Abstract
Background
Venetoclax is a first-in-class targeted therapy option that is an inducer of apoptosis in chronic lymphocytic leukemia (CLL) cells. The open-label phase III CLL14 clinical trial showed that venetoclax combined with obinutuzumab (VEN+O) is superior to obinutuzumab combined with chlorambucil in newly diagnosed patients with CLL. The aim of this study was to assess the health economic value of VEN+O for the frontline treatment of CLL in Canada from a publicly funded healthcare system perspective.
Methods
A partitioned survival analyses model was developed including three health states: progression free, progressed, and death. A cycle length of 28 days and a time horizon of 10 years was assumed. VEN+O treatment for a fixed duration of 12 months was compared to obinutuzumab combined with chlorambucil, fludarabine plus cyclophosphamide plus rituximab, bendamustine plus rituximab, chlorambucil plus rituximab, ibrutinib, and acalabrutinib. The population in the model included both unfit and overall frontline CLL patients, two subgroups were also assessed (patients with del17p/TP53 mutations and patients without del17p/TP53 mutations). Survival data extrapolated from the CLL14 trial were used to populate the model. Uncertainty was assessed via one-way sensitivity analyses, probabilistic analyses, and scenario analyses.
Results
Based on the probabilistic analyses, unfit frontline CLL patients receiving VEN+O were estimated to incur costs of Canadian dollars ($) 217,727 [confidence interval (CI) $170,725, $300,761] (del17p/TP53: $209,102 [CI $159,698, $386,190], non-del17p/TP53: $217,732 [CI $171,232, $299,063]) and accrue 4.96 [CI 4.04, 5.82] quality-adjusted life-years (del17p/TP53: 3.11 [CI 2.00, 4.20], non-del17p/TP53: 5.04 [CI 4.05, 5.92]). Obinutuzumab combined with chlorambucil, bendamustine plus rituximab, chlorambucil plus rituximab, and ibrutinib accrued lower quality-adjusted life-years and higher costs and as such, VEN+O was the dominant treatment option. The full incremental analysis showed that acalabrutinib was more expensive and more efficacious compared with VEN+O with an incremental-cost-effectiveness-ratio of $2,139,180/quality-adjusted life-year versus VEN+O and not a cost-effective option in Canada. Probabilistic analyses show that at a willingness to pay of $50,000/quality-adjusted life-year gained, VEN+O has the greatest probability of being cost effective.
Conclusions
VEN+O is a cost-effective treatment option for unfit frontline CLL patients and provides value for money to healthcare payers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Fixed-duration treatment, such as that offered by venetoclax plus obinutuzumab, has the potential of reducing the substantial health and economic burden of frontline chronic lymphocytic leukemia. |
This cost-effectiveness model shows that, for frontline unfit patients with chronic lymphocytic leukemia, venetoclax plus obinutuzumab as a 12-month fixed-duration treatment offers lower projected costs and more quality-adjusted life-years gained versus relevant treatments, except acalabrutinib, which was not cost effective (incremental cost-effectiveness ratio of $2,139,180/quality-adjusted life-year versus venetoclax plus obinutuzumab). |
At a threshold of $50,000/quality-adjusted life-year gained, venetoclax plus obinutuzumab provides value for money to Canadian jurisdictions compared with existing funded treatments in Canada. |
1 Introduction
Chronic lymphocytic leukemia (CLL) is a clonal disease of unknown etiology and is the most common type of leukemia reported in adults living in Western countries [1]. Chronic lymphocytic leukemia manifests as the uncontrolled growth of B lymphocytes, which accumulate in the blood, bone marrow, lymph nodes, and spleen [2]. The incidence of CLL in the Western world is estimated at 4.2 per 100,000 persons per year and increases by more than ten-fold with age [3]. In Canada, yearly incidence varies between 5.0 and 8.0 per 100,000 persons [4, 5], translating into over 1700 new CLL cases in 2016 and about 600 deaths in 2017 [6].
Chronic lymphocytic leukemia is more common with advanced age, with a median age of diagnosis between 67 and 72 years. Therefore, the majority of patients with CLL also have clinically relevant co-existing medical conditions [7]. The fitness of patients, defined by age and the Cumulative Illness Rating Scale measuring patient comorbidity, has been defined as a key prognostic factor for CLL survival [9]. Important disease-related risk factors influencing CLL prognosis and treatment pathway include the deletion of the short arm of chromosome 17 (del17p) and/or mutations in the tumor suppressor gene TP53 (mTP53), the mutation status of the immunoglobulin heavy-chain variable region (IGVH), b2-microglobulin level, and CLL clinical stage [2, 8]. Presence of del17p/mTP53 indicates a poor prognosis, with resistance to conventional chemoimmunotherapy and median overall survival (OS) of 2–5 years. Overall survival and duration of remissions are also shorter for unmutated IGVH patients owing to a higher risk of genetic instability [3].
The frontline treatment options for patients with CLL (1L CLL) vary with patients’ prognostic risk factors. For patients with a non-del17p/mTP53 status, treatment options depend on the fitness level. Patients aged < 65 years with a Cumulative Illness Rating Scale score < 6, defined as ‘fit’ patients, usually receive fludarabine combined with cyclophosphamide and rituximab (FCR). Patients aged > 65 years with multiple comorbidities, commonly defined as ‘unfit’, cannot tolerate FCR treatment because of the high toxicity and infection rates associated primarily with fludarabine [9]. For the FCR-ineligible previously untreated patients with CLL, obinutuzumab (Gazyvaro® [G]) combined with chlorambucil (GClb) has been considered an effective regimen [3, 10, 11]. Other treatment options for the FCR-ineligible 1L CLL patients include bendamustine and rituximab (BR), chlorambucil and rituximab (Clb+R), and ibrutinib (Ibr). Patients with a del17p/mTP53 status usually have a poor prognosis even after FCR therapy, hence the usual treatments are based on novel inhibitors such as Ibr, which is currently the most frequently used regimen out of the funded novel inhibitors in Canada.
Venetoclax (VEN) is a first-in-class oral selective inhibitor of BCL-2 anti-apoptotic protein that is overexpressed in approximately 95% of patients with CLL. Its unique targeted mechanism of action and fixed treatment duration distinguish it from other available therapies [12]. The recent open-label, phase III, CLL14 clinical trial (NCT02242942) results demonstrated an acceptable safety profile of VEN in combination with obinutuzumab (VEN+O), for the treatment of previously untreated patients with CLL with co-existing medical conditions (unfit patients) [7, 13]. In all patients and across all the major prognostic subgroups analyzed, VEN+O also showed a consistently superior treatment profile compared with the standard of care GClb [7]. Health Canada has issued a notice of compliance for VEN+O for the 1L treatment of patients with previously untreated CLL [14].
