Abstract
Background
Azacitidine is an hypomethylating agent widely adopted for the treatment of acute myeloid leukaemia (AML) in patients who are ineligible for curative-intent chemotherapy. Patients with low bone marrow blast counts (< 30%) experience improved survival with azacitidine, but the benefits are significantly lower in patients with > 30% blasts in the bone marrow. As such, there is uncertainty around the economic benefit of azacitidine in patients with higher blast counts.
Objective
We present a cost-utility analysis of azacitidine in patients with AML with > 30% blasts to determine the economic value of azacitidine in this patient population from the perspective of a third-party payer.
Methods
A Markov model was developed with a time horizon of 25 months divided into 22 cycles of 35 days each. The cost utility of azacitidine was compared with that of conventional care regimens (which include best supportive care, low-dose cytarabine and induction chemotherapy). A Canadian public healthcare system perspective was selected.
Results
In the base case, the incremental cost per quality-adjusted life-year gained (incremental cost-effectiveness ratio [ICER]) for azacitidine compared with conventional care regimens was $Can160,438, year 2018 values. The estimated ICER was insensitive to a longer time horizon but sensitive to the cost of azacitidine and to assumptions relating to survival in both treatment regimens, although the ICER always remained greater than Can$80,000 in all scenarios.
Conclusion
Azacitidine is unlikely to be cost effective given that the estimated ICER exceeds the willingness to pay commonly used in the Canadian healthcare system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Azacitidine offers low value for money in the treatment of acute myeloid leukemia in patients who have more than 30% blasts compared to those who have lower blast counts. |
1 Introduction
Acute myeloid leukaemia (AML) is the most common form of acute leukaemia in adults, with a median patient age of 65 years [1]. It is characterized by the proliferation of malignant cells, called blasts, in the bone marrow. The standard treatment for AML involves repeat cycles of anthracycline and cytarabine chemotherapy, often followed by allogeneic stem cell transplantation in higher-risk individuals. This curative-intent treatment is associated with significant mortality and morbidity, with prolonged hospitalization, and is deemed too risky in older patients, defined in most studies those aged > 65–70 years.
No universally accepted standard treatment exists for older patients with AML. The goal of care in these patients is usually palliative, with a focus on quality of life (QOL) and extending lifespan. Until recently, the conventional care regimen (CCR) in older patients was a choice between (1) standard chemotherapy (similar to that offered to younger patients), (2) low-dose chemotherapy or (3) best supportive care (BSC), consisting of transfusion, treatment of infections and the use of drugs such as hydroxyurea to control leucocytosis. The prognosis among elderly patients is very poor, with a median survival of 3 months and survival of 2 years in < 10% of patients [2, 3].
Azacitidine is a pyrimidine nucleoside analogue of cytidine that targets the aberrant DNA methylation seen in AML. It has been approved for AML treatment in most jurisdictions for patients who are ineligible to receive standard chemotherapy by virtue of their age, comorbidities or personal preference [4].
Treatment with azacitidine is labour intensive; it is given in a medical daycare unit via subcutaneous injection daily for 7 days per cycle, repeated every 4 weeks. The treatment is continued until progression of disease or based on patient’s preference. Azacitidine is generally well-tolerated; its most common side effects include fatigue and gastrointestinal complaints [5]. Improvement in haematological profile is slow and typically takes a minimum of 4 months. Patients undergo a bone marrow aspiration at 6 months, and those who demonstrate either stable or improved disease control are eligible to continue treatment. Non-responders discontinue treatment and are offered BSC.
1.1 Evidence for the Use of Azacitidine in the Treatment of Acute Myeloid Leukaemia (AML)
Dombret et al. [5] published the AZA-AML-001 trial, a phase III, multicentre, international, randomized controlled trial comparing azacitidine and CCR in patients with newly diagnosed AML. That study relied on the former World Health Organization definition of AML, with > 30% blasts in the bone marrow. In total, 445 elderly patients (> 65 years) were randomized to receive either azacitidine or CCR. Patients were followed for a median of 24.4 months, and those receiving azacitidine experienced improved median overall survival (OS; 10.4 vs. 6.5 months, hazard ratio 0.85; 95% confidence interval 0.69–1.03, respectively) and improved 1-year survival rates (46.5 vs. 34.2%). Health-related quality of life (HRQoL) was assessed using the European Organisation for Research and Treatment of Cancer Core Quality of Life Questionnaire (EORTC QLQ-C30). Azacitidine treatment was associated with greater transfusion independence (38.5 vs. 27.6% for red blood cells and 40.6 vs. 29.3% for platelets, respectively). Azacitidine was not associated with worse HRQoL than that observed with CCR. The relative benefits of azacitidine did not extend beyond 25 months, when the survival curves between the two arms merged, suggesting the benefits from azacitidine treatment are derived only during the first 2 years and that the assumption of proportional hazards is unlikely to be justified in this setting. Thus, health gains from azacitidine relate primarily to the accumulated life expectancy gains during the first 2 years, with no life expectancy gains beyond this time horizon.
