Abstract
Background
The National Institute for Health and Care Excellence (NICE) is responsible for ensuring that patients in England and Wales can access clinically and cost-effective treatments. However, NICE’s processes pose significant reimbursement challenges for treatments for rare diseases. While some orphan medicines have been appraised via the highly specialised technology route, most are appraised via the single technology appraisal programme, a route that is expected to be increasingly used given new more restrictive highly specialised technology criteria. This often results in delays to access owing to differences in applicable thresholds and the single technology appraisal approach being ill-equipped to deal with the inevitable decision uncertainty. NICE recently published their updated methods and process manual, which includes a new severity-of-disease modifier and an instruction to be more flexible when considering uncertainty in rare diseases. However, as the threshold gap between the single technology appraisal and highly specialised technology programmes remains, it is unlikely that these changes alone will address the problem.
Objective
We explored the potential impact of quality-adjusted life-year weights in decision making.
Methods
We explored the impact of NICE’s new severity-of-disease modifier weighting and two alternative methods (the use of alternative quality-adjusted life-year weights and the fair rate of return), using three recent single technology appraisals of orphan medicines (caplacizumab, teduglutide and pirfenidone for mild idiopathic pulmonary fibrosis).
Results
Our results suggest NICE’s severity-of-disease modifier would not have affected the recommendations. Using alternative methods, based upon achievement of an incremental cost-effectiveness ratio below standard thresholds, patients could have received access to caplacizumab approximately 5 months earlier, and the appraisals for teduglutide and pirfenidone would have resulted in a positive recommendation following appraisal consultation meeting 1 when neither of these products was available over 5 years from the initial submission.
Conclusion
Ultimately, moving from a restrictive end-of-life modifier to one based on disease severity is a more equitable approach likely to benefit many therapies, including orphan products. However, NICE’s single technology appraisal updates are unlikely to result in faster reimbursement of orphan medicines, nor will they address concerns around market access for orphan medicines in the UK.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
When appraising drugs for severe or rare diseases, the National Institute for Health and Care Excellence’s single technology appraisal approach does not allow for as much flexibility as the highly specialised technology route. |
For treatments that fail to meet specific criteria, standard thresholds apply, resulting in delays to patient access or no access at all. |
The new severity-of-disease modifier introduced by the National Institute for Health and Care Excellence is expected to result in some positive changes. However, the gap in the applicable threshold between the single technology appraisal and highly specialised technology routes remains, and the changes to the highly specialised technology criteria suggest the number of rare disease medicines appraised via the highly specialised technology route will decrease rather than increase. |
While the new decision modifiers and allowance of greater flexibility to tolerate uncertainty may go some way to support reimbursement of treatments for severe conditions, the three case studies in this article indicate NICE’s decisions would be unlikely to change. This article details two alternate methods for assigning modifiers that could be considered in place of the new severity-of-disease modifier. |
1 Introduction
The National Institute for Health and Care Excellence (NICE) plays a vital role in ensuring patients get access to clinically and cost-effective treatments in England and Wales. Most treatments are appraised via NICE’s single technology appraisal (STA) route, where technologies demonstrating plausible incremental cost-effectiveness ratios (ICERs) below the standard willingness-to-pay (WTP) threshold of £20,000 per quality-adjusted life-year (QALY) gained are considered cost effective [1] and are therefore reimbursed. For ICERs between £20,000 and £30,000 per QALY gained, decision making considers the degree of certainty around the ICER and aspects that relate to uncaptured benefits and non-health factors.
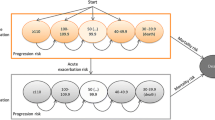
Following NICE’s review of their methods and processes (implemented February 2022), treatments for severe diseases are now given extra consideration (a QALY weight of either 1.2 or 1.7; equivalent to WTP thresholds of £36,000 and £50,000 per QALY, based on the £30,000 current maximum, respectively) based on the most favourable of absolute and proportional QALY shortfall (for measures to quantify the burden of disease, see Fig. 1; explained in more detail later) [1]. This change was implemented in response to concerns that the end-of-life criteria previously used to determine whether a higher threshold was appropriate in the STA programme were too narrow and did not reflect societal preferences [2,3,4,5]. Prior to the new severity modifier, NICE’s binary (yes/no) approach for end-of-life consideration did not capture varying degrees of severity and was considered to emphasise treatments that extend the lifespan at the expense of those that improve quality of life. There were also concerns that treatments for rare diseases could not meet the criteria for acceptance in the STA route, in terms of the uncertainty around efficacy and safety outcomes and the standard of evidence required. The changes to NICE’s approach seek to address these concerns through the introduction of a severity modifier and the allowance of “greater” uncertainty for rare diseases, acknowledging the challenge in the evidence base. These changes align NICE’s methods with those used elsewhere in Europe; countries such as the Netherlands, Norway and Sweden give extra consideration to severe diseases by either adopting shortfall methods or implicitly considering higher thresholds for more severe disease [2].