The health and economic burden of previously untreated CLL is substantial and increasing over time, leading to both considerable decreases in quality of life for 1L CLL patients and high lifetime costs to the healthcare system and caregivers [15]. Randomized controlled trial data suggest that various therapeutic options for 1L CLL are associated with improved efficacy in both unfit and a combination of unfit and fit populations. Nonetheless, the increased number of therapeutic options for patients with CLL requires formal comparisons of efficacy, safety, and economic burden to effectively treat 1L CLL patients.
The objective of the current study was to assess the cost effectiveness of VEN+O for the treatment of 1L CLL patients compared to current and future treatment options in Canada. The model has been parametrized to be consistent with Canadian Agency for Drugs and Technologies in Health (CADTH) guidelines. It also aligns with the National Institute of Health and Care Excellence (NICE) economic evaluation guidelines and the decision analytic modeling best practice recommendations from the International Society for Pharmacoeconomics and Outcomes Research (ISPOR).
2 Methods
A systematic literature review (SLR) was conducted in September 2020 to identify studies assessing the cost effectiveness of 1L CLL treatment options [16]. The SLR identified 17 full-text articles and 27 abstracts [5, 17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59]. Most published models followed a Markov or semi-Markov model. Other modeling approaches were discrete-event simulations or microsimulation models, whereas more recent models adopted a partitioned survival analysis (PartSA) approach.
2.1 Model Structure
The current analysis employed a PartSA model for two main reasons. First, recent pan-Canadian Oncology Drug Review (pCODR) appraisals of CLL treatments have primarily used PartSA models [60,61,62,63]. Second, the PartSA model allows for a clear linkage between the clinical efficacy data from the pivotal trial and the clinical data used in the model.
The PartSA methodology was used to estimate and extrapolate progression-free survival (PFS), post-progression survival (PPS), time to next treatment (TTNT), and OS for up to a 10-year time horizon. Patient distribution among health states (PFS, PPS, and dead) and over time were estimated using the extrapolated survival curves alongside an area-under-the-curve analysis. The model cycle length was 28 days and a half-cycle correction was also applied. It was assumed that the age-adjusted and sex-adjusted mortality hazard rates of patients with CLL were not lower than age-adjusted and sex-adjusted all-cause mortality rates of the general population. To enable a cost-effectiveness analysis, specific utility values and cost profiles were attributed to the different health states of the model.
2.2 Patient Population and Treatment Interventions
The patient population base-case characteristics, including age and sex distributions, were informed by the CLL14 trial population (Table 1). The present study considered two populations, the unfit as the base case and the overall 1L CLL (fit and unfit patients) as a scenario. The unfit 1L CLL population was further subdivided into four subgroups based on del17p/TP53 and IGVH mutation status. The treatment comparators of VEN+O for the 1L CLL unfit patient population were GClb, BR, Clb+R, Ibr, and acalabrutinib monotherapy. For the overall 1L CLL patient population, in addition to all the comparators, VEN+O was also compared to FCR. For all the unfit subgroups, the treatment comparator was GClb, except in the case of the del17p/TP53 subgroup, where Ibr was also used.
2.3 Model Inputs
The model inputs were either identified from the economic and clinical SLRs, estimated from the CLL14 trial, or elicited from clinical experts during an advisory board. Ten local clinical experts validated the model structure and inputs, facilitated by an economic expert. Given the nature of a probabilistic model, expert responses on eight survey questions were used to calculate mean resource use responses along with variances and standard errors. Specifically, health-related quality of life, cost, resource use, and previous economic model data were identified from the economic SLR. Whenever possible, model inputs were informed from Canadian-specific sources and databases. Background mortality was estimated from the latest Canadian life tables published by Statistics Canada [64].
2.3.1 Treatment Efficacy
The primary measures of clinical effectiveness for the 1L CLL treatments were from the CLL14 trial data and included PFS, OS, TTNT, and time on treatment (ToT) curves for VEN+O and GClb treatment arms. The observed survival curves of VEN+O and GClb in the CLL14 trial were parameterized (a) to estimate outcomes beyond the observed trial period, (b) to allow synthesis of outcomes with data from external comparators, and (c) to facilitate a probabilistic analysis. The parameterization methods were guided by the NICE technical support documentation [65]. The dependent and independent models explored included exponential, Weibull, Gompertz, log-normal, log-logistic, gamma, and generalized-gamma distributions. Additionally, spline 1–3 knot models based on the hazards, odds, and probit (or normal) scale were also fitted to the observed time-to-event data. The internal validity of the models was investigated using statistical measures of fit (Akaike’s Information Criterion and the Bayesian Information Criterion), followed by a visual fit inspection. To assess the clinical plausibility and external validity, landmark survival values were discussed with clinical experts and cross-validated with external sources.
The sample of patients with the del17p/TP53 mutation is small. To maximize the predictive power of the CLL14 data, del17p/TP53 was included as a covariate in the time-to-event modeling and the impact of del17p/TP53 status on the scale of the survival curves was estimated. Similarly, to assess the differential effect of IGVH mutation on survival outcomes, IGVH mutation status was included as a covariate in the independent and dependent modeling approaches of OS, PFS, and TTNT to enable parametrization of the survival outcomes of the two subgroups.
A clinical SLR was conducted to inform indirect treatment comparisons (ITC) between VEN+O and the comparators outside the CLL14 trial. Indirect treatment comparisons were made using network meta-analysis (NMA) methods. Two sets of NMAs were performed: unfit only 1L CLL and overall 1L CLL [66]. Hazard ratios (HRs) were generated versus VEN+O for two outcomes (PFS and OS). These NMAs were updated to include acalabrutinib (Tables S1 and S3 of the Electronic Supplementary Material [ESM]). The NMA feasibility assessment had identified heterogeneity in GClb dosing and Clb treatment duration across trials. A scenario analysis was run to adjust for this heterogeneity in the NMA (Table S2 of the ESM). The analysis was not used in the base case because the results favored VEN+O and would introduce further uncertainty in the analysis.
