] 20% liquid). In the largest CIDP clinical trial to date (the PATH study), patients, once stabilized on IVIG, who transitioned to subcutaneous Hizentra (at the eventually approved starting maintenance dose of 0.2 g/kg body weight, once weekly) were associated with maintained freedom from relapse and maintained functionality [39]. The PATH study included patients with definite and probable CIDP, including motor, sensory, and other types of atypical CIDP. Moreover, these CIDP patients either improved or maintained health status, in terms of key domains of the EQ-5D [40], and maintained daily activities and participation, as measured by the Inflammatory Rasch-Built Overall Disability Scale (I-RODS) [41]. In addition, Hizentra self-infusion at home appears to have been preferred over continued maintenance with IVIG [42]. Separately, studies in conditions such as primary immune deficiency reveal that Hizentra reduces the burden of care for some patients by enabling them to self-administer their treatment outside of the hospital setting [43, 44]. Finally, Hizentra is associated with an improved adverse effect profile, and patients with CIDP treated with Hizentra have a lower rate of systemic adverse events (AEs) compared with treatment with IVIG [39].
The objective of this analysis was to develop an economic and budget impact model (BIM) of the costs and budget impact of Hizentra, the only approved subcutaneous immunoglobulin (SCIG) for maintenance treatment of CIDP in the US, by incorporating its documented clinical benefit [39] in comparison with a market share basket of IVIGs in patients receiving IG treatment.
2 Methods
2.1 Model Structure
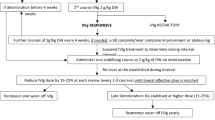
A BIM was developed in accordance with the Academy of Managed Care Pharmacy (AMCP) and International Society for Pharmacoeconomics and Outcomes Research (ISPOR) best practices [45, 46] over a 1-year model time horizon to estimate the budget impact of adding Hizentra to a health plan formulary for the treatment of CIDP. A 1-year time horizon was selected to model out the initial starting doses in CIDP and to capture any titration in dosing due to patient relapse at 24 weeks. The model adopts primarily a US integrated delivery network (IDN) perspective and, secondarily, a commercial perspective. A decision tree approach was employed for the model structure (Fig. 1) in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). An unlocked version of the model, except for US label-based dosing and relapse parameters to be consistent with Hizentra prescribing information, has been provided as electronic supplementary material (ESM).
2.2 Model Inputs
The data inputs for this model were taken from various literature-based sources. These included key clinical studies in CIDP for Hizentra and Gamunex, the two approved maintenance products with evidence on relapse rates in stable CIDP patients, product-specific US labels, published papers describing AEs associated with intravenous infusions (including those related to the use of infusion ports), and various studies providing cost estimates for the management of the AEs. Disease prevalence was based on US epidemiologic data, with a prevalence of 8.9 per 100,000 [2]. Drug costs were obtained from January 2022 Centers for Medicare and Medicaid Services (CMS) average sales price (ASP) and average wholesale price (AWP)/wholesale acquisition cost (WAC) list price sources (Red Book) [47] for both Hizentra and IVIG products (Table 1). A payment mix of ASP (73%), AWP (25%), and WAC (2%) was used in the base case [48].
The dosing of Hizentra and IVIGs was based on an average US adult patient weight of 85.9 kg [49]. The average patient weight was calculated based on the average adult female weight of 77.5 kg and the average adult male weight of 90.6 kg [49]. The average male and female weights were weighted by a gender distribution of 64.7% male and 35.3% female from the ICE and PATH studies [28, 39]. For Hizentra, the initial maintenance weekly dose was 0.2 g/kg body weight, based on US prescribing information (label) and the PATH study [39]. At 24 weeks, patients were evaluated for relapse in the PATH study [39]; those who did not relapse were assumed to continue on the stable 0.2 g/kg body weight dose. For those who did relapse, subsequent dose management was assumed to entail dose up-titration to 0.4 g/kg body weight for an additional 24 weeks, consistent with US prescribing information, and subsequent relapse rates were taken from the PATH extension study [42]. Observed initial and subsequent relapse rates from the placebo-controlled PATH study [39] and PATH extension study [42], respectively, were adjusted in this indirect comparison with IVIGs, as below.
