Abstract
Purpose
We aimed to compare Australian health system costs at 12 months for adjuvant whole-brain radiotherapy (WBRT) treatment after stereotactic radiosurgery (SRS) and/or surgery versus observation among adults with one to three melanoma brain metastases. We hypothesized that treatment with adjuvant WBRT and subsequent healthcare would be more expensive than SRS/surgery alone.
Methods
The analysis was conducted alongside a multicentre, randomized phase III trial. A bespoke cost questionnaire was used to measure healthcare use, including hospitalizations, specialist and primary care visits, imaging, and medicines over 12 months. Mean per-patient costs were calculated based on the quantity of resources used and unit costs, reported in Australian dollars ($AU), year 2018 values. Skewness of cost data was determined using normality tests and censor-adjusted costs reported using the Kaplan–Meier sample average method. The analysis of difference in mean costs at each 2-month time point and at 12 months was performed and checked using Kruskal–Wallis, generalized linear models with gamma distribution and log link, modified Park test, ordinary least squares, and non-parametric bootstrapping.
Results
In total, 89 patients with similar characteristics at baseline were included in the cost analysis (n = 43 WBRT; n = 46 observation). Hospitalization cost was the main cost, ranging from 63 to 89% of total healthcare costs. The unadjusted 12-monthly cost for WBRT was $AU71,138 ± standard deviation 41,475 and for observation $AU69,848 ± 33,233; p = 0.7426. The censor-adjusted 12-monthly cost for WBRT was $AU90,277 ± 36,274 and $AU82,080 ± 34,411 for observation. There was no significant difference in 2-monthly costs between groups (p > 0.30 for all models).
Conclusions
Most costs were related to inpatient hospitalizations associated with disease recurrence. Adding WBRT after local SRS/surgery for patients with one to three melanoma brain metastases did not significantly increase health system costs during the first 12 months.
Trial Registration
ACTRN12607000512426, prospectively registered 14 September 2007.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The 12-monthly cost for whole-brain radiotherapy (WBRT) was Australian dollars ($AU)90,277 ± standard deviation 36,274 and $AU82,080 ± 34,411 for observation. |
Adding WBRT after local stereotactic radiosurgery/surgery for patients with one to three melanoma brain metastases did not significantly increase health system costs during the first 12 months. |
A traditional approach to costing without adjustment for censoring may underestimate total healthcare costs. |
1 Background
Melanoma is one of the most serious forms of skin cancer, with incidence rising worldwide among fair-skinned populations. Brain metastases, a frequent complication of advanced stage IV melanoma and a common cause of death, produce disabling symptoms such as headaches, nausea, vomiting, and seizures and are difficult to treat [1,2,3]. For patients with only a few melanoma brain metastases, a high rate of local control can be achieved with surgery and/or stereotactic radiosurgery (SRS) [4]. However, the risk of progression within the brain during the first 12 months can reach 70%, so an effective therapy is needed to reduce the risk of subsequent failure.
Investigators from the Melanoma Institute Australia designed a trial to investigate the effectiveness of adjuvant whole-brain radiotherapy (WBRT) following SRS/surgery for adults with one to three melanoma brain metastases (ACTRN 12607000512426) [5]. A cost-effectiveness analysis of WBRT at 12 months was planned alongside this trial [6]. The main findings of the trial showed that WBRT did not significantly improve distant intracranial control at 12 months or survival or preserve performance status [7]. Clinical practice is now changing as a result of these findings, with adjuvant WBRT not recommended.
Important economic data, including health system costs for those randomized to WBRT versus observation, were carefully collected alongside this trial. These data are very useful for clinicians and health service managers to understand the healthcare resources and costs of care associated with advanced melanoma, as well as the likely impact on their budgets and any differences in costs between treatment options.
