Abstract
This study investigated the acute effects of the dietary nootropic stack CILTEP®. It contains a combination of ingredients that have been individually reported to improve cognitive performance. Especially, the ingredients luteolin, which is considered a phosphodiesterase type 4 (PDE4) inhibitor, and forskolin, an adenylate cyclase stimulator, were of interest since they can increase the second messenger cAMP and thus also intracellular signaling. Numerous studies have shown that inhibition of PDE4 can improve memory in animals and humans. We examined whether acute dosing of 3 capsules of CILTEP® would improve cognitive function in healthy participants aged 30 to 40 (n = 33). We used a randomized, double-blind, placebo-controlled, two-way cross-over design. Our test battery was aimed at measuring memory performance, attention, and sensorimotor speed. The primary outcome measures were the performance on the verbal learning task and the spatial pattern separation task. Secondary outcomes included other cognitive tests, event-related potentials (ERPs), and assessment of the activity of the enzyme beta-glucuronidase and its effect on the bioavailability of luteolin, heart rate, and blood pressure. No relevant effects of acute CILTEP® treatment were found on any measure of the test battery or ERPs. Blood plasma concentrations of luteolin increased, yet about 2000 times too low to likely exert any PDE4 inhibition. CILTEP® treatment did neither affect heart rate nor blood pressure. In summary, there is no evidence that a single standardized dose of 3 capsules of CILTEP® can improve cognitive function in healthy middle-aged participants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nootropics, also called “smart drugs” or “cognitive enhancers,” are substances that have been alleged to improve cognitive performance. While this research is still emerging and the mechanisms underlying cognition are complex and multifaceted, some nootropics such as, e.g., L-theanine, caffeine, and modafinil, have shown to improve attention (Barbhaiya et al., 2008; Giesbrecht et al., 2010; Einöther et al., 2010), verbal memory (Barbhaiya et al., 2008; Illieva et al., 2015), creativity (Müller et al., 2013), and executive function (Killgore et al., 2009) in cognitively healthy individuals. Nootropics can be divided into three different categories: dietary supplements, synthetic compounds, and prescription drugs (Malik et al., 2007). Most nootropics, especially those that fall under the dietary supplement category, do not need the approval of the Food and Drug Administration (FDA) or Federal Trade Commission (FTC) and are therefore not monitored or scheduled (Howland, 2010). Dietary nootropics are usually not one isolated supplement/ingredient, however, contain a combination of nootropic substances to support the best possible cognitive performance. These are most often called supplement stacks or nootropic stacks. A nootropic stack combines substances that claim to work well together to act as a synergistic substance, i.e., the effect of two or more substances becomes more powerful and better in combination than either of them separately (Jedrejko et al., 2023).
CILTEP®, recently also sold as Neurofuel™, is a dietary nootropic stack, which contains many plant-derived ingredients that have been claimed to improve brain function. One of the ingredients, artichoke extract, contains multiple bioactive compounds of which one is luteolin. Luteolin is a polyphenolic flavone found in multiple herbs, vegetables, and fruits. Luteolin falls under the class of non-selective phosphodiesterase (PDE) inhibitors class 1 to 5 (Yu et al., 2010). In particular, the inhibition of PDE4 is of interest as PDE4 is responsible for breaking down the second messenger cyclic-adenosine monophosphate (cAMP). Thus, by inhibiting PDE4, intracellular cAMP levels increase. Another component of CILTEP® is forskolin, an ingredient that stimulates adenylate cyclase, which produces cAMP, and thereby increases intracellular cAMP levels (Balakrishnan et al., 2016). Intracellular cAMP signaling plays a pivotal role in long-term potentiation, a neurobiological substrate of memory formation (Bollen et al., 2014). Preclinical research by Vanmierlo and colleagues (2016) showed that roflumilast administration, a PDE4 inhibitor, improved memory performance in an object location task and spatial Y-maze (Vanmierlo et al., 2016). A study by Zhang et al. (2004) showed that PDE4 administration led to improved learning and memory in the radial arm maze test, possibly through neuroprotective mechanisms that include anti-inflammatory effects and protection against oxidative stress (Zhang et al., 2004). Recent human clinical studies found that acute administration of roflumilast improved episodic memory performance on the verbal learning test (VLT) in healthy adults and elderly participants (Blokland et al., 2019; M. A. Van Duinen et al., 2018a, 2018b). In line with this, previous research has shown that acute treatment with a PDE4 inhibitor improves memory performance in both animals and humans.
Luteolin and forskolin increase intracellular cAMP levels by different mechanisms of action. It is assumed that together they have a synergistic effect on neuronal cAMP signaling underlying memory processes, so a more powerful effect than either of them separately. Additional ingredients in CILTEP® are vitamin B6 (Bryan et al., 2002; Li et al., 2014) and L-phenylalanine (Eckart et al., 2014), which are both needed for the production of the neurotransmitter dopamine and acetyl-L-carnitine, which increases energy metabolism and mitochondrial function (Chen et al., 2017b; Kobayashi et al., 2010). All three ingredients may further support brain function as well causing synergistic effects on cognition considering that all ingredients of CILTEP® fall within a similar range of peak plasma concentration of between 30 min and 3 h (Li et al., 2013; Nulman & Koren, 2009; Sangeetha et al., 2011; Stegink & Filer Jr, 1984; Stegink et al., 1981; Wittemer et al., 2005).
Employing a double-blind, placebo-controlled, two-way cross-over design, the effect of the dietary nootropic CILTEP® on cognition was investigated in healthy middle-aged participants who had normal cognitive performance according to their age, sex, and education. The primary objective of this study was to investigate the effects of CILTEP® on cognition, with a specific focus on episodic memory, including the verbal learning test and spatial pattern separation test. We selected these cognitive tasks as our primary objectives due to the demonstrated potential of PDE4 inhibitors (e.g., roflumilast), represented by luteolin and forskolin in CILTEP®, to enhance neuronal communication, synaptic plasticity, and overall improve memory formation and retrieval. Specifically, episodic memory and spatial working memory, as assessed by the VLT and the spatial pattern separation task, rely on the integrity of neuronal circuits in the hippocampus (Burgess et al., 2002; Maguire et al., 2000). Within the hippocampus, cAMP signaling pathways are involved in mediating synaptic plasticity, such as long-term potentiation (LTP) and long-term depression (LTD), which are mechanisms underlying learning and memory (Silva et al., 1998). Additionally, compared to other brain structures, PDE4 isoforms are expressed in various cell types in within the hippocampus, including neurons and glial cells (Tibbo et al., 2019). The specific localization of PDE4 isoforms within different cellular compartments may regulate the spatial and temporal dynamics of cAMP signaling, thereby influencing synaptic plasticity and memory processes in distinct ways (Gong et al., 2004). It is assumed that all the individual components of CILTEP® have an additive or even synergistic effect on cognition. Accordingly, it was hypothesized that an acute dose, i.e., a single administration of 3 capsules of CILTEP®, would improve episodic memory in healthy middle-aged participants.
