Abstract
In light of the evolving nature of various diseases, time becomes a crucial factor in diagnosis and identifying the underlying causes. A point-of-care device provides a rapid diagnosis of a disease without using complex and advanced instruments, which are costly and difficult to transport. A paper-based device is a relatively frugal solution wherein the paper is used as a substratum in which the reactions are carried out. These methods are non-invasive, and the sample collection is relatively easy. Saliva is one such body fluid in which various biomarkers are present for numerous diseases. Bioanalysis of saliva has attracted more attention recently due to its non-invasiveness and robustness. Exploiting the discovery of clinical biomarkers from salivary analysis has the potential to revolutionize the healthcare sector by providing crucial information to monitor the health status of individuals and disease progression which enables personalized treatment. This review provides the limitation of the traditional methods in clinical applications and highlights the significance of saliva as a non-invasive biological fluid that is a source of multiple biomarkers associated with various diseases. It also provides insights into the different paper-based colorimetric microfluidic devices developed against salivary biomarkers in the past decade. The major challenges in the point-of-care application and the future prospects have been discussed as well. Further, we also emphasize the importance of this approach in dental disease diagnosis which is least explored and holds potential applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Clinical diagnosis and health monitoring are critical parts of healthcare management, which incites the treatment decision and track of a patient’s health progress [1]. The accuracy and efficiency of clinical diagnosis have improved significantly due to advancements in medical technology. A wide range of diagnostic tools such as blood tests, imaging, wearable devices, remote monitoring, and real-time electronic health records have been developed [2]. Though these tools are more accurate and qualitative, the analysis is time-consuming, requires laboratory setups, expensive sophisticated equipment, and well-trained professional operators. Rapid analysis is a critical factor that could facilitate the timely treatment of patients. Modern biosensor technology tools overcome these limitations [3]. One such potential diagnostic tool that provides prompt and reasonable precise outcomes is paper-based microfluidics [4]. Paper-based devices have numerous benefits, including their affordability, user-friendliness, rapidness, reliability, portability, and most significantly, their biocompatibility [5,6,7,8,9,10,11,12,13,14,15,16]. In addition, thread-based devices were also leveraged for wide range of sensing applications [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]. Generally, invasive and non-invasive are the two types of clinical sample collection methods that are used for bioanalysis [36]. Invasive techniques involve penetration to the skin and body tissues. They have their own set of disadvantages, which include discomfort to the patient, time taken for wound healing in case of incision, potential puss formation and development of infection in the cut area, time taken for result analysis, cost and economics of the equipment used to analyze the sample and poor ease of use [37,38,39,40]. These disadvantages create a void to be filled using a fast, effective, and reliable detection technique where non-invasive sampling and paper-based devices play a significant role [41]. In non-invasive sampling, the samples are collected from the patient without penetrating the dermis or skin layer. The sample collection is usually from saliva, urine, gums and gingival crevicular fluid (GCF), tears, and sweat. Overall, the patient samples containing the biomarkers to be detected undergo appropriate diagnostic procedures, and their levels are compared to those of normal levels. Additionally, to diagnose a health status or condition, healthcare professionals leverage other monitoring and imaging techniques. These include heart rate monitoring, electrocardiograms, blood pressure tracking, magnetic resonance imaging (MRI Scans), computed tomography (CT scans), and other methods [42,43,44,45,46,47].
In general, various conventional methods are used for detecting biomarkers including Electrophoresis [48], Chromatography [49], polymerase chain reaction (PCR) [50], enzyme linked immunosorbent assay (ELISA) [51], Spectrophotometry [52], Chemiluminescence [53], and Electrochemical methods [54]. These methods are the most accurate and reliable in determining the concentration of the biomarkers. Chromatography uses preferential separation of compounds in determining the desired biomarker [55], whereas electrochemical methods use change in the electrical signals in the presence of the target biomarker and signal change is utilized for the quantitative detection of the biomarker [56]. Chemiluminescence and spectrophotometry involve the emission and absorption of light. In chemiluminescence, the biomarker is usually allowed to react with another luminophore that resulting in light emission [57], which is detected by the light sensor. Spectrophotometry involves the determination of compounds using their specific absorption wavelength [58]. ELISA is an immunoassay where the emission of light marks the detection of the compound due to the binding of fluorescent-tagged antibodies to the biomarker [59, 60]. Despite their accuracy and reliability, conventional methods have several drawbacks, including time-consuming processes, high costs, inconvenience, and challenges in transporting and storing samples. Additionally, the required instruments for analysis are often available only at specialized facilities, making the process even more cumbersome [61].
Saliva is a viscous fluid that is secreted by the salivary glands, which are present under the jaw and near the ear [62]. The fluids produced by salivary glands help with the digestion and breakdown of food. They also protect the mouth's lining from becoming dry and eliminate any harmful bacteria that may enter through the mouth by using peroxidases and enzymes [63, 64]. Saliva consists primarily of water with various enzymes including lipases, lysozyme, amylase, maltase etc. [65, 66]. Saliva has an average pH of 6.7, making it slightly acidic. The change in the pH could also be an indicator for diseases such as gingivitis [67]. Saliva also consists of a relatively large number of white blood cells and other electrolytes and antibodies such as IgA [68]. Saliva and urine are two of the most convenient and effective bodily fluids for non-invasive diagnosis, as they both contain numerous biomarkers that are associated with different diseases and their stages. When the levels of biomarkers in the blood increase, they get secreted in the saliva, and hence saliva also contains biomarkers from the blood such as certain viruses, glucose, and lactate [69, 70]. In addition, saliva is constantly secreted, making saliva the most viable source for collecting samples for disease diagnosis using non-invasive methods [71]. In spite of many advantages, using saliva as a biofluid for sample collection has some drawbacks. One of them is the safety of the medical practitioner who handles the sample, as it may be contaminated with a bacterial or viral disease [69]. Proper disposal of the used sample after diagnosis is essential. Improper disposal methods lead to contamination. In cases where patients experience excessive salivation, the collected sample may not be suitable for diagnosis due to the potential for significant dilution of their saliva. The schematic illustration showing invasive and non-invasive methods for sample collection, different salivary biomarkers and conventional methods for detecting the salivary biomarkers is given in Fig. 1.
In recent years, microfluidic paper-based analysis devices (μPADs) have seen remarkable development. Compared to conventional tests, they are economical and can be used to diagnose various diseases [72]. Paper-based microfluidics system that consists of patterned paper, with small volumes of fluid moving through multi-channel designs within the paper substrate, using capillary action [73,74,75,76]. They are compatible with testing biological samples such as serum, urine, whole blood, nasal swabs, and saliva [77, 78]. Paper-based microfluidics use various approaches for detection, namely, colorimetric and electrochemical methods [79,80,81,82,83]. Other methods of detection include electrochemiluminescence and chemiluminescence [84, 85].
The colorimetric method is widely used for detecting myriads of analytes. This approach involves observing a color change when the desired analyte is present [86]. Colorimetry is arguably the simplest form of detection as it does not involve the requirement of any complex instruments to detect the change observed. The ‘color change’, aspect of colorimetry is defined by the laws of physics and the color observed is a result of an electron transition from a higher state to a lower state. The tuning and construction of the color detection algorithm however is a complex process as the creators need to specify the hex value range in which the application should output a set of defined remarks for those ranges. The software not only needs to be able to distinguish color but also be able to detect the intensity of the output which can convey the quantity of the compound that is detected. In colorimetry, the color change is initially plotted on a graph to determine a standard concentration slope, this concentration slope is obtained by testing a set of linearly varying concentration ranges. The standard curve obtained by this method is also known as the calibration curve, using which the detected output is quantified. The minimal concentration of the analyte that can be detected using a device is termed as its limit of detection, below which there would be no visible or significant color change observed. In most devices, the limit of detection is usually less than the lower range specified. This is done to eliminate false positive results that may arise due to impurities present in the sample.
There are two distinct approaches available for creating μPADs; they are physical deposition and chemical modification, respectively. Standard manufacturing methods include polydimethylsiloxane (PDMS), wax printing, inkjet printing, and laser cutting. Other manufacturing methods, such as thermal embossing, photolithography, and hydrophobic silylation, are also present [87, 88]. According to the World Health Organization (WHO), there is an urgent need for diagnostic methods that are not only affordable but also sensitive, specific, user-friendly, robust, rapid, equipment-free, and deliverable to end-users (ASSURED Criteria). Among the various available diagnostic tools, paper-based analytical devices (PADs) have emerged as an up-and-coming option. This is because PADs can meet all the aforementioned criteria, providing an efficient and convenient solution for diagnostic testing. In addition to their ease of use, PADs also offer the advantages of low manufacturing costs and easy disposal, making them an increasingly popular choice in the field of diagnostics [89]. This review article focuses on the application of paper-based devices that deploy colorimetric methods for detecting biomarkers from the salivary fluid. Lateral flow devices for detecting biomarkers in saliva have not been discussed in this paper as it is beyond the scope of the paper, and readers of this article are advised to lean towards other review papers regarding the information on different testing methodologies [90].
