Abstract
Neurofeedback (NF) is an important treatment for attention deficit/hyperactivity disorder (ADHD). In ADHD, cognitive control deficits pose considerable problems to patients. However, NF protocols are not yet optimized to enhance cognitive control alongside with clinical symptoms, partly because they are not driven by basic cognitive neuroscience. In this study, we evaluated different EEG theta and/or beta frequency band NF protocols designed to enhance cognitive control. Participants were n = 157 children and adolescents, n = 129 of them were patients with ADHD (n = 28 typically developing (TD) controls). Patients with ADHD were divided into five groups in the order of referral, with four of them taking part in different NF protocols systematically varying theta and beta power. The fifth ADHD group and the TD group did not undergo NF. All NF protocols resulted in reductions of ADHD symptoms. Importantly, only when beta frequencies were enhanced during NF (without any theta regulation or in combination with theta upregulation), consistent enhancing effects in both response inhibition and conflict control were achieved. The theta/beta NF protocol most widely used in clinical settings revealed comparatively limited effects. Enhancements in beta band activity are key when aiming to improve cognitive control functions in ADHD. This calls for a change in the use of theta/beta NF protocols and shows that protocols differing from the current clinical standard are effective in enhancing important facets of cognitive control in ADHD. Further studies need to examine regulation data within the neurofeedback sessions to provide more information about the mechanisms underlying the observed effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Attention deficit hyperactivity disorder (ADHD) is a prevalent, multi-faceted disorder in childhood and adolescence. Apart from pharmacological treatments using stimulants (e.g. methylphenidate) or non-stimulants (e.g. atomoxetine), non-pharmacological treatment approaches are increasingly applied (dosReis et al., 2017; Ng et al., 2017; Schatz et al., 2015). One major approach is “neurofeedback training” (NF) which has frequently been shown to ameliorate ADHD symptoms and associated problems (Arns & Strehl, 2013; Arns et al., 2009; Bluschke et al., 2016a, 2016b; Gevensleben et al., 2010; Holtmann et al., 2014; Lofthouse et al., 2012; Micoulaud-Franchi et al., 2015). The basic principle behind NF is that EEG-recorded brain activity is visualized and directly fed back to patients, e.g. via simple computer games. Using this feedback, the patient trains to up- or downregulate a predefined aspect of brain activity that is thought to be associated with the desired behavioural outcomes (like increased attention in the case of ADHD, for example). Building on this association, NF is thought to exert therapeutic effects based on the principles of operant conditioning and increasing habituation. There are several forms of NF, the most established form of which (“theta/beta NF”) could be called the “gold standard” of NF. During this approach, the commonly applied procedure in clinical settings is to train the patient to decrease activity in the theta (θ) frequency band and to concomitantly increase activity in the beta (β) frequency band (i.e. a θ↓β↑ protocol). This protocol has been developed on the basis of two considerations: theta frequency activity has commonly been interpreted as an indicator of daydream-like, in-alert states (i.e. this state should be decreased in ADHD), while beta band activity has been thought to reflect a state of focused attention and mental arousal (i.e. this state should be increased in ADHD) (Bluschke et al., 2016a, 2016b).
Although meta-analyses and review articles corroborated that this form of NF ameliorates clinically assessed ADHD symptoms (Enriquez-Geppert et al., 2019; Lambez et al., 2020; Lofthouse et al., 2012; Riesco-Matías et al., 2021; Van Doren et al., 2019; Yan et al., 2019), this procedure is not without problems (Bluschke et al., 2016a, 2016b; Catalá-López et al., 2017; Cortese et al., 2016; Razoki, 2018). Rather than solely relying on rather subjective symptom assessments, it seems useful to employ objective measures of functionality when evaluating the effects of neurofeedback (Berger, 2011; Emser et al., 2018). In the case of ADHD, such objective measures commonly are measures of different domains of executive functions resp. cognitive control (Diamond, 2013) which, albeit not universally deficient in all affected patients (Willcutt et al., 2005), are frequently reported and represent a clinically relevant facet of the disorder (Faedda et al., 2019; Krieger & Amador-Campos, 2018; Krieger et al., 2019; Miklós et al., 2019; Skogli et al., 2017), especially as far as academic functioning (Sibley et al., 2019) and time spent on task (Dekkers et al., 2017) are concerned. Importantly, it has been argued (Bluschke et al., 2016a, 2016b) that the currently widely established θ↓β↑ NF protocol may not be optimal when aiming to ameliorate difficulties in executive functions in ADHD. Especially, it does not seem useful to train patients with ADHD to downregulate theta band activity (as done in the standard θ↓β↑ NF protocol) when aiming to improve cognitive control deficits (e.g. response inhibition and interference control). This presumption is based on the fact that several lines of basic cognitive neuroscience research have shown for high performance in inhibitory control and (attentional) interference control to be associated with a transient upregulation of processes for which theta band activity is relevant (Cavanagh & Frank, 2014; Cohen, 2014; Wascher et al., 2012). From the basic cognitive neuroscience point of view, a training protocol focussing on the increase of theta band activity thus seems more suitable (Bluschke et al., 2016a, 2016b). Concerning beta band activity, its functional relevance and importance in theta/beta NF protocols and its effect on cognitive control processes in ADHD is largely elusive. Basic research suggests that beta band activity is important to allow a high functioning of cognitive (attentional) and sensorimotor systems (Engel & Fries, 2010; Studer et al., 2014; Womelsdorf & Everling, 2015) and enables response regulation processes (Bluschke et al., 2020; Dahan et al., 2018) allowing sensory information to efficiently guide subsequent motor actions (MacKay, 1997). This may be the reason why training to upregulate beta band activity is useful for the enhancement of cognitive control processes (Bluschke et al., 2020, 2016a, 2016b). Concerning ADHD, some data suggest that particularly the modulation of beta band activity may be of importance (Bluschke et al., 2020).