Our secondary objective included the measurement of performance on other cognitive tasks: working memory (n-back task), information processing speed (digit symbol substitution test), response inhibition and focused attention (Stroop task), complex scanning, and visual tracking (the trail making test), and reaction time (simple and choice reaction task). This diverse selection of cognitive tasks was chosen deliberately to provide a thorough assessment covering a spectrum of cognitive abilities, thereby enabling a comprehensive understanding of the potential effects of CILTEP® on cognitive function across multiple domains. An additional secondary objective was to measure event-related potentials (ERPs) during some computerized cognition tasks to investigate task-related brain activity by linking neural activity to behavior, possibly detecting early cognitive processes and differentiating them, as well as possible central effects of CILTEP®. Further, we wanted to verify the activity of the enzyme beta-glucuronidase in blood plasma, as it has a modulating effect on the bioavailability of luteolin. Finally, basic vital parameters such as blood pressure and heart rate were measured to evaluate the effect of CILTEP® on autonomic function which functions as safety monitoring and interplay between cognitive performance and cardiovascular function, as forskolin is known to reduce blood pressure.
Materials and Methods
Study Design and Population
All procedures were approved by the local Medical Research Ethical Committee (Medisch Ethische Toetsingscommissie azM/UM) and were in accordance with the Helsinki Declaration of 1975. The study was monitored and audited by the Clinical Trial Center Maastricht (CTCM) (https://www.ctcm.nl/en). The study was conducted according to a double-blind, placebo-controlled, cross-over design. We performed a within-subjects design, characterized by administering CILTEP® and placebo conditions to the all participants. This approach included two testing sessions for each participant, separated by a one-week wash-out period to mitigate any residual effects of the treatment. Importantly, the sequence in which the treatment and control conditions were administered was randomized across participants to robustly control for potential treatment order effects that could confound our results. Healthy individuals between the ages of 30 and 40 years were recruited through advertisements. Participants were screened and selected based on their memory performance on the VLT. Cognitive performance was considered within the norm when individuals scored within the range of − 1 and + 1 standard deviation (SD) from the predicted normative score (see (Van der Elst et al., 2005)).
Assuming a clinically meaningful minimum effect size of 0.5 (Cohen d), power calculation resulted in a group size of 27 (power 0.8; alpha 0.05). According to Natural Stacks®, approximately 20% of the CILTEP® users do not report any effect (personal communication). Therefore, the group size was increased to 33. Furthermore, in previous acute studies, approximately 9% of the participants dropped out of the study due to personal reasons (Blokland et al., 2019). Therefore, we assumed a dropout rate of 9% and increased the group size to 36. Inclusion was continued until 33 participants were included, i.e., after screening 45 individuals.
Exclusion criteria included major cardiac, hepatic, renal, pulmonary, neurological, gastrointestinal, hematological, endocrinological, or psychiatric illness (depression, bipolar disorder, anxiety disorder, panic disorder, psychosis, attention deficit hyperactivity disorder, and first-degree relative with a psychiatric disorder or history with a psychiatric disorder). Other exclusion criteria included body mass index (BMI) under 18.5 or higher than 30, excessive alcohol consumption (> 20 units of alcohol per week), smoking, pregnancy or currently lactating, use of psychoactive medication, centrally acting beta blockers, use of illicit drugs (e.g., amphetamines, barbiturates, benzodiazepines, cannabinoids, cocaine, and opiates) from 2 weeks before until the completion of the experiment, systolic blood pressure above 160 mmHg, phenylketonuria, and any sensory or motor deficits which could reasonably be expected to affect test performance. Participants using steroids or Sudafed (pseudoephedrine) were also excluded.
Intervention
CILTEP® and placebo capsules were kindly provided by Natural Stacks®. Natural Stacks® is manufactured, packaged, and labeled according to the certified good manufacturing process (GMP) guidelines. The placebo capsules were identical to the CILTEP® capsules and contained rice flour, silica, and ascorbyl palmitate as therapeutically inactive ingredients. On two separate occasions, participants received an oral acute dose of CILTEP® (3 capsules; see Table 1 for the composition of ingredients of one capsule) or a placebo (3 capsules, no active ingredient). The wash-out period between treatment conditions was at least 7 days. Luteolin has a predicted half-life of fewer than 19 h (Liu et al., 2013). The treatment order was performed by counterbalancing. Sixty minutes after capsule intake, participants began the cognitive tasks. This decision is guided by the understanding that key constituents of CILTEP®, namely, luteolin and forskolin, typically attain peak plasma levels in humans within 30 to 60 min post-ingestion, with an elimination half-life ranging between 2 and 3 h (Li et al., 2013; Sangeetha et al., 2011). Before arriving on the test day, participants were asked not to eat, as they received a light meal upon arrival. The light meal did not contain any flavonoids/luteolin to avoid any effect on CILTEP®. After the light meal, participants were not allowed to eat for two hours before the intervention (CILTEP®/placebo). Participants were asked not to consume any caffeinated products or alcohol in the 24 h preceding the test day.
Pharmacokinetics
Two blood samples were collected. One blood sample to determine baseline pre-treatment beta-glucuronidase activity and one blood sample to measure luteolin levels after CILTEP® treatment. The first blood sample was collected in sodium heparin tubes (5 ml) by venipuncture on both test days before administration of treatment (placebo or CILTEP®) at T-30 and the second blood sample (5 ml) was collected after completion of neuropsychological testing at T120. Following collection, blood samples were immediately placed on ice and centrifuged at 3500 rpm at 4 °C for 10 min within 30 min. Plasma was collected and stored at – 80 °C. High-performance liquid chromatography (HPLC) analysis was performed as described previously (Bartholome et al., 2010; Cheruvu et al., 2018; Zheng et al., 2014). The detection range of luteolin was 0.5–5 ng/ml. < LLOQ = below lower limit of quantification (< 0.5 ng/ml). < LOD = below limit of detection.
Neurocognitive Testing
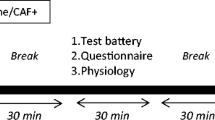
Following a positive medical evaluation and successful cognitive screening participants were familiarized with the setting and the cognitive test battery to minimize learning effects. See Table 2 for an overview of the test day which was identical for each participant. In order to capture participants in their optimal cognitive state, the VLT and spatial pattern separation test have been strategically placed at the beginning of the cognitive task sequence as we anticipate that (most) ingredients of CILTEP® will have reached maximum blood plasma value at this time. This positioning aims to maximize the precision and reliability of our assessments of immediate verbal memory and spatial pattern separation, which serve as primary outcomes in this study. Additionally, the delayed recall of the VLT is performed 30 min after the immediate recall. The same order of cognitive testing for each participant and test day allows for a reliable comparison between CILTEP® and placebo.
Verbal Learning Task (VLT)
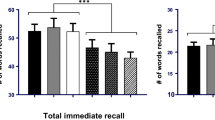
Verbal memory was assessed by an adjusted version of the Rey Auditory Verbal Learning Test (Lezak, 1995): the Visual Verbal Learning Test (Riedel et al., 1999). This test consists of a list of 30 Dutch monosyllabic words (18 nouns, 12 adjectives) of which participants are instructed to recall as many as possible. All words are presented one by one on a computer screen with an interval of one word per three seconds, in three trials with the same word sequence. After each trial, participants are asked to recall as many words as they remember from the list (immediate recall). Thirty minutes after the last and third trials, the participants are asked to recall as many words as possible (delayed recall). Dependent variables obtained from this task include the total correctly recalled words over three trials (VLT trial immediate total) and the number of correctly recalled words in the delayed recall (VLT delayed recall). ERPs obtained from this task included the N400, P300 (P3a and P3b), and P600, for the three immediate recall trials only.