2 Detection of Biomarkers Using Paper-Based Systems
Biological marker or Biomarker is a collective term used for diagnosing any disease or condition in the human body. Biomarkers have many types, such as biochemical, pathogenic and single molecule or ions. These biomarkers are used for diagnosing oral diseases and early prognosis of other conditions such as cancer and periodontitis in the human body. Biochemical markers are chemicals or molecules that are produced or released at the time of disease, either at the initiation of the disease or during its progression [91]. These markers are critical indicators as their levels in the body rise or fall from the normal levels during illness. By measuring the levels of specific biomarkers, both the individual and practitioner can determine the appropriate countermeasures to take. Most of the biochemical biomarkers are present in the blood, while some can be found in tissues of tumors, stool, saliva, and urine [92]. Biomarker diagnosis from urine and saliva is the most easy and non-invasive sampling. Herein, the potential biomarkers were identified from the salivary samples using paper-based assay systems. Various biomarkers such as thiocyanate, nitrite, ammonia, cyanides, aldehydes, lactoferrin, and lactates are specific to wide range of diseases. In this section, we highlight different colorimetric detection approaches used for detecting aforementioned biomarkers (Table 1).
2.1 Thiocyanate
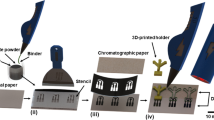
Thiocyanate, or SCN− is a conjugative base of thiocyanic acid. It is an anion, and it functions as a human metabolite [92]. It is often found in human urine and saliva samples and can be used as a biomarker to indicate different disease diagnoses and progression. Thiocyanate is one entity of the dissociation products of glycosinolates and cyanide [93]. Thiocyanate also functions in the host defence mechanism by being a part of the lactoperoxidase microbicidal pathway [94]. Since thiocyanate is an anion, it combines with other cations, such as ammonia, sodium, potassium, etc., to form ionic compounds that may negatively affect the body. For example, prolonged and repeated exposure to ammonium thiocyanate is found to cause nausea, headache, and vomiting. Potassium thiocyanate can cause headaches and, if inhaled, can lead to respiratory tract infection. The presence of thiocyanate in saliva has been associated with different diseases and could serve as a promising biochemical biomarker for detecting them. Paper-based microfluidics provides easy and cheap detection of thiocyanate, and many papers have been published linking thiocyanate to disease progression and their detection using paper-based microfluidics. Pena-Pereira F et al. successfully developed a colorimetric assay for in situ detection of thiocyanate from saliva. The assay uses Fe(NO3)3 as a reagent, when it reacted with thiocyanate in the presence of nitric acid, Fe(NO3)3 turns into Fe(SCN)3 [iron(III)-thiocyanate], a red-colored complex. The wax printing method was used to prepare the hydrophobic areas, and the detection limit was 0.06 mmol/L of thiocyanate [95]. Jirayu Sitanurak et al. improved the economy of mass-producing these paper-based devices by preparing a new and cheap method for printing the hydrophobic barrier in Whatman filter paper using T-shirt ink (Fig. 2). The reagent used was Fe(NO3)3, and the detection limit for thiocyanate was 1 mmol/L [96].
Protocol for simultaneous detection of thiocyanate and nitrite in artificial saliva (reproduced with permission from Ref. [96].
Yu et al. used spraying-based technology for the fabrication of the device. The filter paper was seated on top of a patterned acrylic plate, and later the lacquer was spray painted on the filet paper forming a pattern of the plate. Separate bottom and top layers were fabricated simultaneously, and then they were sandwiched together to form a 3D layer. Ferric nitrate was utilized for the detection, and the limit of detection was 0.074 mmol/L [97]. In another study, Pungjunun et al. developed an improved μpumpPAD, a microcapillary grooved device engraved by LASER capable of dual-mode sensing using both colorimetric and electrochemical detection. These devices have a more comprehensive range of thiocyanate detection, and for colorimetric-based detection, Fe(NO3)3, was used and the limit of detection was 6 μmol/L [98]. The schematic illustration showing the μpumpPAD assembly and assay procedure of μpumpPAD is given in Fig. 2.
2.2 Nitrite
Nitrite is an intermediate in oxidative and reductive processes such as nitrification and denitrification [99]. Nitrite is generally accepted to be the compound responsible for the preservation of foods along with other compounds like table salt. Long-term studies in mice have shown that feeding on a nitrite-deficient diet would lead to metabolic syndrome and cardiovascular death after 22 months [100]. Oral bacteria can convert nitrate into nitrite, which can be absorbed into the digestive system. Nitrites, which are linked to various diseases, can be detected as biomarkers in blood, urine, and saliva samples. Prolonged nitrite exposure has almost similar symptoms to thiocyanate, including nausea, vomiting, headache, and abdominal cramps. Nitrite can be easily detected by Griess Reagent, which is a mixture of naphthyl ethylenediamine dihydrochloride suspended in water and sulphanilamide in phosphoric acid, and a lot of paper-based microfluidic devices have been formulated based on that.
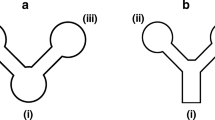
Bhakta et al. is the pioneer in colorimetric detection of nitrite using paper based microfluidic devices. They used wax printing to form the hydrophobic layer and used Griess Reagent to detect nitrite, which turns into a dark pink color when interacting with nitrite. The observed limit of detection was 10 μmol/L [101]. Jayawardane et al. developed the microfluidic device with inkjet printing instead of wax printing (Fig. 3a). They also studied the functionality of the device at different temperature conditions and concluded that it could work in various temperatures ranging from room temperature to – 20 οC. The optimum time allowed for color formation was found to be 5 min using Griess reagent and the limit of detection was 1 μmol/L [102].

Copyright 2014, American Chemical Society). b The schematic illustration showing the μPAD for (1) nitrite, (2) nitrate detection and (3) device after sample placement. The layers are indicated as follows: L1 (top layer of the laminating pouch), L2 (bottom layer of the laminating pouch), E1 (empty layer), G1 (Griess reagent layer), Z (zinc embedded layer), E2 (empty layer), and G2 (Griess reagent layer) (reproduced with permission from Ref. [103]. Copyright 2020, Elsevier)
a Schematic representations of the developed (1)2D, (2) 3D microfluidic paper-based analytical devices (μPADs) and card containing one used (3) 3D μPAD. The components are labelled as follows: DZ (Detection Zone), TC (Transport Channel), RC (Reduction Channel) and IS (Interleaving Sheet, Cellulose Triacetate (reproduced with permission from Ref. [102].
Ferreira et al. improved Jayawardene’s work by developing a paper-based device that is capable of detecting 0.05 μmol/L of nitrite. They used a two-layer approach where one layer consist of Griess reagent, and the other was empty, allowing for more contact to facilitate the reaction (Fig. 3b). These layers were then laminated on both the top and the bottom, which is the most delicate step that decides the reproducibility of the device, as misalignment of the layers would vastly decrease the efficiency of the device. The sample was placed on the top layer, and the color change was photographed for quantification using a software [103]. Yu et al. simultaneously detected thiocyanate and nitrite using the same 3D microfluidic device with Griess reagent as an indicator. The limit of detection for this method was 9.6 μmol/L [97].
Jiang et al. implemented a new fabrication technique for constructing the hydrophilic pattern in the paper. After immersing it in an OTS-hexane solution, they used corona treatment on the filter paper. The device was flower-shaped and has 8 arms to facilitate the simultaneous detection of multiple salivary samples. The results of samples taken from the same person via this device and ion chromatography methods were very much comparable. The limit of detection for this device was 7.8 μmol/L [104]. Noiphung et al. developed a paper-based device using wax printing capable of detecting both pH and nitrite. The device had two arms for nitrite detection and three arms on the opposite end for pH detection. Nitrite detection was done in saliva samples of humans in Australia, and they found a linear correlation between nitrite concentration and concentration of C-reactive protein in saliva. The detection was done using Griess Reagent, and the limit of detection was 0.1 mg/dL [105]. Chiang et al. used a combination of wax printing and 3D printing technology to fabricate the paper-based device, which has multiple advantages like the ability to print any design pattern of the μPAD, higher resolution of the patterns printed and the hydrophilic channels are not vulnerable to organic solvents. Griess reagent was used for the detection of nitrite, and the limit of detection was 14.8 μmol/L [106].
2.3 Ammonia
Ammonia and other nitrogen compounds such as nitrite and nitrate are the critical nutrients from which plants get their nitrogen. Nitrogen cannot be absorbed from the atmosphere and hence it has to be given in different forms, such as ammonia. Although a small quantity of ammonia can be ingested orally, it is typically produced in the gut by bacteria that digest protein compounds to release ammonia. Ammonia has a distinctive foul-smelling odor and a sour taste. The human kidneys are tasked with eliminating ammonia from the body. However, if they are unable to perform this function, an excessive buildup of ammonia can occur, which may eventually lead to the presence of ammonia in the mouth. Elevated ammonia levels in the blood can cause severe complications like brain damage, confusion, tiredness, and even possible coma and death. Ammonia in saliva can be determined by point-of-care devices, which are helpful in the early and cheap diagnosis of any probable kidney diseases. Sheini et al. developed a colorimetric assay for detecting ammonia in biological samples such as saliva. It is an origami paper-based device consisting of silver nanoparticles capped by thiomalic acid. The reaction is a hydrolysis reaction of urea into ammonia and water, and the silver nanoparticles capped with thiomalic acid accumulated ammonia where the color of the mixture turned from yellow to brown. The limit of detection for ammonia was observed to be 0.03 mg/L [107]. Thepchuay and team worked on a paper-based device that had stacks of 3 circular disks for the detection of ammonia where one layer is the sample layer and the second had sodium hydroxide solution, a separation layer that is permeable made of PTFE, and the third layer had bromothymol blue, the detection reagent which changes color to blue when exposed to ammonia. Laser-jet printing was used to print the hydrophobic barriers, and the limit of detection for this device was 0.03 mg/L [108].
