Abstract
Introduction
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is a rare progressive or relapsing inflammatory disease. Intravenous immunoglobulin (IVIG) is recommended as a first-line therapy. The aim of this study was to describe real-world treatment patterns and outcomes of patients with CIDP in the Define initiating IVIG treatment.
Methods
This cohort study used health insurance claims data from the Merative MarketScan Research Databases (2008–2018). Adult patients (≥ 18 years old) with CIDP without prior immunoglobulin treatment were identified using International Statistical Classification of Diseases and Related Health Problems (ICD) codes, and patients subsequently initiating IVIG were included in the analysis. Real-world IVIG treatment patterns and treatment and safety outcomes (assessed via ICD codes) were described.
Results
In total, 3975 patients (median age 58 years) with CIDP who initiated IVIG were identified. After the initial IVIG loading period, patients received IVIG at a median dosing interval of 21 days (quartile [Q]1, Q3: 7, 28), and continued treatment for a median of 129 days (Q1, Q3: 85, 271). After the 2-year follow-up period, 55% of patients had discontinued all IVIG treatment; more than one-half of these discontinuations occurred within 4 months. Diagnoses of impaired functional status were evident in more than 30% of patients at baseline, but at lower rates during follow-up. Rates of new-onset safety outcomes after IVIG treatment were low.
Conclusion
This real-world analysis of IVIG treatment patterns and treatment and safety outcomes of patients with CIDP who initiated IVIG highlights the unmet need for improved long-term management. Further research is needed to evaluate the use of functional status measures as endpoints for immunoglobulin treatment effectiveness.
Plain Language Summary
Chronic inflammatory demyelinating polyradiculoneuropathy, also called CIDP, is a rare disease that causes the body’s immune system to attack its nerves. Treatments for CIDP include antibodies, which are also called immunoglobulins. Immunoglobulins may be given intravenously, meaning they are administered into a vein. Intravenous immunoglobulin, also called IVIG, is recommended as one of the first treatments that patients with CIDP receive in their therapy and involves giving antibodies through a drip into a vein. This study aimed to gather information on the day-to-day use of IVIG by patients with CIDP. Information from 2008 to 2018 was collected from a large health insurance database in the USA. Information was taken from the records of patients aged 18 years or older who had received IVIG during the information collection period. In total, records from 3975 patients with an average age of 58 years were included in the study. On average, patients received IVIG every 21 days for 129 days. By 2 years, 55% of patients had stopped receiving IVIG; most of those patients had stopped within 4 months of first receiving the treatment. In the 6 months before receiving IVIG, over 30% of patients experienced limitations owing to their CIDP that affected their daily lives, although this percentage became smaller once patients started to receive IVIG. In addition, a low number of patients experienced side effects because of their IVIG treatment. This study highlights that improved long-term care for patients with CIDP is needed. Further research into ways of measuring the impact of CIDP on patients’ daily lives is required, which may help doctors to work out how effective IVIG is at treating CIDP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is a rare progressive or relapsing inflammatory disease, for which intravenous immunoglobulin (IVIG) is recommended as a first-line treatment |
Real-world data on IVIG treatment patterns and outcomes are limited; therefore, this cohort study aimed to describe these patterns and outcomes in US patients with CIDP initiating IVIG treatment |
What was learned from the study? |
Although discontinuation of IVIG treatment within the first year of initiation is consistent with clinical evidence and guideline recommendations, there is an unmet need for improved long-term management of patients with CIDP initiating IVIG treatment |
Further research is needed to evaluate the use of functional status measures as endpoints for immunoglobulin treatment effectiveness |
Introduction
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is a rare progressive or relapsing immune-mediated inflammatory disease that causes demyelination and axonal damage to the peripheral nerves [1,2,3]. This manifests as characteristic symptoms of distal- and proximal-limb weakness and sensory dysfunction that affect gait and muscle strength and impair activities of daily living. Fatigue, pain, and anxiety/depression further add to the significant clinical and humanistic burdens of CIDP [4]. Given the diversity of clinical manifestations in the disease, pathogenesis of CIDP is presumed to be heterogeneous [5]. Pathogenesis may include ‘classical’ macrophage-mediated demyelination, or association between immunoglobulin G4 and paranodal junction proteins leading to neuropathy in some patient subpopulations [5].