The P300, in this case, P3b, is probably the most widely used ERP component in cognitive research. From the memory perspective, it has been shown to play a role in working memory, but more generally speaking, it is said to reflect activity related to updating the mental representation of the stimulus context (Polich & Criado, 2006). The N400, on the other hand, is sensitive to stimulus repetition and varies with the amount of context available, commonly showing a less negative amplitude when stimuli are presented in a known context (Dunn et al., 1998; Olichney et al., 2011). The P600 is consistently associated with recognition from memory, showing larger amplitudes for memorized than for new items (e.g., (Addante et al., 2012; Rugg & Curran, 2007)). Finally, there may be functional links between the P300 and P600 in the role they play in memory processing (Fields, 2023).
Spatial Pattern Separation Task
The spatial pattern separation task (Gilbert et al., 1998) assesses episodic memory using a series of 140 color images of everyday neutral objects on a white background. The task consisted of two phases: in the first phase, which is the encoding phase, the participant is asked to decide whether an image belongs to the category “outdoor” or “indoor” by pressing a button (140 items total, 2 s each, and 0.5 s inter-stimulus interval). Immediately following the encoding phase, the test phase was presented, in which participants were instructed regarding a surprise recognition memory test that required participants to accurately identify whether images were in the same location on the screen or not compared to the encoding phase (140 items total, 2 s each, and 0.5 s inter-stimulus interval). Forty images were presented in the same position as in the first phase. The other images were presented in another location on the screen, ranging from close to more distant (4 different distances/locations, 20 images each), and 20 images were presented in an opposing corner. Thus, the similarity of the spatial information varied, creating five levels (lures) of mnemonic interference. The participants had to indicate using the keyboard whether the images were in the same place or a different position on the screen as compared to the first phase. Dependent variables obtained from this task are how many images were correctly identified that stayed in the same location (SPS accuracy repeat), lure one (SPS lure accuracy one), lure two (SPS lure accuracy two), lure three (SPS lure accuracy three), lure four (SPS lure accuracy four), and the corner of the screen (SPS lure accuracy corner).
n-Back Task
The n-back (Owen et al., 2005) task measures the working memory function. Participants are instructed to monitor a sequence of stimuli and respond when the current stimulus matches the one from n-trials previously in the sequence. In this study, we used a 0-back, 1-back, and 2-back task, in which the 0-back was a simple focused attention/speed task, and the 1- and 2-back required retrieving information from working memory, namely, retrieving whether the same item as presented was also presented 1 or 2 stimuli back. Dependent variables obtained from this task include the number of accurate responses for 0-back (N-back accuracy S0L), 1-back (N-back accuracy S1L), and 2-back (N-back accuracy S2L), i.e., the number of times the participant pressed the button when the stimulus matches the one from n steps earlier, and median reaction time (RT) for 0-back (N-back RT S0L), 1-back (N-back RT S1L), and 2-back (N-back RT S2L). ERP obtained from this task was the P300.
Digit-Symbol Substitution Task
The digit-symbol substitution task (Wechsler, 1981) assesses complex scanning and visual tracking. The screen shows a series of 9 numbered symbols that represent a “key.” The participant was presented with a series of parallel boxes that contained a symbol in the top half of the box. The participant had to provide a matching “number” response for the bottom half by referring to the key. The dependent variable was calculated by how many correct responses/matches were made within 90 s (DSST correct score).
Simple and Choice Reaction Time Task
The simple and choice reaction time task (Houx et al., 1993) was divided into two parts. First, the participant is instructed to react as soon as the button lights up in the center of the response box (red button). In the second part, one of three possible buttons lights up. The participant is instructed to respond and push the target button as quickly as possible. Dependent variables obtained from this task included RT for simple (SRT) and choice (CRT), as well as movement time (MT) for simple (SMT) and choice conditions (CMT). RT refers to the time in milliseconds needed to release the red button and MT refers to the milliseconds needed to move from the red button to the target button. The RT and the MT have been log-transformed.
Trail-Making-Test
The trail-making-test (TMT) (Reitan, 1956) was used to examine attention and concept-shifting abilities. It is divided into parts A and part B, both consisting of 25 circles distributed over a sheet of paper. In part A, the circles are numbered 1–25, and the participant is instructed to connect the numbers in ascending order. In part B, the circles include both numbers (1–13) and letters (A–L). Again, the participant is instructed to connect the circles in an ascending pattern, but with the added task of alternating between numbers and letters. In both tasks, the participant is not allowed to lift the pencil off the paper and to connect the circles as fast as possible. The dependent variables are calculated separately by the seconds required to complete the task for parts A (TMT-A) and B (TMT-B).
Stroop Color-Word Task
The Stroop task (Hammes, 1973; Stroop, 1935) assesses response inhibition (interference) and focused attention. In this task, color names are printed in colored ink. In the congruent category, the color name and the color of the ink were the same, in the incongruent category, they were not. Participants are instructed to name the color of the ink, not the word itself. However, interference occurs because of the urge to read the printed words (even if one is asked to ignore them). Since the printed words and ink color differed in the incongruent category, interference is larger in this category than in the congruent category (Jasper, 1958). The colors used in this task are blue, red, green, and yellow. The ink color has to be named by pressing one out of four buttons, each representing one of the colors. Dependent variables obtained from this task included the number of errors made in both the congruent (Stroop misses congruent) and incongruent conditions (Stroop misses incongruent), as well as correct answers given in both the congruent (Stroop hits congruent) and incongruent (Stroop hits incongruent) conditions.
Electroencephalography (Jobert et al.) and Electrooculogram (EOG) Acquisition
The electrophysiological activity was recorded with 32 EEG electrodes and placed according to the international 10–20 system (Jasper, 1958). Reference and ground were placed at the linked mastoids and the forehead, respectively. Eye movements were detected by horizontal and vertical EOG recordings. Before electrode attachment, the positions were slightly scrubbed with a gel to provide a good measurement. Both EEG and EOG were filtered between 0.01 and 100 Hz and sampled at 500 Hz.
Statistical Analyses
Neuropsychological Tests
All data were checked for outliers and then subjected to statistical tests. Statistical analyses were performed using SPSS. For tests with single testing points, we used paired t-tests to compare the performance of the two groups. In the case of multiple measurements on the same parameter, we used a repeated measures analysis. For RT measurements, the median was used as the analysis, except for the simple and choice reaction time task, the RT was log-transformed. Missing data were not considered for analysis. The significance level was set at alpha = 0.05. We used the Holm-Bonferroni approach to correct for multiple pairwise comparisons. In this case, the significance level was set at alpha = 0.0014.