2.4 Cyanide
Cyanide is the name given to a compound that contains the functional group C≡N. The most commonly referred to substance when the term cyanide is generally used is hydrogen cyanide which has a chemical formula of HCN. Hydrogen cyanide is a toxic compound and is fatal to the organism [109, 110]. Cyanide is used in manufacturing industries to manufacture textiles, paper, and plastics and is used in pest extermination. It is a significant health concern for those who are working in these industries must be fully protected before handling cyanide [111]. Cyanide is also a bi-product produced by the organism Pseudomonas aeruginosa which gets secreted in the saliva and can be used as a biomarker for diseases using an easy, non-invasive test [112]. Lvova et al. developed a paper-based sensor to detect hydrogen cyanide in saliva. He used drop-casting on Whatman filter paper using an anion exchange process to form detection spots, using 5,10,15-tritolylcorrolate of Cobalt (III) triphenylphosphine as detecting agent. When it comes to contact with a cyanide group, this reagent change its color from red to light yellow as the concentration increases (at 6500 K temperature). The limit of detection was found to be 0.053 mg/L [113].
2.5 Aldehydes
Aldehydes are chemical compounds with the functional group CHO, where the carbon is double-bonded to an oxygen. These compounds are highly reactive and prone to cause damage to the body if not eliminated properly. Aldehyde dehydrogenase is usually involved in the metabolism of aldehyde. It oxidizes the aldehyde into carboxylic acid, which is less harmful to the body and is later metabolized by the liver and used up by the muscle cells. Blocking of the enzyme via drug uptake and constant exposure to pollution could result in aldehyde accumulation, and these further can cause damage to the cells and cause carcinogenic [114]. Hence, detecting aldehydes in saliva can be helpful in diagnosing salivary aldehyde dehydrogenase activity.
Ramdzan and team fabricated a paper-based device for the detection of aldehydes from saliva using 3-methyl-2- benzothiazolinone hydrazone and iron (III) chloride which is characterized by a blue color due to the formation of formazan dye when reacts with an aldehyde. The device had a 3D design with two overlapping layers where one consisted of 3-methyl-2- benzothiazolinone hydrazone and had 15 detection zones of 8 mm diameter each. The second layer had 15 reagent zones with a 4 mm diameter each and consisted of iron (III) chloride. The sample addition is done at the first layer. The limit of detection was 6.1 μmol/L [115]. The schematic illustration showing the development of a microfluidic paper-based analytical device for the determination of salivary aldehydes, Fig. 4.
Schematic representation of the development process for a microfluidic paper-based analytical device designed for the determination of salivary aldehydes (reproduced with permission from Ref. [115].
2.6 Lactoferrin
Lactoferrin is a protein usually found in milk and helps absorb iron from the digested food to the bloodstream. Lactoferrin from breast milk given to infants also has antimicrobial activity and helps in fighting infection [116]. Lactoferrin in saliva is responsible for the antimicrobial effect of saliva; hence a higher than usual concentration of this protein in salivary sample indicates a probability of having periodontal diseases and other oral diseases. High lactoferrin concentration in saliva can also indicate inflammatory bowel disease as its level correlates with the disease severity. Kudo and his team developed a point-of-care device for detecting lactoferrin in biological samples such as saliva. They used 2-(5-bromo-2-pyridylazo)-5-diethylaminophenol as a reagent, when it reacted with lactoferrin, results in the displacement of Fe3+ ions and hence a color change to dark blue or purple. The complex was encapsulated in poly(styrene-block-vinylpyrrolidone) particles, immobilizing the complex to the paper (Fig. 5). The device was fabricated using wax printing, and the limit of detection for lactoferrin was 110 μg/mL [117].
Fabrication procedure for μPADs for distance-based readout of Lactoferrin (reproduced with permission from Ref. [117].
2.7 Lactate
Lactate or Lactic Acid is a biomolecule that is made by muscle cells and red blood cells and is usually present in low concentrations in the blood, but rises when the oxygen concentration is lower as they get formed by muscle cells as a by-product of glucose oxidation in an oxygen-limited condition [118]. A higher lactate presence in blood could imply poor metabolism by the body as the oxygen is not used correctly for glucose breakdown [119].The condition does not mean a problem with a particular organ, as the failure of different organs also results in a rise in lactate levels in the blood. It implies that there could be a problem with either one or many of these organs, such as the heart, liver, and kidney [120]. The rise in blood lactate level will reflect in the mouth and saliva; hence, saliva can be used for assessing blood lactate levels using point-of-care devices to diagnose various disease conditions. Rossini and team developed a device to detect lactate in saliva samples. They fabricated the device using wax printing. Fluorescent nanoparticles were used as detection probes along with lactate dehydrogenase and horse-radish peroxidase. Carbon dots were used to synthesize these nanoparticles with citric acid and tyramine as precursors. These solutions were mixed with PBS to form the reagent impregnated into the filter paper. The fluorescence quenching was visible after UV light was shown on the paper. The limit of detection for lactate was 0.814 μmol/L [121]. The Schematic illustration showing the paper microfluidic device using carbon dots to detect glucose and lactate as shown in Fig. 6.
Schematic illustration showing paper microfluidic device using carbon dots to detect glucose and lactate (reproduced with permission from Ref. [121].
2.8 Glucose
Glucose is an essential carbohydrate used by the body to generate ATP, which is the energy currency of the cells [122]. Glucose is broken down into carbon dioxide and released by the cells, utilizing the bond break energy to form ATP molecules. Carbohydrates are the primary source of glucose, and they are broken down by different enzymes, such as maltase, to form glucose. Excess glucose in the body is converted into fat, and some are stored as glycogen [123]. In diabetes, there is an elevation in blood glucose levels due to either the ineffectiveness or complete absence of insulin, which is responsible for regulating the levels of glucose in the body. Prolonged elevation of glucose levels in the body can give rise to a range of health-related complications, including heart disease, kidney failure, impaired wound healing, and even loss of consciousness [124]. Hence the diagnosis of high glucose presence in the blood is essential and can be helpful in the early diagnosis of diabetes. Glucose can be easily detected by using Glucose Oxidase and Horse Radish Peroxidase, which is implemented in point-of-care paper-based microfluidic devices for easy detection [125].
Santana-Jiménez et al. developed a low-cost bi-enzymatic paper-based sensor for the detection of glucose. They used glucose oxidase-horse radish peroxidase and 2,4,6-tribromo-3-hydroxy benzoic acid, giving a usable range of detection from 0 to 180 mg/dL of glucose in saliva. Wax printing was used to fabricate the device, and the limit of detection was 0.37 mg/dL [126]. Tansu Gölcez and team integrated an offline smartphone app along with the paper-based device for easy detection and quantification of glucose in saliva. The application can utilize both RAW and JPEG images and converts them into RGB values using MATLAB, which is later used to plot the calibration curve. They used glucose oxidase, horse radish peroxidase, and chitosan for improved detection. They used ink-printed paper towels to form the hydrophobic barriers. The limit of detection was 29.65 μmol/L [127].
Chiang et al., and de Castro et al., detected nitrite and glucose simultaneously μPAD. They used glucose oxidase and horse radish peroxidase as reagents for detection, and their limits of detection were 29.65 μmol/L and 0.3 mmol/L for nitrate and glucose respectively [106, 128]. Mercan et al. coupled the classic Gox-HRP reagent with 3,3’,5,5’-tetramethylbenzidine as a chromogenic agent to help improve detection. The colorimetric output of this paper-based device was then analyzed using a smartphone application that uses a machine learning algorithm trained using multiple data sets of photos from different smartphones. Hence this application can be used on a wide range of mobile devices. A linear LDA correlation of 98.4% was achieved using TMB, and the limit of detection was 47 μmol/L [129]. The schematic illustration showing glucose detection using μPAD and smartphone as depicted in Fig. 7.
Schematic illustration showing glucose detection. The color change in the μPAD detection zones was photographed using a smartphone under fluorescent, sunlight and halogen light sources (reproduced with permission from Ref. [129].