Worldwide, the prevalence and incidence of CIDP is estimated at 0.8 to 10.3 cases per 100,000 people, and 0.2 to 1.6 cases per 100,000 person-years, respectively [6]. The primary goals of CIDP treatment are to reduce symptoms, improve functional status, and, if possible, maintain long-term remission [7]. Early detection and initiation of appropriate therapy may prevent loss of nerve function, and long-term therapy is generally required to maintain treatment response and prevent relapse [8].
Intravenous immunoglobulin (IVIG) treatment and systemic corticosteroids are recommended in the European Academy of Neurology/Peripheral Nerve Society (EAN/PNS) guidelines as first-line treatment options for CIDP with disabling symptoms, both for induction treatment and maintenance of response. Plasma exchange should be considered if IVIG and corticosteroids fail [9]. Studies have shown that chronic use of corticosteroids carries a long‐term risk of serious side effects [10]. In the most recent (2021) update to the EAN/PNS guidelines, immunoglobulin (IG) treatment recommendations have been further extended to allow for use of subcutaneous IG as an alternative maintenance treatment in IVIG-responsive patients with active disease.
IVIG treatment has been the most extensively studied treatment for CIDP for both initiation and maintenance therapy, and appears to be effective in treating macrophage-mediated demyelination in CIDP by modulating Fc receptors on the macrophage surface [5]. IVIG has been shown to be effective in improving impairment and disability scores [11,12,13,14,15,16,17,18,19,20,21,22], with a 2017 Cochrane systematic review that included five trials of IVIG in CIDP finding greater short-term improvement in disability with IVIG than with placebo (53% vs 23% of patients; risk ratio 2.40, 95% confidence interval [CI] 1.72–3.36) [20]. In patients with CIDP, guidelines recommend that IVIG should be initiated with a loading dose divided over 2–5 consecutive days [23, 24], followed by maintenance IVIG infusions every 3 weeks [23,24,25,26]. Dose and interval may be adapted to each patient [9].
Real-world studies evaluating utilization of IVIG treatment in patients with CIDP can provide valuable insight into how current practice aligns with available evidence and guidelines [27]. However, real-world data are limited, particularly in terms of understanding IVIG treatment patterns and outcomes. Although several medical record review studies have evaluated utilization, effectiveness, and safety of IVIG alongside other treatment options for patients with CIDP, the numbers of IVIG-treated patients included have been low (n < 60) [28,29,30]. Therefore, the present study sought to describe the real-world treatment patterns and outcomes of IVIG in a large-scale population of patients in the USA who initiated IVIG for treatment of CIDP during a 10-year period.
Methods
Data Source and Study Period
This cohort study used health insurance claims data between 1 January 2008 and 30 September 2018 from the Merative MarketScan Research Databases. The databases contain information on insurance plan enrolment, outpatient pharmacy dispensing information, and inpatient and outpatient diagnoses and procedures recorded on adjudicated claims for paid treatments. Patient data from the Commercial Claim and Encounters Database, Medicare Supplementary and Coordination of Benefit Database, and Multi-State Medicaid Database within the Merative MarketScan Research Databases were combined into one analytic cohort.
Compliance with Ethics Guidelines
This study did not involve the collection, use, or transmittal of individually identifiable data. The databases used contained fully de-identified/anonymized data and were compliant with the Health Insurance Portability and Accountability Act (HIPAA); this study was, therefore, exempt from institutional review board approval and administrative permission from individuals was not required.
Population
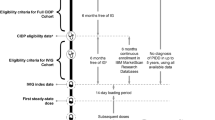
Adult patients (≥ 18 years old) were included in the analysis if they had at least one recorded diagnosis of CIDP, no recorded use of any IG treatment in the preceding 6 months of the diagnosis, and at least one subsequent IVIG claim indicating the initiation of an IVIG product (Gammagard Liquid [Baxalta US Inc., Lexington, MA, USA], Gamunex-C [Grifols Therapeutics LLC, Research Triangle Park, NC, USA], Gammaked [Grifols Therapeutics LLC, Research Triangle Park, NC, USA], Privigen [CSL Behring AG, Bern, Switzerland]; Fig. 1). Patients must have had at least 6 months of continuous enrolment in the MarketScan database before the IVIG index date (defined as the first IVIG product claim after CIDP diagnosis) and no diagnosis of primary immunodeficiency disease in up to 5 years before the IVIG index date to avoid misclassification, as IVIG is also indicated for primary immunodeficiency disease (Fig. 1).