Underlying populations in the PATH and ICE studies were evaluated in terms of their respective relapse rates. In the PATH study, the observed relapse rate for the Hizentra maintenance dose of 0.2 g/kg body weight was 33% and the corresponding placebo relapse rate was 56% [39]. In the ICE study, the observed relapse rate for the Gamunex (immune globulin injection [human] 10% caprylate/chromatography purified) maintenance dose of 1 g/kg body weight was 13% [28, 50] and the placebo relapse rate was 42%. Since the placebo relapse rates were unequal across the two studies, indicative of population heterogeneity, the relapse rate for the Hizentra 0.2 g/kg body weight dose was adjusted as follows:
-
observed relative risk of Hizentra 0.2 g/kg dose versus IVIG (Gamunex) relapse rate = 0.33/0.13 = 2.53
-
adjusted relative risk of Hizentra 0.2 g/kg dose versus IVIG (Gamunex) relapse rate = (0.33/0.56)/(0.13/0.42) = 1.90
-
adjusted relapse rate for Hizentra 0.2 g/kg body weight = (13%) × (1.90) = 24.8%.
The subsequent relapse rate was similarly adjusted for the 24.8% of Hizentra 0.2 g/kg body weight-dosed patients who were projected to relapse at 24 weeks and were uptitrated to the higher 0.4 g/kg body weight dosing, from the observed 8% in the PATH extension study [38] to 6%, as above. Finally, for the 75.2% of patients who did not relapse in the initial 24 weeks and remained on the same stable dose of 0.2 g/kg body weight, the observed relapse rate of 30% in the following 6 months was adjusted to 22.5%.
Maintenance dosing for IVIGs was calculated at 1.0 g/kg body weight, with a post-relapse induction dose of 2.0 g/kg [28, 50]. As noted, the ICE study was the basis of incorporated relapse rates at the initial assessment period (24 weeks) and post-relapse subsequent management [28]. IG maintenance was assumed to involve 15 yearly administrations, based on analysis of US patients from a global Guillain–Barré Syndrome (GBS)/CIDP Foundation patient survey [51]. The market shares of different IVIG products were taken from US data from the aforementioned GBS/CIDP global patient survey (Table 1) [51].
For IVIGs, literature-based estimates were used for costs of IVIG infusions by site of care [37], IVIG site of care distribution [51], costs of IVIG complications (venous access, need for implanted infusion port, implanted infusion port complications), and types of infection and corresponding rates and costs [52,53,54] (Table 2). Hizentra AE rates from the Hizentra prescribing information were assessed for inclusion in the model. The most frequent AEs for patients treated with 0.2g/kg Hizentra were local reactions (19.3%), fatigue (8.8%), headache (7%), and nasopharyngitis (7%) [55]. The vast majority of Hizentra AEs were mild, easily treated, and self-limiting and thus were not included in the model. Similar rates of common minor AEs were seen with Gamunex-C, such as headache (8%), pharyngitis (5%), injection site reaction (5%), and cough increased (7%) [50]. As the rates of minor AEs were similar between Hizentra and IVIG, and costs to treat these AEs were minimal, the model only considered serious AEs. Costs in the model reflect 2022 US dollars. In accordance with ISPOR guidelines and recommendations for budget impact modeling, no discounting was performed [46].
The distribution of site of care (IG administration) was based on the aforementioned GBS/CIDP Foundation survey. Finally, indirect costs for infusion time and caregiver time were included for the IDN perspective (base case). For the model base case from the IDN perspective, the percentage of patients requiring a caregiver was derived from evidence from two studies in a similar neuromuscular condition, myasthenia gravis [67, 68]. As the percentage of patients requiring a caregiver varied by underlying age and gender (higher age and males being associated with greater caregiving) in these studies, this relationship was applied to the age and gender distribution in the CIDP population from the ICE and PATH studies to arrive at an expected caregiver rate of 84% for the model base case [28, 39]. Travel cost per infusion, including fuel cost and parking/tolls, were also included from the IDN perspective.
A one-way sensitivity analysis was conducted by varying the key model input parameters one at a time while holding all other inputs constant to determine the sensitivity and robustness of the model. The base-case variance percentage was set to ±10%. An alternative sensitivity scenario with variance set to ±20% was also evaluated.
Various scenario analyses were also conducted. These consisted of (1) comparing Hizentra with Gamunex-C; (2) Hizentra compared with Panzyga; (3) Hizentra compared with hospital-based IVIG alone; (4) Hizentra compared with home-based IVIG alone; (5) Hizentra compared with IVIG, with all IVIG patients requiring infusion ports; (6) Hizentra compared with IVIG from a commercial perspective, excluding indirect costs; (7) Hizentra compared with IVIG using unadjusted relapse rates; (8) Hizentra compared with IVIG, with AEs excluded; (9) Hizentra versus IVIG using only the ASP; and (10) Hizentra versus IVIG using mean average weights for males and females.