In the del17p/TP53 population, two naïve comparisons and one matching-adjusted indirect comparison (MAIC) was conducted for VEN+O versus Ibr monotherapy [67, 68]. The PFS and OS HRs from the naïve comparison using Mato et al. [67] were combined with the VEN+O PFS and OS curves, respectively, to generate the individual survival curves for Ibr. The naïve comparison using Mato et al. was used in the base case because the results were powered by a larger sample size [16]. All parameter estimations for the treatment effects were performed using maximum likelihood using the R package flexsurv [69].
2.3.2 Model Validation
The OS outcomes from the CLL14 trial were immature for both treatment arms. Due to this and as the proportional hazards assumption could not be rejected, dependent modeling was the most appropriate option. To account for the immature OS data and to explore the validity of the OS CLL14-based extrapolations, PPS curves were generated based on 5-year follow-up data from the GClb arm of the CLL11 trial comparing GClb to Clb+R [70]. During the advisory board, the CLL11 trial was highlighted as a relevant external data source, as GClb was one of the included treatment arms and the trial population inclusion criteria were comparable to CLL14.
Comparing survival estimates with real-world data [71,72,73,74] showed that long-term survival estimates matched better with the relative than with the absolute real-word-based survival values. Brenner et al. [71] for instance, indicate that the absolute 10-year survival in the 1L CLL population in the USA was between 28 and 35%, and the relative between 46 and 55%, whereas the landmark 10-year OS survival prediction from the CLL14 trial data ranged from 57 to 77% in both trial arms (Table S4 of the ESM). Such discrepancies originate from the fact that available real-world data pertain to a treatment era in which efficacious treatment options were lacking. More recent studies indicate higher 10-year OS estimates of 51–64% [72, 73]. Following the exploration of alternative approaches, including validation with external data sources, and consultations with clinical and economic experts, it was concluded that the CLL14 trial was the most appropriate source of evidence for base-case OS predictions. Therefore, the dependent model using the exponential distribution was employed in the base-case scenario, while we assumed no difference in OS between VEN+O and GClb. The latter is a conservative assumption because the CLL14 trial shows that patients progressed slower in the VEN+O arm and post-first-relapse patients were salvaged quite quickly in the CLL14 trial.
For PFS and TTNT survival curves, the proportional hazards assumption was rejected. Therefore, independent models were used to extrapolate beyond the trial duration. The landmark PFS and TTNT estimates from the extrapolations were discussed with clinical experts, who recommended a log-logistic distribution for PFS as the base-case option. However, uncertainty remained in the TTNT extrapolations because of limited follow-up data. Similar to the OS projections, the GClb arm from the CLL11 trial was used as a source for external validation. The 5-year TTNT in the CLL11 trial was 49%, comparable to the CLL14-based extrapolations that varied between 52 and 60% (Table S5 of the ESM). The independent log-logistic distribution also provided the best-fitting distribution for the TTNT extrapolations.
Patients in the VEN+O and GClb treatment arms followed a fixed treatment duration. To inform treatment costs, the number of patients remaining on treatment per cycle length up until the fixed treatment duration point from the CLL14 trial was estimated. Time on treatment was estimated based on discontinuation of therapy using censoring pegged to OS. Due to a fixed treatment duration, no extrapolations of the ToT curve were required. For comparators outside the CLL14 trial, PFS curves were used to determine the number of patients on treatment per cycle.
2.3.3 Modeling Beyond First-Line Therapy
To inform the number of patients receiving subsequent treatment following VEN+O or GClb, the estimated differences between the OS and TTNT from the CLL14 curves were used. For comparators outside the CLL14 trial, the difference between their own OS and PFS curves were used to inform the respective number of patients receiving subsequent treatment. To inform the duration that patients remain on subsequent treatment, previously published data collected from a targeted literature search were used [12, 75,76,77,78,79,80]. To be conservative, the lowest duration on subsequent treatment between the modeled and the literature values was incorporated in the model. See Box 1 in the ESM for further details on the approach to modeling subsequent treatments.
2.3.4 Utility Values and Adverse Events
Health state utilities and adverse event (AE) disutilities were derived from the SLR. Utilities from CLL14 were deemed to be clinically implausible by clinical and economic experts as they were higher than the age-adjusted general population utilities. Therefore, utility values for pre-progression and post-progression states were derived from the most recent NICE submission for 1L CLL trials for obinutuzumab (TA343 and TA174, see Table S8 in the ESM) [32, 81,82,83], and were also adjusted for age-related deterioration as recommended by the NICE Decision Support Unit [65].
Adverse event disutility values and duration estimates were used to assess the impact of AEs on quality-adjusted life-years (QALYs). The parameters for each AE were sourced from previous NICE technology appraisals and the literature (see Table S9 in the ESM) [84,85,86,87,88,89]. For VEN+O and GClb, the incidence of AEs was informed from CLL14. Neutropenia, febrile neutropenia, pneumonia, sepsis, and thrombocytopenia were the most common serious AEs. Grade 3–4 AEs with at least a 2% difference in the rate between the treatment arms were also included. For other comparators, the most recent publications (including NICE appraisals) with the longest follow-up data were used to inform respective AE incidence (Table S6 of the ESM). In accordance with other oncology models, AEs were assumed to occur within the first cycle. Because of a lack of granular data, the same approach was also taken for treat-to-progress regimens (e.g., Ibr and acalabrutinib), which was a conservative assumption.
2.3.5 Cost and Resource Use Data
Total costs consisted of CLL active treatment (drug and administration) costs, routine care and monitoring costs, treatment-specific monitoring costs, costs related to AEs, disease progression, and end-of-life care and were taken from various sources (Table S7 of the ESM). All costs were inflated to 2020 Canadian dollars using the healthcare component of the consumer price index [90].
2.4 Model Analyses
The key outcomes of the cost-effectiveness analysis were total life-years (LYs), QALYs, and costs over a 10-year time horizon, as well as incremental LYs, QALYs, costs and incremental cost-effectiveness ratios (ICERs), representing the cost or per QALY gained. Analysis was conducted using a pan-Canadian healthcare system perspective. The overall analytic structure includes both a probabilistic and deterministic model. Consistent with CADTH guidelines, the base-case analysis results were derived using the probabilistic model. In the probabilistic analyses, a simulation of a certain number of iterations generated a mean output with associated upper and lower confidence intervals (CIs). For each individual iteration, the model parameters were simultaneously sampled from pre-defined distributions: the gamma distribution was selected for cost parameters, the beta distribution (bound between 0 and 1) for utilities and proportions, and log-normal distributions for hazard ratios. The method of Hatswell et al. [91] suggested that 1000 iterations were enough to generate stable results, but the base-case outcomes were generated from 5000 iterations to adhere to CADTH guidelines. Outcomes and costs were discounted at 1.5% per year [92].