Patient eligibility and treatment timeline. aIVIG treatments were identified through procedural (inpatient and outpatient Healthcare Common Procedure Coding System) and pharmacy dispensing (National Drug Code) codes. bPatients were aged ≥ 18 years on the CIDP eligibility date. CIDP chronic inflammatory demyelinating polyradiculoneuropathy, IG immunoglobulin, IVIG intravenous immunoglobulin, PIDD primary immunodeficiency disease
CIDP diagnosis was identified by International Statistical Classification of Diseases and Related Health Problems (ICD), Ninth Revision, Clinical Modification diagnosis code 357.81 or ICD, Tenth Revision, Clinical Modification diagnosis code G61.81, and IVIG product administration and dispensing was identified by outpatient or inpatient Healthcare Common Procedure Coding System and pharmacy dispensing codes (National Drug Codes).
The IVIG product initiated for each patient was defined as the index IVIG product. The follow-up period spanned from the IVIG index date to the end of the study period (30 September 2018), disenrolment from the database, or 2 years after the IVIG index date, whichever event occurred first.
IVIG Use
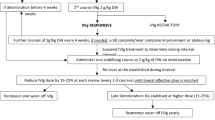
To evaluate periods of continuous IVIG treatment, each patient was considered exposed to an IVIG product for 12 weeks after an IVIG administration to allow for longer-spaced dosing schedules and potentially missed or skipped doses. Patients were considered continuously exposed if they received a subsequent administration of the same IVIG product within the 12-week period (if not, they were considered to have discontinued the IVIG product; Fig. 2). A sensitivity analysis was conducted by defining continuous exposure with 6-week and 9-week periods to evaluate the effect of shorter assumed exposure durations.
Determining periods of continuous use of IVIG. This is an example of a patient health and treatment timeline depicting how continuous IVIG use period is determined for a patient. IVIG use was considered to be continuous if each administration occurred within 12 weeks of the previous one. aIVIG treatments were identified through procedural (inpatient and outpatient Healthcare Common Procedure Coding System) and pharmacy dispensing (National Drug Code) codes. IVIG intravenous immunoglobulin
To account for the variability in dosing occurring immediately after initiation, we defined the first 14 days after the IVIG index date as a loading period, after which initial tolerance, titration, and loading dosing were assumed to be complete. The first administration of the index IVIG product occurring after the 14-day loading period was defined as the steady-state dose. The index IVIG treatment period was defined as a patient’s time using the index IVIG product.
Treatment Outcomes
Healthcare utilization data assessed during the index IVIG treatment period included hospitalizations, emergency department visits, clinic visits, and all healthcare encounters (with multiple occurrences per patient considered).
Rates of discontinuation, switching between IVIG products, initiation of other non-IVIG CIDP treatments, and initiation of opioids were identified during follow-up. Initiation of each non-IVIG CIDP treatment was evaluated individually in outcome-specific cohorts in which patients who had previous use of the non-IVIG CIDP treatments before the IVIG index date were excluded. For example, patients with previous use of high-dose systemic corticosteroids were excluded from evaluation of high-dose systemic corticosteroids initiation after IVIG, but not excluded from evaluation of immunosuppressant/immunomodulatory therapy initiation. This was to ensure that initiation of non-IVIG CIDP treatments after IVIG initiation could be identified. Initiation of opioids was included as an indirect measure of CIDP pain, which is an important contributor to the clinical burden of patients with CIDP [31,32,33].
Potential markers of disease severity or burden were evaluated before or on the IVIG index date and during follow-up with diagnosis coding. These markers included diagnoses of nerve dysfunction (e.g. abnormal reflex, abnormal response to nerve stimulation), which can be evaluated by healthcare professionals with electrodiagnostic testing during patient examinations, and markers of impaired functional status (e.g. difficulty walking, weakness), which have been associated with activities of daily living and frailty [34]. Diagnoses of these four measures of nerve or functional status were evaluated separately at the IVIG index date and during the 1-year and 2-year follow-up of the index IVIG treatment period.
IVIG treatment safety and tolerability was assessed by occurrence of the following a priori identified safety endpoints (identified using diagnosis coding) during the index IVIG treatment period: renal failure, hyperproteinaemia and hyponatraemia, ischaemic stroke, intracranial haemorrhage, thrombosis/thromboembolism (other than stroke), aseptic meningitis, haemolysis, hypertension, and anaphylaxis. To ensure identification of new cases of the safety outcomes, patients who had a prior occurrence of one of these safety outcomes in the 6 months before the IVIG index date were excluded from the analysis for that specific safety outcome.