3 Results
3.1 Budget Impact
Based on the US-labeled starting dose of 0.2 g/kg for maintenance treatment of CIDP, the output of the model revealed that Hizentra is expected to result in annual cost savings of about US$32,000 per patient compared with a market basket of IVIGs (Fig. 2). As seen, slightly over US$10,000 of the per-patient expected savings are attributable to drug costs and the remaining approximately US$22,000 are attributable to non-drug (infusion and AE) costs. For a hypothetical 25-million-member IDN, the budget impact of a 10% market share shift from IVIG to Hizentra results in annual expected savings of about US$2.29 million (Fig. 3). Assuming a 5% market share shift from IVIG to Hizentra results in an annual expected savings of US$1.15 million, while assuming a 20% market share shift from IVIG to Hizentra results in an annual expected savings of US$4.59 million. When evaluated in relation to Gamunex, the originally approved IVIG that is the basis for the efficacy outcomes (relapse rates) used in this model, the annual expected savings per patient are US$26,488, with a budget savings of about US$1.87 million. By contrast, savings in relation to the most costly IVIG, Panzyga (immune globulin intravenous [human]-ifas 10% liquid preparation), are an expected US$63,733 per patient and a favorable budget impact of US$4.51 million for the healthcare plan (Table 3).
The savings and budget impact savings seen in the model are expected to be more favorable for a shift from IVIGs to Hizentra under certain conditions or for specific subgroups. The first scenario is a greater share of hospital-based and infusion center-based IVIG infusions, as opposed to home administration of IVIG. This is especially so for hospital-based IVIG infusions. Thus, for patients transitioning from hospital-based IVIG infusion to Hizentra self-infusion, the expected annual savings for each such patient is US$53,773 and US$3.81 million if all IVIG patients were receiving hospital infusions. Nevertheless, even for patients who may choose self-infusion over home-based IVIG infusion with a healthcare professional, our model projected annual savings of about US$16,881 per patient and US$1.19 million at the organization level.
A second group for whom expected savings are likely to be greater is patients who require implantable ports for IVIG infusions. For them, because of the excess incidence of AEs and associated costs for patients on implantable infusion ports, the expected savings from a transition to Hizentra subcutaneous infusion would be US$46,552 and US$3.29 million for the organization if all patients receiving IVIG required implantable ports.
In addition to variability in patient-level costs, the model predicts additional opportunity for an IDN to increase projected cost savings with a larger shift of the IVIG population to Hizentra. Thus, a shift of 15% (from an assumed current Hizentra share of 10% to 25% in 1 year) would yield a projected cost savings of about US$3.44 million.
Under other scenarios, overall expected savings and budget impact from an IVIG to Hizentra switch may be more modest. First, if indirect costs such as patient and caregiver time were not valued as in the commercial perspective, the annual cost savings per patient would be smaller, at US$30,896 per patient compared with the market basket of IVIGs, and US$2.19 million for a commercial health plan that is not responsible for patient and caregiver time costs.
Second, if we did not adjust for severity differences in the underlying populations of the Hizentra clinical study (PATH) [more severe as reflected in a higher placebo relapse rate] and the Gamunex clinical study (ICE) [less severe with a lower placebo relapse rate], the expected annual cost savings per patient would be US$27,400 and the organizational annual budget savings would be around US$1.94 million.
Third, if the IVIG AEs were not included (i.e., were assumed to be negligible), then the expected annual cost savings per patient would be about US$28,113 and the annual budget savings would be limited to US$1.99 million. Finally, we included a scenario using only the ASP; the expected savings per patient would be about US$32,949 and the budget impact savings would be US$2.33 million, a slight increase from the model base case.
3.2 Sensitivity Analysis
The output for the one-way sensitivity analysis is the budget impact with a base-case value of US$2,296,235. The results of the sensitivity analysis suggest that the model parameters with the largest impact on the budget impact results were, in order of decreasing impact, the number of yearly IVIG administrations, the IVIG maintenance dose (g/kg), the number of yearly Hizentra administrations, and the Hizentra price (ASP). The sensitivity analysis showed a maximum expected annual budget impact of US$3,458,586 when the number of yearly IVIG administrations was increased by 10%, and a minimum expected budget impact of US$1,133,884 when the number of yearly IVIG administrations was reduced by 10% (Fig. 4). When adjusting the input variables by ±20%, the key drivers of the model did not vary, but the budget impact ranged from US$4,620,936 to US$28,466.
4 Discussion
Due to its progressive neuromuscular pathophysiology, the journey of a patient with CIDP is typically characterized by decreasing physical function and quality of life [69] and high disability levels [70, 71]. Going beyond diagnosis and early treatments with immunosuppressives, including corticosteroids, IGs have been demonstrated to be variously associated with improvement from acutely dysfunctional to stable physical function and subsequent maintained relapse-free state for most patients [27, 28, 39]. In addition, maintenance treatment with IGs, specifically Hizentra, has been shown to be associated with maintenance of patient-reported health status and quality of life [40].