3 Detection of COVID-19 Biomarkers Using Paper-Based Systems
As of September 2022, the COVID-19 pandemic is responsible for over 610 million confirmed cases and over 6,508,521 deaths worldwide (As per the WHO’s COVID-19 Dashboard). SARS-CoV-2 coronavirus is the causative organism of the pandemic [130]. The subfamily and family of coronaviruses is Coronavirinae and Coronaviridae, respectively [131]. In March 2022, the World Health Organization declared the outbreak a global pandemic [132, 133]. Owing to the pandemic, a tremendous impact has been seen on global health. Additionally, a significant social and economic impact was seen owing to several lockdowns and closures worldwide. The virus is known to transmit rapidly by respiratory droplets and aerosols; early detection is crucial [134]. Fast, accurate, low-cost diagnostic tools have become a prerequisite to tackling the COVID-19 pandemic. The nucleic acids present in the samples are analyzed using various molecular detection methods, which in turn aids in identifying the virus. Commonly, laboratories worldwide have been using the method of real-time reverse transcriptase (RT-PCR) for the detection of the coronavirus [135, 136]. A primary disadvantage of using RT-PCR as a detection method is the possible occurrence of false negative cases [86]. Apart from nucleic acid tests, bioanalytical detection methods such as immunoassays work on quantifying antigen–antibody interaction. However, they have low specificity when compared to tests that recognize the RNA sequences of the virus [137]. Lateral flow immunoassays (LFIAs) and enzyme-linked immunosorbent assays (ELISAs) are the most extensively used methods to detect antibodies against SARS-CoV-2 [138]. Recent studies have shown that the CRISPR-Cas 13-based assay has a higher detection capacity compared to the RT-PCR assay [139]. However, the test’s sensitivity might be hindered as the viral load keeps changing and is not constant [86]. The pandemic saw µPADs being established to provide an efficient and low-cost solution to point-of-care (POC) necessities. This was obtained by combining microfluidics with various paper-based approaches [140]. The detection of nucleic acids or protein targets usually makes use of fluorescence or colorimetric detection approaches [141]. There are different detection targets for SARS-CoV-2, namely, antigens, virus genes, and antibodies. The present paper-based diagnostic methods can be classified under the above-mentioned detection targets. Present viral infections are detected by viral antigen and gene tests. Serological tests account for the detection of prior infections [142, 143].
Saliva-based detection, nasopharyngeal swab, and serum are frequently used to detect SARS-CoV-2. However, sensitivity poses to be a challenge in saliva-based detection [144]. To tackle this, Fabiani et al. established a paper-based immunoassay using magnetic beads. The platform uses 96-well wax-printed paper to support the immunological chain. The method uses color images aided by a smartphone with Spotxel free-charge app for visualization. The effectiveness was ensured by collecting the nasopharyngeal swab specimens of the respective patients and testing them using the RT-PCR method. The assay could also detect the delta variant of SAR-CoV-2 by the NGS method. A colorimetric approach was used, with the limit of detection being 0.1 µg/mL [145]. The Scheme of the smartphone-assisted paper-based device for detecting SARS-coV-2 in saliva samples is depicted in Fig. 8.
Scheme of the smartphone-assisted paper-based device for detecting SARS-coV-2 in saliva samples (reproduced with permission from Ref. [145].
In the case of SARS-CoV-2, point-of-care tests are upcoming, however, they are associated with some key disadvantages, such as reduced scalability and the requirement to process the samples, which could prove to be time-consuming [146]. Davidson et al. developed a paper-based device for detecting nucleic acids present in samples with the help of loop-mediated isothermal amplification (LAMP). It produced a colorimetric response. It was then optimized for SAR-CoV-2 detection in humans’ saliva, which does not require pre-processing. RT-LAMP is a technique characterized by the amplification of nucleic acids and is conducted at a constant temperature [147]. The device consisted of Grade 222 chromatography paper. It was 100% specific with a limit of detection of 200 genomic copies/μL. It could also detect multiple targets and pathogen detection by just varying the primer sets of LAMP [148].
4 Conclusion and Future Prospects
Saliva is one of the potential body fluids to monitor the diseases and overall health of the body. This non-invasive diagnosis of biomarkers from salivary samples are simple, easy, and low-cost. This review outlines the constraints of conventional methods (Table 2) in clinical applications while emphasizing the importance of saliva as a non-invasive biological fluid that contains numerous biomarkers linked to different diseases This review highlights the state of the art of recent advancements in the diagnosis of salivary biomarkers using different methodologies. Additionally, it offers an overview of the advancements made in the past decade regarding paper-based colorimetric microfluidic devices designed for detecting salivary biomarkers. The focus of this paper was on the salivary biomarkers as well and their detection using paper-based systems. As the area of paper-based electronics advances, we may anticipate more completely integrated and reusable sensor systems. Paper-based devices have a great and mostly unexplored potential, making them an attractive and cost-effective alternative for future applications. Compared to the small molecule and nucleic acid-based biomarkers, identification, and characterization of new proteome composition and human saliva would help find more possible protein biomarkers. Protonic biomarker diagnostic would integrate the biomarker into medication, therapeutic followed by ultimate progression of the diseases. Detection of these biomarkers is more convenient, cost-effective, easy, and rapid. The bioanalysis report would be an alarm to the patients to have a possible treatment before it reaches the advanced stage. Despite many advantages, such as being easily associable for point-of-care testing and assured, they have still many challenges to overcome for the real sample analysis. such as non-specific interaction, low sensitivity, and low detection limit. However, the sensitivity of the sensors can in tuned for better performance by integrating the advanced nanomaterial into the detection systems. On the other hand, loop-mediated isothermal amplification ( LAMP) methods can be used to improve the sensitivity of the nucleic acid-based biomarkers enabling point-of-care testing with more sensitivity. Identification of disease-specific new biomarkers such as microRNA (miRNA) would facilitate easy diagnosis of the diseases. For example, a simple pH test paper-based sensor has been developed for the sensitive detection of miR by Feng et al. by rolling circle amplification (RCA) technique with minimal laboratory setting [149].
The emergence of 3D printing technologies has revolutionized the fabrication of these devices, moving beyond the conventional 2D design to incorporate three-dimensional structures. This advancement allows for the inclusion of features such as detecting multiple samples and multiple biomarkers from a single sample. Furthermore, significant progress has been made in various fabrication techniques employed for developing these devices. In the past, wax printing and inkjet printing methods were commonly employed in paper-based device manufacturing due to their cost-effectiveness and simplicity of production. However, these methods have certain limitations, such as uneven sample penetration through the paper, resulting in incomplete filling of the detection zone. Additionally, they may face challenges in detecting certain biomarkers, such as pathogens and antibodies, which may not be compatible with wax or inkjet printing techniques. To overcome these limitations, novel techniques have been developed that combine the traditional wax and inkjet printing methods with the implementation of capillary membrane action, as well as the utilization of aptamers and nanoparticles. These innovative approaches aim to enhance the detection limit and enable the detection of previously untargeted biomarkers.
While numerous paper-based devices rely on smartphones for result interpretation, it is important to note that the majority of these devices are utilized in developing nations and under-developing regions. Consequently, it is crucial for these devices to be designed in a way that allows them to function independently without the need for external equipment such as smartphones or computers for result interpretation. One approach to achieving this is by incorporating a grading sheet into the device, which displays the range of colors it can generate and their corresponding values. This can be accomplished by including a separate sheet alongside the device that outlines all possible colors and their associated values, providing a quantification of the biomarker concentration in the sample. However, creating an accurate grading sheet requires multiple standard graphs and analyses to be conducted beforehand. It is important to acknowledge that human error can significantly impact the interpretation of color, potentially leading to incorrect diagnoses. Consequently, scientists often rely on smartphones or computer-based result analysis to mitigate these errors. Further research and development are needed to address these challenges and enhance the usability of paper-based devices without external equipment. The current use of paper-based devices deployed in identifying biomarkers in saliva can be extended to study and analyze biomarkers in Gingival Crevicular Fluid or GCF. GCF is a fluid secreted by the gums, and it mainly contains antibodies, serum, diseased cells, and microbes [150,151,152]. It also has various biomarkers such as nitrite, C C-reactive proteins, and uric acid, to name a few. Analyzing this fluid can help identify any prevalent gum diseases, such as periodontitis and gingivitis. These diseases appear to have similar biomarkers to those detected in the saliva, like nitrite and nitric oxide [153, 154]. Hence, they can be used to diagnose dental diseases using GCF as a biofluid instead of saliva. Dental plaques and other oral microbial disorders can also be detected and analyzed using paper-based devices as the advancement in the area of 3D printing and that of integrating aptamers and anti-antibodies, which are tagged with a dye into the paper combined with lysing reagents have enabled them to detect DNA and RNA. Comprehensive investigations into symptom-based approaches for the detection of dental diseases are yet to be conducted. Exploring the predominant symptoms and utilizing biomarker detection can assist in localizing or narrowing down the specific type of acquired disease. For instance, periodontitis manifests in various symptoms, including tooth sensitivity, gum pain or bleeding, altered taste sensation, halitosis (bad breath), and gum swelling, among others. By combining these symptoms with the detection of relevant biomarkers like nitrite and nitric oxide, the diagnosis of the disease can be facilitated. The primary advantages of this approach lie in its simplicity and rapid detection capability. Further research in these areas has the potential to yield significant benefits.
Though the sensitivity of many biosensors is high from the proof of concept report, it is highly challenging to reproduce the same sensitivity from the real sample due to the counter interaction of the biological component in the fluid with the sensing elements, which leads to false conclusions. Therefore, bioanalysis data from a single biomarker is not sufficient for a physician to make a decision. Bioanalysis of multiple biomarkers from the same sample using multiplex sensors facilitates improvement in the accuracy of the report [155, 156]. At this stage, these biosensing methods are immature, and more experimental research has to be carried out to improve their sensitivity, specificity, and multiplex capacity for the performance of sensors for real sample applications.
Data availability
No datasets were generated or analyzed during the current study.
References
Dash S, Shakyawar SK, Sharma M, Kaushik S. Big data in healthcare: Management, analysis and future prospects. J Big Data. 2019;6:54.