Statistical Analysis
Continuous variables were described with means and standard deviations. Categorical and binary variables were described with counts and percentages. Outcomes were analysed with incidence rates (IRs) expressed as events per 100 person-years with 95% CI.
A Sankey diagram was constructed to display a patient’s treatment trajectory [35] for up to 2 years of follow-up from the date of the steady-state dose. Treatment status was assessed and plotted at 4-month intervals.
All statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA). A glossary of the study terms defined in the methods is shown in in the electronic supplementary material.
Results
Baseline
In total, 3975 patients with CIDP who initiated IVIG between January 2008 and September 2018 were identified from the Merative MarketScan Research Databases (Fig. 3). The median age of the patients on the IVIG index date was 58 years (quartile [Q]1, Q3: 49, 65), and 59.3% of patients were men.
Demographics and baseline characteristics. aAssessed on the IVIG index date. bAssessed in the 5 years before the IVIG index date. The Charlson Comorbidity Index is a summary measure of conditions present in an individual. Select conditions are assigned a score from 1 to 6, with 6 representing the most severe morbidity, and the summation of the weighted comorbidity scores results in a summary score. cAssessed in the 6 months before the IVIG index date. CIDP chronic inflammatory demyelinating polyradiculoneuropathy, IG immunoglobulin, IVIG intravenous immunoglobulin, Q quartile, SD standard deviation
IVIG Use
Patients continued their index IVIG treatment for a median of 129 days (Q1, Q3: 85, 271). The median IVIG dosing interval was 21 days (Q1, Q3: 7, 28) following the 14-day loading period; the median number of doses in this period was 1 (Q1, Q3: 1, 3). By the end of the 2-year follow-up period, 55% of patients had discontinued their index IVIG treatment, 12% switched to another IG treatment, and 33% were lost to follow-up. More than 60% of patients who would eventually discontinue the index IVIG product had done so by 4 months and over 80% of patients had discontinued by the eighth month of treatment (Fig. 4).
Sankey diagram showing status of index IVIG treatment at 4-month intervals during follow-up. Note: SS dose is the first IG dose received more than 14 days after the index IVIG date, to account for loading dosing of the index IVIG. Faded arcs represent transition of patients from one treatment status to another. The width of the arcs is proportional to the percentage of patients who transitioned. Of the patients who transitioned from index IVIG (faded dark-blue arcs), a high proportion discontinued the index IVIG, whereas a smaller proportion switched to another IG or different IVIG. IG immunoglobulin, IVIG intravenous immunoglobulin, SS steady state
Discontinuing the index IVIG product without switching to another IG treatment was much more common than switching to a different IG treatment (Fig. 4). The percentage of patients who switched from index IVIG product to other IG treatment increased the most between the steady-state dose (1%) and 4 months after the steady-state dose (3%), suggesting that the highest rate of IVIG product switching occurred during this time. The rate of discontinuing the index IVIG product was 107.95 events/100 person-years, and the rate of switching to a different IG treatment was 10.24 events/100 person-years.
Initiation of high-dose systemic corticosteroids and opioids after IVIG initiation was common (IR 44.0 and 17.6 events/100 person-years, respectively), while use of immunosuppressant/immunomodulatory therapies and plasma exchange/plasmapheresis therapy was infrequent (IR 8.1 and 2.3 events/100 person-years, respectively; Table 1). The highest rates of initiation of corticosteroids, prior plasma exchange/plasmapheresis, immunosuppressant/immunomodulatory therapy, or opioids occurred in the first 3 months of IVIG treatment (data not shown).
Predictably, the sensitivity analysis with shorter assumed exposure durations (6-week and 9-week periods) resulted in shorter periods of continuous IVIG exposure.
Treatment Outcomes
Clinic visits comprised 92.3% of all healthcare encounters during follow-up. Compared with clinic visits, the rates for emergency department visits and hospitalization were approximately 32-fold and 17-fold lower, respectively (Table 1). Markers of impaired functional status (e.g. difficulty walking and weakness) were evident in more than 30% of patients at baseline, but at lower rates during follow-up (Table 2).
The analyses of safety endpoints during the index IVIG treatment period indicated that the rates of new-onset safety outcomes after IVIG treatment were generally low (Table 3). All safety outcomes had an IR of at most 6 cases/100 person-years except for new-onset hypertension (IR 37.8 cases/100 person-years).