Occasionally, NICE appraises treatments for rare, severely disabling diseases with a high unmet need via the highly specialised technology (HST) route, using significantly higher WTP thresholds equivalent to £100,000–300,000 per QALY gained (dependent on the magnitude of benefit to undiscounted QALYs) and with greater levels of uncertainty accepted [6]. Specific and extensive criteria preclude the acceptance of most technologies into the HST programme [7], and many treatments for rare diseases are ineligible because of the narrow criteria required for entry (e.g. routing criteria 2: normally, no more than 300 people in England are eligible for the technology in its licensed indication, and no more than 500 are eligible across all its indications). They are thus appraised via the STA process, where the maximum WTP threshold is considerably lower and the methods used less well equipped for such treatments. Out of 797 technology appraisals conducted by NICE to date [8], 21 have been HSTs. A review of NICE appraisals between 2015 and 2020 found that only a third of orphan medicines appraised had been reviewed via the HST route, with the remainder going through the standard STA process [9]. The review found that orphan medicines were subject to a significantly longer mean time in the NICE STA process than non-orphan medicines (370 [n = 44] vs 277 [n = 118] days; p < 0.0001) and that orphan medicines in the STA process were disadvantaged by worse outcomes with respect to positive recommendations than those of orphan medicines assessed by HST (100% of HSTs recommended in full vs 73% of orphan STAs). Zamora et al. demonstrated that fewer European Medicines Agency-approved orphan medicines are reimbursed in England than in Spain, Germany, France and Italy [10].
The NICE process consultation document noted that “there is an important societal interest in rare diseases” and acknowledged that “patients with serious ultra-rare conditions where there is vulnerability, substantial unmet needs, or very limited, not very effective treatment options” may be disadvantaged by an appraisal undertaken via standard processes. This is due to not only the difficulties in producing an STA-standard evidence base with limited patient numbers, and, in many cases, an evolving understanding of the disease and limited applicability of standard measures such as the EQ-5D questionnaire, particularly in paediatric diseases, but also the need to recoup research and development costs to fund future innovation [11].
NICE states that “the number of HST topics is not expected to change as a result of the revised wording” of the HST entry criteria, with key changes being the introduction of limits across indications and the removal of the requirement for treatment to be life-long [12]. The implication of this is that the majority of rare disease medicines will continue to be routed via the STA process. In addition, all five NICE Committees (four STAs and the HST Committee) may now assess products for diseases routed to HST rather than the assessment only being conducted by the HST Committee. The STA Committees have traditionally been less lenient when faced with the limited data available in rare diseases [9]. This underscores the importance of both ensuring consistency in the approach to the assessment of rare diseases across committees and closing the gap between HST and STA processes and applicable WTP thresholds for products that narrowly miss the bar for HST (see Sect. 5 for further exploration of these thresholds).
2 Objectives
This paper explores the impact of the new NICE severity modifier and two alternative approaches to QALY modifiers for rare severe disease medicines in the STA programme. We compare three methods to calculate QALY weights and adjusted ICERs for three case studies of orphan medicines recently appraised by NICE via the STA route: caplacizumab for acquired thrombotic thrombocytopenic purpura, teduglutide for short bowel syndrome and pirfenidone for mild idiopathic pulmonary fibrosis. These three case studies were chosen as examples of appraisals of treatments that narrowly missed out on entry to the HST programme under the old criteria and for which QALY data were available unredacted. By comparing unweighted and weighted estimates of cost effectiveness, our aim was to determine how much sooner patients may have received access to treatment under each of the different weighting systems.
3 Methods to Derive QALY Weights
A variety of methods for the formal incorporation of decision modifiers into cost-effectiveness analyses are discussed in the literature. Modifiers may be based on age (e.g. the “fair innings” approach), severity (e.g. the use of absolute or proportional shortfall approaches) or rarity (e.g. the approach proposed by Berdud and colleagues to determine a fair rate of return for rare disease therapeutics) [13,14,15]. As the selected examples are for rare and/or severe diseases, the absolute shortfall, proportional shortfall and fair rate of return approaches were considered.
3.1 Absolute Shortfall
The absolute shortfall method calculates the total amount of future health a patient is expected to lose because of their disease. The absolute shortfall method is favoured by the Norwegian Medicines Agency, based on the rationale that technologies for diseases resulting in greater absolute losses should be given greater weight [16].
In our analysis, for England and Wales expected lifetime QALYs for a person without the disease were calculated using life tables to estimate survival, weighted by expected utility at each age by sex, starting from the mean age at onset [17]. Quality-of-life data were taken from a published, widely used UK regression analysis [18]. Total QALYs for patients with the disease were taken from results reported in each appraisal for the existing standard of care. Absolute QALY shortfall was calculated as the difference between these two values.
3.2 Proportional Shortfall
Proportional shortfall builds on the concept of absolute shortfall but is instead defined as the total QALYs lost because of a disease as a proportion of the expected lifetime QALYs, for an average member of the general population of the same age. For this method, absolute shortfall is calculated in the same way and divided by the total lifetime QALYs for an average patient without the disease (i.e. the general population).
Once proportional shortfall (%) is determined, the next step is to assign appropriate QALY weights. Although proportional shortfall methods are discussed extensively in the literature, there is a lack of consensus on the appropriate QALY weights to apply based on this method. Proportional shortfall weights are used in the Netherlands as an equity approach that combines aspects of the severity of illness and fair innings approaches [19,20,21].