Gandrup J, Ali SM, McBeth J, van der Veer SN, Dixon WG. Remote symptom monitoring integrated into electronic health records: A systematic review. J Am Med Inform Assoc. 2020;27:1752–63.
Naresh V, Lee N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors. 2021;21(4):1109.
Anushka A, Bandopadhyay PK. Das, Paper based microfluidic devices: a review of fabrication techniques and applications. Eur Phys J Spec Top. 2023;232:781–815.
Prabhu A, Giri Nandagopal MS, Peralam Yegneswaran P, Singhal HR, Mani NK. Inkjet printing of paraffin on paper allows low-cost point-of-care diagnostics for pathogenic fungi. Cellulose. 2020;27:7691–701.
Kelkar N, Prabhu A, Prabhu A, Giri Nandagopal MS, Mani NK. Sensing of body fluid hormones using paper-based analytical devices. Microchem J. 2022;174:107069.
Zhang Y, Li Y-L, Cui S-H, Wen C-Y, Li P, Yu J-F, Tang S-M, Zeng J-B. Distance-based detection of Ag+ with gold nanoparticles-coated microfluidic paper. J Anal Test. 2021;5:11–8.
Ray R, Prabhu A, Prasad D, Kumar Garlapati V, Aminabhavi TM, Mani NK, Simal-Gandara J. Paper-based microfluidic devices for food adulterants: Cost-effective technological monitoring systems. Food Chem. 2022;390:133173.
Tao KJ, Castleman MD, Tao S. Reagent-loaded annulus-shaped reactor on filter-paper with virtual colorimeter for onsite quick detection of chlorogenic acid. J Anal Test. 2023;7:25–32.
Liu Z-G, Xiao M, Yang R-Z, Zhou Q-Q, Ye H-F, Yi C-Q. Multiplexed detection of Fe3+, cobalamin and folate using fluorescent nanoprobe-based microarrays and a smartphone. J Anal Test. 2021;5:19–29.
Hou Y, Lv CC, Guo YL. Recent advances and applications in paper-based devices for point-of-care testing. J Anal Test. 2022;6:247–73.
Mani NK, Das SS, Dawn S, Chakraborty S. Electro-kinetically driven route for highly sensitive blood pathology on a paper-based device. Electrophoresis. 2020;41:615–20.
Hasandka A, Prabhu A, Prabhu A, Singhal HR, Nandagopal MSG, Shenoy R, Mani NK. “Scratch it out”: carbon copy based paper devices for microbial assays and liver disease diagnosis. Anal Methods. 2021;13:3172–3180.
Ray R, Goyal A, Prabhu A, Parekkh S, Maddasani S, Mani NK. Paper-based dots and smartphone for detecting counterfeit country eggs. Food Chem. 2023;403:134484.
Sudarsan S, Prabhu A, Prasad D, Mani NK. DNA compaction enhances the sensitivity of fluorescence-based nucleic acid assays: a game changer in point of care sensors? Analyst. 2023;148:2295–307.
Sudarsan S, Shetty P, Chinnappan R, Mani NK. Tuning hydrophobicity of paper substrates for effective colorimetric detection of glucose and nucleic acids. Anal Bioanal Chem. 2023;415:6449–60.
Prabhu A, Nandagopal MSG, Peralam Yegneswaran P, Prabhu V, Verma U, Mani NK. Thread integrated smart-phone imaging facilitates early turning point colorimetric assay for microbes. RSC Adv. 2020;10:26853–61.
Prabhu A, Singhal H, Giri Nandagopal MS, Kulal R, Peralam Yegneswaran P, Mani NK. Knitting thread devices: detecting candida albicans using napkins and tampons. ACS Omega. 2021;6:12667–75.
Selvam GS, Dheivasigamani T, Prabhu A, Mani NK. Embellishing 2-D MoS2 nanosheets on lotus thread devices for enhanced hydrophobicity and antimicrobial activity. ACS Omega. 2022;7:24606–13.
Agustini D, Bergamini MF, Marcolino-Junior LH. Tear glucose detection combining microfluidic thread based device, amperometric biosensor and microflow injection analysis. Biosens Bioelectron. 2017;98:161–7.
Cabot JM, Breadmore MC, Paull B. Thread based electrofluidic platform for direct metabolite analysis in complex samples. Anal Chim Acta. 2018;1000:283–92.
Gonzalez A, Gaines M, Gomez FA. Thread-based microfluidic chips as a platform to assess acetylcholinesterase activity. Electrophoresis. 2017;38:996–1001.
Malon RSP, Heng LY, Córcoles EP. Recent developments in microflluidic paper-, cloth-, and thread-based electrochemical devices for analytical chemistry. Rev Anal Chem. 2017;36:1–19.
Nilghaz A, Zhang L, Li M, Ballerini DR, Shen W. Understanding thread properties for red blood cell antigen assays: Weak ABO blood typing. ACS Appl Mater Interfaces. 2014;6:22209–15.
Terse-Thakoor T, Punjiya M, Matharu Z, Lyu B, Ahmad M, Giles GE, Owyeung R, Alaimo F, Shojaei Baghini M, Brunyé TT, Sonkusale S. Thread-based multiplexed sensor patch for real-time sweat monitoring, Npj Flex. Electron. 2020;4(1):159–68.
Sateanchok S, Wangkarn S, Saenjum C, Grudpan K. A cost-effective assay for antioxidant using simple cotton thread combining paper based device with mobile phone detection. Talanta. 2018;177:171–5.
Xiao G, He J, Chen X, Qiao Y, Wang F, Xia Q, Yu L, Lu Z. A wearable, cotton thread/paper-based microfluidic device coupled with smartphone for sweat glucose sensing. Cellulose. 2019;26:4553–62.
Choi JR, Nilghaz A, Chen L, Chou KC, Lu X. Modification of thread-based microfluidic device with polysiloxanes for the development of a sensitive and selective immunoassay. Sens Actuat B Chem. 2018;260:1043–51.
Suarez WT, Franco MOK, Capitán-Vallvey LF, Erenas MM. Chitosan-modified cotton thread for the preconcentration and colorimetric trace determination of Co(II). Microchem J. 2020;158: 105137.
Weng X, Kang Y, Guo Q, Peng B, Jiang H. Recent advances in thread-based microfluidics for diagnostic applications. Biosens Bioelectron. 2019;132:171–85.
Gonzalez A, Gaines M, Gallegos LY, Guevara R, Gomez FA. Enzyme-linked immunosorbent assays (ELISA) based on thread, paper, and fabric. Electrophoresis. 2018;39:476–84.
Arroyo MJ, Erenas MM, de Orbe-Payá I, Cantrell K, Dobado JA, Ballester P, Blondeau P, Salinas-Castillo A, Capitán-Vallvey LF. Thread based microfluidic platform for urinary creatinine analysis. Sens Actuat B Chem. 2020;305: 127407.
Jiang N, Tansukawat ND, Gonzalez-Macia L, Ates HC, Dincer C, Güder F, Tasoglu S, Yetisen AK. Low-cost optical assays for point-of-care diagnosis in resource-limited settings. ACS Sensors. 2021;6:2108–24.
Tomazelli Coltro WK, Cheng C-MM, Carrilho E, de Jesus DP. Recent advances in low-cost microfluidic platforms for diagnostic applications. Electrophoresis. 2014;35:2309–24.
Li YD, Li WY, Chai HH, Fang C, Kang YJ, Li CM, Yu L. Chitosan functionalization to prolong stable hydrophilicity of cotton thread for thread-based analytical device application. Cellulose. 2018;25:4831–40.
Cosgun Y, Yildirim A, Yucel M, Karakoc AE, Koca G, Gonultas A, Gursoy G, Ustun H, Korkmaz M. Evaluation of invasive and noninvasive methods for the diagnosis of helicobacter pylori infection. Asian Pac J Cancer Prev. 2016;17:5265–72.
Andari MVC, Bussamra SLC, Tedesco TGD, Peixoto PAB, Pares PDBS, Braga A, Araujo Júnior E, Aoki T. Noninvasive prenatal testing: benefits and limitations of the available tests. Ces Gynekol. 2020;85:41–8.
Tooley KL, Howarth GS, Butler RN. Mucositis and non-invasive markers of small intestinal function. Cancer Biol Ther. 2009;8:753–8.
Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20:475–85.
Ranzani OT, Senussi T, Idone F, Ceccato A, Li Bassi G, Ferrer M, Torres A. Invasive and non-invasive diagnostic approaches for microbiological diagnosis of hospital-acquired pneumonia. Crit Care. 2019;23:51.
Li Z, You M, Bai Y, Gong Y, Xu F. Equipment-free quantitative readout in paper-based point-of-care testing. Small Methods. 2020;4:1900459.
Nabi Z, Karyampudi A, Reddy DN (2020) Chapter 23-Biliary motility and sphincter of Oddi disorders. In: S.S.C. Rao, Y.Y. Lee, U.C.B.T.-C. and B.N. and M. Ghoshal (Eds.) Academic Press, p. 331–342. https://doi.org/10.1016/B978-0-12-813037-7.00023-6
Wroblewski LE, Peek RMJ, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713–39.