No study has yet presented a systematic comparison of NF protocols modulating theta and/or beta frequencies. Specifically, a comparison against the θ↓β↑ protocol reflecting the “gold standard” of NF concerning the effectiveness in modulating different cognitive control functions seems necessary. The current study closes this gap. Specifically, it aims to examine the effects of theoretically driven manipulations of the classical NF protocol on objective performance measures assessing cognitive control functions bearing a significant relevance for the day-to-day functioning of patients with ADHD. As primary outcome measures, we thus focus on response inhibition/impulsivity examined by a Go/Nogo task and on (attentional) interference control examined by a flanker task (see “Methods” section for details). We compare various ADHD groups receiving different NF protocols: (i) a group receiving a standard θ↓β↑ NF protocol (θ↓β↑), (ii) a group receiving a NF protocol to upregulate both theta and beta frequency activity (θ↑β↑), (iii) a group receiving a NF protocol to only upregulate theta band activity (θ↑) and (iv) a group receiving a NF protocol to only upregulate beta band activity (β↑). Aside from the direction of modulation of theta and/or beta band activity, the NF procedure was identical in all groups (see “Methods” section for details). Since basic neuroscience research suggests that cognitive control requires enhanced theta and beta oscillations (Bluschke et al., 2020, 2016a, 2016b), this study did not include protocols which only aimed at training participants to decrease power in both or one of these frequency bands. Before and after NF, the same Go/Nogo and flanker tasks were used to test response inhibition and interference control performance. In addition to the ADHD groups treated with NF, a group of patients with ADHD not taking part in NF was examined to control for retest effects (no NF). Similarly, a group of typically developing healthy controls (TD) not receiving an intervention was examined.
We hypothesize that changes in task performance before vs. after NF training (in the Go/Nogo and the flanker task) will be stronger in NF protocols training the upregulation of theta and/or beta frequency bands than it is the case for the standard θ↓β↑ NF protocol. Concerning the Go/Nogo task, we hypothesize that specific effects will occur in inhibition errors (i.e. Nogo false alarms), since demands on cognitive control depending on theta/beta oscillations are particularly high in these trials. For similar reasons, we expect specific effects in the flanker task due to modulations in the incompatible condition (i.e. where flanker and target stimuli point into opposite directions). We hypothesize that patients with ADHD and typically developing controls not receiving an intervention between testing time points will not show any changes in performance in the Go/Nogo and the flanker task. At present, no clear-cut hypotheses can be stated as to whether stronger or more consistent effects of the examined NF protocols will be evident for the different examined cognitive control functions. Further, it is important to stress that our design has not been conceptualized in order to compare the effects of different NF protocols on clinical parameters or even their effectiveness. Instead, the goal was to examine the effects of theoretically driven manipulations of the common NF protocol on objective performance measures by assessing cognitive control functions which bear a significant relevance for the day-to-day functioning of patients with ADHD. To provide a basic estimate of symptom severity and changes within it, we additionally report parent-rated ADHD symptoms as measured using the AD(H)D Symptom Checklist (Döpfner et al., 2008) (secondary outcome measure). Further, it will also be examined whether any changes in the power of the frequency bands at rest (i.e. during the baseline measurements) and during training (i.e. “training success”) actually occur between the first and the last neurofeedback session.
Methods
This study was conducted in a naturalistic setting in the outpatient department of the Clinic for Child and Adolescent Psychiatry and Psychotherapy of a large university hospital. Overall, n = 231 children and adolescents were included in the study between April 2014 and October 2020.