3.3 NICE’s Approach: Combination of Absolute and Proportional QALY Shortfall
In the STA programme, NICE applies QALY weights based upon the most favourable of the absolute and proportional shortfall methods using discounted QALYs. The weights applied are 1.7× for a proportional QALY shortfall of ≥ 0.95 or an absolute QALY shortfall of ≥ 18; and 1.2× for a proportional QALY shortfall of between 0.85 and 0.95 or an absolute QALY shortfall of 12–18 [1]. Because of a lack of information from societal preference research, these weights were defined based upon an “opportunity-cost-neutral” approach, aiming to reallocate additional weighting previously applied to end-of-life treatments using data from appraisals conducted between 2011 and 2021.
To apply this method, we extracted information on the discounted QALYs with the comparator standard of care treatment from the documentation for each of our case studies. We then calculated the potential discounted QALYs for a healthy population using the mean age and percentage of female individuals reported for each case study.
3.4 A Modified NICE Approach: Alternate QALY Weights
We also provide an alternate analysis using a different QALY weighting system to NICE’s to demonstrate the impact of the weighting system used. This “modified NICE approach” uses a weight of 1 for a proportional shortfall of 0%, corresponding to the standard threshold of £20,000/QALY, and increases on a sliding scale to a weight of 5 for a proportional shortfall of 100%, corresponding to the lower HST threshold of £100,000. We look at the absolute shortfall in the same manner: a weight of 1 is given for a loss of 0 corresponding to the lower NICE STA threshold of £20,000/QALY, increasing on a sliding scale to a weight of 5 for an absolute shortfall of 30 QALYs corresponding to the lower HST threshold of £100,000. This bridges the gap between the thresholds used for STA and HST, eliminating the current “cliff-edge” between thresholds, and equates to a marginal effect of £2667 per QALY using the absolute shortfall method and £800 per 1% using the proportional shortfall method. The same data were used within the calculation as when NICE’s severity modifier was applied.
3.5 Fair Rate of Return
Berdud and colleagues proposed that orphan drug pricing should be based on the proposition that rates of return for investments in developing orphan drugs should not be greater than the industry average [13]. Using the NICE standard cost-effectiveness threshold of £20,000 per QALY gained as an anchor and adjusting by research and development costs and expected market revenue, the authors estimated adjusted WTP thresholds of £39,100 and £937,100 for orphan (population size: ≤ 25 per 50,000) and ultra-orphan (population size: ≤ 1 per 50,000) drugs, respectively. The calculation equates to a marginal effect of £3734 per 1 in 1,000,000 decrease in prevalence after the orphan population size cut-off. To apply this method, we extracted information on prevalence in England from each case study and used the thresholds proposed by Berdud et al. to determine the appropriate threshold (i.e. QALY weights) for each example via a linear interpolation between the proposed thresholds for orphan and ultra-orphan populations. In line with NICE’s approach and the data available for the case studies, we use discounted QALYs when applying this method.
4 Case Studies
Details of the three case studies are provided in Table 1. Table 1 in the Electronic Supplementary Material (ESM) demonstrates that each of the case studies narrowly missed out on entry to the HST programme under the old criteria. While TA667 may have met the new criteria (how the criteria around severity will be applied is currently unclear), it is unlikely either of the other two case studies would meet the new criteria.
5 Results
Table 2 presents the QALY weights based on the absolute shortfall, proportional shortfall and fair rate of return methods for each population considered within the three appraisals. Table 2 also presents the parameters required in the calculations, such as mean age and percentage of female individuals for the absolute and proportional shortfall methods, and prevalence per 100,000 (in England) for the fair rate of return method.
The highest proportional and absolute shortfall results were observed for children with short bowel syndrome (79% and 25.2 discounted QALYs), based on potential lifetime QALYs of 25.2 (based on a mean age of onset of 4 years), but only 5.4 QALYs on current treatment. The greatest QALY weights occur for the most severely ill populations. Using the NICE method, a weighting of 1.7× would be applied; if our alternate scale is considered, this would be 4.1×. The use of NICE’s severity modifiers resulted in a modifier of 1 for both acquired thrombotic thrombocytopenic purpura and mild idiopathic pulmonary fibrosis. The use of the weightings from the modified NICE approach demonstrates the high level of sensitivity to the system used, with weights that range from 3.0 to 4.1 using a proportional QALY shortfall and from 1.5 to 3.4 using an absolute QALY shortfall. In all cases, a higher weight would have been assigned than that granted under NICE’s current method.
For the fair rate of return method, which calculates QALY weights based on rarity using estimates of prevalence in England, the greatest weights naturally result from the diseases with the lowest prevalence. For caplacizumab in particular, the adjusted WTP threshold using this method is £1,001,909, corresponding to a QALY weight of 50.1.
Figures 2, 3, 4 and 5 present the evolution of the ICER over the duration of the appraisal process. Considering the technology appraisal for caplacizumab (Fig. 2a, b), the most plausible ICERs based on both the company submission and the Evidence Review Group (ERG) assessment were around £30,000/QALY. However, the NICE technical team’s preferred ICER (N1) exceeded £170,000, only reducing to £30,000 at the time of publication of the final appraisal determination. The ICERs generated using QALY weightings based on a fair rate of return are much lower. Using a modifier based on rarity, based upon achievement of an ICER below standard thresholds, it is likely that caplacizumab would have been reimbursed 5 months earlier immediately following appraisal consultation meeting 1.