Sarandi E, Thanasoula M, Anamaterou C, Papakonstantinou E, Geraci F, Papamichael MM, Itsiopoulos C, Tsoukalas D. Metabolic profiling of organic and fatty acids in chronic and autoimmune diseases. Adv Clin Chem. 2021;101:169–229.
C. Smith, Chapter 26 - Traumatic brain injury, in: M.J. Zigmond, C.A. Wiley, M.-F Chesselet. Neurobiology of Brain Disorders (Second Edition). Academic Press, 2023. pp. 443–455. https://doi.org/10.1016/B978-0-323-85654-6.00010-1.
Bonati LH, Jansen O, de Borst GJ, Brown MM. Management of atherosclerotic extracranial carotid artery stenosis. Lancet Neurol. 2022;21:273–83.
Ruiz-Bañobre J, Goel A. Genomic and epigenomic biomarkers in colorectal cancer: From diagnosis to therapy. Adv Cancer Res. 2021;151:231–304.
Zhang Q, Maddukuri N, Gong M. A direct and rapid method to determine cyanide in urine by capillary electrophoresis. J Chromatogr A. 2015;1414:158–62.
Jedličková V, Paluch Z, Alušík Š. Determination of nitrate and nitrite by high-performance liquid chromatography in human plasma. J Chromatogr B. 2002;780:193–7.
Zhang Y, Isaacman DJ, Wadowsky RM, Rydquist-White J, Post JC, Ehrlich GD. Detection of Streptococcus pneumoniae in whole blood by PCR. J Clin Microbiol. 1995;33:596–601.
Yilmaz Ö, Sen N, Küpelioǧlu AA, Simsel I. detection of H pylori infection by ELISA and western blot techniques and evaluation of anti CagA seropositivity in adult Turkish dyspeptic patients. World J Gastroenterol. 2006;12:5375–8.
Liang Y, Yan C, Guo Q, Xu J, Hu H. Spectrophotometric determination of ammonia nitrogen in water by flow injection analysis based on NH3- o-phthalaldehyde -Na2SO3 reaction. Anal Chem Res. 2016;10:1–8.
Nagababu E, Rifkind JM. Measurement of plasma nitrite by chemiluminescence. Methods Mol Biol. 2010;610:41–9.
Idris A, Saleh TA, Sanhoob MA, Muraza O, Al-Betar AR. Electrochemical detection of thiocyanate using phosphate-modified zeolite carbon paste electrodes. J Taiwan Inst Chem Eng. 2017;72:236–43.
Coskun O. Separation techniques: Chromatography. North Clin Istanbul. 2016;3:156–60.
Zhu X, Shi L. Electrochemistry, Nano-Inspired Biosens. Protein Assay with Clin Appl. 2019. https://doi.org/10.1016/B978-0-12-815053-5.00009-X.
Yan Y, Shi P, Song W, Bi S. Chemiluminescence and bioluminescence imaging for biosensing and therapy: in vitro and in vivo perspectives. Theranostics. 2019;9:4047.
Bachmann LM, Miller WG. Chapter 7 - Spectrophotometry, Contemporary Practice in Clinical Chemistry (Fourth Edition). Academic Press, 2020. p. 119–133.
Clark MF, Lister RM, Bar-Joseph M. ELISA techniques. Methods Enzymol. 1986;118:742–66.
Wisdom GB. Enzyme-immunoassay. Clin Chem. 1976;22:1243–55.
Nimse SB, Sonawane MD, Song KS, Kim T. Biomarker detection technologies and future directions. Analyst. 2016;141:740–55.
Pomili T, Donati P, Pompa PP. Paper-based multiplexed colorimetric device for the simultaneous detection of salivary biomarkers. Biosensors. 2021;11:443.
Banerjee RK, Datta AG. Salivary peroxidases. Mol Cell Biochem. 1986;70:21–9.
Ilea A, Andrei V, Feurdean CN, Bǎbtan AM, Petrescu NB, Câmpian RS, Bosca AB, Ciui B, Tertis M, Sǎndulescu R, Cristea C. Saliva, a Magic biofluid available for multilevel assessment and a mirror of general health—a systematic review. Biosens. 2019;9:27.
Humphrey SP, Williamson RT. A review of saliva: Normal composition, flow, and function. J Prosthet Dent. 2001;85:162–9.
Dawes C. Circadian rhythms in human salivary flow rate and composition. J Physiol. 1972;220:529–45.
Baliga S, Muglikar S, Kale R. Salivary pH: A diagnostic biomarker. J Indian Soc Periodontol. 2013;17:461.
Tenovuo J. Antimicrobial agents in saliva—protection for the whole body. J Dent Res. 2002;81:807–9.
Hassaneen M, Maron JL. Salivary diagnostics in pediatrics: Applicability, translatability, and limitations. Front Public Heal. 2017;5: 230463.
Yoshizawa JM, Schafer CA, Schafer JJ, Farrell JJ, Paster BJ, Wong DTW. Salivary biomarkers: Toward future clinical and diagnostic utilities. Clin Microbiol Rev. 2013;26:781–91.
Jasim H, Carlsson A, Hedenberg-Magnusson B, Ghafouri B, Ernberg M. Saliva as a medium to detect and measure biomarkers related to pain. Sci Rep. 2018;8:3220.
Noviana E, Ozer T, Carrell CS, Link JS, McMahon C, Jang I, Henry CS. Microfluidic paper-based analytical devices: From design to applications. Chem Rev. 2021;121:11835–85.
Loo JFC, Ho AHP, Turner APF, Mak WC. Integrated printed microfluidic biosensors. Trends Biotechnol. 2019;37:1104–20.
Rivas L, Medina-Sánchez M, De La Escosura-Muñiz A, Merkoçi A. Improving sensitivity of gold nanoparticle-based lateral flow assays by using wax-printed pillars as delay barriers of microfluidics. Lab Chip. 2014;14:4406–14.
Xia Y, Si J, Li Z. Fabrication techniques for microfluidic paper-based analytical devices and their applications for biological testing: A review. Biosens Bioelectron. 2016;77:774–89.
Kim TH, Hahn YK, Kim MS. Recent advances of fluid manipulation technologies in microfluidic paper-based analytical devices (μPADs) toward multi-step assays. Micromachines. 2020;11:269.
Rodriguez NM, Linnes JC, Fan A, Ellenson CK, Pollock NR, Klapperich CM. Paper-based RNA extraction, in situ isothermal amplification, and lateral flow detection for low-cost, rapid diagnosis of Influenza A (H1N1) from clinical specimens. Anal Chem. 2015;87:7872–9.
Fu LM, Wang YN. Detection methods and applications of microfluidic paper-based analytical devices. Trends Anal Chem. 2018;107:196–211.
Shi F, Xu J, Hu Z, Ren C, Xue Y, Zhang Y, Li J, Wang C, Yang Z. Bird nest-like zinc oxide nanostructures for sensitive electrochemical glucose biosensor. Chinese Chem Lett. 2021;32:3185–8.
Yadav M, Singh G, Lata S. Revisiting some recently developed conducting polymer@metal oxide nanostructures for electrochemical sensing of vital biomolecules: A review. J Anal Test. 2022;6:274–95.
Shi F, Xue Y, Hong L, Cao J, Li J, Jiang M, Hu X, Yang Z, Shen M. Synthesis of a novel hedgehog-shaped Bi2S3 nanostructure for a sensitive electrochemical glucose biosensor. New J Chem. 2021;45:18387–91.
Shi F, Li J, Xiao J, Ma X, Xue Y, Li J, Shen M, Yang Z. Urchin-like PtNPs@Bi2S3: synthesis and application in electrochemical biosensor. Analyst. 2022;147:430–5.
Zhang Y, Li S, Liu H, Shi F, Li J, Hu X, Yang Z. Dual-strategy biosensing of glucose based on multifunctional CuWO4 nanoparticles. Analyst. 2022;147:4049–54.
Lei KF, Huang CH, Kuo RL, Chang CK, Chen KF, Tsao KC, Tsang NM. Paper-based enzyme-free immunoassay for rapid detection and subtyping of influenza A H1N1 and H3N2 viruses. Anal Chim Acta. 2015;883:37–44.
Liu H, Zhao P, Wang Y, Li S, Zhang L, Zhang Y, Ge S, Yu J. Paper-based sandwich type SERS sensor based on silver nanoparticles and biomimetic recognizer. Sens Actuat B Chem. 2020;313:127989.
Singh B, Datta B, Ashish A, Dutta G. A comprehensive review on current COVID-19 detection methods: From lab care to point of care diagnosis. Sensors Int. 2021;2:100119.
Mahmoudi T, de la Guardia M, Shirdel B, Mokhtarzadeh A, Baradaran B. Recent advancements in structural improvements of lateral flow assays towards point-of-care testing. Trends Anal Chem. 2019;116:13–30.
Ruecha N, Shin K, Chailapakul O, Rodthongkum N. Label-free paper-based electrochemical impedance immunosensor for human interferon gamma detection. Sens Actuat B Chem. 2019;279:298–304.
Liu L, Yang D, Liu G. Signal amplification strategies for paper-based analytical devices. Biosens Bioelectron. 2019;136:60–75.
Miočević O, Cole CR, Laughlin MJ, Buck RL, Slowey PD, Shirtcliff EA. Quantitative lateral flow assays for salivary biomarker assessment: a review. Front Public Heal. 2017. https://doi.org/10.3389/fpubh.2017.00133.