Based on the clinical benefits of azacitidine, the drug is approved by Health Canada for the treatment of AML and funded by provincial health agencies for the treatment of AML in patients who are ineligible for high-dose chemotherapy. Most of these patients are elderly (> 65 years) [4].
1.2 Economic Analysis of Azacitidine for the Treatment of Myelodysplasia and AML
Levy et al. [6] performed an economic analysis of azacitidine in the treatment of both myelodysplasia and AML and found that azacitidine for patients with AML was within the willingness-to-pay threshold for third-party public payers for patients with AML in Canada, with an incremental cost-effectiveness ratio (ICER) of $Can86.182 per quality-adjusted life-year (QALY) [6]. However, the Levy et al. [6] study results were only valid for patients with < 30% blasts in the bone marrow, since patients with > 30% blast counts in the blood marrow were excluded from this study. Since these patients do not derive the same clinical benefit as patients with lesser blast counts [5], it is important for third-party payers to determine whether these patients derive the best value for money. This is particularly relevant since most elderly patients with AML have > 30% blasts in the bone marrow. This study proposes to address the decision problem facing policy makers by evaluating the economic value of azacitidine in patients with > 30% blasts, thereby informing third-party payers on reimbursement decisions for this patient population.
2 Method
2.1 Type of Economic Analysis
We performed both a cost-effectiveness analysis and a cost-utility analysis where the ICER gained was derived from estimated life-years and QALYs gained and direct healthcare costs. Costs are expressed in Canadian dollars, year 2018 values. The cost-utility analysis was performed in accordance with established guidelines for economic evaluations [7] and adopting a Canadian provincial healthcare system perspective [8].
2.2 Patient Population
The patient population from the AZA-AML-001 clinical trial [5] served as the basis for the economic analysis. Specifically, these were patients aged ≥ 65 years with a new diagnosis of de novo AML with > 30% blasts in the bone marrow who were not considered candidates for allogeneic stem cell transplantation.
Treatment with azacitidine is compared with CCR, namely the three accepted therapies for AML in transplant-ineligible patients: BSC, low-dose cytarabine (LDAC) and standard-dose chemotherapy (SDC). In the AZA-AML-001 trial, patients were first assigned by their treating physician to receive the CCR that best suited them. Patients were then randomized in a 1:1 ratio to receive either azacitidine or the CCR to which they had been assigned. Patients remained stratified according to their pre-randomization arm to ensure that similar patient groups were compared.
2.3 Model Structure and Outcomes
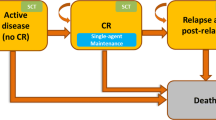
We developed a Markov model to evaluate the health outcomes and costs of azacitidine compared with each CCR option (Fig. 1). The model was developed using Microsoft Excel and underwent verification and validation consistent with recommendations by Vemer et al. [9] and the International Society for Pharmacoeconomics and Outcomes Research Task Force [10]. Hypothetical patients entered the model in the AML state (> 30% blasts in the bone marrow) at age 69 years, thus matching the AZA-AML-001 population and transited either to complete remission (CR) or death (absorbing state). Patients in CR could in turn transit back to the AML state or progress to the absorbing state (Fig. 1). Response evaluations in the AZA-AML-001 trial were scored as per the International Working Group in AML [11], with CR defined as ≤ 5% blasts in the bone marrow with absolute neutrophil counts ≥ 1 × 109/L and platelets ≥ 100 × 109/L.
The primary endpoints were OS, QALYs and direct medical costs and are expressed as ICERs. OS and QALYs were selected as outcomes since they are most clinically relevant in patients with AML.
In the model, patients were assumed to receive treatment for AML until the end of the treatment cycle, after which they could either remain on treatment or have the treatment put on hold (Fig. 1).