A Priori Sample Size Estimation
In previous studies on the effects of NF in ADHD, we reported effect sizes of the treatment of approx. ηp2 = 0.1 (Bluschke et al., 2018, 2016a, 2016b). This estimate was used to calculate the required sample size in the current study to detect an effect of NF treatment with a power of at least 95% (alpha level = 5%, 6 groups, 2 measurement time points, conservative estimation of the correlation among the repeated measures of r = 0.2). The power calculation using G*Power revealed a total necessary sample size of n = 84; i.e. n = 14 patients per group. To ensure an even higher robustness of the effects, we nearly doubled the sample size (n = 157) and no included group was smaller than n = 21 patients (Table 1).
Sample Description and Study Design
N = 38 of the initially included participants did not complete their participation and were thus not included in further analyses (see Table 1). Of those who completed participation, n = 28 were typically developing controls who were recruited via advertisements and an in house data base. The other n = 169 participants were children and adolescents with ADHD, in whom ADHD diagnoses had been determined according to standard clinical guidelines by a team of experienced child and adolescent psychiatrists and psychologists (incl. family and school interviews and questionnaires, IQ and attention testing, exclusion of possible somatic differential diagnoses via blood analyses, EEG, audiometry and vision testing). To match the characteristics of the groups in regard to age, IQ, medication status and group size, some participants were subsequently excluded from analysis (see Table 1). This was done purely based on demographic data and irrespective of any outcome measures. In total, n = 129 patients with ADHD were eventually included in the analyses. All participants had been diagnosed in the outpatient clinic and fulfilled criteria for ADHD according to ICD-10 criteria. N = 14 had an axis I comorbidity (n = 1 OCD, n = 2 adjustment disorder, n = 6 tic disorder, n = 3 CD/ODD, n = 2 emotional problems). N = 22 of the ADHD patients had received an additional axis II diagnosis. Patients were only considered for participation if ADHD was the main diagnosis and if the clinical indication for neurofeedback was confirmed by the treating clinicians. The n = 129 patients eventually included in analyses belonged to five groups (for details, see Fig. 1/Table 2). Group allocation to the different neurofeedback groups took place in the order of referral (order: no NF, θ↓β↑, θ↑β↑, θ↑ and β↑). All participants took part in two assessments (T1 and T2) in the space of 8 weeks. These took place independent of the NF sessions and were conducted by different members of staff who were unaware of any contents of the NF training. Due to the COVID-19 pandemic, a slightly larger number of participants from θ↑ and β↑ groups did not complete their training and/or the T2 assessment (Table 1).
Participants included in four of these groups took part in different forms of NF (details see below and Fig. 1). One ADHD group was trained to downregulate theta and upregulate beta power (i.e. ratio training) (θ↓β↑). Another ADHD group was trained to upregulate both theta and beta power (θ↑β↑). Regulation was rewarded when either theta or beta power was increased successfully. A third ADHD group trained to upregulate theta only (θ↑), while a fourth ADHD group only trained beta upregulation (β↑). The fifth group of patients with ADHD did not take part in any training (no NF). Further, we included a group of typically developing control children (TD). A semi-structured telephone interview was used to confirm the absence of any other neurological or psychiatric conditions. The absence of ADHD symptoms in the TD group was confirmed using the AD(H)D Symptom Checklist (Döpfner et al., 2008) (details below). This instrument assesses ADHD criteria in the domains of inattention, hyperactivity and impulsivity. Its psychometric properties are comparable to those of the internationally commonly used Conners rating scale (Erhart et al., 2008). All participants took part in two testings (Fig. 1). The T1 testing took place before the start of NF in the four treated groups. The T2 testing took place after 8 weeks of NF. In the no NF group and the TD group, T1 and T2 also took place 8 weeks apart. During this time, no intervention took place in these groups. Any ongoing treatment for ADHD in any of the participants remained constant and no new interventions were initiated. At both time points, participants completed two computer tasks measuring varying theta-/beta-related aspects of executive functions. The Go/Nogo task was used to assess behavioural inhibition while the flanker task measured conflict processing (details see below). After each of these appointments, participant families received 5 Euros as compensation. Parents were asked to complete the ADHD Symptom Checklist (Döpfner et al., 2008) at both time points. All groups do not differ from each other in terms of age (F(5,149) = 2.01; p = 0.08; ηp2 = 0.06) or IQ (F(5,142) = 1.28; p = 0.28; ηp2 = 0.05) as assessed by the WISC-IV or V (Table 2). There were no differences between the five included ADHD groups regarding medication status (χ2(4) = 3.6, p = 0.47) (Table 2). Medication intake was kept stable across the duration of study participation in all groups. Both laboratory testings were conducted in the afternoons to ensure comparability between the T1 and T2 appointment.
Using the AD(H)D Symptom Checklist (Döpfner et al., 2008), parents rated their children on a scale of 0 (no problems) to 3 (severe problems) in regard to the ADHD core symptoms of inattention, hyperactivity and impulsivity. Due to compliance problems, complete questionnaire data (i.e. both at T1 and T2) were only available for n = 99 subjects. ADHD symptom levels at T1 or T2 did not differ significantly between the five ADHD groups (see below). Typically developing controls presented with significantly lower ratings than patients with ADHD in all domains (all t ≥ 5.3; all p ≤ 0.001) (see Table 3 for details and descriptive data).