TA667, caplacizumab in acquired thrombotic thrombocytopenic purpura, evolution of the incremental cost-effectiveness ratio (ICER) during the appraisal process. ACD appraisal consultation document, C1 company submission, C2 company response to NICE technical report, C3 company response to ACD1, CE cost-effectiveness, ERG , E1 ERG report, E2 ERG critique of company response to NICE technical report, FAD final appraisal determination, N1 NICE technical report (technical team), N2 NICE Committee-preferred ICER (ACD1), N3 NICE Committee-preferred ICER (ACD2), N4 NICE Committee-preferred ICER (FAD NICE National Institute for Health and Care Excellence, PAS patient access scheme, pricing agreement to improve cost effectiveness, QALY quality-adjusted life-year. Error bars correspond to ICER ranges discussed in NICE documentation. All ICERs presented incorporate the PAS available at that stage in the appraisal process. As a weight of 1 would be applied under NICE’s new severity modifiers, the no-QALY-weight figures also correspond to the NICE severity weight being applied
TA690, teduglutide for short bowel syndrome, adult subpopulation, evolution of the incremental cost-effectiveness ratio (ICER) during the appraisal process, no quality-adjusted life-year (QALY) weights applied. ACD appraisal consultation document, ACM appraisal committee meeting, C1 company submission, C2 company response to ACD1, C3 company-submitted evidence for ACM3, C4 company-submitted evidence for ACM4, C5 company-submitted additional evidence for ACM4, CE cost-effectiveness, DSU Decision Support Unit, E1 ERG report, E2 ERG critique of company response to ACD1, E3 DSU report following C3, E4 DSU report following C4, E5 DSU report following C5, ERG Evidence Review Group, NICE National Institute for Health and Care Excellence, PAS patient access scheme, pricing agreement to improve cost effectiveness. Error bars correspond to ICER ranges discussed in NICE documentation. All ICERs presented incorporate the PAS available at that stage in the appraisal process. PAS-price (decision-making) ICERs were not reported in NICE documentation and are therefore not included within the figures presented
TA690 teduglutide for short bowel syndrome, child subpopulation, evolution of the incremental cost-effectiveness ratio (ICER) during the appraisal process. ACD appraisal consultation document, ACM appraisal committee meeting, C1 company submission, C2 company response to ACD1, C3 company-submitted evidence for ACM3, C4 company-submitted evidence for ACM4, C5 company-submitted additional evidence for ACM4, CE cost-effectiveness, DSU Decision Support Unit, E1 ERG report, E2 ERG critique of company response to ACD1, E3 DSU report following C3, E4 DSU report following C4, E5 DSU report following C5 (uses adult data in child model), ERG Evidence Review Group, NICE National Institute for Health and Care Excellence, PAS patient access scheme, pricing agreement to improve cost-effectiveness, QALY quality-adjusted life-year. Error bars correspond to ICER ranges discussed in NICE documentation. All ICERs presented incorporate the PAS available at that stage in the appraisal process. PAS-price (decision-making) ICERs were not reported in NICE documentation and are therefore not included within the figures presented
TA504, pirfenidone for idiopathic pulmonary fibrosis, mild disease population, evolution of the incremental cost-effectiveness ratio (ICER) during the appraisal process. ACD appraisal consultation document, C1 company submission, C2 company response to NICE ACD (ICER for ITT population, ICER for mild population not reported), CE cost-effectiveness, E1 ERG report, ERG Evidence Review Group, ITT intention to treat, N1 NICE ACD1, NICE National Institute for Health and Care Excellence, PAS patient access scheme, pricing agreement to improve cost-effectiveness, QALY quality-adjusted life-year. Error bars correspond to ICER ranges discussed in NICE documentation. All ICERs presented incorporate the PAS available at that stage in the appraisal process. As a weight of 1 would be applied under NICE’s new severity modifiers, the no-QALY-weight figures also correspond to the NICE severity weight being applied
For teduglutide, considering both adult and paediatric patients with short bowel syndrome (Figs. 3a–c, 4a–c), none of the most plausible ICERs cited by the ERG/Decision Support Unit (DSU) fall below £30,000/QALY, resulting in the current negative reimbursement status of teduglutide. Even with NICE’s new severity modifier applied, ERG/DSU-preferred ICERs never fall below standard thresholds. Applying weights based on a fair rate of return may have resulted in teduglutide being recommended in both adult and paediatric patients at appraisal consultation meeting 1 in October 2017, as all ERG/DSU ICER estimates are below the accepted cost-effectiveness threshold.
For pirfenidone, considering patients with mild idiopathic pulmonary fibrosis (Fig. 5a, b), ERG/NICE Committee-preferred ICERs are well in excess of standard thresholds, even at the lower end of the cited range. Using weights based on a fair rate of return would likely have resulted in reimbursement following appraisal consultation meeting 1 on 5 May, 2016, as the ICERs are dramatically reduced.
In all cases, allowing severity weights to bridge the gap between the STA and HST programmes would also have resulted in positive recommendations early on in the process (Figs. 1–8 of the ESM). Within all of the case studies, the use of a proportional (rather than absolute) shortfall “modified NICE approach” favoured the intervention, in all cases, with a material difference in the timing of recommendation being likely for caplacizumab and teduglutide in the adult population.
6 Discussion
In early 2021, the Department of Health and Social Care published the UK Rare Diseases Framework [35]. One of the high-level priorities over 5 years is to improve access to specialist care and treatments for those living with rare diseases. Changes to the NICE processes and methods were to form a first step in achieving this aim. Moving from a restrictive end-of-life modifier to one based on severity of disease for the STA programme, in a similar vein to the HST programme, aligns better with the preferences of the general public and represents a more equitable approach. However, based upon our results, the new severity modifier alone is unlikely to have much impact on the large delays and restrictions to access for orphan and ultra-orphan products that fall into the gap between the STA and HST programmes.