In melanoma, economic evaluation studies are mostly model based, with the cost inputs obtained from published literature or government reports [8,9,10,11]. Taking aggregated costs from published literature without taking into account censoring (i.e., due to study withdrawal or death) can result in underestimating or overestimating costs, biasing comparative assessments. Some costing studies have been undertaken alongside melanoma randomized controlled trials [12,13,14,15,16]; however, to our knowledge, no studies have been undertaken alongside randomized trials examining the costs among patients with melanoma brain metastases. We therefore aimed to assess the differences in healthcare costs over a 12-month period for adults with melanoma brain metastases using traditional (unadjusted) and censor-adjusted methods.
2 Methods
2.1 Trial Design and Participants
This costing study was undertaken alongside a prospective multinational centre, open-label, stratified, two-arm randomized controlled phase III trial comparing adjuvant WBRT with observation following local treatment with SRS/surgery of one to three melanoma brain metastases [17]. The trial was registered with the Australian and New Zealand Clinical Trials Registry (ANZCTR; #12607000512426). The protocol was approved by the Cancer Institute NSW Clinical Research Ethics Committee #2007C/11/032 and relevant hospital ethics committees in each trial centre. Patients were eligible if they were aged ≥ 18 years, had one to three melanoma brain metastases treated by SRS/surgery, and had an Eastern Cooperative Oncology Group (ECOG) performance status between 0 and 2. WBRT was a multicentre, internationally recruiting (Australia, Norway, and USA), prospective, open-label, phase III randomized controlled trial designed to investigate the safety and efficacy of using WBRT to treat one to three brain metastases in patients with advanced melanoma. The study design has been described in detail [5]. Primary findings indicated that WBRT did not improve survival, distant intracranial control, and performance status and was not recommended as a standard treatment for one to three brain metastases following local treatment [7].
An economic evaluation was approved by the trial management committee and added to the protocol amendment in 2014, to enrol all prospectively randomized participants in the costing substudy. Between 2009 and 2017, 215 patients with one to three melanoma brain metastases were enrolled in the trial after surgery and/or SRS; 107 were randomly allocated to the WBRT group and 108 were allocated to observation alone. After the protocol amendment, 89 consecutive patients were randomized and included in the costing substudy (43 to WBRT, 46 to observation).
2.2 Costing
The costing analysis was conducted from a health system perspective and reported according to the relevant items on the CHEERS checklist for economic evaluations. Final results were inflated to Australian dollars ($AU), year 2018 values using the consumer price index (https://www.abs.gov.au/statistics/economy/price-indexes-and-inflation/consumer-price-index-australia/latest-release). A purpose-built cost questionnaire was designed to capture inpatient and outpatient costs, from randomization to 12 months, administered at each 2-monthly follow-up visit up (see Appendix S8 in the electronic supplementary material [ESM]). The questionnaire was submitted to the Database of Instruments for Resource Use Measurement website: https://www.dirum.org/. The cost questionnaire consisted of multiple items that identified outpatient visits, procedures and imaging, and inpatient hospitalizations, including length of stay. Based on case report forms and questionnaire responses, Medical Benefits Schedule (MBS) items and diagnosis-related groups (DRGs) were assigned by trained study staff in consultation with clinicians. For activities that required intensive care unit (ICU) stays, an ICU adjustor was used to calculate hospitalization costs [18]. Any differences in coding items were resolved through consensus between clinicians and researchers.
Outpatient costs were calculated based on the 2018 MBS items [19]. Inpatient costs were calculated based on the DRG items and length of stay reported in trial records. The inlier weight costs, national weighted activity unit, short- and long-stay weight were taken from the Independent Hospital Pricing Authority 2018 Australian National Efficiency Price [18]. Inlier weight is the weight of the hospitalization cost when the length of stay is within the average length of stay. Any 2-monthly visits with a missing count for healthcare use were excluded from the analysis as costs could not be calculated. Data imputation for costs was not undertaken, and discounting was not required.