All participants and their parents or legal guardians provided written informed consent before any study procedure was commenced. The study was conducted according to the Declaration of Helsinki and approved by the ethics committee of the Medical Faculty of the TU Dresden.
Neurofeedback Protocols and Training Procedures
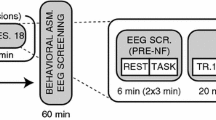
The different NF protocols and study groups are schematically illustrated in Fig. 1.
For all groups, NF training took place in two weekly sessions (1 h each) over a period of 8 weeks. NF was administered by a small team of trained psychologists in the outpatient clinic. To the participating children and adolescents, neurofeedback was introduced as an “attention training on the computer” during which they were supposed to make a cartoon character/race car move on the screen. Apart from pointing out the relevance of attention in general, no instructions on how to achieve this were provided. Participants’ strategies were to be collected during the sessions in order to increase transferability to day-to-day life. Apart from the differences in the trained frequency band(s), all procedures and elements of the treatment were kept constant across participants. The EEG in the theta (4–8 Hz) and/or beta frequency range (13–20 Hz) were recorded at electrode Cz. Eye movements were recorded from electrodes above and below the left eye. These recordings were used to correct for motion artefacts online since these could alter the results if not accounted for. The reference electrode was placed on the left mastoid and an electrode on the forehead was used as the ground electrode. Theta and beta frequency ranges were shown to participants via a custom-made software (“Self-regulation and Attention Management” (“SAM”), University of Erlangen). Time intervals containing artefacts occurring as a result of excessive movement were discarded online and were not included in the feedback procedure. Strong movement artefacts, as well as blinking artefacts, were removed online since they may affect the estimation of theta activity (max 100 µV). In case of excessive movements, children were presented with a sad smiley face in order to remind them to reduce movements. This was also shown in case of activity below a lower threshold (5 µV), in which case the connection of the electrode to the skull was checked and re-established by the psychologist if needed. For artefacts in the frequency range of 25–35 Hz, the bound was defined at 10 μV. Theta and beta power at rest was recorded in a 2-min interval at the beginning of each session (baseline). This data was analysed in order to examine whether any changes had occurred as a result of the training (see “Neurofeedback Baseline and Training Data” section for details). Unfortunately, due to technical issues (one irreparable laptop used for neurofeedback training and data recording), baseline and training data is only available for n = 73 of the n = 106 participants who had taken part in the neurofeedback training. During training, children were tasked with trying to move a cartoon character on the screen by regulating the frequencies as required (immediate and continuous feedback). Within the animation, frequency changes were shown to participants via moving bars on the screen. In each session, three to six NF blocks were conducted (5–10-min duration).
From neurofeedback session 4 or 5 onwards, transfer blocks were introduced (delayed feedback). Here, children were required to perform a task (e.g. attention game, reading, schoolwork) without being given direct feedback. Instead, they received delayed feedback showing them in how far they were able to regulate the theta and/or beta power in the required direction. Performance was reviewed with the participant at the end of each block (with immediate or delayed feedback). To ensure comparability with standard protocols (Gevensleben et al., 2010, 2009a, 2009b), NF additionally contained elements of behavioural therapy (e.g. psychoeducation, homework, token system). Strategies on how to achieve control over the feedback animation were not provided to participants. However, during the course of training, participants were asked about any strategies they employed during neurofeedback. As part of the homework, participants were tasked with applying these individual strategies (e.g. “focus on one thing only”) in attention-demanding situations in school or at home.
Due to the nature of the experimental variation (i.e. group allocation in order of referral), it was not feasible to conduct the neurofeedback training in a blinded fashion. However, staff conducting testings at time point T1 and T2 and analysing behavioural data were unaware of group membership. In addition, although participants had been informed about the use of different neurofeedback protocols within the study, this was of no practical implication to them during training since they were simply instructed as to which frequency bands needed to be regulated in which direction.
Tasks
During the Go/Nogo task (Beste et al., 2011; Chmielewski et al., 2015), either the word “DRÜCK” (German for “PRESS”; Go stimulus) or “STOP” (Nogo stimulus) was presented for 300 ms in white font on a black background. Participants were required to perform a button press with the right index finger as fast as possible (i.e. within 500 ms) after seeing the Go stimulus. In contrast, when seeing the Nogo stimulus, participants were asked to refrain from responding. The inter-trial interval (ITI) was jittered between 1600 and 1800 ms. In total, the experiment consisted of 248 Go trials and 112 Nogo trials presented in four blocks a pseudo-randomized order. The task lasted approximately 20 min. For the analysis, we examined the rate of Nogo false alarms, the rate of correct Go hits as well as reaction times in the Go trials.