Discussion
In this real-world study of health insurance claims data from 2008 to 2018, patients who initiated IVIG treatment stayed on the index IVIG product for a median duration of 129 days; the majority of (55%) patients discontinued IVIG treatment by the end of the 2-year follow-up period. Patients were more likely to discontinue the index IVIG product (107.95 events/100 person-years) than to switch to a different IG treatment (10.24 events/100 person-years). The majority of IVIG discontinuations occurred by the eighth month; thereafter, less discontinuation happened. Add-on therapies were minimal with IVIG treatment.
Observed patterns of initial IVIG treatment among presumed IG-naive patients were consistent with clinical practice, including early discontinuation of treatment. The discontinuation pattern was consistent with findings from Latov et al., suggesting that most patients who will benefit from IG treatment will do so within the first 24 weeks [36]. In a study using administrative claims data in the USA, Williams et al. (2018) found a high rate (65%) of discontinuation of IVIG among patients receiving IVIG for CIDP within the 6-month to 2-year follow-up period [37]; this is consistent with the high rate of discontinuation found in our study (55%) by the end of the 2-year follow-up period. The discontinuation pattern also aligns with the EAN/PNS guideline on CIDP management, which recommends evaluating response after a few months of treatment and tapering off IVIG after the patient experiences improvement or when there is no response to the loading dose [9]. However, as the claims data used in the current study did not include reasons for discontinuation, the conclusions that may be drawn around patient response or non-response to IVIG are limited for this analysis.
The low proportions of diagnoses of nerve dysfunction found in patients with CIDP at baseline and during follow-up suggest that measures of nerve dysfunction were not well captured in this health insurance claims data set as a clinical outcome measure. In contrast, functional status was reported at higher rates and seemed to be more consistently captured. During follow-up, the proportion of the population with diagnoses related to measures of nerve function or functional status appeared to decrease over time. More reliable and granular data on functional status may be recorded in patient electronic health records. Further research is needed to evaluate the use of functional status measures as possible endpoints for IG treatment effectiveness, such as difficulty walking, weakness, falls, discharge to skilled nursing facilities/hospice, admission to intensive care units, and use of wheelchair, cane, or walker.
The analyses of safety endpoints during the index IVIG treatment period indicated that the rate of new-onset safety outcomes after IVIG treatment was generally low. These safety findings may be a result of baseline comorbidities and high burden of disease among patients with CIDP, rather than being associated with IVIG treatment. Although the rate of ischaemic stroke was found to be 3.87 events/100 person-years in the present study, it is important to recognize that this rate may be high because of the inclusion of transient ischaemic attacks. This claim is supported by studies using large databases to assess IG safety, reporting that serious adverse events associated with IG treatment, including acute renal failure, thromboembolic events, and haemolysis, were found to occur infrequently (< 1.4 events/100 persons) [38,39,40,41]. The low rates of new-onset safety outcomes, consistent with results from other studies, suggest that IG treatment discontinuation patterns observed in this study are likely unrelated to the safety profile.
Strengths and Limitations
Key strengths of this study include the large population of patients initiating IVIG included in the analysis and the longitudinal data providing rare insight into real-world IVIG treatment patterns and outcomes. To the best of our knowledge, no such study has been undertaken, and past real-world studies were limited by low numbers of IVIG-treated patients included with a mix of prevalent and incident IVIG users [28,29,30].
This study contains several limitations given the nature of the claims-based database analyses. Firstly, dosage of IVIG is not reliably recorded in administrative billing data. Therefore, dose titration and weaning could not be identified. Additionally, administrative healthcare claims data are intended for billing purposes and lack the level of granularity in patient electronic health records. For example, the reasons for discontinuation (e.g. treatment success or failure) could not be elucidated in administrative healthcare claims data alone. The inclusion criterion of 6 months of continuous enrolment in Merative MarketScan Research Databases also could have introduced biases in patient selection because Medicare tends to have more stable enrolment compared with Medicaid or commercial health insurance plans. However, these biases should be minimal as the continuous enrolment criterion required a period of only 6 months.
Outcome measures, such as nerve dysfunction identified by electrodiagnostic testing, were also not routinely used for patients with CIDP, evidenced by lower-than-expected frequencies of recorded diagnoses of nerve dysfunction at baseline and subsequent assessment points. Although a large proportion of the cohort received electrodiagnostic testing in the 5 years before IVIG initiation, test results are not available in claims data and explicitly recorded diagnoses of nerve dysfunction were rare. Conversely, ischaemic strokes and intracranial haemorrhages may have been over-recorded in the safety outcomes analysis because of inclusion of transient ischaemic attacks.