Within the class of IG treatments, additional benefit in terms of patient-reported treatment satisfaction has been shown to occur with a switch from healthcare professional administration of IVIGs to self-infusion with SCIGs in a number of clinical studies [38, 72, 73] in another indication for IG treatment: primary immune deficiency. Furthermore, a clinical study of patients with CIDP also demonstrated a clear patient preference for SCIG administration over IVIGs [42].
In addition to improvement in patient health status and treatment satisfaction, an improvement in infusion process-related AEs [29,30,31,32] may result from a switch from IVIGs to an SCIG such as Hizentra [74]. This switch has the potential for both a clinical and an economic benefit for CIDP patients.
For this study, we utilized literature-based inputs to develop a BIM of the introduction of Hizentra for maintenance treatment of CIDP to a formulary to inform corresponding economic and financial implications. This BIM suggests that the introduction of Hizentra administration in CIDP maintenance is expected to produce cost savings. Specifically, a switch from Hizentra was projected to save over US$30,000 per patient switched from IVIG and about US$2.3 million for a 25-million-member IDN that valued its members’ time and out-of-pocket infusion costs. For health plans that do not reimburse patients and caregivers for time and out-of-pocket expenses related to travel for any infusion (including IVIG) [65, 66], savings are expected to nevertheless be as much as US$31,000 per patient and about US$2.2 million at the plan level, in addition to the intangible benefit of reducing patient and caregiver burden. Organizational-level savings were projected based on an assumption of a 10% shift (increase) in Hizentra market share, offset by a corresponding reduction in IVIG market share. Of note, a larger increase in Hizentra market share was expected to result in a proportionate increase in organization-level savings and may help offset any training and structural costs associated with a shift from IVIG infusions to SCIG infusions.
Our projected savings from a switch from IVIG to SCIG infusions, especially when the former are administered in a hospital setting [75], are consistent with similar conclusions with regard to use of self-injected biologics in other conditions, such as rheumatoid arthritis [76, 77]. Although some savings are admittedly possible with a shift from the hospital to home setting for IVIG infusions, our study showed optimal cost savings with self-administered subcutaneous infusions.
Our budget impact findings are supported by those of an Australian cost-utility analysis of IVIG versus SCIG, which found that SCIG is cost effective in 93.2% of simulations, given a willingness-to-pay threshold of A$50,000 per quality-adjusted life-year [78]. Additionally, from a cost-minimization perspective, the analysis found that IVIG and SCIG were associated with average cost values of A$297,547 and A$251,713, respectively [78]. Although based on a related indication of primary immune deficiency, other economic evidence is consistent with the findings of our novel analysis in CIDP. According to economic analyses performed in Sweden, Germany, the UK, Canada, and France, home-based SCIG was found to be 25–75% less costly to the healthcare system than hospital-based IVIG [79,80,81,82,83]. While findings on economic analyses on other approved indications and other countries are not directly comparable with our estimated expected BIM from a US plan perspective, especially when considering regional healthcare differences and product prices, the directionality and similarity of magnitude of cost benefit in each suggest that SCIG is more financially appealing than IVIG.
The recent coronavirus disease 2019 (COVID-19) pandemic has prompted fundamental shifts in drug infusions from real or perceived high-risk encounters in healthcare settings to the home environment [84]. It is expected that these trends may continue with greater patient independence and caution, even after the pandemic has subsided, thus providing continued impetus to health plans for encouraging such shifts in care.
4.1 Limitations
As with any economic model, the validity of the results is dependent on the inputs and assumptions made within the model. Model inputs are based on a variety of assumptions, including the size of the target population, pharmacy costs, medical costs, and market shares. While we employed methodologically sound modeling techniques and tested our assumptions with sensitivity analyses, the inputs and assumptions used in this model may not be appropriate for all healthcare plans.
Additionally, we assumed that all patients administered Hizentra within the home setting would be self-administering therapy. In real-world settings, Hizentra administration may be facilitated via an infusion nurse for select patients.
5 Conclusion
This budget impact analysis strongly suggests that Hizentra is expected to be associated with favorable economic benefits compared with IVIG in the management of CIDP. As such, there may be economic value in switching patients from IVIG to an SCIG, in addition to intangible benefits in terms of greater treatment satisfaction for appropriate patients. Clearly, such a transition must be supported by adequate self-infusion training for patients. Other necessary training may revolve around alleviating any modifiable patient-level factors that could decrease the chance of successful and sustained uptake to SCIG. Furthermore, choice of IVIG versus SCIG and corresponding choice of site of administration are predicated upon a variety of patient-level and patient-influencing factors, including but not limited to those revolving around the COVID-19 pandemic, which may have influenced greater and perhaps enduring patient independence worldwide.