In the flanker task, vertically arranged white arrowheads were presented in the middle of the screen on a black background. Target stimuli pointing to the left or right were displayed in the centre of the screen. Two hundred milliseconds before target onset, two arrowheads (i.e. flanker stimuli) were presented above and below the target. These flanker stimuli were pointing in the same (compatible, 67% of trials) or opposite (incompatible, 33% of trials) direction as the target arrow. Target stimuli were presented for 300 ms. Flanker stimuli were displayed until target onset. The response-stimulus interval was pseudo-randomized between 1400 and 1800 ms. In order to enhance the conflict, time pressure was administered by asking participants to respond within 450 ms after target onset. A warning tone (1000 Hz, 60 dB SPL) was presented 1200 ms after the response if it had occurred more than 450 ms after the target. The flanker task consisted of four blocks of 120 stimuli each (i.e. 480 trials in total) and lasted approximately 20 min. For the analysis, we examined the reaction times and error rates in compatible and incompatible trials. Due to a technical error, T2 flanker data is missing for one participant in the θ↓β↑ group and the θ↑β↑ group.
Results and Statistical Analyses
Statistical Analysis
Mixed-effects ANOVAs including the within-subject factor Time Point (T1 vs. T2) and the between-subjects factor Group (θ↓β↑ vs. θ↑β↑ vs. θ↑ vs. β↑ vs. no NF vs. TD) were used to examine the effects of the four NF protocols on task performance. Analyses in the flanker task additionally included the within-subject factor Compatibility (compatible vs. incompatible trials). Any significant interactions were followed up by ANOVAs and/or independent/paired-sample t-tests. Greenhouse–Geisser correction was applied and post hoc tests were Bonferroni-corrected as necessary.
Go/Nogo Task
Behavioural parameters showing performance in the Go/Nogo task are shown in Fig. 2.
Descriptive statistics (mean ± SE) for Go hits (A), Go reaction times (B) and Nogo false alarms (C). Blue bars represent testing time point T1; orange bars show testing time point T2. Data is shown for four groups of patients with ADHD treated with four different neurofeedback protocols (θ↓β↑: trained to downregulate theta and upregulate beta power, θ↑β↑: trained to upregulate both theta and beta power, θ↑: trained to upregulate theta only, β↑: trained to upregulate beta only) and for two control groups (no NF: patients with ADHD who did not take part in any training, TD: typically developing control children). Asterisks indicate level of significance of conducted post hoc t-tests
Data concerning the Go trials (accuracy and reaction times) revealed no effects and is presented in the supplemental material. Descriptive data are shown in Fig. 2A and B.
Concerning Nogo false alarms, we found a significant main effect of Time (F(1,149) = 29.8; p ≤ 0.001; ηp2 = 0.17; T1: 40.8 ± 1.7%; T2: 34.9 ± 1.7%), indicating a general performance improvement at time point T2. In addition, we also found a significant main effect of Group (F(5,149) = 5.1; p ≤ 0.001; ηp2 = 0.15; θ↓β↑: 44.1 ± 3.7%, θ↑β↑: 32.8 ± 3.8%, θ↑: 34.8 ± 4.4%, β↑: 37.8 ± 3.8%, no NF: 51.8 ± 4.2%, TD: 25.8 ± 3.9%), indicating general group differences in performance. Most importantly, the Time*Group interaction was also significant (F(5,149) = 2.4; p = 0.04; ηp2 = 0.07). We thus opted to not analyse or interpret the main effect of Group any further. Descriptive data are shown in Fig. 2C. To analyse this interaction more closely, we first examined group differences at the two measurement times. A significant main effect of Group was present both at T1 (F(5,149) = 4.6; p = 0.001; ηp2 = 0.14) and at T2 (F(5,149) = 5.0; p ≤ 0.001; ηp2 = 0.14).
Bonferroni-corrected post hoc tests showed significantly lower false alarm rates in the TD group compared to the no NF group (p = 0.001) and the θ↓β↑ group (p = 0.003) at T1 (Fig. 2C). At time point T2, only the patients with ADHD not taking part in NF (no NF group) still presented with more false alarms than the TD (p ≤ 0.001) (Fig. 2C). In all four groups treated with NF, false alarm rates did not differ from the error rate in the TD group (θ↓β↑: p = 0.18; θ↑β↑: p ≥ 0.99; θ↑: p ≥ 0.99; β↑: p ≥ 0.99). Mirroring this effect, the false alarm rate in three of the four NF groups was now significantly lower than the false alarm rate in the no NF group (θ↑β↑: p = 0.003; θ↑: p = 0.012; β↑: p = 0.03). Interestingly, it was particularly the θ↓β↑ group, i.e. containing patients taking part in the “traditional” NF protocol, that did not differ from the untreated (no NF) group at time point T2 (p = 0.58).