2.4 Efficacy, Safety and Adverse Events
The base-case model inputs and probability distribution are shown in Table 1. Probabilities of outcomes in each treatment arm were derived from the AZA-AML-001 clinical trial [5]. The AZA-AML-001 study enrolled 488 patients with de novo AML; these patients were then followed for a median of 24.4 months [5].
The transition probabilities to the absorbing state of each cycle were extracted from the Kaplan–Meier curves of the AZA-AML-001 study using the WebPlot Digitizer web app v3.10 [12] [see Electronic Supplementary Material (ESM) 1]. The transition probabilities from the time of randomisation to the CR state and from the CR to the AML state for both the azacitidine and the CCR arms were derived from the AZA-AML-001 randomized study comparing the two arms [5] (see ESM 2 for details). The measurement of the transition probabilities was made relative to the randomisation and not from the time of diagnosis since the delay between diagnosis and randomisation was not available to the authors.
For the model, we assumed that patients with AML treated with azacitidine transitioned to the CR state between cycles 4 and 6. This reflected what has been documented in the literature [13, 14]. To reflect clinical experience, in the CCR arm, we assumed that only patients who received induction chemotherapy could achieve CR; only 2–3% of patients treated with LDAC achieved CR, and none of the patients managed with BSC achieved CR [15]. In the model, the transition to a CR state was assumed to occur between cycles 2 and 6. This assumption was made based on the observation that patients assigned to receive induction chemotherapy most commonly need to receive sequential cycles of chemotherapy before achieving remission and that the treatment cycles are frequently delayed by 1–3 months (corresponding to the duration of 1–3 cycles). This implies that patients assigned to the induction chemotherapy arm had a chance of achieving CR only after 2 cycles until cycle 6.
Baseline utility values were obtained from results presented in the AZA-AML-001 study [5]. The subsequent changes in utility scores were obtained after converting the EORTC QLQ-C30 scores collected alongside the AZA-AML-001 clinical trial at cycles 3, 5, 7 and 9, thus ensuring optimal internal validity. Moreover, trial participants included adult Canadian patients with AML with > 30% blasts. These health scores were converted to utility scores using the mapping algorithm described in McKenzie and van der Pol [16]. We conducted a linear regression analysis to derive the impact on utilities of both treatment arms and whether the patient achieved complete response (see ESM 3). This allowed estimation of the utility values for the whole time horizon of the model based on the proportion of patients for each treatment that remained with complete response. Adverse events were not specifically modelled since they were explicitly included in the QOL questionnaire.
2.5 Costs
Direct medical costs were adjusted to year 2018 values using the Bank of Canada inflation calculator [17]. Healthcare costs were derived from the Levy et al. [6] study, in which the health costs were derived from a Canadian panel of four clinical experts who estimated the costs associated with health resource utilization borne to the Ministry of Health and Long-Term Care in Ontario, Canada. These costs included medication, human health resource, routine physician follow-up, hospitalization and laboratory investigations. The cost of 1 cycle of azacitidine in year 2018 values was estimated at $Can8701.25, with a unit price of azacitidine of $Can662 (average dose of 1.34 vials; Celgene Ltd). For the CCR comparators, the following assumptions were made: BSC is exclusively received as an outpatient, LDAC is administered 50% of the time by an outpatient clinic nurse and induction chemotherapy is received exclusively as an inpatient [6].
2.6 Sensitivity Analysis
Uncertainty in model parameters was characterized by probability distribution using simulations, and 5000 iterations were used to include parameter uncertainty in the results. In addition, to examine the effects of individual parameter uncertainty, one-way sensitivity analysis varying each parameter included in the model was performed, with parameters varied by 20%, giving a range of values that we felt was reasonable. Results of the one-way sensitivity analysis are presented in a tornado diagram.
2.7 Time Horizon
The cycle duration was 35 days, corresponding to the average treatment cycle duration in the AZA-AML-001 clinical trial. A time horizon of 25 months was selected for our base case as the Kaplan–Meier survival curves for azacitidine compared with CCR converged at this point, with no difference in survival beyond this time horizon. Thus, expanding the time horizon is unnecessary as it likely would have little impact and introduce greater uncertainty because of the need to extrapolate beyond the known clinical data [5]. However, we conducted a further analysis to assess the impact of adopting a lifetime or 10-year horizon using survival data beyond 25 months from the same study. For this analysis, an exponential model was fitted to the data between 24 months and 28 months and then used to extrapolate for the estimation of lifetime outcomes. The exponential model provided the best fit for the available data. The analysis found both no difference in survival between the two regimens post 24 months and a consistent probability of death across the time period (see ESM 4).