EEG
EEG data were analyzed using Brain Vision Analyzer 2 (Brain Products, GmbH) software. High-pass (1 Hz) and low-pass (30 Hz) filters were applied offline. Next, EOG activity was removed from the signal using the Gratton and Coles method in Vision Analyzer. Subsequently, segments were made from 100 ms before stimulus onset until 1000 ms after onset, using the last 100 ms before stimulus onset as a baseline. The segments were baseline corrected and then checked for artifacts and excluded if an artifact occurred during the first 1000 ms after stimulus presentation. Next, averages were calculated for each stimulus type and treatment. The grand average was used to determine the ERP components. For the VLT, peak detection windows were defined as the most positive or negative value between the following intervals: P3a (210–290 ms), P3b (290–360 ms), N400 (340–470 ms), and P600 (450–700 ms). For the n-back, the P300 (210–350 ms) was determined. The analyses were performed for three different electrodes (frontal (Fz), central (Cz), and parietal (Pz)). The peaks of both groups were compared with an ANOVA with repeated measures.
Blood Plasma Measurements
No outlier tests were possible for the luteolin measurements as too many samples were below the limit of the detection (Glodny et al., 2000). Therefore, a non-parametrical test was performed for the statistical analysis. In addition, the absolute number of luteolin measurements was so low, that paired testing would result in a loss of power. Therefore, the non-parametric non-paired Mann–Whitney U test was applied to all plasma measurements.
Results
Socio-demographic Characteristics
Participants’ ages ranged from 30 to 40 years (M = 33.7, SD = 3.1) of which 51% identified as female. Out of all participants, 79% had a high level of education and 21% had a medium level of education (based on the education level according to Verhage (De Bie & Vegter, 1987; Verhage, 1964)).
Physiological and Neuropsychological Tests
Two significant effects were found, namely, a slower motion time in the simple reaction time task and an increased spatial separation accuracy repeat post-CILTEP® treatment; however, after correcting for multiple comparisons with the Holm-Bonferroni approach, these two significant results were not significant anymore. The rest of the physiological measurements and neuropsychological tests did not show a significant difference between placebo and CILTEP®. An overview of the different physiological and neuropsychological tests is shown in Tables 3 and 4.
Verbal Learning Task (VLT)-EEG
The data of the different components of the ERPs during the 3 successive presentations of the words in the VLT task are presented in Table 5. Although the main effect of electrode position was found for the P3a amplitude (F(2, 64) = 2.64, p < 0.01), no treatment effects were found (all associated F-values < 0.40, n.s.). For the P3b, a stimulus repetition effect was found for amplitude (F(2, 64) = 8.38, p < 0.01) indicating that this amplitude increased across the three trials. No treatment effects were found (all associated F-values < 1.0, n.s.). The analysis of the N400 showed that this peak became less negative with repeated presentations (F(2, 64) = 24.18, p < 0.01). Additionally, there were differential effects for the electrodes (F(2,64) = 7.55, p < 0.01). No treatment effects were observed for the N400 (all associated F-values < 2.2, n.s.). The P600 was affected by the electrode position (F(2, 64) = 68.87, p < 0.001) and the trials (F(2, 64) = 11.57, p < 0.001). No treatment effects were observed for the P600 (all associated F-values < , n.s.) The peak increased with repeated presentation of the stimuli. There were no differences for latencies (all associated F-values < 1, n.s.).
n-Back-EEG
Comparable to the different components in the VLT task, the P300 component in the n-back task was affected by the electrode position (F(2, 64) = 9.41, p < 0.01; see Table 6). With increasing difficulty in this task the amplitude of the P300 decreased in both treatment groups equally (F(2, 64) = 51.91, p < 0.01). No treatment effects were found (all associated F-values < 1, n.s.). There were no differences found for the latencies in the different conditions (all associated F-values < 1, n.s.).
Beta-glucuronidase and Luteolin
We could not obtain blood from 8 participants divided over both pre- and post-treatment. In addition, luteolin measurements were below the lower limit of detection for 26 participants of the placebo group and 1 participant of the CILTEP® group. Consequently, these samples were not used for analyses. As can be seen from Table 7, there was no difference in plasma baseline beta-glucuronidase activity before placebo and CILTEP® treatment. CILTEP® treatment resulted in a 20-fold increase in the luteolin concentration compared to placebo treatment. To investigate a possible relationship between beta-glucuronidase activity at baseline and plasma luteolin concentration, beta-glucuronidase activity before CILTEP® treatment was correlated with plasma luteolin concentration after CILTEP® treatment. No correlation was found (Pearson’s correlation coefficients − 0.07, n.s., respectively).
Discussion
This study aimed to investigate the acute treatment effects of CILTEP® on cognitive performance with a test battery that taps into different domains of cognition. The acute effects were assessed in a group of middle-aged participants. In addition, we measured ERPs in some tasks to see whether brain activity was altered by CILTEP® treatment. Lastly, baseline beta-glucuronidase activity and pre-treatment and luteolin concentration in blood plasma post-treatment were measured, as well as the effects of CILTEP® on heart rate and blood pressure.
We assessed neuropsychological performance in different cognitive domains. Based on our previous findings with acute treatment with the PDE4 inhibitor roflumilast, we expected an improvement in verbal learning performance, specifically the delayed recall in the VLT task (Blokland et al., 2019; M. Van Duinen et al., 2018a, 2018b). However, although CILTEP® is assumed to exert PDE4 inhibition, we did not observe any effect on verbal learning performance. The other neurocognitive tests also showed no effects of CILTEP®, except a slower response of MT in a simple reaction time task and an increased spatial separation accuracy repeat post-CILTEP® treatment; however, after correcting for multiple comparisons with the Holm-Bonferroni approach, these two significant results were not significant anymore. Accordingly, we argue that these are not relevant effects. EEG measurements, specifically ERP components, are indicative of a central effect of treatment and are usually known to be more sensitive to treatment effects (Jobert et al., 2012). In this study, we examined the effects of CILTEP® treatment on different ERP components in the VLT and n-back tasks. There was no indication that CILTEP® had any effect on these EEG measures. Lastly, we measured heart rate and blood pressure before and after the intake of CILTEP®, however, found no significant effects on heart rate or blood pressure compared to placebo.
As expected, the baseline levels of beta-glucuronidase activity before treatment were not different between placebo and CILTEP® administration. This suggests that baseline glucuronidase activity is unlikely to affect luteolin metabolism after CILTEP® administration. To investigate a possible relationship between beta-glucuronidase activity at baseline and plasma luteolin concentration, beta-glucuronidase activity before CILTEP® treatment was correlated with plasma luteolin concentration after CILTEP® treatment. No correlation was found. Plasma luteolin concentration was higher after CILTEP® administration compared to placebo administration. It is interesting to note that after placebo administration, almost all luteolin plasma concentrations were below the LOD. Only 5 of the 31 participants had measurable luteolin concentrations. In fact, in all but one of these placebo samples, measurements were still below the lower limit of quantification, i.e., the lowest value of the standard curve. This indicates that luteolin concentrations in the plasma before CILTEP® treatment are very low and usually not detectable. In contrast, the luteolin concentration after CILTEP® treatment could be reliably determined (approximately a 20-fold increase), clearly indicating that CILTEP® treatment increases blood plasma luteolin concentrations. Calculation of the plasma luteolin concentration in molarities results in a concentration of 4.22 nM. luteolin inhibits PDE1-5 with IC50 values of 10 μM or higher (Ayoub & Melzig, 2006; Rohrig et al., 2017). Thus, the plasma concentration of luteolin is still at least 2370-fold lower than the IC50 of any PDE type. Consequently, PDE inhibition is unlikely due to increased luteolin concentrations in plasma (or brain) after CILTEP® administration.