In addition, we examined the presence of any treatment effects (effects of Time) in all of the groups separately. Significant main effects of time were found in all patients with ADHD treated with either of the four NF protocols (θ↓β↑: t(28) = 3.4, p = 0.002, d = 0.63; θ↑β↑: t(27) = 2.4, p = 0.025, d = 0.45; θ↑: t(20) = 3.1, p = 0.005, d = 0.69; β↑: t(27) = 3.6, p = 0.001, d = 0.68). In contrast, no such effects were found in the no NF group (t(22) = − 0.01, p = 0.99, d = − 0.003) and the TD group (t(25) = 0.73, p = 0.47, d = 0.14). For descriptive data see Fig. 2C.
Flanker Task
Behavioural parameters showing performance in the flanker task are shown in Fig. 3.
Descriptive statistics (mean ± SE) for flanker reaction times (A) and flanker error rates (B) for compatible (pale colours) and incompatible (bright colours) trials. Blue bars represent testing time point T1; orange bars show testing time point T2. Data is shown for four groups of patients with ADHD treated with four different neurofeedback protocols (θ↓β↑: trained to downregulate theta and upregulate beta power, θ↑β↑: trained to upregulate both theta and beta power, θ↑: trained to upregulate theta only, β↑: trained to upregulate beta only) and for two control groups (no NF: patients with ADHD who did not take part in any training, TD: typically developing control children). Asterisks indicate level of significance of conducted post hoc t-tests
An analysis of the reaction times (Fig. 3A) is shown in the supplemental material. Concerning error rates (Fig. 3B), we found significant main effects of Time (F(1,147) = 13.2; p ≤ 0.001; ηp2 = 0.08; T1: 30.1 ± 1.1%; T2: 26.7 ± 1.0%) and Compatibility (F(1,147) = 427.5; p ≤ 0.001; ηp2 = 0.74; compatible trials: 15.6 ± 1.0%; incompatible trials: 41.2 ± 1.2%). The main effect of Group was not significant (F(5,147) = 2.1; p = 0.07; ηp2 = 0.07). Importantly, the Time*Compatibility*Group interaction was significant (F(5,147) = 2.3; p = 0.049; ηp2 = 0.07). To tease the effects apart, compatible and incompatible trials were examined separately. No significant interaction of Time*Group was found within compatible trials (F(5,147) = 0.91; p = 0.48; ηp2 = 0.03). Within the incompatible trials, however, this interaction was significant (F(5,147) = 3.3; p = 0.008; ηp2 = 0.1). Dividing this up further, we first found significant main effects of Group at time point T1 (F(5,147) = 2.7; p = 0.02; ηp2 = 0.08) and at time point T2 (F(5,147) = 5.3; p ≤ 0.001; ηp2 = 0.15). For incompatible trials at T1, Bonferroni-corrected post hoc analyses revealed significantly lower error rates in θ↑β↑ group than in the no NF group (p = 0.04). After treatment (i.e. at time point T2), this difference increased even further (p ≤ 0.001). In addition, error rates in incompatible trials were now significantly different between the no NF and the TD group (p = 0.004) and β↑ group (p = 0.01). When analysing the effects of Time within each group separately (see Fig. 3B for descriptive data), the θ↑β↑ group (t(26) = 2.1, p = 0.05, d = 0.40), β↑ group (t(27) = 2.8, p = 0.01, d = 0.52) and TD group (t(25) = 3.1, p = 0.005, d = 0.60) revealed significant differences. This was not the case for the other two NF groups (θ↓β↑ (t(28) = 1.1, p = 0.30, d = 0.20), θ↑ (t(20) = − 1.1, p = 0.29, d = − 0.24)) or the no NF group (t(21) = − 1.2, p = 0.26, d = − 0.25).
Post Hoc Power Analysis
To obtain a post hoc estimate of the robustness of our results in the Go/Nogo and the flanker task, we conducted a post hoc power analysis in addition to the a priori power analysis detailed in the “Methods” section. Our obtained effect size reached approximately ηp2 = 0.1, as it was the case in previous studies (Bluschke et al., 2018, 2016a, 2016b). The outcome measures showing the relevant effects (i.e. Nogo false alarm rate and error rate in incompatible flanker trials) showed repeated measure correlations of r ≥ 0.63 (all p ≤ 0.001). Taking into consideration our sample size of n = 157 (6 groups, 2 measurement time points, alpha level = 5%), the power calculation using G*Power revealed an achieved power ≥ 90%.