2.5 Sensitivity Analyses
Variables for which values were uncertain were tested in a one-way sensitivity analysis (OWSA). The OWSA was conducted based on a pairwise comparison between two treatments examining the impact of different model parameter values (i.e., based upon the 95% CI) on incremental costs, incremental QALYs, and ICERs. Using the net monetary benefit approach, the probability of each treatment being cost effective at different willingness-to-pay (WTP) levels was investigated in a cost-effectiveness acceptability curve (CEAC). Last, extensive scenario analyses were conducted to examine the impact on outcomes of alternative modeling assumptions or alternative data sets (e.g., utility values).
3 Results
3.1 Incremental Costs and QALYs
Table 2 presents the per-patient costs across the different categories. All comparators resulted in higher costs than VEN+O. For treat to progression-based regimens, the high costs were driven by the high drug acquisition costs that were accrued for these comparators. For non-treat to progression-based comparators, the high costs were driven by the subsequent treatment costs that were accrued because of the larger proportion of patients remaining in the PPS period compared with VEN+O.
Table 3 shows the 10-year expected per-patient LYs (undiscounted) and QALYs (discounted), averaged across the 5000 simulations, by the pre-progression and post-progression periods, AE disutilities, and by treatment for the unfit and the overall 1L CLL patient population. Acalabrutinib accrued the highest health gains at 5.27 [95% CI 4.25, 6.25] QALYs, followed by VEN+O, and GClb, with 4.96 [95% CI 4.04, 5.82] and 4.75 [95% CI 4.03, 5.45] QALYs, respectively. VEN+O accrued most of its QALYs during the progression-free stage, reflecting the improved PFS for VEN+O compared with other treatments. For unfit 1L CLL patients with the del17p/TP53 mutation, Ibr accrued 0.26 more QALYs and was $375,061 more expensive than VEN+O. For the IGVH mutation subgroup, VEN+O resulted in higher QALYs and less costs compared with GClb.
3.2 Cost-Effectiveness Analysis Results
Figure 1 presents the cost-effectiveness frontier for the different treatments of the base-case unfit 1L CLL patient population included in the model. The figure shows that VEN+O is on the frontier with acalabrutinib. Acalabrutinib is not cost effective and compared with VEN+O shows an ICER of $2,139,180 per QALY.
Cost-effectiveness frontier for the unfit frontline chronic lymphocytic leukemia population. Acala acalabrutinib, BR bendamustine plus rituximab, Clb chlorambucil, CR chlorambucil plus rituximab, GClb Gazyvaro (obinutuzumab) plus chlorambucil, Ibr ibrutinib, QALYs quality-adjusted life-years, Ven+G venetoclax plus Gazyvaro (obinutuzumab)
Table 4 presents the full incremental analysis for the unfit and overall 1L CLL population and the subgroups included in the analyses. When comparing to Ibr for the del17p/TP53 subgroup, Ibr accrued more QALYs but also incurred more costs than VEN+O. For all other subgroups, VEN+O was dominant.
3.3 Sensitivity Analyses on the Unfit 1L CLL Population
The OWSA demonstrates that variances in the OS and PFS hazard ratios from the NMA and from the unadjusted comparison have the biggest influence on key model results across all comparisons, including the subgroup analyses for with and without del17p/TP53. Tornado plots for incremental costs and incremental QALYs, for all pairwise comparisons of VEN+O versus GClb (all populations) or Ibr (for del17p/TP53 subgroup), can be found in the Figs. S1–6 of the ESM.
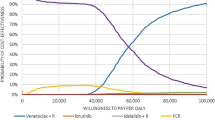
As shown in Fig. 2, PA results following 5000 iterations remained stable and in accordance with the deterministic results conveying that the dominance of VEN+O over GClb and most other comparators is robust. The total cost and QALY estimates were comparable between the deterministic and PA. Cost estimates revealed higher uncertainty (<5% for all other interventions) compared with the QALY estimates (≤4% both intervention and comparator treatment arms). The CEAC shows that at $50,000 WTP threshold, VEN+O has an over 90% probability of being cost effective (Fig. 3).
Scenario analyses are presented in Tables 5 and 6. Despite variations in expected costs and expected QALYs, during pairwise comparisons, VEN+O remained dominant over all four treatment comparators (apart from vs acalabrutinib) across almost all the scenarios. The three exceptions were the scenario assuming a 5-year time horizon, applying the PPS CLL11 data, and applying alternative HRs from the NMA. In the first two scenarios, VEN+O was predicted to cost $311,691 and $316,165/QALY gained compared to GClb, respectively. When applying Clb dosing-adjusted HRs from the NMA, VEN+O was dominant versus all comparators including acalabrutinib. Reducing discount rates improved incremental QALYs for the comparators that accrued lower QALYs than VEN+O. Changing utility values had a large impact on the incremental QALYs but did not alter conclusions. Finally, relaxing the assumption of difference in OS between VEN+O and GClb did not significantly alter expected costs or QALYs relative to the base case.
4 Discussion
The identification of effective 1L CLL treatments with limited toxicity that can be tolerated by older or unfit patients remains a challenge in the management of newly diagnosed FCR-ineligible CLL patients. This study estimated the health economic impact of VEN+O in the frontline treatment of CLL in Canada. The CLL14 trial demonstrated that VEN+O improved PFS in previously untreated CLL older and less fit patients when compared with chemoimmunotherapy, GClb. The results showed the improved treatment potential of VEN+O translated into improved long-term health outcomes in CLL patients ineligible for FCR. VEN+O showed better economic outcomes as it was more clinically effective and less costly than most comparators owing to its fixed treatment duration. A probabilistic analysis showed that at a WTP of approximately $50,000 per QALY, VEN+O had an over 90% probability of being the most cost-effective treatment. This was acknowledged by CADTH in the final recommendation on VEN+O in 1L CLL, which mentioned a 97% probability that VEN+O is cost effective at a WTP of $50,000 per QALY. At the time of submission, acalabrutinib was not yet recommended by CADTH [93].
VEN+O consistently showed better outcomes for PFS compared with all comparators across the total and the unfit 1L CLL population including the IGVH mutated population, except compared to acalabrutinib for the unfit and total 1L CLL patient population and compared to Ibr for the patient population with the del17p/TP53 mutation. The OWSA and several scenario analyses conveyed the robustness of the results. Within the scenario analysis where Clb dosing-adjusted HRs were used, VEN+O remained the dominant treatment option across all comparators including acalabrutinib.