2.3 Statistical Analysis
Costs were calculated based on the quantity of resource used and their unit cost ($AU, year 2018 values), using the formula cost = resource use × unit cost. Costs of inpatient and outpatient services were calculated and tabulated for the WBRT and observation groups. Total costs were calculated using the sum of inpatient and outpatient costs. The skewness of cost data was determined using several normality tests, including Shapiro–Wilk, Kolmogorov–Smirnov, Cramer–von Mises, and Anderson–Darling. The 2-monthly costs were reported using the traditional method (unadjusted) and a censor-adjusted method using the Kaplan–Meier sample average (KMSA) approach. Unadjusted annual costs were calculated by summing all the costs from month 2 to month 12 for each patient. If a patient died or was censored at month 8, the total annual cost was the sum of total costs in month 2, month 4, and month 6. The total annual cost was then calculated by averaging the annual cost of all patients in the cohort.
In KMSA, the follow-up period is divided into intervals. Total costs in each interval are multiplied by the individual patient’s survival probability at the beginning of each interval and then summed across the intervals [20]. KMSA produces estimates more reflective of actual patient-level healthcare costs compared with traditional methods, by accounting for the effect of censoring [20]. For example, the adjusted cost at month 2 = S(t) × average unadjusted cost at month 2. S(t) is probability of survival at time t. The adjusted annual cost was the sum of the adjusted monthly cost exported from the KMSA, from month 2 to month 12 [20].
The analysis of difference in costs between groups was performed using a number of statistical methods to examine the consistency in findings, including (1) Kruskal–Wallis test for the difference in mean costs at 12 months, (2) generalized linear models (GLM) with gamma distribution and log link, (3) modified Park test, (4) ordinary least squares method (OLS), and (5) non-parametric bootstrapping for difference in 2-monthly costs over 12 months. The five methods were chosen because they are popular methods to model costing data [20].
3 Results
3.1 Patient Characteristics
Patient baseline characteristics were balanced in both groups and are reported in Table 1. The mean age was 62 years, and the majority were males (61% for WBRT and 65% for observation). Patient characteristics in the costing substudy were not significantly different from those in the whole trial except for ECOG performance status and Breslow thickness of the primary lesion (Table S1 in the ESM). Specifically, compared with the whole trial, patients in the costing substudy receiving WBRT had significantly better ECOG performance status (p = 0.05), and patients in the costing substudy receiving SRS/surgery had significantly lower Breslow thickness (p = 0.002) (Table S1 in the ESM).
3.2 Health Resource Use and Cost Every 2 Months
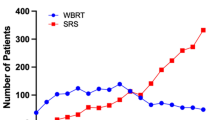
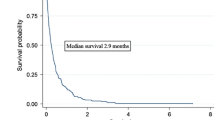
Healthcare resource use is presented in Table S7 in the ESM. Costs are presented and compared between the two groups at months 2, 4, 6, 8, 10, and 12 and are presented in Table 2 and Fig. 1. Most of the costs were incurred during the first few months and at the time of intracranial recurrence. The skewness of costs for the follow-up period is shown in Fig. S1 in the ESM and indicates substantial positive skewness.
Between 60 and 90% of the total healthcare costs were related to inpatient hospitalizations, 2–6% related to outpatient consultations, 4–20% related to surgical or radiotherapy procedures and diagnostic imaging, and 5–7% related to prescribed medicines. Treatment-related costs associated with intracranial recurrence were higher in the first 2 months after randomization for both groups than in later months (Table 2).
The 2-monthly unadjusted costs for patients in the WBRT group over the follow-up period ranged between $AU17,754 and 22,679 (mean 19,466). The 2-monthly unadjusted costs for patients in the observation group ranged between $AU16,163 and 20,733 (mean 18,517) (Table S2 in the ESM). The GLM indicated that there was no significant difference in costs between the two groups at each time point (p > 0.05) (Table S3 in the ESM). Other statistical analyses, including the modified Park test, OLS, and non-parametric bootstrapping, showed no significant difference between groups (p > 0.30) (Tables S3–S6 in the ESM).