Parent-Rated ADHD Symptoms
Parent-rated levels of ADHD symptoms (Table 3) did not differ significantly between the five ADHD groups at T1 or T2 (all F ≤ 1.0, all p ≥ 0.44, all ηp2 ≤ 0.043). All patients with ADHD demonstrated significantly stronger ADHD symptoms than children in the TD group (all t ≥ 5.3; all p ≤ 0.001). In all three core symptoms of ADHD, we found significant main effects of Time (all F ≥ 5.2, all p ≤ 0.025; all ηp2 ≥ 0.05), indicating generally reduced symptoms at T2 compared to T1 across groups (see Table 3). Further, we found a main effect of Group (all F ≥ 7.8, all p ≤ 0.001; all ηp2 = 0.28) concerning all three symptom domains. Regarding inattention (all p ≤ 0.001) and impulsivity (all p ≤ 0.04), Bonferroni-corrected post hoc tests demonstrated that this effect was driven by the difference in symptom levels between TD and all ADHD groups together. Hyperactivity levels, however, did not differ significantly between TD and patients included in the θ↑ group (p = 0.2) or in the β↑ group (p = 0.1). We found no significant Time*Group interactions (all F ≤ 1.5, all p ≥ 0.2; all ηp2 ≤ 0.07).
Neurofeedback Baseline and Training Data
Data are presented in Table 4.
We examined whether there were any changes in the power (resting state) of the trained frequency bands in the four groups treated with neurofeedback. We did not observe any changes in the theta/beta frequency band power in either of the four groups (all t ≤ 1.53; all p ≥ 0.14). Further, we examined “training success” by comparing the increases/decreases achieved in power from the first to the last NF session. Here, we found a significant increase of theta power in the θ↑ group as well as a significant increase of beta power in the β↑ group. No such effects were found in the two groups training up-/downregulation of both frequency bands in parallel. In the classic NF protocol (θ↓β↑), beta power was even regulated in the opposite direction, resulting in lower beta power after completion of the NF sessions.
Discussion
The current study compared the effects of different NF protocols modulating theta and/or beta frequency bands on two instances of cognitive control functions in children and adolescents with ADHD. It aimed to examine the effects of theoretically driven manipulations of the classical NF protocol on objective performance measures by assessing cognitive control functions which bear a significant relevance for the day-to-day functioning of patients with ADHD. We chose a Go/Nogo task measuring response inhibition and a flanker task measuring interference control. Additionally, we examined effects on parent-rated ADHD symptoms as measured by the AD(H)D Symptom Checklist as well as changes occurring in the trained neurofeedback parameters.
The results show that the “gold standard” NF protocol (i.e. θ↓β↑) only enhanced response inhibition performance and led to reductions of false alarms in the Go/Nogo task, corroborating previous results using the same task (Bluschke et al., 2018). Regarding interference control, no changes between testing time points were evident applying the standard θ↓β↑ protocol. This shows that the NF protocol being most commonly used in ADHD treatment only modulates some aspects of cognitive control (i.e. response inhibition or impulsivity), while other important aspects of cognitive control are not affected (i.e. interference control). Inhibitory control processes are enhanced by all of the tested NF protocols. Opposed to this, interference control was only enhanced when theta and beta activity was upregulated (θ↑β↑), or when only the upregulation of beta band activity was trained (β↑). Therefore, these two protocols are more powerful than the other ones in enhancing cognitive control processes in ADHD. The finding that protocols entailing a modulation of beta band activity (i.e. not the θ↑ protocol) led to most consistent effects in ADHD suggests that beta band activity is particularly important when aiming to enhance both response inhibition and interference control processes in ADHD. Theta oscillations do not seem to be as important as beta oscillations for the cognitive control processes being examined in ADHD despite several studies having shown that theta oscillations are important during response inhibition (Chmielewski et al., 2016; Mückschel et al., 2017) and interference control (Cohen, 2014; Nigbur et al., 2012), as well as in ADHD in general (Yordanova et al., 2013; Zhang et al., 2017). To explain this pattern of results, it is important to consider the possible functional roles of theta and beta oscillations in cognitive control.
There is some conceptual overlap in functions between theta and beta oscillations in terms of continuous comparison processes between a desired/expected and the actually perceived information, or the desired and the achieved response outcome. This is relevant in both tasks. Theta oscillations have been suggested to be important for cognitive control since such slow oscillation processes allow the organization of brain activity across remote areas (i.e. from basic perceptual processes to aspects of motor response execution) (Buzsáki, 2006; Cavanagh & Frank, 2014). This may particularly be the case for medial frontal theta oscillations (Cavanagh & Frank, 2014), which may reflect a “surprise signal”. Whenever expectancies, particularly of one’s own behaviour (e.g. response in a task), are not fulfilled, the continuous comparison process between expected and factual outcome is resulting in such a “surprise signal”. Thus, the theta frequency band is related to continuous comparison processes. In the sense of a sensory sampling for relevant information and the maintenance of the status quo (Engel & Fries, 2010), such comparison processes related to beta frequency oscillations are necessary when directing attention towards upcoming motor tasks (Fetz, 2013; Khanna & Carmena, 2015; MacKay, 1997; Saleh et al., 2010; Seki & Fetz, 2012). This conceptualization suggests that beta band activity is stronger when the maintenance of the status quo is likely than when a change is expected. Again, this requires a comparison process. Yet, whereas theta oscillations seem to be enhanced when there is a violation of expectancies, beta oscillations seem to be particularly pronounced when this is not the case and a current state is expected to be maintained.