For the unfit 1L CLL population, the best source of evidence for the VEN+O arm and the GClb arm was utilized from the CLL14 trial. Demonstrating robust evidence for the cost effectiveness of VEN+O for the treatment of 1L CLL patients was challenging for two reasons. First, the CLL14 trial data were still immature because the median OS was not reached in both treatment arms, while median PFS was only reached for GClb and not for VEN+O. Second, data limitations exist for the comparator study versus Ibr for the del17p population.
In the absence of mature data from the CLL14 patient population, long-term OS results are uncertain. To validate the extrapolated results from the CLL14 patient population, the 5-year follow-up data from the CLL11 trial were incorporated within a scenario. The CLL11 serves as a conservative scenario as the post-progression period has limited innovative treatment regimens that were provided to the CLL14 trial population. Therefore, the OS estimates from CLL11 are likely underestimated given the current treatment landscape and poorly fit the VEN+O as well as the GClb treatment arms in CLL14.
5 Conclusions
This study supports that VEN+O is an effective fixed-duration treatment option for the treatment of unfit 1L CLL patients demonstrating potential cost savings for Canadian jurisdictions compared with existing funded treatments in Canada. This is in line with the recently released CADTH CLL provisional funding algorithm used to provide advice to the Canadian public-participating drug programs on implementation issues in CLL, which raised the concept of affordability as an important factor to consider when assessing the relative place in therapy for the different treatment options in the first-line setting [94].
References
Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975–2017, National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/csr/1975_2017/, based on November 2019 SEER data submission, posted to the SEER web site, April 2020. Accessed 1 Sept 2021.
Hallek M. Chronic lymphocytic leukemia: 2017 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2017;92(9):946–65.
Eichhorst B, Robak T, Montserrat E, et al. Chronic lymphocytic leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl._5):v78–84.
Seftel M, Demers AA, Banerji V, et al. High incidence of chronic lymphocytic leukemia (CLL) diagnosed by immunophenotyping: a population-based Canadian cohort. Leuk Res. 2009;33(11):1463–8.
Herring W, Pearson I, Purser M, et al. Cost effectiveness of ofatumumab plus chlorambucil in first-line chronic lymphocytic leukaemia in Canada. Pharmacoeconomics. 2016;34(1):77–90.
Canadian Cancer Society. Chronic lymphocytic leukemia statistics. https://www.cancer.ca/en/cancer-information/cancer-type/leukemia-chronic-lymphocytic-cll/statistics. Accessed 7 Aug 2020.
Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380(23):2225–36.
Mistry H, Nduka C, Connock M, et al. Venetoclax for treating chronic lymphocytic leukaemia: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2018;36(4):399–406.
Eichhorst B, Fink AM, Busch R, et al. Frontline chemoimmunotherapy with fludarabine (F), cyclophosphamide (C), and rituximab (R)(FCR) shows superior efficacy in comparison to bendamustine (B) and rituximab (BR) in previously untreated and physically fit patients (pts) with advanced chronic lymphocytic leukemia (CLL): final analysis of an international, randomized study of the German CLL Study Group (GCLLSG) (CLL10 Study). Washington, DC: American Society of Hematology; 2014.
Goede V, Fischer K, Engelke A, et al. Obinutuzumab as frontline treatment of chronic lymphocytic leukemia: updated results of the CLL11 study. Leukemia. 2015;29(7):1602–4.
Wierda WG, Byrd JC, Abramson JS, et al. Chronic lymphocytic leukemia/small lymphocytic lymphoma, version 4.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(2):185–217.
Kater AP, Seymour JF, Hillmen P, et al. Fixed duration of venetoclax-rituximab in relapsed/refractory chronic lymphocytic leukemia eradicates minimal residual disease and prolongs survival: post-treatment follow-up of the MURANO phase III study. J Clin Oncol. 2019;34(4):269–77.
Fischer K, Al-Sawaf O, Fink AM, et al. Venetoclax and obinutuzumab in chronic lymphocytic leukemia. Blood. 2017;129(19):2702–5.
pCODR Expert Review Committee (pERC). Venetoclax (Venclexta) in combo with obinutuzumab for CLL: details. 2020. https://cadth.ca/venetoclax-venclexta-combo-obinutuzumab-cll-details. Accessed 11 Oct 2020.
Lafeuille M-H, Vekeman F, Wang ST, et al. Lifetime costs to Medicare of providing care to patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53(6):1146–54.
National Institute for Health and Care Excellence. Single technology appraisal, Venetoclax with obinutuzumab for untreated chronic lymphocytic leukaemia [ID1402]. Committee Papers. 2020.
Adena M, Houltram J, Mulligan SP, et al. Modelling the cost effectiveness of rituximab in chronic lymphocytic leukaemia in first-line therapy and following relapse. Pharmacoeconomics. 2014;32(2):193–207.
Barnes J, Divi V, Begaye A, et al. Cost-effectiveness of ibrutinib as first-line therapy for chronic lymphocytic leukemia in older adults without deletion 17p. Blood Adv. 2018;2(15):1946–56.
Batty A, Thompson GJ, Maroudas PA, et al. Estimating cost-effectiveness based on results of uncontrolled clinical trials: ofatumumab for the treatment of fludarabine- and alemtuzumab-refractory chronic lymphocytic leukemia. Value Health. 2010;13(7):A328–9.
Becker U, Briggs AH, Moreno SG, et al. Cost-effectiveness model for chemoimmunotherapy options in patients with previously untreated chronic lymphocytic leukemia unsuitable for full-dose fludarabine-based therapy. Value Health. 2016;19(4):374–82.
Bertwistle D, Munakata J, Wehleret E, al. Cost-effectiveness of bendamustine versus fludarabine for first-line treatment of chronic lymphocytic leukemia (Cll) in Colombia. Value Health. 2013;16(7):A413.
Bertwistle D, Munakata J, Wehler E, et al. The cost-effectiveness of bendamustine versus fludarabine for the first-line treatment of chronic lymphocytic leukemia (Cll) in Mexico. Value Health. 2013;16(7):A409.
Bosch F, Casado LF, Garcia-Marcoet JA, et al. Pcn103 pharmacoeconomic analysis of the addition of rituximab to fludarabine-cyclophosphamide regimen in the first-line treatment of chronic lymphocytic leukaemia patients in Spain. Value Health. 2009;2(7):A276.