Finally, a large proportion of the cohort was excluded for having prior diagnosis of hypertension when evaluating new-onset hypertension. New-onset hypertension is difficult to identify with administrative healthcare claims data alone. Worsening of existing hypertension also was not evaluated because it likely would not be identified in administrative healthcare claims data.
Conclusion
This large-scale analysis of claims data using the Merative MarketScan Research Database revealed real-world treatment patterns and outcomes of patients with CIDP who initiated an IVIG product. Although discontinuation of IVIG treatment within the first year of initiation is consistent with clinical evidence and guideline recommendations, improved management of this progressive disease is needed to provide patients effective, long-term treatments. Further research is needed to evaluate the use of functional status measures as endpoints for IG treatment effectiveness in real-world data sources.
References
Boukhris S, Magy L, Gallouedec G, et al. Fatigue as the main presenting symptom of chronic inflammatory demyelinating polyradiculoneuropathy: a study of 11 cases. J Peripher Nerv Syst. 2005;10(3):329–37. https://doi.org/10.1111/j.1085-9489.2005.10311.x.
Guptill JT, Bromberg MB, Zhu L, et al. Patient demographics and health plan paid costs in chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 2014;50(1):47–51. https://doi.org/10.1002/mus.24109.
Merkies IS, Kieseier BC. Fatigue, pain, anxiety and depression in Guillain-Barre syndrome and chronic inflammatory demyelinating polyradiculoneuropathy. Eur Neurol. 2016;75(3–4):199–206. https://doi.org/10.1159/000445347.
Westblad ME, Forsberg A, Press R. Disability and health status in patients with chronic inflammatory demyelinating polyneuropathy. Disabil Rehabil. 2009;31(9):720–5. https://doi.org/10.1080/09638280802306497.
Koike H, Nishi R, Ikeda S, et al. Ultrastructural mechanisms of macrophage-induced demyelination in CIDP. Neurology. 2018;91(23):1051–60.
Querol L, Crabtree M, Herepath M, et al. Systematic literature review of burden of illness in chronic inflammatory demyelinating polyneuropathy (CIDP). J Neurol. 2020;268:3706–16. https://doi.org/10.1007/s00415-020-09998-8.
Gorson KC. An update on the management of chronic inflammatory demyelinating polyneuropathy. Ther Adv Neurol Disord. 2012;5(6):359–73. https://doi.org/10.1177/1756285612457215.
Chan YC, Wilder-Smith E. Predicting treatment response in chronic, acquired demyelinating neuropathies. Expert Rev Neurother. 2006;6(10):1545–53. https://doi.org/10.1586/14737175.6.10.1545.
Van den Bergh PY, Hadden RD, Bouche P, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society - first revision. Eur J Neurol. 2010;17(3):356–63. https://doi.org/10.1111/j.1468-1331.2009.02930.x.
Yasir M, Goyal A, Bansal P, et al. Corticosteroid adverse effects. [Updated 2020 Jul 4]. Treasure Island (FL): StatesPearls; 2020. https://www.ncbi.nlm.nih.gov/books/NBK531462/.
Eftimov F, Winer JB, Vermeulen M, et al. Intravenous immunoglobulin for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2013; 12:CD001797; doi: https://doi.org/10.1002/14651858.CD001797.pub3.
Harbo T, Andersen H, Hess A, et al. Subcutaneous versus intravenous immunoglobulin in multifocal motor neuropathy: a randomized, single-blinded cross-over trial. Eur J Neurol. 2009;16(5):631–8. https://doi.org/10.1111/j.1468-1331.2009.02568.x.
Hughes R, Bensa S, Willison H, et al. Randomized controlled trial of intravenous immunoglobulin versus oral prednisolone in chronic inflammatory demyelinating polyradiculoneuropathy. Ann Neurol. 2001;50(2):195–201. https://doi.org/10.1002/ana.1088.
Hughes RA. Systematic reviews of treatment for chronic inflammatory demyelinating neuropathy. Rev Neurol (Paris). 2002;158(12 Pt 2):S32–6.
Hughes RA, Donofrio P, Bril V, et al. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo-controlled trial. Lancet Neurol. 2008;7(2):136–44. https://doi.org/10.1016/S1474-4422(07)70329-0.
Kuitwaard K, van den Berg LH, Vermeulen M, et al. Randomised controlled trial comparing two different intravenous immunoglobulins in chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol Neurosurg Psychiatry. 2010;81(12):1374–9. https://doi.org/10.1136/jnnp.2010.206599.