3.3 Annual Costs
The unadjusted annual cost for the WBRT group was $AU71,138 ± standard deviation 41,475 and for the SRS/surgery group was $AU69,848 ± 33,233; p = 0.74. The censor-adjusted annual cost for WBRT was $AU90,277 ± 36,274 and $AU82,080 ± 34,411 for SRS/surgery (Table 3).
4 Discussion
Our study showed no significant difference in mean per patient healthcare costs for those receiving adjuvant WBRT or observation after SRS/surgery up to 12 months after randomization. Treatment-related costs associated with recurrence were higher in the first 2 months after randomization than in the latter months in both groups, and the majority of healthcare costs were associated with hospitalizations. Therefore, the cost of hospitalization will be an important consideration alongside drug costs in the overall assessment of the economic burden of metastatic melanoma. Total healthcare costs were somewhat lower using a traditional (unadjusted) approach than a censor-adjusted approach, likely underestimating true costs.
Although we hypothesized that people receiving adjuvant WBRT would incur higher healthcare costs at 12 months than those in the observation group, we found the rate of inpatient hospitalizations was the major contributor to health system costs (63–89%) and not the upfront WBRT. The mean annual costs per patient were somewhat lower in our study than previously reported in an Australian decision analytic study [10]. In that study, the mean annual cost per patient for stage III unresectable or stage IV melanoma was $AU115,109 in 2017 (equal to ~ $AU117,307 in year 2018 values). Our study captured the costs after randomization, therefore costs for the initial management of brain metastases (i.e., SRS/surgery) were not included in this analysis. It should also be noted that our study among patients with stage IV melanoma brain metastases used actual patient-level data and adjusted for patient survival, whereas the abovementioned study included patients with stage III cancer with a better prognosis (hence longer survival time to accrue costs) and used a decision analytic model with model inputs from secondary data sources.
We reported both unadjusted and censor-adjusted costs at 12 months. Unadjusted costs could lead to a downward-biased mean estimate of costs [20]. KMSA, using non-parametric interval methods with the assumption that, at any follow-up time, the probability of censoring is independent of the future outcomes of patients, therefore, this method can address the bias associated with unadjusted costs. Although there are other ways to manage censoring, including approaches published by Lin et al. [21], Baker et al. [22], and Bang and Tsiatis [23], the KMSA approach is the most widely used.
Our study has a number of strengths. It was conducted as part of a randomized controlled trial among multiple sites, providing the highest-quality data without confounding. We conducted robust statistical analyses with various statistical tests that demonstrated consistency in findings. Healthcare use was verified by on-site clinicians who provided treatment to the trial patients, meaning the accuracy is likely higher than a patient recall of healthcare use using diaries. Our study also had some limitations. First, because the costing study was added after the trial had commenced, we recruited less than half of the total participants. Second, patients in the costing substudy were likely to be less sick than patients in the whole trial (i.e., better ECOG performance status and better Breslow thickness of primary lesion). Therefore, the costs in the costing substudy might underestimate the real costs. Third, we received some missing data for healthcare use, particularly if patients were lost to follow-up or died shortly after their most recent study visit. We tried to minimize the impact of this missing data by using the KMSA method, in which the average costs were adjusted for censoring, e.g., loss to follow-up or death. Fourth, the MBS items and DRG codes assigned for each documented test or procedure during the trial may have differed slightly from the codes used in actual practice. To overcome this, trial coordinators were intensively trained to assign accurate codes from a master list. Authors ADT and RLM independently checked the Medicare coding, and any inconsistencies were discussed and resolved by consensus through in-person meetings between researchers and the clinical team. Finally, it would be helpful to report on differences in salvage treatments utilized as well as potential differences in magnetic resonance imaging (MRI) surveillance frequency between the two groups. However, we categorized the costs by service type, i.e., inpatient or outpatient, but not by treatment purpose, i.e., salvage treatment, palliative treatment, or best supportive care. Similarly, we did not separate MRI as a separate costing in the procedure costing category. We therefore suggest examining differences in salvage treatment and MRI frequencies for future studies.