Alternative approaches, such as extending the upper limit of the modifier to align with the lower limit of the threshold applied within the HST programme for products that narrowly miss out on entry or applying a rarity-based modifier such as that proposed in Berdud et al. [13], would prove considerably more favourable for orphan and ultra-orphan products. Using these alternative methods, based upon achievement of an ICER below standard thresholds, patients could have received access to caplacizumab approximately 5 months earlier, and the appraisals for teduglutide and pirfenidone would have resulted in a positive recommendation following appraisal consultation meeting 1 when neither of these products is available at the time of writing, over 5 years from initial submission. Our results align with other recent work, which showed that orphan medicines were subject to a significantly longer mean time in the NICE process than non-orphan medicines: 370 versus 277 days [9]. The majority of pharmaceutical companies have a global presence and need to make decisions on where to launch and when. Continued unpredictability, delays and restricted access for products for rare diseases, which represent a substantial proportion of the current clinical development pipeline, risks making England an unattractive option for an early launch (or even not an option at all). The analysis using the work of Berdud et al. [13] demonstrates that the use of societal preference to set WTP thresholds leads to an under-return on investment for orphan diseases (compared with other disease areas). For caplacizumab in particular, the adjusted WTP threshold using this method is approximately £1 million: a reflection of the rarity of acquired thrombotic thrombocytopenic purpura.
The analysis posed several challenges; none of which relates to the calculations themselves, which are simple to perform. First, the identification of appropriate case studies was hindered by the lack of reported patient-access scheme-price (decision-making) ICERs in NICE documentation. However, within the three case studies chosen, the companies’ presented patient-access scheme-price ICERs that were available for all meetings, the ERG’s ICERs were available for most meetings, and a preferred ICER from NICE for some meetings. Second, at many stages of the appraisal processes, NICE did not specify a preferred ICER, making it difficult to draw conclusions about the likely reimbursement date when weightings were applied. Third, it is not clear whether QALY weights should be applied to patients and carers or patients only. For example, in the teduglutide appraisal, ICERs were calculated based on both patient and carer QALYs; however, in our analysis, we assumed only patient QALYs would be upweighted.
Fourth, all other processes using severity modifiers use undiscounted QALYs to calculate the QALY shortfall [14, 16, 19]. While the use of undiscounted QALYs aligns with NICE’s HST programme, NICE has chosen to opt for discounted QALYs for STAs. In line with existing methodology, our modified NICE approach considered undiscounted QALYs where these were available; however, only discounted QALYs were reported within the appraisals for teduglutide and pirfenidone. Use of discounted QALYs makes it more difficult for many treatments to qualify for severity weighting, as discounting incrementally reduces the value of the healthy QALYs used for comparison. Additionally, all other things being equal, the magnitude of impact of discounting on health outcomes rises as age falls, meaning that conditions affecting the youngest patients are most disadvantaged by using discounted results.
Fifth, the QALY weightings used in the modified NICE approach, the lower STA and HST thresholds that they correspond with, and NICE’s new severity modifiers are all arbitrarily chosen parameters. NICE intends to initiate research to address the arbitrary nature of the QALY weighting system; however, similar efforts conducted to inform a potential value-based pricing system back in 2012–13 demonstrate how difficult producing an evidence-based weighting system is likely to be [36, 37]. For example, multiple studies have found evidence of preferences consistent with aversion to differences in life expectancy but not quality-adjusted life expectancy [38,39,40].
Sixth, the Berdud et al. study [13] used for the fair rate of return approach does not provide guidance on assumptions for population sizes smaller than the ultra-orphan cut-off. Here, we have assumed that the trajectory of the segment between the orphan mid-point and ultra-orphan cut off is extended in the caplacizumab case study, we note however that as the population size is close to the ultra-orphan cut-off, this has little impact on the results.
Finally, committee decision making is not based upon ICERs alone. Each committee makes decisions according to their own application of NICE’s methods and processes to the evidence base and clinical and cost-effectiveness case for each decision problem. Our analysis therefore indicates when ICERs would fall below standard thresholds, rather than being able to state definitively that a different decision may have been made earlier in the process.
When considering the exact approach to modifiers to be used, various value judgements need to be made, many of which are discussed in NICE’s task and finish reports [8, 41]. They are:
-
What is the HTA body trying to achieve by applying a decision modifier? For example:
-
To reflect societal preferences (and the evidence available for that);
-
To stimulate innovation;
-
To stimulate investment in disease areas that have historically been underinvested in, or difficult to justify investing in under standard business models;
-
To allow the capture of elements of value not included within current QALY calculations.
-
-
Should modifiers apply to technologies that are less effective than current practice, but free up sufficient resources that can be reinvested in the system (southwest quadrant on the cost-effectiveness plane)?
-
The overlap between potential systems of modifiers.
-
Whether to implement quantitively or qualitatively within deliberations.
-
How to implement any weighting system: sliding scale or stepped approach.
-
How the use of modifiers affects the base threshold and the opportunity costs associated with the implementation of a new modifier system.