References
Bunschoten C, Jacobs BC, Van den Bergh PYK, Cornblath DR, van Doorn PA. Progress in diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy. Lancet Neurol. 2019;18(8):784–94.
Laughlin RS, Dyck PJ, Melton LJ 3rd, Leibson C, Ransom J, Dyck PJB. Incidence and prevalence of CIDP and the association of diabetes mellitus. Neurology. 2009;73(1):39–45.
Viala K, Maisonobe T, Stojkovic T, et al. A current view of the diagnosis, clinical variants, response to treatment and prognosis of chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. 2010;15(1):50–6.
Kieseier BC, Dalakas MC, Hartung H-P. Immune mechanisms in chronic inflammatory demyelinating neuropathy. Neurology. 2002;59(12 Suppl 6):S7–12.
Bunschoten C, Blomkwist-Markens PH, Horemans A, van Doorn PA, Jacobs BC. Clinical factors, diagnostic delay, and residual deficits in chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. 2019;24(3):253–9.
Chiò A, Cocito D, Bottacchi E, et al. Idiopathic chronic inflammatory demyelinating polyneuropathy: an epidemiological study in Italy. J Neurol Neurosurg Psychiatry. 2007;78(12):1349–53.
Gorson KC, van Schaik IN, Merkies ISJ, et al. Chronic inflammatory demyelinating polyneuropathy disease activity status: recommendations for clinical research standards and use in clinical practice. J Peripher Nerv Syst. 2010;15(4):326–33.
dos Santos PL, Nogueira de Almeida-Ribeiro GA, Daoud Silva DM, Marques W Jr, Barreira AA. Chronic inflammatory demyelinating polyneuropathy: quality of life, sociodemographic profile and physical complaints. Arq Neuropsiquiatr. 2014;72(3):179–83.
Dyck PJB, Tracy JA. History, diagnosis, and management of chronic inflammatory demyelinating polyradiculoneuropathy. Mayo Clin Proc. 2018;93(6):777–93.
Taylor T. Chronic inflammatory demyelinating polyradiculoneuropathy: in a remote northern Ontario hospital. Can Fam Physician. 2013;59(4):368–71.
Eftimov F, van Schaik I. Chronic inflammatory demyelinating polyradiculoneuropathy: update on clinical features, phenotypes and treatment options. Curr Opin Neurol. 2013;26(5):496–502.
Eftimov F, Winer JB, Vermeulen M, de Haan R, van Schaik IN. Intravenous immunoglobulin for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2013;(12):CD001797. https://doi.org/10.1002/14651858.CD001797.pub3.
Van Lieverloo GGA, Peric S, Doneddu PE, et al. A retrospective, multicentre study, comparing efficacy and safety of daily prednisolone, pulsed dexamethasone, and pulsed intravenous methylprednisolone. J Neurol. 2018;265(9):2052–9.
Hughes RA, Mehndiratta MM, Rajabally YA. Corticosteroids for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2017;11(11):CD002062.
Van den Bergh PY, Hadden RDM, Bouche P, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—first revision. Eur J Neurol. 2010;17(3):356–63.
Nobile-Orazio E, Cocito D, Jann S, et al. Intravenous immunoglobulin versus intravenous methylprednisolone for chronic inflammatory demyelinating polyradiculoneuropathy: a randomised controlled trial. Lancet Neurol. 2012;11(6):493–502.
Mahdi-Rogers M, McCrone P, Hughes RA. Economic costs and quality of life in chronic inflammatory neuropathies in southeast England. Eur J Neurol. 2014;21(1):34–9.
Hughes RA, Swan AV, van Doorn PA. Cytotoxic drugs and interferons for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2003;(1):CD003280. https://doi.org/10.1002/14651858.CD003280.
Kuitwaard K, van Doorn PA. Newer therapeutic options for chronic inflammatory demyelinating polyradiculoneuropathy. Drugs. 2009;69(8):987–1001.
Dyck PJ, Daube J, O’Brien P, et al. Plasma exchange in chronic inflammatory demyelinating polyradiculoneuropathy. N Engl J Med. 1986;314(8):461–5.
Hahn AF, Bolton CF, Pillay N, et al. Plasma-exchange therapy in chronic inflammatory demyelinating polyradiculoneuropathy. A double-blind, sham-controlled, crossover study. Brain. 1996;119(Pt 4):1055–66.
Donofrio PD, Berger A, Brannagan TH 3rd, et al. Consensus statement: the use of intravenous immunoglobulin in the treatment of neuromuscular conditions report of the AANEM ad hoc committee. Muscle Nerve. 2009;40(5):890–900.