Leger JM, De Bleecker JL, Sommer C, et al. Efficacy and safety of Privigen® in patients with chronic inflammatory demyelinating polyneuropathy: results of a prospective, single-arm, open-label phase III study (the PRIMA study). J Peripher Nerv Syst. 2013;18(2):130–40. https://doi.org/10.1111/jns5.12017.
Mendell JR, Barohn RJ, Freimer ML, et al. Randomized controlled trial of IVIg in untreated chronic inflammatory demyelinating polyradiculoneuropathy. Neurology. 2001;56(4):445–9. https://doi.org/10.1212/wnl.56.4.445.
Nobile-Orazio E, Cocito D, Jann S, et al. Intravenous immunoglobulin versus intravenous methylprednisolone for chronic inflammatory demyelinating polyradiculoneuropathy: a randomised controlled trial. Lancet Neurol. 2012;11(6):493–502. https://doi.org/10.1016/S1474-4422(12)70093-5.
Oaklander AL, Lunn MP, Hughes RA, van Schaik IN, Frost C, Chalk CH. Treatments for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): an overview of systematic reviews. Cochrane Database Syst Rev. 2017;1:CD010369. https://doi.org/10.1002/14651858.CD010369.pub2.
Patwa HS, Chaudhry V, Katzberg H, et al. Evidence-based guideline: intravenous immunoglobulin in the treatment of neuromuscular disorders: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2012;78(13):1009–15. https://doi.org/10.1212/WNL.0b013e31824de293.
Van Schaik IN, Winer JB, De Haan R, et al. Intravenous immunoglobulin for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2002;2:CD001797. https://doi.org/10.1002/14651858.CD001797.
Privigen prescribing information. 2019. http://labeling.cslbehring.com/PI/US/Privigen/EN/Privigen-Prescribing-Information.pdf. Accessed 6 Aug 2020.
Van den Bergh PYK, van Doorn PA, Hadden RDM, et al. European Academy of Neurology/Peripheral Nerve Society Guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint Task Force - Second Revision. J Peripher Nerv Syst. 2021;26(3):242–68. https://doi.org/10.1111/jns.12455.
Gammaked prescribing information. 2017. http://www.gammaked.com/clientuploads/2017%20PI/GM-0559-00-2018B_GAMMAKED_Promotional_PI_3045813-3045814_Web_21031A.pdf. Accessed 6 Aug 2020.
Gamunex-C prescribing information. 2020. https://www.gamunex-c.com/documents/27482625/27482925/Gamunex-C+Prescribing+Information.pdf/9258bd0f-4205-47e1-ab80-540304c1ff8e. Accessed 6 Aug 2020.
Guptill JT, Runken MC, Eaddy M, et al. Treatment patterns and costs of chronic inflammatory demyelinating polyneuropathy: a claims database analysis. Am Health Drug Benefits. 2019;12(3):127–35.
Lopate G, Pestronk A, Al-Lozi M. Treatment of chronic inflammatory demyelinating polyneuropathy with high-dose intermittent intravenous methylprednisolone. Arch Neurol. 2005;62(2):249–54. https://doi.org/10.1001/archneur.62.2.249.
Lucke IM, Adrichem ME, Wieske L, et al. Intravenous immunoglobulins in patients with clinically suspected chronic immune-mediated neuropathy. J Neurol Sci. 2019;397:141–5. https://doi.org/10.1016/j.jns.2018.12.036.
Viala K, Maisonobe T, Stojkovic T, et al. A current view of the diagnosis, clinical variants, response to treatment and prognosis of chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. 2010;15(1):50–6. https://doi.org/10.1111/j.1529-8027.2010.00251.x.
Divino V, Mallick R, DeKoven M, et al. The economic burden of CIDP in the United States: a case-control study. PLoS One. 2018;13(10):e0206205. https://doi.org/10.1371/journal.pone.0206205.
Michaelides A, Hadden RDM, Sarrigiannis PG, Hadjivassiliou M, Zis P. Pain in chronic inflammatory demyelinating polyradiculoneuropathy: a systematic review and meta-analysis. Pain Ther. 2019;8(2):177–85. https://doi.org/10.1007/s40122-019-0128-y.
Zis P, Sarrigiannis PG, Rao DG, et al. Chronic idiopathic axonal polyneuropathy: prevalence of pain and impact on quality of life. Brain Behav. 2019;9(1):e01171. https://doi.org/10.1002/brb3.1171.