It is important to note that the population chosen for this study, namely, healthy highly educated middle-aged adults, warrants cognitive and physiological exceptions. Age-related peaks in various cognitive abilities are heterogeneous and complex; thus, floor or ceiling effects related to some cognitive tasks cannot be ruled out (Hartshorne & Germine, 2015). However, individuals were selected for participation based on their performance on the VLT, i.e., the main outcome. The interpretation of performance was based on normative scores (z-scores), where individuals were only admitted to participation if they performed within a normal range (1 SD above or below the norm) based on their age, sex, and education, thus reducing a floor and ceiling effect specific to episodic memory. However, selecting an older population (i.e., above the age of 65), a population that is cognitively impaired, or a population that also includes low education could potentially result in different outcomes in terms of performance on cognitive tasks and/or physiological measurements such as ERPs, heart rate, and blood pressure.
This study investigated the acute effects of CILTEP® on cognitive performance. This inherently raises the question of whether chronic and long-term administration of CILTEP® could result in different outcomes. As discussed earlier, in our acute findings, luteolin plasma concentration levels were too low, 2370-fold, to inhibit any PDE type. Consequently, we hypothesize that chronic administration of CILTEP® would not be able to improve cognition by increasing luteolin. B6, another ingredient in CILTEP®, is an essential nutrient. Vitamin B6 deficiency is hyperactive in the noradrenergic system, which can lead to cognitive impairment (Toriumi et al., 2021). This suggests that in adults who are B6 deficient, CILTEP could enhance cognition, as one CILTEP capsule includes 384% of the daily requirement of B6. However, a Cochrane review showed that there was no evidence of benefit from vitamin B6 supplementation on the mood or cognition of older people with normal vitamin B6 status or with vitamin B6 deficiency (Malouf & Grimley Evans, 2003). A meta-analysis by Zhang and colleagues has shown that high B6 concentrations in elderly populations had no benefit on cognition or dementia risk (Zhang et al., 2020).
CILTEP® also includes L-phenylalanine. L-phenylalanine is not known to have a direct impact on cognition, but it modulates the metabolism of dopamine, norepinephrine, and epinephrine, which in turn affects mood, anxiety, attentiveness, and motivation (van Ruitenbeek et al., 2009). One study found a positive correlation between l-phenylalanine and cognitive assessment scores in patients with amnestic mild cognitive impairment (aMCI) (Ravaglia et al., 2004). There has been much research on l-phenylalanine as an anti-depressant or as an intervention for individuals with ADHD (Akram et al., 2020). However, no studies (to our knowledge) have been conducted with healthy participants. Accordingly, no suggestions can be made as to whether chronic administration of l-phenylalanine would be able to modulate dopamine and thus increase cognition, mood, anxiety, attentiveness, and motivation.
Acetyl-L-carnitine (ALC), the second most prominent ingredient in CILTEP®, is a widely studied supplement related to cognition and cognitive impairment, and its underlying mechanisms have been shown to restore cell membranes and synaptic function, enhance cholinergic activity, promote mitochondrial energy metabolism, protect against toxins, and exert neurotrophic and nootropic effects in (Pennisi et al., 2020) in healthy elderly and patients with AD or MCI. However, differences in methodology and assessment tools make it difficult to compare existing studies. Thus, available evidence and the role of ALC are still subject to debate, but the findings have been promising. Suggestions from critical reviews published in 2017 (Chen et al., 2017a) and 2020 (Pennisi et al., 2020) suggested that future studies should focus on large samples, higher doses, and prolonged treatments in healthy, elderly, and individuals with cognitive impairment.
Note that each ingredient in CILTEP® may exhibit distinct pharmacokinetic profiles and mechanisms of action, leading to variations in the onset, duration, and magnitude of their effects. Furthermore, interactions between ingredients within the stack may influence the time course of cognitive enhancement. For example, synergistic interactions between L-phenylalanine and acetyl-L-carnitine could potentiate neurotransmitter synthesis and mitochondrial function, leading to sustained improvements in cognitive performance over time. Considering these factors, it is anticipated that the time course of the intervention’s effects may be multifaceted, with some ingredients exerting rapid, acute effects, while others may contribute to more gradual, sustained improvements in cognitive function. Future studies utilizing longitudinal assessments and pharmacokinetic analyses could provide valuable insights into the dynamics of cognitive enhancement following ingestion of CILTEP®.
In conclusion, acute treatment with 3 capsules of CILTEP® does not improve cognitive performance in healthy middle-aged participants compared to placebo. Linked to this, ERP measurements do not indicate an effect of acute treatment of CILTEP® on brain activity. Plasma luteolin levels after CILTEP® treatment were below IC50 levels of PDE4 inhibition and therefore unlikely to have any effect on cognitive performance. It remains to be determined whether chronic CILTEP® administration or CILTEP® administration in an elderly or cognitively impaired population may show positive effects on cognitive performance.
Data Availability
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Addante, R. J., Ranganath, C., & Yonelinas, A. P. (2012). Examining ERP correlates of recognition memory: Evidence of accurate source recognition without recollection. Neuroimage, 62(1), 439–450. https://doi.org/10.1016/j.neuroimage.2012.04.031
Akram, M., Daniyal, M., Ali, A., Zainab, R., Shah, S. M. A., Munir, N., & Tahir, I. M. (2020). Role of phenylalanine and its metabolites in health and neurological disorders. In Synucleins-Biochemistry and Role in Diseases. IntechOpen. https://doi.org/10.5772/intechopen.83648
Ayoub, S., & Melzig, M. F. (2006). Induction effects of apigenin, luteolin and vinpocetin on neutral endopeptidase (NEP) and angiotensin-converting enzyme activity (ACE) of SK-N-SH cells. Natural Product Communications, 1(8), 633–639. <Go to ISI>://WOS:000249006500007.