Patwa HS, Chaudhry V, Katzberg H, Rae-Grant AD, So YT. Evidence-based guideline: intravenous immunoglobulin in the treatment of neuromuscular disorders: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2012;78(13):1009–15.
European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—First Revision. J Peripher Nerv Syst. 2010;15(1):1–9.
Van den Bergh P, van Doorn P, Hadden R, et al. European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force-second revision. J Peripher Nerv Syst. 2021;26:242–68.
Léger JM, De Bleecker JL, Sommer C, et al. Efficacy and safety of Privigen in patients with chronic inflammatory demyelinating polyneuropathy: results of a prospective, single-arm, open-label phase III study (the PRIMA study). J Peripher Nerv Syst. 2013;18(2):130–40.
Merkies ISJ, Bril V, Dalakas MC, et al. Health-related quality-of-life improvements in CIDP with immune globulin IV 10%: the ICE study. Neurology. 2009;72(15):1337–44.
Hughes RA, Donofrio P, Bril V, et al. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo-controlled trial. Lancet Neurol. 2008;7(2):136–44.
Ramírez E, Romero-Garrido JA, López-Granados E, et al. Symptomatic thromboembolic events in patients treated with intravenous-immunoglobulins: results from a retrospective cohort study. Thromb Res. 2014;133(6):1045–51.
Totzeck A, Stettner M, Hagenacker T. Early platelet and leukocyte decline in patients with neuroinflammatory disorders after intravenous immunoglobulins. Eur J Neurol. 2017;24(4):638–44.
Sekul EA, Cupler EJ, Dalakas MC. Aseptic meningitis associated with high-dose intravenous immunoglobulin therapy: frequency and risk factors. Ann Intern Med. 1994;121(4):259–62.
Dychter SS, Gold DA, Carson D, Haller M. Intravenous therapy: a review of complications and economic considerations of peripheral access. J Infus Nurs. 2012;35(2):84–91.
Harbo T, Andersen H, Hess A, Hansen K, Sindrup SH, Jakobsen J. Subcutaneous versus intravenous immunoglobulin in multifocal motor neuropathy: a randomized, single-blinded cross-over trial. Eur J Neurol. 2009;16(5):631–8.
Misbah SA. Effective dosing strategies for therapeutic immunoglobulin: managing wear-off effects in antibody replacement to immunomodulation. Clin Exp Immunol. 2014;178(Suppl 1):70–1.
Allen J, Pasnoor M, Burns T, et al. Intravenous immunoglobulin (IVIg) treatment-related fluctuations in chronic inflammatory demyelinating polyneuropathy (CIDP) patients using daily grip strength measurements (GRIPPER): study design and progress update (P2.269). Neurology. 2016;86(16 Suppl):P2.269.
Slen B. Infused therapies: costs savings benefits through home infusion. Spec Pharm Times. 2014;5(1):24–5.
Luthra R, Quimbo R, Iver R, Luo M. An analysis of intravenous immunoglobulin site of care: home vs outpatient hospital. Am J Pharm Benefits. 2014;6(a):e41–9.
Nicolay U, Kiessling P, Berger M, et al. Health-related quality of life and treatment satisfaction in North American patients with primary immunedeficiency diseases receiving subcutaneous IgG self-infusions at home. J Clin Immunol. 2006;26(1):65–72.
van Schaik IN, Bril V, van Geloven N, et al. Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (PATH): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2018;17(1):35–46.
Hartung H-P, Mallick R, Bril V, et al. Patient-reported outcomes with subcutaneous immunoglobulin in chronic inflammatory demyelinating polyneuropathy: the PATH study. Eur J Neurol. 2020;27(1):196–203.
Merkies ISJ, Mallick R, Haudrich A, et al. Impact of CIDP on daily activity and participation: I-RODS analysis from a US patient survey. In: Presented at the Annual Meeting of the Peripheral Nerve Society; June 2018; Baltimore, MD.
van Schaik IN, Mielke O, Bril V, et al. Long-term safety and efficacy of subcutaneous immunoglobulin IgPro20 in CIDP PATH extension study. Neurol Neuroimmunol Neuroinflamm. 2019;6(5): e590.
Cocito D, Merola A, Romagnolo A, et al. Subcutaneous immunoglobulin in CIDP and MMN: a different long-term clinical response? J Neurol Neurosurg Psychiatry. 2016;87(7):791–3.
Hadden RD, Marreno F. Switch from intravenous to subcutaneous immunoglobulin in CIDP and MMN: improved tolerability and patient satisfaction. Ther Adv Neurol Disord. 2015;8(1):14–9.