Zhai G. Clinical relevance of biochemical and metabolic changes in osteoarthritis. Adv Clin Chem. 2021;101:95–120.
Atkinson AJ, Colburn WA, DeGruttola VG, DeMets DL, Downing GJ, Hoth DF, Oates JA, Peck CC, Schooley RT, Spilker BA, Woodcock J, Zeger SL. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95.
Eisenschmidt-Bönn D, Schneegans N, Backenköhler A, Wittstock U, Brandt W. Structural diversification during glucosinolate breakdown: mechanisms of thiocyanate, epithionitrile and simple nitrile formation. Plant J. 2019;99:329–43.
Chandler JD, Day BJ. THIOCYANATE: A potentially useful therapeutic agent with host defense and antioxidant properties. Biochem Pharmacol. 2012;84:1381.
Pena-Pereira F, Lavilla I, Bendicho C. Paper-based analytical device for instrumental-free detection of thiocyanate in saliva as a biomarker of tobacco smoke exposure. Talanta. 2016;147:390–6.
Sitanurak J, Fukanaa N, Wongpakdeea T, Thepchuaya Y, Ratanawimarnwong N, Amornsakchai T, Nacapricha D. T-shirt ink for one-step screen-printing of hydrophobic barriers for 2D- and 3D-microfluidic paper-based analytical devices. Talanta. 2019;205: 120113.
Yu P, Deng M, Yang Y, Nie B, Zhao S. 3D microfluidic devices in a single piece of paper for the simultaneous determination of nitrite and thiocyanate. Sensors. 2020;20:4118.
Pungjunun K, Yakoh A, Chaiyo S, Praphairaksit N, Siangproh W, Kalcher K, Chailapakul O. Laser engraved microapillary pump paper-based microfluidic device for colorimetric and electrochemical detection of salivary thiocyanate. Microchim Acta. 2021;188:1–11.
Sigman DM, Casciotti KL. Nitrogen isotopes in the ocean. Encycl Ocean Sci. 2001;1884–94. https://doi.org/10.1006/rwos.2001.0172.
Kina-Tanada M, Sakanashi M, Tanimoto A, Kaname T, Matsuzaki T, Noguchi K, Uchida T, Nakasone J, Kozuka C, Ishida M, Kubota H, Taira Y, Totsuka Y, Ichiro Kina S, Sunakawa H, Omura J, Satoh K, Shimokawa H, Yanagihara N, Maeda S, Ohya Y, Matsushita M, Masuzaki H, Arasaki A, Tsutsui M. Long-term dietary nitrite and nitrate deficiency causes the metabolic syndrome, endothelial dysfunction and cardiovascular death in mice. Diabetologia. 2017;60:1138–51.
Bhakta SA, Borba R, Taba M, Garcia CD, Carrilho E. Determination of nitrite in saliva using microfluidic paper-based analytical devices. Anal Chim Acta. 2014;809:117–22.
Jayawardane BM, Wei S, McKelvie ID, Kolev SD. Microfluidic paper-based analytical device for the determination of nitrite and nitrate. Anal Chem. 2014;86:7274–9.
Ferreira FTSM, Mesquita RBR, Rangel AOSS. Novel microfluidic paper-based analytical devices (μPADs) for the determination of nitrate and nitrite in human saliva. Talanta. 2020;219:121183.
Jiang Y, Hao Z, He Q, Chen H. A simple method for fabrication of microfluidic paper-based analytical devices and on-device fluid control with a portable corona generator. RSC Adv. 2016;6:2888–94.
Noiphung J, Nguyen MP, Punyadeera C, Wan Y, Laiwattanapaisal W, Henry CS. Development of paper-based analytical devices for minimizing the viscosity effect in human saliva. Theranostics. 2018;8:3797.
Chiang CK, Kurniawan A, Kao CY, Wang MJ. Single step and mask-free 3D wax printing of microfluidic paper-based analytical devices for glucose and nitrite assays. Talanta. 2019;194:837–45.
Sheini A. A paper-based device for the colorimetric determination of ammonia and carbon dioxide using thiomalic acid and maltol functionalized silver nanoparticles: application to the enzymatic determination of urea in saliva and blood. Microchim Acta. 2020;187:1–11.
Thepchuay Y, Mesquita RBR, Nacapricha D, Rangel AOSS. Micro-PAD card for measuring total ammonia nitrogen in saliva. Anal Bioanal Chem. 2020;412:3167–76.
Kester M, Karpa KD, Vrana KE. Toxicology. Elsevier’s Integr Rev Pharmacol. 2012;29–39. https://doi.org/10.1016/B978-0-323-07445-2.00003-3.
Cai Z. Cyanide. Encyclopedia of Toxicology (Second Edition). Elsevier: 2005. p. 698–701. https://doi.org/10.1016/B0-12-369400-0/00276-3.
Coppock RW. Threats to wildlife by chemical warfare agents. Handb Toxicol Chem Warf Agents. Academic Press: 2009. p.747–751. https://doi.org/10.1016/B978-012374484-5.00049-3.
Enderby B, Smith D, Carroll W, Lenney W. Hydrogen cyanide as a biomarker for Pseudomonas aeruginosa in the breath of children with cystic fibrosis. Pediatr Pulmonol. 2009;44:142–7.
Lvova L, Pomarico G, Mandoj F, Caroleo F, Di Natale C, Kadish KM, Nardis S. Smartphone coupled with a paper-based optode: Towards a selective cyanide detection. J Porphyr Phthalocyanines. 2020;24:964–72.
Giebultowicz J, Wroczyński P, Samolczyk-Wanyura D. Can lower aldehyde dehydrogenase activity in saliva be a risk factor for oral cavity cancer? Oral Dis. 2013;19:763–6.
Ramdzan AN, Almeida MIGS, McCullough MJ, Kolev SD. Development of a microfluidic paper-based analytical device for the determination of salivary aldehydes. Anal Chim Acta. 2016;919:47–54.
Orsi N. The antimicrobial activity of lactoferrin: current status and perspectives. Biometals. 2004;17:189–96.
Kudo H, Maejima K, Hiruta Y, Citterio D. Microfluidic paper-based analytical devices for colorimetric detection of lactoferrin. SLAS Technol. 2020;25:47–57.
V.V.A.M. Schreurs, G. Schaafsma. Lactic acid and lactates. Nutrafoods. 2010;91(9):7–16.
Rabinowitz JD, Enerbäck S. Lactate: the ugly duckling of energy metabolism. Nat Metab. 2020;27(2):566–71.
Bakker J, Gris P, Coffernils M, Kahn RJ, Vincent JL. Serial blood lactate levels can predict the development of multiple organ failure following septic shock. Am J Surg. 1996;171:221–6.
Rossini EL, Milani MI, Lima LS, Pezza HR. Paper microfluidic device using carbon dots to detect glucose and lactate in saliva samples, Spectrochim. Acta Part A Mol Biomol Spectrosc. 2021;248: 119285.
Cherkas A, Holota S, Mdzinarashvili T, Gabbianelli R, Zarkovic N. Glucose as a major antioxidant: When, what for and why it fails? Antioxidants. 2020. https://doi.org/10.3390/antiox9020140.
Björntorp P, Sjöström L. Carbohydrate storage in man: Speculations and some quantitative considerations. Metabolism. 1978;27:1853–65.
Kaplan NM. The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch Intern Med. 1989;149:1514–20.
Lan D, Li B, Zhang Z. Chemiluminescence flow biosensor for glucose based on gold nanoparticle-enhanced activities of glucose oxidase and horseradish peroxidase. Biosens Bioelectron. 2008;24:934–8.
Santana-Jiménez LA, Márquez-Lucero A, Osuna V, Estrada-Moreno I, Dominguez RB. Naked-eye detection of glucose in saliva with bienzymatic paper-based sensor. Sensors. 2018;18:1071.
Gölcez T, Kiliç V, Şen M. A portable smartphone-based platform with an offline image-processing tool for the rapid paper-based colorimetric detection of glucose in artificial saliva. Anal Sci. 2021;37:561–7.
de Castro LF, de Freitas SV, Duarte LC, de Souza JAC, Paixão TRLC, Coltro WKT. Salivary diagnostics on paper microfluidic devices and their use as wearable sensors for glucose monitoring. Anal Bioanal Chem. 2019;411:4919–28.
Mercan ÖB, Kılıç V, Şen M. Machine learning-based colorimetric determination of glucose in artificial saliva with different reagents using a smartphone coupled μPAD. Sens Actuat B Chem. 2021;329:129037.
Khan S, Siddique R, Bai Q, Shabana Y, Liu M, Xue G, Nabi JL. Coronaviruses disease 2019 (COVID-19): causative agent, mental health concerns, and potential management options. J Infect Public Health. 2019;13(2020):1840.
Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–92.
Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L, Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–20.
Matsuyama S, Nao N, Shirato K, Kawase M, Saito S, Takayama I, Nagata N, Sekizuka T, Katoh H, Kato F, Sakata M, Tahara M, Kutsuna S, Ohmagari N, Kuroda M, Suzuki T, Kageyama T, Takeda M. Enhanced isolation of SARS-CoV-2 by TMPRSS2- expressing cells. Proc Natl Acad Sci U S A. 2020;117:7001–3.