The model assumed that patients received the assigned treatment until the end of the treatment cycle, after which they could either remain on treatment or have the treatment put on hold (Table 2).
Costs and benefits are discounted at 1.5% per annum, with sensitivity analysis adopting rates of 0 and 3% [8].
3 Results
3.1 Base-Case Analysis
Table 3 presents the results of the probabilistic base-case analysis comparing azacitidine with CCR. The incremental cost per life-year gained was $Can96,755, and the ICER per QALY gained was $Can160,438. The purchased cost of the treatment drug accounted for most of the incremental cost of the treatment with azacitidine compared with CCR.
At a willingness-to-pay threshold of $Can100,000, the probability that azacitidine was cost effective was only 11%; at a willingness-to-pay threshold of $Can50,000, the probability was < 1% (Fig. 2). This probability increased to 50% at a willingness-to-pay threshold of $Can160,000.
3.2 Sensitivity and Scenario Analysis
Figure 3 is a tornado diagram presenting the results of the univariate analysis. Only the variables that affected the ICER by > 2% are illustrated. The ranges for the univariate sensitivity analysis included the 20% change for the clinical, utility and cost parameters. The ICER was highly sensitive to the cost of azacitidine, ranging between $Can103,709 and 217,167 for azacitidine compared with the ranges seen with variations in the cost of the CCR treatment options (SDC $Can109,711–184,248; LDAC $Can131,921–160,422). There was only a very small variation in the ICER with changes in the cost of BSC ($Can159,190–161,708). The probability of death in both the CCR and the azacitidine arm was associated with a significant impact on the ICER, with a range of $Can101,240–220,902 for azacitidine and $Can99,853–215,605 for CCR.
Univariate sensitivity analysis. The ICER is most sensitive to the therapeutic effects of azacitidine and the CCR. It is also quite sensitive to the cost of azacitidine and to the utilities in both the azacitidine and conventional care regimen. Legend: Prob Dying CCR probability of death when treated with CCR, Prob Dying AZA probability of death when treated with azacitidine, Cost AZA cost of treatment with azacitidine; Cost CCR: conventional care regimen
A change in the baseline utility value at the time of diagnosis was associated with large ICER variations of $Can132,679–202,884 for azacitidine and $Can134,983197,735 for CCR. Variations in the transition probability to a CR state, the various utility gains for the various treatment options, cost per cycle of treatment and probability of being off treatment were not associated with significant variations in the ICER.
The scenario analysis considering a lifetime horizon found only a small difference in the estimated ICER, with the incremental cost per QALY gained for azacitidine versus CCR increasing to $Can160,373 (Table 3). The probability that azacitidine was cost effective at willingness-to-pay thresholds of $Can50,000 and 100,000 were 0% and 15%, respectively.
4 Discussion
This is the first study to investigate the economic value of azacitidine in adult patients with AML who are ineligible for transplant and who have > 30% blasts in the bone marrow. In Canada, thresholds of around $Can50,000–140,000 per QALY gained have typically been observed with respect to reimbursement decisions for pharmaceuticals [18,19,20]. This study found that azacitidine has an ICER of $Can160,438 per QALY gained compared with CCR and, as such, is above the typically accepted willingness-to-pay threshold for a QALY in Canada.
Our results complement those of Levy et al. [6], where the effect of azacitidine was measured against the same comparator in patients with AML. However, in the Levy et al. [6] study, patients had < 30% blasts in the bone marrow, and azacitidine had better economic value than that found in the present study, with a lower ICER of $Can86,182 per QALY gained. The significantly lower economic value of azacitidine in patients with > 30% blasts in the bone marrow reflects the lower clinical efficacy of the drug among patients with higher blast counts. Our study provides a better understanding of where azacitidine is placed as a therapeutic option in patients with higher blast counts in the bone marrow, with a decrease in the economic value of azacitidine in patients with higher blast counts. Studies to stratify populations that would benefit from azacytidine would be helpful. The increased ICER seen in the higher-risk patient population with AML reflects a common trend in oncology, where therapies usually offer lower clinical effectiveness with higher-risk disease. This highlights that third-party payers involved in price negotiations and health technology assessment agencies formulating funding recommendations should consider the fact that the cost effectiveness of therapies can vary for a given disease as a function of specific disease characteristics affecting the risk features.