-
The effect of a particular system of modifiers on equity, examples being:
-
How the use of proportional shortfall tends to favour interventions for older populations, whereas absolute shortfall tends to favour younger populations.
-
How modifiers based on rarity could be used to solve issues with equity in access to treatment compared with more common diseases and the flip-side of the potential impact of rarity-based modifiers on access for patients with more common diseases.
-
When looking at assessment in jurisdictions that already implement QALY modifiers, only Sweden has assessed the three case studies considered here. For caplacizumab, recommendation was gained in June 2021 [42]. Without cost effectiveness having been assessed because of a lack of demonstration of quality-of-life benefit, following appraisal in 2014, teduglutide was not recommended [43]. In 2012, pirfenidone was given a conditional recommendation, excluding the mild disease population initially, but then including it on reappraisal in 2015 [44, 45]. In all three cases, a shorter appraisal process was followed compared with the NICE timelines; a severity modifier application was not formally discussed (documentation is considerably less detailed than for NICE).
For rare diseases, a “cliff-edge” remains between the thresholds used to appraise technologies considered for the HST programme and the standard thresholds used for STA, with no indication from NICE that this is likely to change. Given this, it would appear logical that medicines for orphan indications should be routed to the HST process, as this was made to be suitable for the purpose of assessing treatments for rare diseases. The legislation underpinning the creation of the HST programme defines a “highly specialised health technology” as “a health technology intended for use in the provision of services for rare and very rare conditions”. If this is not possible because of capacity constraints, alternative proposals for addressing the STA-HST threshold gap could be considered. As outlined in this paper, one option is to use a combination of severity and rarity QALY modifiers. Decision makers could consider the wider application of managed access agreements outside of oncology, using the Innovative Medicines Fund, and take a less risk-averse approach to the handling of uncertainty in the case of rare diseases. Other ideas may also be explored, such as allowing for a period of access prior to assessment to enable additional data collection or applying a more holistic value framework within the TA programme. Whilst empirical evidence does not support UK citizens placing a higher value on rarity alone [46], use of a modifier aimed at reducing inequity because of rarity may also serve to incentivise investment in rare diseases in the UK, as per the rationale for HST.
7 Conclusions
While the adoption of new decision modifiers and the allowance of greater flexibility to tolerate uncertainty go some way to supporting the reimbursement of treatments for severe conditions, based upon our results, the new NICE severity modifier may not significantly improve access to medicines for rare diseases. We propose alternative approaches that could be used by NICE in subsequent updates to their methods that would improve access to rare disease medicines in the UK and ensure continued investment in rare diseases by global pharmaceutical companies.
Change history
20 November 2022
The presentation of bulletpoints was incorrect in the original version of this article.
References
National Institute for Health and Care Excellence (NICE). [PMG36] NICE health technology evaluations: the manual. 2022. Updated: 31 January 2022. Available from: https://www.nice.org.uk/process/pmg36/chapter/introduction-to-health-technology-evaluation. Accessed 17 Feb 2022.
National Institute for Health and Care Excellence (NICE). Reviewing our methods for health technology evaluation: consultation. 2020. Available from: https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/chte-methods-consultation. Accessed 29 Mar 2022.
Rizzardo S, Bansback N, Dragojlovic N, Douglas C, Li KH, Mitton C, et al. Evaluating Canadians’ values for drug coverage decision making. Value Health. 2019;22(3):362–9. https://doi.org/10.1016/j.jval.2018.08.008.
Chim L, Salkeld G, Kelly P, Lipworth W, Hughes DA, Stockler MR. Societal perspective on access to publicly subsidised medicines: a cross sectional survey of 3080 adults in Australia. PLoS ONE. 2017;12(3): e0172971. https://doi.org/10.1371/journal.pone.0172971.
Gu Y, Lancsar E, Ghijben P, Butler JR, Donaldson C. Attributes and weights in health care priority setting: a systematic review of what counts and to what extent. Soc Sci Med. 2015;146:41–52. https://doi.org/10.1016/j.socscimed.2015.10.005.
National Institute for Health and Care Excellence (NICE). Changes to NICE drug appraisals: what you need to know. 2017. Available from: https://www.nice.org.uk/news/feature/changes-to-nice-drug-appraisals-what-you-need-to-know. Accessed 29 Mar 2022.
National Institute for Health and Care Excellence (NICE). Changes we're making to health technology evaluation. 2022. Available from: https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-technology-appraisal-guidance/changes-to-health-technology-evaluation. Accessed 4 Mar 2022.
National Institute for Health and Care Excellence (NICE). CHTE methods review: decision making. Task and Finish Group report. 2020. Available from: https://www.nice.org.uk/Media/Default/About/what-we-do/our-programmes/nice-guidance/chte-methods-consultation/Decision-making-task-and-finish-group-report.docx. Accessed 12 Aug 2022.
Clarke S, Ellis M, Brownrigg J. The impact of rarity in NICE’s health technology appraisals. Orphanet J Rare Dis. 2021;16(1):218. https://doi.org/10.1186/s13023-021-01845-x.
Zamora B, Maignen F, O’Neill P, Mestre-Ferrandiz J, Garau M. Comparing access to orphan medicinal products in Europe. Orphanet J Rare Dis. 2019;14(1):95. https://doi.org/10.1186/s13023-019-1078-5.