Academy of Managed Care Pharmacy. The AMCP Format for Formulary Submissions Version 4.1. 2020. https://www.amcp.org/sites/default/files/2019-12/AMCP_Format%204.1_1219_final.pdf. Accessed 5 May 2022.
Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis—principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014;17(1):5–14.
Red Book, IBM Micromedex. 2021: Copyright IBM Corporation.
Academy of Managed Care Pharmacy. AMCP guide to pharmaceutical payment methods, 2013 update. Version 3.0. Published April 2013.
Fryar CD, Carroll MD, Gu Q, Afful J, Ogden CL. Anthropometric reference data for children and adults: United States, 2015–2018. National Center for Health Statistics. Vital Health Stat., series 3, number 46; 2021, p. 1–441.
Gamunex-C prescribing information. Research Triangle Park, NC: Grifols Therapeutics LLC; 2017.
GBS/CIPD Foundation patient reported outcomes and treatment survey 2018. 2018 Peripheral Nerve Society Annual Meeting 21–25 July, 2018, Baltimore, Maryland. J Peripher Nerv Syst. 2018;23(4):249–405.
Barton AJ, Danek G, Johns P, Coons M. Improving patient outcomes through CQI: vascular access planning. J Nurs Care Qual. 1998;13(2):77–85.
Kokotis K. Cost containment and infusion services. J Infus Nurs. 2005;28(3 Suppl):S22–32.
Dimick JB, Pelz RK, Consunji R, Swoboda SM, Hendrix CW, Lipsett PA. Increased resource use associated with catheter-related bloodstream infection in the surgical intensive care unit. Arch Surg. 2001;136(2):229–34.
Hizentra prescribing information. Kanakee, IL: CSL Behring; 2022.
Find-a-code. CPT 98960. https://www.findacode.com/cpt/98960-cpt-code.html. Accessed 4 Feb 2021.
United States Bureau of Labor Statistics. Economic news release. Table B-3: Average hourly and weekly earnings of all employees on private nonfarm payrolls by industry sector, seasonally adjusted. https://www.bls.gov/news.release/empsit.t19.htm. Accessed 5 May 2022.
Pierce J, Baker J. A nursing process model: quantifying infusion therapy resource consumption. J Inf Nurs. 2004;27(4):232–44.
Agency for Healthcare Research and Quality (AHRQ). Healthcare Cost and Utilization Project (HCUP). https://hcupnet.ahrq.gov/#setup. Accessed 5 May 2022.
Maki D, Kluger D, Crnich C, et al. Risk of bloodstream infection in adults with different intra-vascular devices: a systematic review of 200 published prospective studies. Mayo Clinic Proc. 2006;81(9):1159–71.
Hadaway L. Infiltration and extravasation: preventing a complication of IV catheterization. Am J Nurs. 2007;107(8):64–72.
Grosse S, Nelson R, Nyarko K, et al. The economic burden of incident venous thromboembolism in the United States: a review of estimated attributable healthcare costs. Thromb Res. 2016;137:3–10.
Bharath V, Eckert K, Kang M, et al. Incidence and natural history of IVIG-induced aseptic meningitis: a retrospective review at a single tertiary care centre. Blood. 2014;124(21):2884.
Daw Z, Padmore R, Neurath D, et al. Hemolytic transfusion reactions after administration of intravenous immune (gamma) globulin: a case series analysis. Transfusion. 2008;48:1598–601.
Weiss AJ, Pickens G, Roemer M. Methods for calculating patient travel distance to hospital in HCUP data. HCUP Methods Series Report # 2021-02 Online. December 6, 2021. www.hcup-us.ahrq.gov/reports/methods/methods.jsp. Accessed 14 Oct 2022.
Premnath N, Grewal U, Gupta A. Park the parking. JCO Oncol Pract. 2020;16(5):215–7.
Kleman M, Myren K, Tursunova S, et al. Impact of generalised myasthenia gravis on activities of daily living and employment for patients and caregivers. Eur J Neurol. 2022;29(Suppl 1):612.
Jacob S, Dewilde S, Qi C, et al. Productivity losses for generalized myasthenia gravis patients and their caregivers: association with disease severity. J Neuromuscul Dis. 2022;9(Suppl 1):S245–6.
Merkies IS, Hughes RA, Donofrio P, et al. Understanding the consequences of chronic inflammatory demyelinating polyradiculoneuropathy from impairments to activity and participation restrictions and reduced quality of life: the ICE study. J Peripher Nerv Syst. 2010;15(3):208–15.
Lunn MP, Manji H, Choudhary PP, Hughes RA, Thomas PK. Chronic inflammatory demyelinating polyradiculoneuropathy: a prevalence study in south east England. J Neurol Neurosurg Psychiatry. 1999;66(5):677–80.