Balakrishnan, S., Niebert, M., & Richter, D. W. (2016). Rescue of cyclic AMP mediated long term potentiation impairment in the hippocampus of Mecp2 knockout (Mecp2(-/y) ) mice by rolipram. Frontiers in Cellular Neuroscience, 10, 15. https://doi.org/10.3389/fncel.2016.00015
Barbhaiya, H. C., Desai, R. P., Saxena, V. S., Pravina, K., Wasim, P., Geetharani, P., ... & Amit, A. J. J. P. T. (2008). Efficacy and tolerability of BacoMind® on memory improvement in elderly participants-a double blind placebo controlled study. https://doi.org/10.3923/jpt.2008.425.434
Bartholome, R., Haenen, G., Hollman, C. H., Bast, A., Dagnelie, P. C., Roos, D., Keijer, J., Kroon, P. A., Needs, P. W., & Arts, I. C. (2010). Deconjugation kinetics of glucuronidated phase II flavonoid metabolites by beta-glucuronidase from neutrophils. Drug Metabolism and Pharmacokinetics, 25(4), 379–387. https://doi.org/10.2133/dmpk.dmpk-10-rg-002
Blokland, A., Van Duinen, M. A., Sambeth, A., Heckman, P. R. A., Tsai, M., Lahu, G., Uz, T., & Prickaerts, J. (2019). Acute treatment with the PDE4 inhibitor roflumilast improves verbal word memory in healthy old individuals: A double-blind placebo-controlled study. Neurobiology of Aging, 77, 37–43. https://doi.org/10.1016/j.neurobiolaging.2019.01.014
Bollen, E., Puzzo, D., Rutten, K., Privitera, L., De Vry, J., Vanmierlo, T., Kenis, G., Palmeri, A., D’Hooge, R., Balschun, D., Steinbusch, H. M., Blokland, A., & Prickaerts, J. (2014). Improved long-term memory via enhancing cGMP-PKG signaling requires cAMP-PKA signaling. Neuropsychopharmacology, 39(11), 2497–2505. https://doi.org/10.1038/npp.2014.106
Bryan, J., Calvaresi, E., & Hughes, D. (2002). Short-term folate, vitamin B-12 or vitamin B-6 supplementation slightly affects memory performance but not mood in women of various ages. The Journal of Nutrition, 132(6), 1345–1356. https://doi.org/10.1093/jn/132.6.1345
Burgess, N., Maguire, E. A., & O’Keefe, J. (2002). The human hippocampus and spatial and episodic memory. Neuron, 35(4), 625–641. https://doi.org/10.1016/s0896-6273(02)00830-9
Chen, N., Yang, M., Zhou, M., Xiao, J., Guo, J., & He, L. (2017a). L-carnitine for cognitive enhancement in people without cognitive impairment. Cochrane Database of Systematic Reviews, 3(3), CD009374. https://doi.org/10.1002/14651858.CD009374.pub3
Chen, N., Yang, M., Zhou, M., Xiao, J., Guo, J., & He, L. (2017b). L-carnitine for cognitive enhancement in people without cognitive impairment. Cochrane Database of Systematic Reviews, 3, CD009374. https://doi.org/10.1002/14651858.CD009374.pub3
Cheruvu, H. S., Yadav, N. K., Valicherla, G. R., Arya, R. K., Hussain, Z., Sharma, C., Arya, K. R., Singh, R. K., Datta, D., & Gayen, J. R. (2018). LC-MS/MS method for the simultaneous quantification of luteolin, wedelolactone and apigenin in mice plasma using hansen solubility parameters for liquid-liquid extraction: Application to pharmacokinetics of Eclipta alba chloroform fraction. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences, 1081, 80–90. https://doi.org/10.1016/j.jchromb.2018.01.035
De Bie, S. D., & Vegter, J. (1987). Standaardvragen 1987 voorstellen voor uniformering van vraagstellingen naar achtergrondkenmerken in interviews. Vereniging van onderzoek instituten (VOI). Retrieved 28 February 2024, from: https://books.google.nl/books/about/Standaardvragen_1987.html?id=hBBuAAAACAAJ&redir_esc=y
Dunn, B. R., Dunn, D. A., Languis, M., & Andrews, D. (1998). The relation of ERP components to complex memory processing. Brain and Cognition, 36(3), 355–376. https://doi.org/10.1006/brcg.1998.0998
Eckart, C., Fuentemilla, L., Bauch, E. M., & Bunzeck, N. (2014). Dopaminergic stimulation facilitates working memory and differentially affects prefrontal low theta oscillations. NeuroImage, 94, 185–192. https://doi.org/10.1016/j.neuroimage.2014.03.011
Einöther, S. J., Martens, V. E., Rycroft, J. A., & De Bruin, E. A. (2010). L-theanine and caffeine improve task switching but not intersensory attention or subjective alertness. Appetite, 54(2), 406–409. https://doi.org/10.1016/j.appet.2010.01.003
Fields, E. C. (2023). The P300, the LPP, context updating, and memory: What is the functional significance of the emotion-related late positive potential? International Journal of Psychophysiology, 192, 43–52. https://doi.org/10.1016/j.ijpsycho.2023.08.005
Gilbert, P. E., Kesner, R. P., & DeCoteau, W. E. (1998). Memory for spatial location: Role of the hippocampus in mediating spatial pattern separation. Journal of Neuroscience, 18(2), 804–810. https://doi.org/10.1523/JNEUROSCI.18-02-00804.1998
Giesbrecht, T., Rycroft, J., Rowson, M., & De Bruin, E. (2010). The combination of L-theanine and caffeine improves cognitive performance and increases subjective alertness. Nutritional neuroscience, 13(6), 283–290. https://doi.org/10.1179/147683010x12611460764840
Glodny, B., Kuhle, C., Cromme, S., Brockmann, J., & Winde, G. (2000). An assessment of diagnostic procedures preparatory to retroperitoneoscopic removal of adenoma in cases of primary hyperaldosteronism. Endocrine Journal, 47(6), 657–665. https://doi.org/10.1507/endocrj.47.657
Gong, B., Vitolo, O. V., Trinchese, F., Liu, S., Shelanski, M., & Arancio, O. (2004). Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. The Journal of Clinical Investigation, 114(11), 1624–1634. https://doi.org/10.1172/JCI22831
Hammes, J. (1973). The STROOP color-word test: manual. https://doi.org/10.1007/springerreference_183455
Hartshorne, J. K., & Germine, L. T. (2015). When does cognitive functioning peak? The asynchronous rise and fall of different cognitive abilities across the life span. Psychological Science, 26(4), 433–443. https://doi.org/10.1177/0956797614567339
Houx, P. J., Jolles, J., & Vreeling, F. W. (1993). Stroop interference: Aging effects assessed with the Stroop Color-Word Test. Experimental Aging Research, 19(3), 209–224. https://doi.org/10.1080/03610739308253934
Howland, R. H. (2010). Drug therapies for cognitive impairment and dementia. Journal of Psychosocial Nursing and Mental Health Services, 48(4), 11–14. https://doi.org/10.3928/02793695-20100311-01
Ilieva, I. P., Hook, C. J., & Farah, M. J. (2015). Prescription stimulants’ effects on healthy inhibitory control, working memory, and episodic memory: a meta-analysis. Journal of cognitive neuroscience, 27(6), 1069–1089. https://doi.org/10.1162/jocn_a_00776