Although WBRT is unlikely to be prescribed in the adjuvant setting following SRS/surgery, it is occasionally prescribed following further intracranial recurrence. Given the large proportion of people with advanced melanoma who develop brain metastases, these cost estimates are particularly useful to inform health services about the subsequent healthcare use and likely costs of care. Healthcare costs in this study could also be used to update our published pre-trial model [24].
5 Conclusion
Healthcare costs in the first 12 months following local treatment for one to three melanoma brain metastases is substantial, in the order of $AU82,080–90,277 per patient. The largest costs were related to inpatient hospitalizations associated with disease recurrence. Our study found the provision of adjuvant WBRT did not significantly increase health system costs and that a traditional approach to costing without adjustment for censoring may underestimate total healthcare costs.
References
Bafaloukos D, Gogas H. The treatment of brain metastases in melanoma patients. Cancer Treat Rev. 2004;30(6):515–20.
Bottoni U, Clerico R, Paolino G, et al. Predictors and survival in patients with melanoma brain metastases. Med Oncol. 2013;30(1):466.
Chukwueke U, Batchelor T, Brastianos P. Management of brain metastases in patients with melanoma. J Oncol Pract. 2016;12(6):536–42.
Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401–9.
Fogarty G, Morton RL, Vardy J, et al. Whole brain radiotherapy after local treatment of brain metastases in melanoma patients—a randomised phase III trial. BMC Cancer. 2011;11:142.
Lo SN, Hong AM, Haydu LE, et al. Whole brain radiotherapy (WBRT) after local treatment of brain metastases in melanoma patients: statistical analysis plan. Trials. 2019;20(1):477.
Hong AM, Fogarty GB, Dolven-Jacobsen K, et al. Adjuvant whole-brain radiation therapy compared with observation after local treatment of melanoma brain metastases: a multicenter, randomized phase III trial. J Clin Oncol. 2019;37(33):3132–41.
Jensen IS, Zacherle E, Blanchette CM, et al. Evaluating cost benefits of combination therapies for advanced melanoma. Drugs Context. 2016;5:212297–212297.
Meng Y, Hertel N, Ellis J, et al. The cost-effectiveness of nivolumab monotherapy for the treatment of advanced melanoma patients in England. Eur J Health Econ. 2018;19(8):1163–72.
Elliott TM, Whiteman DC, Olsen CM, et al. Estimated healthcare costs of melanoma in Australia over 3 years post-diagnosis. Appl Health Econ Health Policy. 2017;15(6):805–16.
Mooney MM, Mettlin C, Michalek AM, et al. Life-long screening of patients with intermediate-thickness cutaneous melanoma for asymptomatic pulmonary recurrences: a cost-effectiveness analysis. Cancer Interdiscip Int J Am Cancer Soc. 1997;80(6):1052–64.
Agnese DM, Abdessalam SF, Burak WE Jr, et al. Cost-effectiveness of sentinel lymph node biopsy in thin melanomas. Surgery. 2003;134(4):542–7.
Crott R, Ali F, Burdette-Radoux S. Cost–utility of adjuvant high-dose interferon alpha therapy in stage III cutaneous melanoma in Québec. Value Health. 2004;7(4):423–32.
Hillner BE, Agarwala S, Middleton MR. Post hoc economic analysis of temozolomide versus dacarbazine in the treatment of advanced metastatic melanoma. J Clin Oncol. 2000;18(7):1474–80.
Hofmann U, Szedlak M, Rittgen W, et al. Primary staging and follow-up in melanoma patients–monocenter evaluation of methods, costs and patient survival. Br J Cancer. 2002;87(2):151–7.
Deckers EA, Hoekstra-Weebers JE, Damude S, et al. The MELFO study: a multicenter, prospective, randomized clinical trial on the effects of a reduced stage-adjusted follow-up schedule on cutaneous melanoma IB–IIC patients—results after 3 years. Ann Surg Oncol. 2020;27(5):1407–17.