A major strength of this study is that the parameters used in the model for transition probability for OS and utility scores were derived from a randomized controlled trial that influenced clinical practice. AZA-AML-001 was a methodologically sound, multicentre phase III study in which the same comparators were tested in a patient population that reflected both the Canadian clinical setting [5] and what was modelled in this study. Extending the time horizon beyond 25 months was not expected to change the base results from this study. The utility scores used in this model were mapped from HRQoL data collected alongside the AZA-AML-001 study. Furthermore, the unit costs used in this study reflected those that are relevant to the third-party payer system in the Canadian setting. This ensures both the internal and the external validity of the study and confirms the relevance of the results of this study for third-party payers.
A set time horizon of 25 months was chosen in this study as opposed to a longer time horizon typically seen in pharmacoeconomic studies because the trial found the therapeutic impact of azacitidine on OS was limited to the first 25 months after treatment initiation, after which no statistically significant impact on survival was found [5]. As such, the survival outcomes in this study were derived from the Kaplan–Meier curve, and we had no need to rely on parametric extrapolation, reducing the uncertainty around the therapeutic impact of azacitidine and eliminating the uncertainty inherent when extrapolating results beyond those collected in the clinical trial. A scenario analysis adopting a lifetime horizon found only a modest increase in the estimated ICER, suggesting no change to the interpretation of the study results.
The results in this study are most sensitive to the probability of death in both the azacitidine and the CCR arms, reflecting the lower therapeutic benefit derived from treatments among patients with a higher percentage of blast counts and who are deemed to have higher-risk myelodysplasia. The results were also highly sensitive to the costs of azacitidine, so a substantially discounted price can alter the ICER enough to make azacitidine treatment more attractive in that patient population. However, in all instances, the incremental cost per QALY gained associated with azacitidine remained greater than $Can80,000 per QALY gained.
One of the limitations of the study is that the resource utilization costs were derived from a survey [6]. We chose this source because no other valid source was available from which to extract costs. Further research will be necessary to better define the cost structure associated with the treatment of AML in the Canadian setting. We assumed in the model that the transition probabilities to CR between cycles 4 and 6 for azacitidine and between cycles 2 and 6 for CCR remained constant between these intervals. We acknowledge that this is unlikely between consecutive cycles over time, but we are not aware of available data that could help us better refine these transition probabilities, and varying the transition probabilities to CR and loss of CR in the sensitivity analysis did not have an impact on the ICER.
The measurement of the transition probabilities was made relative to the time at randomisation since the delay between diagnosis and randomisation was not available to the authors. Although this measurement should ideally have been made relative to the time at diagnosis, this discrepancy is unlikely to have had a significant impact on the results since AML is typically managed urgently [21], within days, so the time at diagnosis and treatment are very close together.
It should be noted that uncertainty exists over the appropriate willingness-to-pay threshold for a QALY within the Canadian public healthcare system. Advisory pan-Canadian bodies such as the pan-Canadian Oncology Drug Review and the Committee to Evaluate Drugs do not approve drugs for coverage but provide recommendations to provincial ministries. Evidence suggests that such ministries adopt a much lower threshold of between $Can40,000 and 60,000 and that a true threshold representing the opportunity cost of funding new technologies may be as low as $Can30,000 per QALY if Canada were similar to other jurisdictions [19].
This paper leaves some important questions unanswered. The cost effectiveness of azacitidine was compared with the aggregate of the CCR, but azacitidine was not compared with the individual CCR options. In our opinion, the outcome data available from the Levy et al. [6] clinical trial or elsewhere in the literature do not allow for a comparative analysis on the individual strategies of BSC, low-dose cytarabine and induction chemotherapy. Given that the individual CCR options are associated with different costs and effects, it would be valuable to articulate their individual economic and clinical value and refine recommendations for funding and treatment strategies. We have commented on the ICER of azacitidine with patients with > 30% blasts; it would be valuable to conduct studies to stratify populations of patients with AML according to clinical risk factors and response to azacytidine to better inform third-party payers.
5 Conclusion
This study examines the value for money of azacitidine in patients with AML with > 30% blasts in the bone marrow compared with CCR from the public payer perspective. Azacitidine was a significantly less valuable option at the current price compared with CCRs, which brings into question the value of publicly funding this therapeutic option for this patient population. The number of available therapeutic options for non-curative-intent patients with AML are increasing, and these results will be helpful in determining how azacitidine is placed in the management of patients with AML.