Faurot KR, Jonsson Funk M, Pate V, et al. Using claims data to predict dependency in activities of daily living as a proxy for frailty. Pharmacoepidemiol Drug Saf. 2015;24(1):59–66. https://doi.org/10.1002/pds.3719.
Thomas S, Chirila C, Ritchey M. Visualization of patient electronic records to support exploratory analysis and variable derivation of categorical data. Presented at the 25th Annual Southeast SASI Users Group (SESUG) Conference; November 2017. Cary, NC.
Latov N, Deng C, Dalakas MC, et al. Timing and course of clinical response to intravenous immunoglobulin in chronic inflammatory demyelinating polyradiculoneuropathy. Arch Neurol. 2010;67(7):802–7. https://doi.org/10.1001/archneurol.2010.105.
Williams T, Polsen M, Runken M. Real-world use of IVIG in US regional healthcare plans. J Managed Care Spec Pharm. 2018;24:S103.
Daniel GW, Menis M, Sridhar G, et al. Immune globulins and thrombotic adverse events as recorded in a large administrative database in 2008 through 2010. Transfusion. 2012;52(10):2113–21. https://doi.org/10.1111/j.1537-2995.2012.03589.x.
Ekezue BF, Sridhar G, Forshee RA, et al. Occurrence of acute renal failure on the same day as immune globulin product administrations during 2008 to 2014. Transfusion. 2017;57(12):2977–86. https://doi.org/10.1111/trf.14330.
Sridhar G, Ekezue BF, Izurieta HS, et al. Occurrence of hemolytic reactions on the same day as immune globulin product administrations during 2008 to 2014. Transfusion. 2018;58(1):70–80. https://doi.org/10.1111/trf.14384.
Sridhar G, Ekezue BF, Izurieta HS, et al. Immune globulins and same-day thrombotic events as recorded in a large health care database during 2008 to 2012. Transfusion. 2014;54(10):2553–65. https://doi.org/10.1111/trf.12663.
Acknowledgements
Funding
This work was conducted by researchers from RTI Health Solutions and Takeda, with funding from Takeda Development Center Americas, Inc. The rapid service fee was funded by Takeda Pharmaceuticals International AG.
Medical Writing and/or Editorial Assistance
Medical writing services provided by Katherine Chu, PharmD, of Oxford PharmaGenesis Inc. were funded by Takeda Development Center Americas, Inc and Takeda Pharmaceuticals International AG.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Colin Anderson-Smits, Mary E. Ritchey, Hakan Ay, and J. Bradley Layton contributed to the conception and design of the study, analysis of data, and interpretation of the data. Zhongwen Huang contributed to the analysis of data and interpretation of the data. Shailesh Chavan and Nizar Souayah contributed to the conception and design of the study and interpretation of the data. All authors have contributed to drafting of the manuscript or revising it critically for important intellectual content and have reviewed and approved the final manuscript for submission.
Disclosures
Colin Anderson-Smits, Zhongwen Huang, and Hakan Ay are employees of Takeda Development Center Americas, Inc., and are Takeda shareholders. Mary E. Ritchey and Nizar Souayah have acted as consultants to Takeda for work outside of this manuscript. Mary E. Ritchey is a former employee of RTI Health Solutions and is currently affiliated with Med Tech Epi, LLC, Philadelphia, PA, USA, and Center for Pharmacoepidemiology and Treatment Sciences, Rutgers University, New Brunswick, NJ, USA. Shailesh Chavan was an employee of Takeda Development Center Americas, Inc., at the time of the study and is currently affiliated with Veloxis Pharmaceuticals, Cambridge, MA, USA. J. Bradley Layton is an employee of RTI Health Solutions, an organization funded by Takeda to conduct this research.
Compliance with Ethics Guidelines
This study did not involve the collection, use, or transmittal of individually identifiable data. The databases used contained fully de-identified/anonymized data and were compliant with the Health Insurance Portability and Accountability Act (HIPAA); this study was, therefore, exempt from institutional review board approval and administrative permission from individuals was not required.
Data Availability
The data that support the findings of this study are available from Merative® MarketScan®. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of Merative® MarketScan®.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Anderson-Smits, C., Ritchey, M.E., Huang, Z. et al. Intravenous Immunoglobulin Treatment Patterns and Outcomes in Patients with Chronic Inflammatory Demyelinating Polyradiculoneuropathy: A US Claims Database Analysis. Neurol Ther 12, 1119–1132 (2023). https://doi.org/10.1007/s40120-023-00478-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-023-00478-5