Cocito D, Paolasso I, Antonini G, et al. A nationwide retrospective analysis on the effect of immune therapies in patients with chronic inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol. 2010;17(2):289–94.
Gardulf A, Nicolay U, Math D, et al. Children and adults with primary antibody deficiencies gain quality of life by subcutaneous IgG self-infusions at home. J Allergy Clin Immunol. 2004;114(4):936–42.
Mallick R, Jolles S, Kanegane H, Agbor-Tarh D, Rojavin M. Treatment satisfaction with subcutaneous immunoglobulin replacement therapy in patients with primary immunodeficiency: a pooled analysis of six Hizentra® studies. J Clin Immunol. 2018;38(8):886–97.
Guo Y, Tian X, Wang X, Xiao Z. Adverse effects of immunoglobulin therapy. Front Immunol. 2018;9:1299.
Himmelstein DU, Jun M, Busse R, et al. A comparison of hospital administrative costs in eight nations: US costs exceed all others by far. Health Aff (Millwood). 2014;33(9):1586–94.
Yazdany J, Dudley RA, Chen R, Lin GA, Tseng CW. Coverage for high-cost specialty drugs for rheumatoid arthritis in Medicare Part D. Arthritis Rheumatol. 2015;67(6):1474–80.
Schmeir J, Ogden K, Nickman N, et al. Costs of providing infusion therapy for rheumatoid arthritis in a hospital-based infusion center setting. Clin Ther. 2017;39(8):1600–17.
Windegger TM, Nghiem S, Nguyen KH, Fung YL, Scuffham PA, et al. Primary immunodeficiency disease: a cost-utility analysis comparing intravenous vs subcutaneous immunoglobulin replacement therapy in Australia. Blood Transfus. 2020;18(2):96–105.
Gardulf A, Jonsson G. A comparison of the patient-borne costs of therapy with gamma globulin given at the hospital or at home. Int J Technol Assess Health Care. 1995;11(2):345–53.
Högy B, Keinecke HO, Borte M. Pharmacoeconomic evaluation of immunoglobulin treatment in patients with antibody deficiencies from the perspective of the German statutory health insurance. Eur J Health Econ. 2005;6(1):24–9.
Liu Z, Albon E, Hyde C. The effectiveness and cost effectiveness of immunoglobulin replacement therapy for primary immunodeficiency and chronic lymphocytic leukaemia: a systematic review and economic evaluation. University of Birmingham. 2005. https://www.birmingham.ac.uk/Documents/college-mds/haps/projects/WMHTAC/REPreports/2005/IgRT.pdf. Accessed 12 Oct 2020.
Martin A, Lavoie L, Goetghebeur M, Schellenberg R. Economic benefits of subcutaneous rapid push versus intravenous immunoglobulin therapy in adult patients with primary immune deficiency. Transfus Med. 2013;23(1):55–60. https://doi.org/10.1111/j.1365-3148.2012.01201.x.
Beauté J, Levy P, Millet V, et al. Economic evaluation of immunoglobulin replacement in patients with primary antibody deficiencies. Clin Exp Immunol. 2010;160(2):240–5.
Flaherty L. Covid infusion therapy shifts to the home. https://www.hmenews.com/article/covid-infusion-therapy-shifts-to-the-home. Accessed 29 Apr 2022.
Acknowledgments
The authors would like to acknowledge Kylie Matthews from Xcenda for copyediting and manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by CSL Behring.
Conflicts of interest
Rajiv Mallick is an employee of CSL Behring and holds stock options. Rashad Carlton and Joris Van Stiphout are employees of Xcenda, which received funding from CSL Behring for this analysis.
Data availability
Data for the model were obtained from a variety of sources, including published studies, publicly available costs and databases, and assumptions as necessary.
Ethics approval
As this was an economic analysis that did not use any identifiable patient data, no ethics approval was required.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
An unlocked version of the model has been provided as ESM. The input parameters specific to the US-approved labeling for Hizentra, including dosing and relapse parameters, are not modifiable. The VBA coding is proprietary to the model developers and is not publicly available.
Author contributions
Conceptualization: RM, RC, and JVS. Methodology: RM, RC, and JVS. Formal analysis: RM, RC, and JVS. Writing, review, and editing: RM, RC, and JVS.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Mallick, R., Carlton, R. & Van Stiphout, J. A Budget Impact Model of Maintenance Treatment of Chronic Inflammatory Demyelinating Polyneuropathy with IgPro20 (Hizentra) Relative to Intravenous Immunoglobulin in the United States. PharmacoEconomics Open 7, 243–255 (2023). https://doi.org/10.1007/s41669-023-00386-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-023-00386-2