Abstract
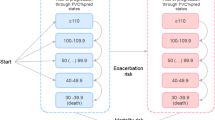
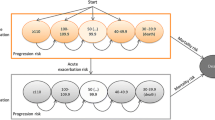
The National Institute for Health and Care Excellence (NICE) published guidance on the use of pirfenidone (Esbriet®, Roche) for the treatment of mild to moderate idiopathic pulmonary fibrosis (IPF) in 2013. NICE decided to review existing guidance following publication of an additional clinical trial, and invited the manufacturer of pirfenidone to submit evidence of its clinical and cost effectiveness for the treatment of mild to moderate IPF when compared with best supportive care (BSC) or nintedanib; nintedanib was a comparator only for moderate IPF. An independent Evidence Review Group (ERG) critiqued the company submission and this paper summarises their report and subsequent NICE guidance. The key clinical effectiveness evidence was based on three randomised controlled trials (RCTs) and an open-label extension study. Supportive data were provided from two additional RCTs conducted in Japan, while one additional open-label study was included for safety outcomes. Meta-analysis of the three key RCTs found pirfenidone to be effective at reducing disease progression compared with placebo, but statistically significant differences were not identified in all of the RCTs. A statistically significant reduction in all-cause mortality was only demonstrated when pooling data across studies. The treatment effects of pirfenidone and nintedanib were broadly similar, based on an indirect comparison using network meta-analysis, although they have slightly different adverse event profiles. There remains considerable uncertainty in the cost-effectiveness estimates for pirfenidone versus BSC, particularly due to uncertainty regarding the duration of treatment effect and the method used to implement the stopping rule within the economic model.
Similar content being viewed by others
References
National Institute for Health and Care Excellence. Guide to the processes of technology appraisal. London: National Institute for Health and Care Excellence; 2014. https://www.nice.org.uk/process/pmg19/. Accessed 9 Sep 2018.
National Institute for Health and Care Excellence. Pirfenidone for treating idiopathic pulmonary fibrosis (TA282). London: The National Institute for Health and Care Excellence; 2013. https://www.nice.org.uk/guidance/ta282. Accessed 9 Sep 2018.
National Institute for Health and Care Excellence. Pirfenidone for treating idiopathic pulmonary fibrosis (TA504). London: National Institute for Health and Care Excellence; 2018. https://www.nice.org.uk/guidance/ta504. Accessed 7 Feb 2018.
National Institute for Health and Care Excellence. Pirfenidone for treating idiopathic pulmonary fibrosis (TA504). London: NICE Technology Appraisals. National Institute for Health and Care Excellence. https://www.nice.org.uk/guidance/TA504/history. Accessed 7 Feb 2018.
Roche Products Ltd. Esbriet 267 mg hard capsules, summary of product characteristics. London: European Medicines Agency; 2015. https://www.medicines.org.uk/emc/product/3705/smpc. Accessed 9 Sep 2018.
Boehringer Ingelheim Limited. Ofev 150 mg soft capsules, summary of product characteristics. London: European Medicines Agency; 2015. https://www.medicines.org.uk/emc/product/1786/smpc. Accessed 9 Sep 2018.
National Institute for Health and Care Excellence. Nintedanib for treating idiopathic pulmonary fibrosis (TA379). London: National Institute for Health and Care Excellence; 2016. https://www.nice.org.uk/guidance/ta379. Accessed 9 Sep 2018.
National Institute for Health and Care Excellence. Pirfenidone for the treatment of idiopathic pulmonary fibrosis (review of TA282) [ID837])—final scope. London: National Institute for Health and Care Excellence; 2015. https://www.nice.org.uk/guidance/ta504/documents/final-scope. Accessed 9 Sep 2018.
Roche Product Limited. Pirfenidone for the treatment of adults with idiopathic pulmonary fibrosis: Company evidence submission. London: The National Institute for Health and Care Excellence; 2016. https://www.nice.org.uk/guidance/ta504/documents/committee-papers. Accessed 9 Sep 2018.
King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–92. https://doi.org/10.1056/NEJMoa1402582.
Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377(9779):1760–9. https://doi.org/10.1016/S0140-6736(11)60405-4.
Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171(9):1040–7. https://doi.org/10.1164/rccm.200404-571OC.
Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35(4):821–9. https://doi.org/10.1183/09031936.00005209.
Costabel U, Albera C, Bradford W, Hormel P, King TJ, Noble P, et al. Analysis of lung function and survival in RECAP: an open-label extension study of pirfenidone in patients with idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31(3):198–205.
Participation Program for Pulmonary Fibrosis. P3 F Registry; 2016. https://www.nationaljewish.org/Participation-Program-for-Pulmonary-Fibrosis/P%E2%82%83F-Registry. Accessed 9 Mar 2016 (database on the Internet).
Martinez FJ, de Andrade JA, Anstrom KJ, King TE, Raghu G. Randomized trial of N-acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2093–101. https://doi.org/10.1056/NEJMoa1401739.
Raghu G, The Idiopathic Pulmonary Fibrosis Clinical Research Network. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366:1968–77. https://doi.org/10.1056/NEJMoa1113354.
Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365(12):1079–87. https://doi.org/10.1056/NEJMoa1103690.
Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–82. https://doi.org/10.1056/NEJMoa1402584.
Huang H, Dai HP, Kang J, Chen BY, Sun TY, Xu ZJ. Double-blind randomized trial of pirfenidone in Chinese idiopathic pulmonary fibrosis patients. Medicine (Baltimore). 2015;94(42):e1600. https://doi.org/10.1097/MD.0000000000001600.
Harari S, Caminati A. Idiopathic pulmonary fibrosis: from clinical trials to real-life experiences. Eur Respir Rev. 2015;24(137):420–7. https://doi.org/10.1183/16000617.0042-2015.
Roche Products Ltd. Pirfenidone for the treatment of adults with idiopathic pulmonary fibrosis: response to clarification letter. London: National Institute for Health and Care Excellence; 2016. https://www.nice.org.uk/guidance/ta504/documents/committee-papers. Accessed 9 Sep 2018.
Roche Product Limited. Pirfenidone for the treatment of adults with idiopathic pulmonary fibrosis: ACD response. London: National Institute for Health and Care Excellence; 2016. https://www.nice.org.uk/guidance/ta504/documents/committee-papers-2. Accessed 9 Sep 2018.
Albera C, Costabel U, Fagan E, Glassberg M, Gorina E, Lancaster L, et al. Efficacy of pirfenidone in patients with idiopathic pulmonary fibrosis with more preserved lung function. Eur Respir J. 2016;48(3):843–51. https://doi.org/10.1183/13993003.01966-2015.
CAPACITY 1 ClinicalTrials.gov. Safety and efficacy of pirfenidone in patients with idiopathic pulmonary fibrosis. ClinicalTrials.gov; 2016.https://clinicaltrials.gov/ct2/show/record/NCT00287729.Accessed 9 Mar 2016 (database on the Internet).
Acknowledgements
The authors wish to thank Dr. Stephen Bianchi and Professor David Thickett for providing clinical advice and commenting on draft materials during the project.
Author information
Authors and Affiliations
Contributions
SD, CC, JH (formerly named Jean Sanderson), ME and RR drafted the manuscript and take responsibility as guarantors of the content. All authors have given their approval for the final version to be published. This summary has not been externally reviewed by PharmacoEconomics
Corresponding author
Ethics declarations
Funding
This project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment Programme (project number 142/06/02 STA); see the HTA programme website for further project information (http://www.hta.ac.uk). This summary of the ERG report was compiled after NICE issued guidance. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of NICE or the Department of Health.
Conflict of Interest
SD, RR, CC, JH and ME have no potential conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Davis, S., Rafia, R., Carroll, C. et al. Pirfenidone for Treating Idiopathic Pulmonary Fibrosis: An Evidence Review Group Perspective of a NICE Single Technology Appraisal. PharmacoEconomics 37, 763–775 (2019). https://doi.org/10.1007/s40273-018-0727-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-018-0727-1