Chan AYL, Chan VKY, Olsson S, Fan M, Jit M, Gong M, et al. Access and unmet needs of orphan drugs in 194 countries and 6 Aaeas: a global policy review with content analysis. Value Health. 2020;23(12):1580–91. https://doi.org/10.1016/j.jval.2020.06.020.
National Institute for Health and Care Excellence (NICE). Reviewing our process for health technology evaluation: consultation. 2021. Available from: https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/reviewing-our-process-for-health-technology-evaluation--consultation. Accessed 29 Mar 2022.
Berdud M, Drummond M, Towse A. Establishing a reasonable price for an orphan drug. Cost Eff Resour Alloc. 2020;18(1):31. https://doi.org/10.1186/s12962-020-00223-x.
Carlson JJ, Brouwer ED, Kim E, Wright P, McQueen RB. Alternative approaches to quality-adjusted life-year estimation within standard cost-effectiveness models: literature review, feasibility assessment, and impact evaluation. Value Health. 2020;23(12):1523–33. https://doi.org/10.1016/j.jval.2020.08.2092.
Office of Health Economics (OHE). Clarifying meanings of absolute and proportional shortfall with examples. 2013. Available from: https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-technology-appraisals/OHE-Note-on-proportional-versus-absolute-shortfall.pdf. Accessed 29 Mar 2022.
Norwegian Ministry of Health and Care Services. Principles for priority setting in health care. 2017. Available from: https://www.regjeringen.no/contentassets/439a420e01914a18b21f351143ccc6af/en-gb/pdfs/stm201520160034000engpdfs.pdf. Accessed 29 Mar 2022.
Office for National Statistics (ONS). National life tables: England and Wales. 2019. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesenglandandwalesreferencetables. Accessed 29 Mar 2022.
Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health. 2010;13(5):509–18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
Zorginstituut Nederland (ZIN). Kosteneffectiviteit in de praktijk. 2015. Available from: Dutch: https://www.zorginstituutnederland.nl/publicaties/rapport/2015/06/26/kosteneffectiviteit-in-de-praktijk. English: https://english.zorginstituutnederland.nl/publications/reports/2015/06/16/cost-effectiveness-in-practice. Accessed 29 Mar 2022.
Association of the British Pharmaceutical Industry (ABPI). Extended value appraisal (EVA): a proposal for the NICE methods review. 2020. Available from: https://www.abpi.org.uk/media/8105/abpi-extended-value-appraisal-proposal-for-the-nice-methods-review.pdf. Accessed 9 Aug 2021.
Reckers-Droog VT, van Exel NJA, Brouwer WBF. Looking back and moving forward: on the application of proportional shortfall in healthcare priority setting in the Netherlands. Health Policy. 2018;122(6):621–9. https://doi.org/10.1016/j.healthpol.2018.04.001.
European Medicines Agency (EMA). Cablivi: assessment report. 2018. Available from: https://www.ema.europa.eu/en/documents/assessment-report/cablivi-epar-public-assessment-report_en.pdf. Accessed 25 Jun 2019.
Peyvandi F, Scully M, Kremer Hovinga JA, Cataland S, Knöbl P, Wu H, et al. Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2016;374(6):511–22. https://doi.org/10.1056/NEJMoa1505533.
Scully M, Cataland SR, Peyvandi F, Coppo P, Knöbl P, Kremer Hovinga JA, et al. Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2019;380(4):335–46. https://doi.org/10.1056/NEJMoa1806311.
NHS England. Service specification 1668. Schedule 2: the services. Draft for consultation. Available from: https://www.engage.england.nhs.uk/consultation/thrombocytopenic-purpura/user_uploads/thrombotic-thrombocytopenic-purpura-service-specification.pdf. Accessed 29 Mar 2022.
National Institute for Health and Care Excellence (NICE). TA667: Caplacizumab with plasma exchange and immunosuppression for treating acute acquired thrombotic thrombocytopenic purpura. 2020. Available from: https://www.nice.org.uk/guidance/ta667. Accessed 29 Mar 2022.
National Institute for Health and Care Excellence (NICE). Guide to the processes of technology appraisal. 2018. Available from: https://www.nice.org.uk/process/pmg19/chapter/the-appraisal-process#table-3-expected-timelines-for-the-appraisal-process-starting-the-process-preparing-the-erg-report. Accessed 29 Mar 2022.
European Medicines Agency (EMA). Revestive: assessment report. 2012. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/revestive. Accessed 16 Mar 2021.
Vipperla K, O’Keefe SJ. Study of teduglutide effectiveness in parenteral nutrition-dependent short-bowel syndrome subjects. Expert Rev Gastroenterol Hepatol. 2013;7(8):683–7. https://doi.org/10.1586/17474124.2013.842894.
Carter BA, Cohran VC, Cole CR, Corkins MR, Dimmitt RA, Duggan C, et al. Outcomes from a 12-week, open-label, multicenter clinical trial of teduglutide in pediatric short bowel syndrome. J Pediatr. 2017;181:102-11.e5. https://doi.org/10.1016/j.jpeds.2016.10.027.
National Institute for Health and Care Excellence (NICE). ID885: teduglutide for short bowel syndrome (terminated appraisal). 2019. Available from: https://www.nice.org.uk/guidance/indevelopment/gid-ta10048/documents; https://www.nice.org.uk/guidance/ta690/resources. Accessed 15 Mar 2021.