Cameron H, Thompson M, Marino JP, et al. Obinutuzumab and chlorambucil versus chlorambucil monotherapy for treatment of previously untreated chronic lymphocytic leukemia where fludarabine-based chemotherapy is considered inappropriate: a Canadian cost-utility analysis. Blood. 2014;124(21):1288.
Casado L, Burgos A, González-Haba E, et al. Economic evaluation of obinutuzumab in combination with chlorambucil in first-line treatment of patients with chronic lymphocytic leukemia in Spain. Clinicoecon Outcomes Res. 2016;8:475–84.
Chen Q, Jain N, Ayer T, et al. Economic burden of chronic lymphocytic leukemia in the era of oral targeted therapies in the United States. J Clin Oncol. 2017;35(2):166–74.
Chiattone C, Borges L, Santos E. Pcn70 cost-effectiveness analysis of rituximab therapy in patients with previously untreated chronic lymphocytic leukemia (Cll) in Brazil. Value Health. 2010;13(7):A264.
Davids M, Chatterjee A, Ravelo A, et al. Cost-effectiveness of a 12-month fixed duration of venetoclax in combination with obinutuzumab in first-line chronic lymphocytic leukemia in the United States. Blood. 2019;134:4741.
Dervaux B, Lenne X, Theis D, et al. Cost effectiveness of oral fludarabine in chronic lymphocytic leukaemia: the French case. J Med Econ. 2008;10(4):339–54.
Ho R, Rufino C, Biondo J, et al. Cost-effectiveness analysis of obinutuzumab associated to chlorambucil for the treatment of slow-go patients with chronic lymphocytic leukemia (CLL) under the Brazilian public healthcare system. Value Health. 2017;20(9):A855.
Holtzer-Goor K, Vandekerckhove S, van den Steen D, et al. Modelling the 10-year cost effectiveness of bendamustine as first line treatment for chronic lymphocytic leukaemia in the Netherlands. Haematologica. 2012;97:443–4.
Hornberger J, Reyes C, Shewade A, et al. Cost-effectiveness of adding rituximab to fludarabine and cyclophosphamide for the treatment of previously untreated chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53(2):225–34.
Hornberger J, Reyes C, Shewade A, et al. Cost-effectiveness of rituximab, fludarabine, and cyclophosphamide (R-Fc) in patients with previously untreated chronic lymphocytic leukemia (Cll). J Clin Oncol. 2010;28:e16500-e.
Kapedanovska A, Sterjev Z, Naumovska Z, et al. Cost effectiveness of obinutuzumab as frontline treatment for unfit patients with chronic lymphocytic leukemia in Republic of Macedonia. Value Health. 2017;20(9):A736.
Kawalec P, Stawowczyk E, Russel-Szymczyk M, et al. Pcn88 an economic evaluation of rituximab versus other first-line treatments for patients with chronic lymphocytic leukemia in Poland. Value Health. 2009;12(7):A273.
Kongnakorn T, Sterchele JA, Salvador CG, et al. Economic implications of using bendamustine, alemtuzumab, or chlorambucil as a first-line therapy for chronic lymphocytic leukemia in the US: a cost-effectiveness analysis. Clinicoecon Outcomes Res. 2014;6:141–9.
Kousoulakou H, Krivasi T, Caporis X, et al. A cost effectiveness analysis of obinutuzumab in the management of chronic lymphocytic leukemia in Greece. Value Health. 2017;20:A555.
Lupu A, et al. Cost-effectiveness of treatment with rituximab in previously untreated patients with chronic lymphocytic leukemia in Romania. Haematologica. 2010;95:195.
Malin J, Kongnakorn T, Sterchele J, et al. Pcn93 cost-effectiveness analysis of bendamustine compared to alemtuzumab and chlorambucil for chronic lymphocytic leukemia in a treatment-naive population. Value Health. 2010;13(3):A41.
Mandrik O, Corro Ramos I, Knies S, et al. Cost-effectiveness of adding rituximab to fludarabine and cyclophosphamide for treatment of chronic lymphocytic leukemia in Ukraine. Cancer Manag Res. 2015;7:279–89.
Muller D, Fischer K, Kaiser P, et al. Cost-effectiveness of rituximab in addition to fludarabine and cyclophosphamide (R-Fc) for the first-line treatment of chronic lymphocytic leukemia. Leuk Lymphoma. 2016;57(5):1130–9.
Paquete A, Miguel LS, Becker U, et al. Cost-effectiveness analysis of obinutuzumab for previously untreated chronic lymphocytic leukaemia in Portuguese patients who are unsuitable for full-dose fludarabine-based therapy. Appl Health Econ Health Policy. 2017;15(4):501–12.
Pearson IV, Hawe E, Zuluaga S, et al. Cost-effectiveness of ofatumumab plus chlorambucil in first-line chronic lymphocytic leukemia in the United Kingdom. Value Health. 2015;18(7):A455.
Plommet N, Boissard F. Cost-effectiveness analysis of Gazyvaroro in association to chlorambucil for patients with chronic lymphocytic leukemia when full-dose fludarabine is unsuitable in France. Value Health. 2016;19(7):A739.
Pompen M, ten Hoor G, Carret E, et al. Pcn109 cost-effectiveness of rituximab in previously untreated patients with chronic lymphocytic leukemia in the Dutch setting. Value Health. 2009;12(7):A277.
Reyes C, Gazauskas G, Becker U, et al. Cost-effectiveness analysis of obinutuzumab versus ofatumumab for previously untreated chronic lymphocytic leukemia (Cll). Blood. 2014;124(21):1324.
Reyes C, Veenstra D, Ramsey S. Is obinutuzumab cost-effective for previously untreated chronic lymphocytic leukemia in the United States? Haematologica. 2014;99:488.
Roussel M, Troussard X, Delmer A, et al. PCN71 cost-effectiveness of rituximab combined with fludarabine and cyclophosphamide in previously untreated patients with chronic lymphocytic leukemia in France. Value Health. 2009;12(7):A270.
Saenz AS. Is obinituzumab the most cost-effective biological therapy for unfit patients with previously untreated chronic lymphocytic leukemia in Colombia? Value Health. 2016;19(3):A152.
Singh M, Mealing S, Baculea S, et al. Impact of novel agents on patient-relevant outcomes in patients with previously untreated chronic lymphocytic leukemia who are not eligible for fludarabine-based therapy. J Med Econ. 2017;20(10):1066–73.
Singh M, et al. Novel therapies in chronic lymphocytic leukaemia (Cll): quantifying the impact on patient relevant outcome measures. Eur J Cancer. 2017;72:S99-100.