Hong AM, Fogarty GB, Dolven-Jacobsen K, et al. Adjuvant whole-brain radiation therapy compared with observation after local treatment of melanoma brain metastases: a multicenter, randomized phase III trial. J Clin Oncol. 2019:JCO1901414.
Authority IHP. National Efficient Price (NEP) and National Efficient Cost (NEC) determinations for Australian public hospital services for 2018–19; 2018. Australia.
Australian Government Department of Health. The November 2018 Medicare Benefits Schedule. 2018, Department of Health: Canberra.
Gray AM, Clarke PM, Wolstenholme JL, et al. Applied methods of cost-effectiveness analysis in healthcare, vol. 3. Oxford: Oxford University Press; 2011.
Lin D, Feuer E, Etzioni R, et al. Estimating medical costs from incomplete follow-up data. Biometrics. 1997;419–34.
Baker MS, Kessler LG, Urban N, et al. Estimating the treatment costs of breast and lung cancer. Med. Care. 40–9.
Bang H, Tsiatis AA. Estimating medical costs with censored data. Biometrika. 2000;87(2):329–43.
Tran AD, Fogarty G, Nowak AK, et al. Cost-effectiveness of subsequent whole-brain radiotherapy or hippocampal-avoidant whole-brain radiotherapy versus stereotactic radiosurgery or surgery alone for treatment of melanoma brain metastases. Appl Health Econ Health Policy. 2020;18(5):679–87.
Acknowledgements
This work was funded by a Cancer Australia, Priority-driven Collaborative Cancer Research Scheme (project #1046923) and an NHMRC Centre of Research Excellence in Melanoma (#1135285). Rachael L. Morton is supported by an NHMRC investigator Grant (#1194703) and a University of Sydney Robinson Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Cancer Australia, Priority-driven Collaborative Cancer Research Scheme project #1046923. Rachael L. Morton is supported by a NHMRC investigator fellowship (#1194703) and a University of Sydney Robinson Fellowship.
Conflict of interest
Angela M Hong has received consultancy fees and honorarium from Amgen and Bayer. Anh D Tran, Mai TH Nguyen, Gerald Fogarty, Victoria Steel, Elizabeth Paton, and Rachael L Morton have no conflicts of interest that are directly relevant to the content of this article.
Availability of data and material
The datasets generated during and/or analysed during the current study are not publicly available because individual trial participants have not provided consent for the sharing of this information. A de-identified dataset may be made available from the corresponding author on reasonable request with a research proposal.
Code availability
Not applicable.
Authors’ contributions
ADT designed the analysis, conducted data analysis, and wrote the manuscript. MTHN wrote part of the manuscript and presented the tables. VS and EP undertook Medicare coding, cleaned the data, and commented on the manuscript. AH, GF, and RLM designed the study, interpreted findings, wrote and critically reviewed the manuscript, and supervised the research team.
Ethics approval
The study protocol was approved by the Cancer Institute New South Wales Ethics Committee (reference 2007C/11/032) and the Sydney Local Health District Ethics Committee (reference X13-0329 and HREC/13/RPAH/465) as well as by institutional review boards at each site. The trial was registered with the Australian Clinical Trials Registry (ACTRN12607000512426) and ClinicalTrials.gov.
Consent to participate
Patients provided their consent to participate prior to participation via ethically approved specific consent forms.
Consent for publication
The consent form also specified that the anonymized results of the study (primary, secondary and tertiary endpoints) would be published, and all patients who consented using the form also agreed in principle to the publication arrangement.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tran, A.D., Hong, A.M., Nguyen, M.T.H. et al. Cost Analysis of Adjuvant Whole-Brain Radiotherapy Treatment Versus No Whole-Brain Radiotherapy After Stereotactic Radiosurgery and/or Surgery Among Adults with One to Three Melanoma Brain Metastases: Results from a Randomized Trial. PharmacoEconomics Open 6, 587–594 (2022). https://doi.org/10.1007/s41669-022-00332-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-022-00332-8