Wang L, Wang Y, Ye D, Liu Q. Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence. Int J Antimicrob Agents. 2020;55:105948.
Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, Bleicker T, Brünink S, Schneider J, Schmidt ML, Mulders DGJC, Haagmans BL, Van Der Veer B, Van Den Brink S, Wijsman L, Goderski G, Romette JL, Ellis J, Zambon M, Peiris M, Goossens H, Reusken C, Koopmans MPG, Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25:1.
Mackay IM, Arden KE, Nitsche A. Real-time PCR in virology. Nucleic Acids Res. 2002;30:1292–305.
Giri B, Pandey S, Shrestha R, Pokharel K, Ligler FS, Neupane BB. Review of analytical performance of COVID-19 detection methods. Anal Bioanal Chem. 2021;413:35–48.
Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10:102–8.
Ji T, Liu Z, Wang GQ, Guo X, Akbar Khan S, Lai C, Chen H, Huang S, Xia S, Chen B, Jia H, Chen Y, Zhou Q. Detection of COVID-19: A review of the current literature and future perspectives. Biosens Bioelectron. 2020;166:112455.
Hartanto H, Wu M, Lam ML, Chen TH. Microfluidic immunoassay for detection of serological antibodies: A potential tool for rapid evaluation of immunity against SARS-CoV-2. Biomicrofluidics. 2020. https://doi.org/10.1063/5.0031521/1024894.
Choi JR. Development of point-of-care biosensors for COVID-19. Front Chem. 2020;8: 556443.
Jia Y, Sun H, Tian J, Song Q, Zhang W. Paper-based point-of-care testing of SARS-CoV-2. Front Bioeng Biotechnol. 2021;9: 773304.
Dong G, Guo X, Sun Y, Zhang Z, Du L, Li M. Diagnostic techniques for COVID-19: A mini-review of early diagnostic methods. J Anal Test. 2021;5:314–26.
Miranda-Ortiz H, Fernández-Figueroa EA, Ruíz-García EB, Muñoz-Rivas A, Méndez-Pérez A, Méndez-Galván J, Astudillo-De la Vega H, Gabiño-López B, Nava-Monroy R, a la Torre ALF, Anaya TLV, Vilar-Compte D, Coquis-Navarrete U, Valdés-Reyes M, Sánchez-Montes S, Becker I. Development of an alternative saliva test for diagnosis of SARS-CoV-2 using TRIzol: Adapting to countries with lower incomes looking for a large-scale detection program. PLoS One. 2021;16:e0255807.
Fabiani L, Mazzaracchio V, Moscone D, Fillo S, De Santis R, Monte A, Amatore D, Lista F, Arduini F. Paper-based immunoassay based on 96-well wax-printed paper plate combined with magnetic beads and colorimetric smartphone-assisted measure for reliable detection of SARS-CoV-2 in saliva. Biosens Bioelectron. 2022;200: 113909.
Parupudi T, Panchagnula N, Muthukumar S, Prasad S. Evidence-based point-of-care technology development during the COVID-19 pandemic. Biotechniques. 2020;70:59–67.
Inaba M, Higashimoto Y, Toyama Y, Horiguchi T, Hibino M, Iwata M, Imaizumi K, Doi Y. Diagnostic accuracy of LAMP versus PCR over the course of SARS-CoV-2 infection. Int J Infect Dis. 2021;107:195–200.
Davidson JL, Wang J, Maruthamuthu MK, Dextre A, Pascual-Garrigos A, Mohan S, Putikam SVS, Osman FOI, McChesney D, Seville J, Verma MS. A paper-based colorimetric molecular test for SARS-CoV-2 in saliva. Biosens Bioelectron X. 2021;9: 100076.
Feng C, Mao X, Shi H, Bo B, Chen X, Chen T, Zhu X, Li G. Detection of microRNA: A point-of-care testing method based on a pH-responsive and highly efficient isothermal amplification. Anal Chem. 2017;89:6631–6.
Sculley DV, Langley-Evans SC. Salivary antioxidants and periodontal disease status. Proc Nutr Soc. 2002;61:137–43.
Subbarao K, Nattuthurai G, Sundararajan S, Sujith I, Joseph J, Syedshah Y. Gingival crevicular fluid: An overview. J Pharm Bioallied Sci. 2019;11:S135–9.
Papagerakis P, Zheng L, Kim D, Said R, Ehlert AA, Chung KKM, Papagerakis S. Saliva and gingival crevicular fluid (GCF) collection for biomarker screening. Methods Mol Biol. 1922;2019:549–62.
Barros SP, Williams R, Offenbacher S, Morelli T. Gingival crevicular fluid as a source of biomarkers for periodontitis. Periodontol. 2016;2000(70):53–64.
Poorsattar Bejeh-Mir A, Parsian H, Akbari Khoram M, Ghasemi N, Bijani A, Khosravi-Samani M. Diagnostic role of salivary and GCF nitrite, nitrate and nitric oxide to distinguish healthy periodontium from gingivitis and periodontitis. Int J Mol Cell Med. 2014;3:138–45.
Jet T, Gines G, Rondelez Y, Taly V. Advances in multiplexed techniques for the detection and quantification of microRNAs. Chem Soc Rev. 2021;50:4141–61.
Liu B, Li Y, Wan H, Wang L, Xu W, Zhu S, Liang Y, Zhang B, Lou J, Dai H, Qian K. High performance, multiplexed lung cancer biomarker detection on a plasmonic gold chip. Adv Funct Mater. 2016;26:7994–8002.
Umeizudike KA, Lähteenmäki H, Räisänen IT, Taylor JJ, Preshaw PM, Bissett SM, Tervahartiala T, Nwhator SO, Pärnänen P, Sorsa T. Ability of matrix metalloproteinase-8 biosensor, IFMA, and ELISA immunoassays to differentiate between periodontal health, gingivitis, and periodontitis. J Periodontal Res. 2022;57:558–67.
Bostanci N, Mitsakakis K, Afacan B, Bao K, Johannsen B, Baumgartner D, Müller L, Kotolová H, Emingil G, Karpíšek M. Validation and verification of predictive salivary biomarkers for oral health. Sci Reports. 2021;111(11):1–12.
Shahi S, Zununi Vahed S, Fathi N, Sharifi S. Polymerase chain reaction (PCR)-based methods: Promising molecular tools in dentistry. Int J Biol Macromol. 2018;117:983–92.
Shirmohammadi A, Babaloo A, Maleki Dizaj S, Lotfipour F, Sharifi S, Ghavimi MA, Khezri K. A view on polymerase chain reaction as an outstanding molecular diagnostic technique in periodontology. Biomed Res Int. 2021;9979948. https://doi.org/10.1155/2021/9979948.
Nascimento MM, Gordan VV, Garvan CW, Browngardt CM, Burne RA. Correlations of oral bacterial arginine and urea catabolism with caries experience. Oral Microbiol Immunol. 2009;24:89–95.
Wahjudi PN, Patterson ME, Lim S, Yee JK, Mao CS, Lee WNP. Measurement of glucose and fructose in clinical samples using gas chromatography/mass spectrometry. Clin Biochem. 2010;43:198–207.
Tonzetich J, Catherall DM. Metabolism of [35S]-thiosulphate and [35S]-thiocyanate by human saliva and dental plaque. Arch Oral Biol. 1976;21:451–8.
Banderas-Tarabay JA, Zacarías-D’Oleire IG, Garduño-Estrada R, Aceves-Luna E, González-Begné M. Electrophoretic analysis of whole saliva and prevalence of dental caries. A study in Mexican dental students. Arch Med Res. 2002;33:499–505.
Bhalla S, Tandon S, Satyamoorthy K. Salivary proteins and early childhood caries: A gel electrophoretic analysis. Contemp Clin Dent. 2010;1:17.
Chinnappan R, Al Faraj A, Abdel Rahman AM, Abu-Salah KM, Mouffouk F, Zourob M. Anti-VCAM-1 and anti-IL4Rα aptamer-conjugated super paramagnetic iron oxide nanoparticles for enhanced breast cancer diagnosis and therapy. Molecules. 2020;25:3437.
Ballesta-Claver J, Valencia-Mirón MC, Capitán-Vallvey LF. One-shot lactate chemiluminescent biosensor. Anal Chim Acta. 2008;629:136–44.
Chapple ILC, Mason GI, Garner I, Matthews JB, Thorpe GH, Maxwell SRJ, Whitehead TP. Enhanced chemiluminescent assay for measuring the total antioxidant capacity of serum. Saliva Crevicular Fluid. 1997;34:412–21.
Acknowledgements
NKM acknowledges the financial support received from Science and Engineering Research Board (SERB), Department of Science and Technology, Govt of India under Core Research Grant (CRG) Scheme (File number CRG/ 2020/003060). We extend our special thanks to Department of Biotechnology, Manipal Institute of Technology. We also acknowledge the help of Dr. Anusha Prabhu for proof-reading the manuscript and for her fruitful discussion. We also thank Mr. Sujesh S for assisting us during figure preparation.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Narasimhan, A., Jain, H., Muniandy, K. et al. Bio-analysis of Saliva Using Paper Devices and Colorimetric Assays. J. Anal. Test. 8, 114–132 (2024). https://doi.org/10.1007/s41664-023-00282-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41664-023-00282-y