Sinha R, Redekop W. Cost-effectiveness of ibrutinib compared with obinutuzumab with chlorambucil in untreated chronic lymphocytic leukemia patients with comorbidities in the United Kingdom. Clin Lymphoma Myeloma Leuk. 2018;18(2):e131–42.
Smet A, Peng S, Dorman E, et al. Cost-effectiveness of ibrutinib as frontline treatment for adult patients with chronic lymphocytic leukemia in Belgium. Presented at 33rd General Annual Meeting, Belgium Hematology Society, February 2–3, 2018.
Soini E, Hautala A, Poikonen E, et al. Cost-effectiveness of first-line chronic lymphocytic leukemia treatments when full-dose fludarabine is unsuitable. Clin Ther. 2016;38(4):889–904.e14.
Vandekerckhove S, Holtzer-Goor K, van den Steen D, et al. Pcn90 cost effectiveness analysis of bendamustine as first line treatment for chronic lymphocytic leukaemia in the Netherlands. Value Health. 2012;15(7):A425.
Veenstra D, Reyes C, Ramsey S. Is obinutuzumab cost-effective in the first-line treatment of Cll? American Society of Clinical Oncology; 2014.
Walzer S, Becker U, Samanta K, et al. The potential cost-effectiveness of obinutuzumab (Ga101) in combination with chlorambucil in chronic lymphocytic leukemia. Value Health. 2013;16(7):A412.
Woods B, Hawkins N, Dunlop W, et al. Bendamustine versus chlorambucil for the first-line treatment of chronic lymphocytic leukemia in England and Wales: a cost-utility analysis. Value Health. 2012;15(5):759–70.
Yagudina R, Kulikov A, Babiy V. Cost-effectiveness analysis of obinutuzumab/chlorambucil vs rituximab/chlorambucil in treatment of chronic lymphocytic leukemia. Value Health. 2015;18(7):A460.
pan Canadian Oncology Drug Review (pCODR). Final economic guidance report: obinutuzumab (Gazyvaro) for chronic lymphocytic leukemia. 2015. https://www.cadth.ca/sites/default/files/pcodr/pcodr-gazyvaro-cll-fn-egr.pdf. Accessed 11 Oct 2022.
pan Canadian Oncology Drug Review (pCODR). Final economic guidance report: ibrutinib (Imbruvica) for chronic lymphocytic leukemia or small lymphocytic lymphoma (previously untreated). 2016. https://www.cadth.ca/sites/default/files/pcodr/pcodr-ibrutinib-cll-sll-fn-egr.pdf. Accessed 11 Oct 2022.
pan Canadian Oncology Drug Review (pCODR). Final economic guidance report: venetoclax (Venclexta) for chronic lymphocytic leukemia 2018.
pan Canadian Oncology Drug Review (pCODR). Final economic guidance report: venetoclax (Venclexta) rituximab for chronic lymphocytic leukemia. 2019. https://www.cadth.ca/sites/default/files/pcodr/Reviews2019/10162VenetoclaxRituximabCLL_fnEGR_NOREDACT-ABBREV_31May2019_final.pdf. Accessed 11 Oct 2022.
Statistics Canada. Life expectancy and other elements of the life table, Canada, 2016/18. 2020. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310011401. Accessed 11 Oct 2022.
Latimer N. NICE DSU technical support document 14: survival analysis for economic evaluations alongside clinical trials-extrapolation with patient-level data. Sheffield: Report by the Decision Support Unit, 2011; 2013.
Sudhapalli P, Piena M, Palaka A, Mato A, van de Wetering G, Manzoor BS, et al. Systematic literature review and network meta-analysis comparing therapies for treatment naïve patients with chronic lymphocytic leukemia. Poster presented at EHA 2020; #EP7252020.
Mato AR, Roeker LE, Allan JN, et al. Outcomes of front-line ibrutinib treated CLL patients excluded from landmark clinical trial. Am J Hematol. 2018;93(11):1394–401.
Ahn IE, Farooqui MZH, Tian X, et al. Depth and durability of response to ibrutinib in CLL: 5-year follow-up of a phase 2 study. Blood. 2018;131(21):2357–66.
Jackson CH. flexsurv: A platform for parametric survival modeling in R. J Stat Softw. 2016;70: i08.
Goede V, Fischer K, Dyer M, et al. Overall survival benefit of obinutuzumab over rituximab when combined with chlorambucil in patients with chronic lymphocytic leukemia and comorbidities: final survival analysis of the CLL11 study. EHA Learning Center. 2018;215923:S151.
Brenner H, Gondos A, Pulte D. Trends in long-term survival of patients with chronic lymphocytic leukemia from the 1980s to the early 21st century. Blood. 2008;111(10):4916–21.
Pulte D, Castro FA, Jansen L, et al. Trends in survival of chronic lymphocytic leukemia patients in Germany and the USA in the first decade of the twenty-first century. J Hematol Oncol. 2016;9(1):28.
Bista A, Uprety D, Ghimire S, et al. Long-term survival in chronic lymphocytic leukemia after first primary malignancy in the United States. J Clin Oncol. 2017;35(15_suppl):e18090–90.
Shvidel L, Braester A, Bairey O, et al. Survival trends among 1,325 patients with chronic lymphocytic leukemia seen over the past 40 years in Israel. Am J Hematol. 2011;86(12):985–92.
Seymour JF, Kipps TJ, Eichhorst BF, et al. MURANO trial establishes feasibility of time-limited venetoclax-rituximab (VenR) combination therapy in relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL). Blood. 2018;132(Suppl. 1):184.
O’Brien S, Furman RR, Coutre S, et al. Single-agent ibrutinib in treatment-naïve and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood. 2018;131(17):1910–9.
Byrd JC, Hillmen P, O’Brien S, et al. Long-term follow-up of the RESONATE phase 3 trial of ibrutinib vs ofatumumab. Blood. 2019;133(19):2031–42.
Davids MS, Hallek M, Wierda W, et al. Comprehensive safety analysis of venetoclax monotherapy for patients with relapsed/refractory chronic lymphocytic leukemia. Clin Cancer Res. 2018;24(18):4371–9.
Jones JA, Mato AR, Wierda WG, et al. Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol. 2018;19(1):65–75.
Coutre S, Choi M, Furman RR, et al. Venetoclax for patients with chronic lymphocytic leukemia who progressed during or after idelalisib therapy. Blood. 2018;131(15):1704–11.