Jasper, H. H. (1958). Ten-twenty electrode system of the international federation. Electroencephalography and Clinical Neurophysiology, 10, 371–375. https://doi.org/10.1080/00029238.1961.11080571
Jedrejko, K., Catlin, O., Stewart, T., Anderson, A., Muszynska, B., & Catlin, D. H. (2023). Unauthorized ingredients in “nootropic” dietary supplements: A review of the history, pharmacology, prevalence, international regulations, and potential as doping agents. Drug Testing and Analysis. https://doi.org/10.1002/dta.3529
Jobert, M., Wilson, F. J., Ruigt, G. S., Brunovsky, M., Prichep, L. S., Drinkenburg, W. H., & Committee, I.P.-E.G. (2012). Guidelines for the recording and evaluation of pharmaco-EEG data in man: The International Pharmaco-EEG Society (IPEG). Neuropsychobiology, 66(4), 201–220. https://doi.org/10.1159/000343478
Killgore, W. D., Kahn-Greene, E. T., Grugle, N. L., Killgore, D. B., & Balkin, T. J. (2009). Sustaining executive functions during sleep deprivation: a comparison of caffeine, dextroamphetamine, and modafinil. Sleep, 32(2), 205–216. https://doi.org/10.5665/sleep/32.2.205
Kobayashi, S., Iwamoto, M., Kon, K., Waki, H., Ando, S., & Tanaka, Y. (2010). Acetyl-L-carnitine improves aged brain function. Geriatrics & Gerontology International, 10(Suppl 1), S99-106. https://doi.org/10.1111/j.1447-0594.2010.00595.x
Lezak, M. D. (1995). Neuropsychological assessment. Oxford University Press, USA. https://doi.org/10.1017/s1355617700000758
Li, L. P., Wu, X. D., Chen, Z. J., Sun, S. Y., Ye, J. F., Zeng, S., & Jiang, H. D. (2013). Interspecies difference of luteolin and apigenin after oral administration of Chrysanthemum morifolium extract and prediction of human pharmacokinetics. Pharmazie, 68(3), 195–200. https://doi.org/10.1691/ph.2013.2744
Li, M. M., Yu, J. T., Wang, H. F., Jiang, T., Wang, J., Meng, X. F., Tan, C. C., Wang, C., & Tan, L. (2014). Efficacy of vitamins B supplementation on mild cognitive impairment and Alzheimer’s disease: A systematic review and meta-analysis. Current Alzheimer Research, 11(9), 844–852. https://doi.org/10.2174/1567205011666141001114140
Liu, Y., Gou, L. S., Tian, X., Fu, X. B., Ling, X., Sun, L. Y., Lan, N., Li, S., & Yin, X. X. (2013). Protective effects of luteolin on cognitive impairments induced by psychological stress in mice. Experimental Biology and Medicine (Maywood), 238(4), 418–425. https://doi.org/10.1177/1535370213477985
Maguire, E. A., Gadian, D. G., Johnsrude, I. S., Good, C. D., Ashburner, J., Frackowiak, R. S., & Frith, C. D. (2000). Navigation-related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences, 97(8), 4398–4403. https://doi.org/10.1073/pnas.070039597
Malik, R., Sangwan, A., Saihgal, R., Jindal, D. P., & Piplani, P. (2007). Towards better brain management: Nootropics. Current Medicinal Chemistry, 14(2), 123–131. https://doi.org/10.2174/092986707779313408
Malouf, R., & Grimley Evans, J. (2003). The effect of vitamin B6 on cognition. Cochrane Database of Systematic Reviews, (4), CD004393. https://doi.org/10.1002/14651858.CD004393
Müller, U., Rowe, J. B., Rittman, T., Lewis, C., Robbins, T. W., & Sahakian, B. J. (2013). Effects of modafinil on non-verbal cognition, task enjoyment and creative thinking in healthy volunteers. Neuropharmacology, 64, 490–495. https://doi.org/10.1016/j.neuropharm.2012.07.009
Nulman, I., & Koren, G. (2009). Pharmacokinetic comparison of a delayed-release combination of doxylamine succinate and pyridoxine hydrocholoride (Diclectin) and oral solutions of these drugs in healthy women of childbearing age. Canadian Journal of Clinical Pharmacology, 16(3), e400-406. Retrieved 28 February 2024, from: https://jptcp.com/index.php/jptcp/article/view/546
Olichney, J. M., Yang, J. C., Taylor, J., & Kutas, M. (2011). Cognitive event-related potentials: biomarkers of synaptic dysfunction across the stages of Alzheimer′s disease. Journal of Alzheimer’s Disease, 26 Suppl 3(0 3), 215–228. https://doi.org/10.3233/JAD-2011-0047
Owen, A. M., McMillan, K. M., Laird, A. R., & Bullmore, E. (2005). N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human Brain Mapping, 25(1), 46–59. https://doi.org/10.1002/hbm.20131
Pennisi, M., Lanza, G., Cantone, M., D'Amico, E., Fisicaro, F., Puglisi, V., Vinciguerra, L., Bella, R., Vicari, E., & Malaguarnera, G. (2020). Acetyl-L-carnitine in dementia and other cognitive disorders: A critical update. Nutrients, 12(5). https://doi.org/10.3390/nu12051389
Polich, J., & Criado, J. R. (2006). Neuropsychology and neuropharmacology of P3a and P3b. International Journal of Psychophysiology, 60(2), 172–185. https://doi.org/10.1016/j.ijpsycho.2005.12.012
Ravaglia, G., Forti, P., Maioli, F., Bianchi, G., Martelli, M., Talerico, T., Servadei, L., Zoli, M., & Mariani, E. (2004). Plasma amino acid concentrations in patients with amnestic mild cognitive impairment or Alzheimer disease. The American Journal of Clinical Nutrition, 80(2), 483–488. https://doi.org/10.1093/ajcn/80.2.483
Reitan, R. M. (1956). Investigation of relationships between psychometric and biological intelligence. The Journal of Nervous and Mental Disease, 123(6), 536–541. https://doi.org/10.1097/00005053-195606000-00004
Riedel, W. J., Klaassen, T., Deutz, N. E., van Someren, A., & van Praag, H. M. (1999). Tryptophan depletion in normal volunteers produces selective impairment in memory consolidation. Psychopharmacology (Berl), 141(4), 362–369. https://doi.org/10.1007/s002130050845
Rohrig, T., Pacjuk, O., Hernandez-Huguet, S., Korner, J., Scherer, K., & Richling, E. (2017). Inhibition of cyclic adenosine monophosphate-specific phosphodiesterase by various food plant-derived phytotherapeutic agents. Medicines (Basel), 4(4). https://doi.org/10.3390/medicines4040080
Rugg, M. D., & Curran, T. (2007). Event-related potentials and recognition memory. Trends in Cognitive Sciences, 11(6), 251–257. https://doi.org/10.1016/j.tics.2007.04.004
Sangeetha, S., Samanta, M., Manjunatha, N., & Tiwari, S. (2011). Establishment of pharmacokinetic parameters for the herbal drug containing forskolin. Journal of Pharmacy Research, 4(7), 2303–2306. Retrieved 28 February 2024, from: https://d1wqtxts1xzle7.cloudfront.net/43804280/98-libre.pdf?1458189092=&response-content-disposition=inline%3B+filename%3DEstablishment_of_Pharmacokinetic_Paramet.pdf&Expires=1709155414&Signature=IMV~D2iwpmMUSXajaVbUQWvuQqGAZS9bIkhr1mT6ex6la8Ii11OOfoDcNzIY-XDtsGVSDgjCXCiKK4w41MPWSzGkNX5B-AA0WcQtWnkb2YoqRucezG7oBuFyUDDcQJtLwmHcB~s5~-vKEd84kmGonjfXxxzgcfbzGRG4j~hoBZ~E2Eva2Sq2EgjaYM4WQunaaUES~0emfMbyDnHAwAP0nbR1N-36wj-5qL1M-ca037-SZliOV7N4JPzdI82oWnjb2ZdKcIBkmIrjLlmQAfT1tE3rqnSgYt5h7n2xj0Is~rZdYGdjF9CzZqJwtWR~vA5HAaXPjtRwgXL87H~zr0~LcQ__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA
Silva, A. J., Kogan, J. H., Frankland, P. W., & Kida, S. (1998). CREB and memory. Annual Review of Neuroscience, 21(1), 127–148. https://doi.org/10.1146/annurev.neuro.21.1.127
Stegink, L. D., Filer, L. J., Jr., & Baker, G. L. (1981). Plasma and erythrocyte concentrations of free amino acids in adult humans administered abuse doses of aspartame. Journal of Toxicology and Environmental Health, 7(2), 291–305. https://doi.org/10.1080/15287398109529980
Stegink, L. D., & Filer Jr, L. (1984). Aspartame. In Physiology and Biochemistry. New York: M. Dekker Inc. https://doi.org/10.1201/9781003065289
Stroop, J. R. (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 18(6), 643. https://doi.org/10.1037/h0054651
Tibbo, A. J., Tejeda, G. S., & Baillie, G. S. (2019). Understanding PDE4’s function in Alzheimer’s disease; a target for novel therapeutic approaches. Biochemical Society Transactions, 47(5), 1557–1565. https://doi.org/10.1042/BST20190763
Toriumi, K., Miyashita, M., Suzuki, K., Yamasaki, N., Yasumura, M., Horiuchi, Y., Yoshikawa, A., Asakura, M., Usui, N., Itokawa, M., & Arai, M. (2021). Vitamin B6 deficiency hyperactivates the noradrenergic system, leading to social deficits and cognitive impairment. Translational Psychiatry, 11(1), 262. https://doi.org/10.1038/s41398-021-01381-z
Van der Elst, W., van Boxtel, M. P., van Breukelen, G. J., & Jolles, J. (2005). Rey’s verbal learning test: Normative data for 1855 healthy participants aged 24–81 years and the influence of age, sex, education, and mode of presentation. Journal of the International Neuropsychological Society, 11(3), 290–302. https://doi.org/10.1017/S1355617705050344
Van Duinen, M., Sambeth, A., Heckman, P., Smit, S., Tsai, M., Lahu, G., Uz, T., Blokland, A., & Prickaerts, J. (2018a). Acute administration of roflumilast enhances immediate recall of verbal word memory in healthy young adults. Neuropharmacology, 131, 31–38. https://doi.org/10.1016/j.neuropharm.2017.12.019
Van Duinen, M. A., Sambeth, A., Heckman, P. R. A., Smit, S., Tsai, M., Lahu, G., Uz, T., Blokland, A., & Prickaerts, J. (2018b). Acute administration of roflumilast enhances immediate recall of verbal word memory in healthy young adults. Neuropharmacology, 131, 31–38. https://doi.org/10.1016/j.neuropharm.2017.12.019
van Ruitenbeek, P., Sambeth, A., Vermeeren, A., Young, S. N., & Riedel, W. J. (2009). Effects of L-histidine depletion and L-tyrosine/L-phenylalanine depletion on sensory and motor processes in healthy volunteers. British Journal of Pharmacology, 157(1), 92–103. https://doi.org/10.1111/j.1476-5381.2009.00203.x
Vanmierlo, T., Creemers, P., Akkerman, S., van Duinen, M., Sambeth, A., De Vry, J., Uz, T., Blokland, A., & Prickaerts, J. (2016). The PDE4 inhibitor roflumilast improves memory in rodents at non-emetic doses. Behavioural Brain Research, 303, 26–33. https://doi.org/10.1016/j.bbr.2016.01.031
Verhage, F. (1964). Intelligentie en leeftijd bij volwassenen en bejaarden. Retrieved 28 February 2024, from: https://pure.rug.nl/ws/portalfiles/portal/3465406/intelligentieenleeftijd.pdf
Wechsler, D. (1981). The psychometric tradition: developing the wechsler adult intelligence scale. Contemporary Educational Psychology. https://doi.org/10.1016/0361-476x(81)90035-7
Wittemer, S. M., Ploch, M., Windeck, T., Muller, S. C., Drewelow, B., Derendorf, H., & Veit, M. (2005). Bioavailability and pharmacokinetics of caffeoylquinic acids and flavonoids after oral administration of Artichoke leaf extracts in humans. Phytomedicine, 12(1–2), 28–38. https://doi.org/10.1016/j.phymed.2003.11.002
Yu, M. C., Chen, J. H., Lai, C. Y., Han, C. Y., & Ko, W. C. (2010). Luteolin, a non-selective competitive inhibitor of phosphodiesterases 1–5, displaced [3H]-rolipram from high-affinity rolipram binding sites and reversed xylazine/ketamine-induced anesthesia. European Journal of Pharmacology, 627(1–3), 269–275. https://doi.org/10.1016/j.ejphar.2009.10.031
Zhang, H.-T., Zhao, Y., Huang, Y., Dorairaj, N. R., Chandler, L. J., & O’Donnell, J. M. (2004). Inhibition of the phosphodiesterase 4 (PDE4) enzyme reverses memory deficits produced by infusion of the MEK inhibitor U0126 into the CA1 subregion of the rat hippocampus. Neuropsychopharmacology, 29(8), 1432–1439. https://doi.org/10.1038/sj.npp.1300440
Zhang, C., Luo, J., Yuan, C., & Ding, D. (2020). Vitamin B12, B6, or folate and cognitive function in community-dwelling older adults: A systematic review and meta-analysis. Journal of Alzheimer’s Disease, 77(2), 781–794. https://doi.org/10.3233/JAD-200534
Zheng, S. R., Ma, Z. Y., Han, H. X., Ye, J. F., Wang, R. W., Cai, S., Zhou, H., Yu, L. S., Zeng, S., & Jiang, H. D. (2014). Post-column mobile phase adjustment: A strategy to eliminate the contradiction between liquid chromatography and mass spectrometry in the determination of flavonoids in rat plasma. Journal of Pharmaceutical and Biomedical Analysis, 95, 176–183. https://doi.org/10.1016/j.jpba.2014.02.024
Funding
This project was financed by the University funding of AB and partly supported by Natural Stacks®.
Author information
Authors and Affiliations
Contributions
NP: investigation, project administration, data curation, formal analysis, visualization, writing—original draft, and writing—review and editing. SC: conceptualization and investigation, methodology, and project administration. AS: conceptualization, software, funding acquisition, methodology, supervision, and validation. AB: conceptualization, funding acquisition, resources, methodology, supervision, and validation.
Corresponding author
Ethics declarations
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
AB and AS have a proprietary interest in the PDE4 inhibitor roflumilast for the treatment of cognitive impairment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Possemis, N., Caldenhove, S., Sambeth, A. et al. Acute Treatment with the Nootropic CILTEP® Does Not Improve Cognitive Performance in Healthy Middle-Aged Participants. J Cogn Enhanc 8, 95–106 (2024). https://doi.org/10.1007/s41465-024-00288-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41465-024-00288-z