Data Availability
The excel file used to generate the model is available for review. The model was developed using Excel (2016, v. 16.0). The data were extracted from Dombret et al. [5].
References
Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341(14):1051–62.
Kantarjian H, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116(22):4422–9.
Frohling S, et al. Cytogenetics and age are major determinants of outcome in intensively treated acute myeloid leukemia patients older than 60 years: results from AMLSG trial AML HD98-B. Blood. 2006;108(10):3280–8.
Health Canada, Drug Product Database online query. 2019. https://health-products.canada.ca/dpd-bdpp/dispatch-repartition.do.
Dombret H, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with > 30% blasts. Blood. 2015;126(3):291–9.
Levy AR, et al. Cost-effectiveness in Canada of azacitidine for the treatment of higher-risk myelodysplastic syndromes. Curr Oncol. 2014;21(1):e29–40.
Garrison LP Jr, et al. Performance-based risk-sharing arrangements-good practices for design, implementation, and evaluation: report of the ISPOR good practices for performance-based risk-sharing arrangements task force. Value Health. 2013;16(5):703–19.
Lee KM, McCarron CE, Bryan S, Coyle D, Krahn M, McCabe C. CADTH methods and guidelines: guidelines for the economic evaluation of health technologies, 4th edn. Canada: CADTH; 2017. https://www.cadth.ca/dv/guidelines-economic-evaluation-health-technologies-canada-4th-edition.
Vemer P, et al. AdViSHE: a validation-assessment tool of health-economic models for decision makers and model users. Pharmacoeconomics. 2016;34(4):349–61.
Weinstein MC, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR task force on good research practices-modeling studies. Value Health. 2003;6(1):9–17.
Cheson BD, et al. Revised recommendations of the International Working Group for Diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21(24):4642–9.
Rohatgi A. WebPlotDigitizer; HTML5 based online tool to extract numerical data from plot images. Version 3. 2012. WWW document URL http://arohatgi.info/webPlotDigitizer/. Access Jan 2017.
Fenaux P, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223–32.
Fenaux P, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28(4):562–9.
Cortes JE, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33(2):379–89.
McKenzie L, van der Pol M. Mapping the EORTC QLQ C-30 onto the EQ-5D instrument: the potential to estimate QALYs without generic preference data. Value Health. 2009;12(1):167–71.
Bank of Canada. Bank of Canada Inflation Calculator. 2018. https://www.bankofcanada.ca/rates/related/inflation-calculator/.
Lemieux J, Audet S. Value assessment in oncology drugs: funding of drugs for metastatic breast cancer in Canada. Curr Oncol. 2018;25(Suppl 1):S161–70.
Sapsfort R. A vast injustice. Ombudsman report. 2009. https://www.ombudsman.on.ca/Files/sitemedia/Documents/Investigations/SORT%20Investigations/avastinweb-en_1.pdf.
Skedgel C, Wranik D, Hu M. The relative importance of clinical, economic, patient values and feasibility criteria in cancer drug reimbursement in canada: a revealed preferences analysis of recommendations of the Pan-Canadian Oncology Drug Review 2011–2017. Pharmacoeconomics. 2018;36(4):467–75.
Ostgard LS, et al. Impact of chemotherapy delay on short- and long-term survival in younger and older AML patients: a Danish population-based cohort study. Leukemia. 2014;28(9):1926–9.
Acknowledgements
The model used in this study was provided to the journal’s peer reviewers for their reference when reviewing the manuscript. The authors thank Dr. Nisha Thampi for reviewing the manuscript.
Author information
Authors and Affiliations
Contributions
PAJV and DC designed and build the model, conducted the analyses and interpreted the data. Both authors contributed to the drafting of the manuscript and approved the final version for submission.
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
The authors, D. Coyle and P. Villeneuve declare that they have no conflict of interest.
Ethical approval
No formal consent was required for this study. The study was not submitted to an independent ethic committee or institutional review board.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Coyle, D., Villeneuve, P.J.A. Economic Evaluation of Azacitidine in Elderly Patients with Acute Myeloid Leukemia with High Blast Counts. PharmacoEconomics Open 4, 297–305 (2020). https://doi.org/10.1007/s41669-019-00180-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-019-00180-z