National Institute for Health and Care Excellence (NICE). [ID3937] Teduglutide for treating short bowel syndrome. In development [GID-TA10842]. 2022. Updated: 19 April 2021. Available from: https://www.nice.org.uk/guidance/indevelopment/gid-ta10842. Accessed 4 Mar 2022.
National Institute for Health and Care Excellence (NICE). TA504: pirfenidone for treating idiopathic pulmonary fibrosis. 2016. Available from: https://www.nice.org.uk/guidance/ta504/history. Accessed 29 Mar 2022.
National Institute for Health and Care Excellence (NICE). Appeal hearing: advice on pirfenidone for treating idiopathic pulmonary fibrosis (review of TA282) [ID837]. 2016. Available from: https://www.nice.org.uk/guidance/ta504/history. Accessed 4 Mar 2022.
Department of Health and Social Care. UK Rare Diseases Framework. 2021. Available from: https://www.gov.uk/government/publications/uk-rare-diseases-framework. Accessed 16 Mar 2021.
Rowen D, Brazier J, Mukuria C, Keetharuth A, Hole AR, Tsuchiya A, et al. EEPRU research report. Update: eliciting societal preferences for weighting QALYs according to burden of illness, size of gain and end of life. 2014. Available from: https://eprints.whiterose.ac.uk/99494/1/EEPRU%20%20weights%20update%20-%20018.pdf. Accessed 29 Mar 2022.
National Institute for Health and Care Excellence Decision Support Unit (NICE DSU). Value based pricing/assessment. 2014. Available from: https://nicedsu.sites.sheffield.ac.uk/methods-development/value-based-pricingassessment. Accessed 29 Mar 2022.
McNamara S, Holmes J, Stevely AK, Tsuchiya A. How averse are the UK general public to inequalities in health between socioeconomic groups? A systematic review. Eur J Health Econ. 2020;21(2):275–85. https://doi.org/10.1007/s10198-019-01126-2.
Dolan P, Tsuchiya A. Health priorities and public preferences: the relative importance of past health experience and future health prospects. J Health Econ. 2005;24(4):703–14. https://doi.org/10.1016/j.jhealeco.2004.11.007.
Rowen D, Brazier J, Mukuria C, Keetharuth A, Risa Hole A, Tsuchiya A, et al. Eliciting societal preferences for weighting QALYs for burden of illness and end of life. Med Decis Mak. 2016;36(2):210–22. https://doi.org/10.1177/0272989x15619389.
National Institute for Health and Care Excellence (NICE). CHTE methods review. Modifiers. Task and Finish Group report. 2020. Available from: https://www.nice.org.uk/Media/Default/About/what-we-do/our-programmes/nice-guidance/chte-methods-consultation/Modifiers-task-and-finish-group-report.docx. Accessed 12 Aug 2022.
Tandvårds-och Läkemedelsförmånsverket (TLV). Hälsoekonomisk bedömning av Cablivi (kaplacizumab). 2021. Available from: https://www.tlv.se/download/18.1a8d0c34179efdbb7af4de91/1623673527756/bes210601_he_bed_cablivi.pdf%20(In%20Swedish. Accessed 15 Aug 2022.
Tandvårds- och Läkemedelsförmånsverket (TLV). Underlag för beslut i landstingen. Revestive (teduglutid). 2014. Available from: https://www.tlv.se/download/18.467926b615d084471ac3399e/1510316400286/Kunskapsunderlag_revestive.pdf. Accessed 15 Aug 2022.
Tandvårds-och Läkemedelsförmånsverket (TLV). Esbriet (Dnr 3977/2011). Uppföljning av beslut inom läkemedelsförmånerna. 2015. Available from: https://www.tlv.se/download/18.467926b615d084471ac3038a/1510316357239/bes150521-esbriet-uppfoljning.pdf. Accessed 15 Aug 2022.
Tandvårds-och Läkemedelsförmånsverket (TLV). Esbriet (pirfenidon). 2015. Available from: https://www.tlv.se/download/18.467926b615d084471ac30394/1510316357265/underlag-beslut-esbriet.pdf. Accessed 15 Aug 2022.
NICE Citizens Council. NICE Citizens Council reports. Quality adjusted life years (QALYs) and the severity of illness. London: National Institute for Health and Care Excellence (NICE); 2008.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Sanofi.
Conflict of interest
Grant McCarthy is employed by Lumanity and Dawn Lee was employed by Lumanity at the time of writing. Lumanity was reimbursed by Sanofi as a consultancy for time spent developing this article. Rachel Allen, Fleur Chandler, Kinga Malottki and Omar Saeed are employees of Sanofi, the manufacturer of caplacizumab and may hold shares and/or stock options in the company. Omar Saeed was previously employed by Shire, the (previous) manufacturer of teduglutide.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All data were taken from publicly available materials that are referenced in the article.
Code availability
Calculations may be shared on request.
Author contributions
All authors were involved in the design and execution of the analysis, interpretation of the results, and the drafting and revision of the manuscript, and provided final approval of the version to be published. All authors vouch for the accuracy of the content included in the full article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lee, D., McCarthy, G., Saeed, O. et al. The Challenge for Orphan Drugs Remains: Three Case Studies Demonstrating the Impact of Changes to NICE Methods and Processes and Alternative Mechanisms to Value Orphan Products. PharmacoEconomics Open 7, 175–187 (2023). https://doi.org/10.1007/s41669-022-00378-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-022-00378-8