National Institute for Health and Care Excellence. TA343: obinutuzumab in combination with chlorambucil for untreated chronic lymphocytic leukaemia. 2015. https://www.nice.org.uk/guidance/ta343. Accessed 11 Oct 2022.
National Institute for Health and Care Excellence. TA174: rituximab for the first-line treatment of chronic lymphocytic leukaemia. 2009. https://www.nice.org.uk/guidance/ta174. Accessed 11 Oct 2022.
Beusterien KM, Davies J, Leach M, et al. Population preference values for treatment outcomes in chronic lymphocytic leukaemia: a cross-sectional utility study. Health Qual Life Outcomes. 2010;8(1):50.
National Institute for Health and Care Excellence. TA306: pixantrone monotherapy for treating multiply relapsed or refractory aggressive non-Hodgkin’s B‑cell lymphoma. 2014. https://www.nice.org.uk/guidance/ta306. Accessed 11 Oct 2022.
Lloyd A, Nafees B, Narewska J, et al. Health state utilities for metastatic breast cancer. Br J Cancer. 2006;95(6):683–90.
National Institute for Health and Care Excellence. TA216: bendamustine for the first-line treatment of chronic lymphocytic leukaemia. 2011. https://www.nice.org.uk/guidance/ta216. Accessed 11 Oct 2022.
National Institute for Health and Care Excellence. TA344: ofatumumab in combination with chlorambucil or bendamustine for untreated chronic lymphocytic leukaemia. 2015. Available from: https://www.nice.org.uk/guidance/ta344. [Accessed 11 Oct 2022].
Nafees B, Stafford M, Gavriel S, et al. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6(1):84.
Tolley K, Goad C, Yi Y, et al. Utility elicitation study in the UK general public for late-stage chronic lymphocytic leukaemia. Eur Jo Health Econ. 2013;14(5):749–59.
Statistics Canada. Consumer price index, health and personal care, by province (monthly): Government of Canada; 2020. http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/cpis13a-eng.htm. Accessed 20 Feb 2020.
Hatswell AJ, Bullement A, Briggs A, et al. Probabilistic sensitivity analysis in cost-effectiveness models: determining model convergence in cohort models. Pharmacoeconomics. 2018;36(12):1421–6.
Canadian Agency for Drugs and Technologies in Health (CADTH). Guidelines for the economic evaluation of health technologies: Canada. 4th ed. 2017: p. 2.
pCODR Expert Review Committee (pERC). Final recommendation, final clinical guidance report & final economic guidance report. 2020.
CADTH. Reimbursement review. Provisional funding algorithm for chronic lymphocytic leukemia. Final. 2021.
Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–74.
Eichhorst B, Fink A-M, Bahlo J, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, openlabel, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17(7):928–42.
Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101–10.
Barr PM, Robak T, Owen C, et al. Sustained efficacy and detailed clinical follow-up of first-line ibrutinib treatment in older patients with chronic lymphocytic leukemia: extended phase 3 results from RESONATE-2. Haematologica. 2018;103(9):1502–10.
Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278–91.
Tam V, Ko YJ, Mittmann N, et al. Cost-effectiveness of systemic therapies for metastatic pancreatic cancer. Curr Oncol. 2013;20:e90-106.
Indeed. Pharmacy technician salaries in Canada. 2020.
Millar D, Corrie P, Hill M, et al. PCN74 a service evaluation to compare secondary care resource use between xelox and folfox-6 regimens in the treatment of metastatic colorectal cancer (MCRC) from a UK national health service (NHS) perspective. Value Health. 2008;11(6):A483-A.
Registered Nurses Association of Ontario. Dollars and sense: what are nurses paid? Toronto (ON): Ontario Ministry of Health; 2018.
MaRS Discovery District. Employee benefits and benefits packages: what Ontario employers should know. 2015.
Rituxan Hycela™. Prescribing information. Cambridge (MA): Biogen Genentech USA Inc.; 2017.
Hillhouse E, et al. Costs associated with the management of relapsed/refractory chronic lymphocytic leukemia: an updated costing report. Commissioned report for AbbVie Canada. 2019; p. 1–36.
Ontario Ministry of Health and Long Term Care (MoHLTC). Schedule of benefits for laboratory services. 2020.
Ontario Ministry of Health and Long Term Care (MoHLTC). Physician services under the Health Insurance Act. Schedule of benefits. 2019.
Mittmann N, Hassan S, Seung SJ, et al. Health care resource utilization in the management of chronic lymphocytic leukemia at an Ontario cancer centre. Value Health. 2014;17(A79):464.
Ontario Ministry of Health and Long-Term Care. Ontario case costing inititative: OCCI costing analysis tool 2017. 2017. https://hsim.health.gov.on.ca/hdbportal/. Accessed 11 Oct 2022.
Acknowledgments
The authors thank Poojee Sudhapalli, PharmD and Martina Bianco, MSc, both former employees of OPEN Health, for their work on the economic model, as well as Venetia Qendri, PhD and Ana Moura, PhD, employee of OPEN Health, for medical writing assistance and editorial support for this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
AbbVie Inc. provided financial support for the study. Venetoclax is being developed in a collaboration between AbbVie Inc. and Genentech Inc. No honoraria or payments were made for authorship.
Conflicts of interest/Competing interests
Anuja Chatterjee: consultancy (AbbVie); Gijs van de Wetering: consultancy (AbbVie); Ron Goeree: consultancy (AbbVie); CO: honoraria (Abbvie, Astrazeneca, Janssen, Roche, Merck, Incyte); Anne Marie Desbois: employment and ownership interests (AbbVie); Stephane Barakat: employment and ownership interests (AbbVie); Beenish S. Manzoor: employment and ownership interests (AbbVie); Kavita Sail: employment and ownership interests (AbbVie).
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Input data for the generation of the results are available in this article and its Electronic Supplementary Material. Additional datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. The MS Excel model cannot be shared because of confidentiality reasons.
Code availability
Not applicable.
Authors’ contributions
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. AbbVie and Pharmerit, an OPEN Health Company participated in the design, study conduct, analysis and interpretation of data, as well as the writing, review, and approval of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chatterjee, A., van de Wetering, G., Goeree, R. et al. A Probabilistic Cost-Effectiveness Analysis of Venetoclax and Obinutuzumab as a First-Line Therapy in Chronic Lymphocytic Leukemia in Canada. PharmacoEconomics Open 7, 199–216 (2023). https://doi.org/10.1007/s41669-022-00375-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-022-00375-x