In the flanker task, especially sensory sampling is more complex than in the Go/Nogo task because here (i) more complex stimuli have to be captured in less time (flanker SOA) and because there is (ii) also a choice between different responses instead of the demand to inhibit one particular response. Therefore, for the Go/Nogo task the modulation of beta band activity seems to be less crucial, possibly because demands on stimulus sampling and the closely related response selection are lower. In this context, it is important to consider that the effects of beta band upregulation are not independent of concurrent processes taking place in the theta frequency range. This is evident when comparing the effects of the standard NF protocol (i.e. θ↓β↑) to those of protocols training the upregulation of theta and beta activity (i.e. θ↑β↑) or only beta (i.e. β↑). When theta is modulated in the direction opposite to beta activity, there is no improvement in the control of interference processes as measured by the flanker task, indicating that the enhancement of beta activity is not sufficient in order to handle the more complex sensory sampling demands of the flanker compared to the Go/Nogo task. Thus, our findings demonstrate that an NF-based increase in beta band only has a consistent effect when theta activity is modulated in the same direction but not when theta and beta band activity are modulated in opposite directions. Thus, it seems that the effects of theta and beta somewhat cancel each other out when both frequency bands are modulated simultaneously in opposite directions in the NF protocol. However, this mutual cancellation of effects is most likely incomplete since positive effects of a θ↓β↑ NF protocol were still present in the Go/Nogo task with its lower demands on sensory sampling. Accordingly, an improvement in Go/Nogo performance is still possible through the application of a θ↓β↑ NF protocol. This is not the case for interference control in the flanker task which poses higher demands on sensory sampling. However, such an explanation is only plausible if theta and beta band activity are at least partially based on common overlapping neural mechanisms. Crucially, this is the case since the functional processes coded by theta and beta oscillations both relate to a continuous comparison process (see above).
This concept of different levels at which NF-related changes may or may not occur is also supported by our finding that neither of the trained neurofeedback parameters significantly de- or increased between the first and last NF session in either of the four groups when considering baseline power levels. Concerning the actual changes in the ability to up-/downregulate the required frequency band power according to the respective NF protocol (i.e. “trainings success”), we did find specific effects in the groups of participants training the upregulation of either theta or beta power. No such effects were found in the groups of participants required to train the modulation of both these frequency bands simultaneously. Overall, these findings support existing knowledge (Pscherer et al., 2019, 2020, 2022) showing that resting state power carries a different functional relevance than it is the case for task-related oscillatory power and outcomes measured on the behavioural level. This further strongly depends on age and the cognitive function in question (Pscherer et al., 2019, 2021). It is thus not surprising that the pattern of results differs depending on the analysed outcome parameter. Future research would need to examine these different coding levels and their functional relevance further. Specifically, to arrive at an in-depth understanding of how, why and when neurofeedback works, it would be relevant to also consider effects on neurophysiological measures including event-related potentials, time–frequency decompositions and source localizations. Overall, however, our results show that particularly the protocols focusing on training the modulation of one (instead of both) of the frequency bands result in increased power values at the end of the NF training. The upregulation of theta power alone was associated with measureable increases in theta power as well as improved inhibition performance. The upregulation of beta power alone, however, additionally led to improved interference control.
The obtained results differentiate previous findings by showing that beneficial effects of theta/beta ratio NF protocols on cognitive control processes are mainly due to one component of the currently used protocol, namely, the increase in beta band activity (Bluschke et al., 2016a, 2016b). Thus, previous finding showing only limited evidence for the effectiveness of neurofeedback (Catalá-López et al., 2017; Cortese et al., 2016; Razoki, 2018) might be the result of non-optimized protocols. As argued previously (Bluschke et al., 2016a, 2016b), the current practice of the θ↓β↑ NF protocol in clinical settings is based on historically routed misconceptions about the role of theta oscillations for cognition (Bluschke et al., 2016a, 2016b). In these frameworks, theta frequency activity has commonly been interpreted as an indicator of daydream-like, in-alert states, while beta band activity has been thought to reflect focused attention and mental arousal (Hammond, 2011). The current data suggest the need for a change in clinical practice and underline criticisms concerning the relative lack of a neuroscientific rationale for the application of θ↓β↑ NF protocols in clinical settings (Heinrich et al., 2014; Holtmann et al., 2014; Saad & Kohn, 2015). Previous clinical studies on ADHD and NF have focused almost exclusively on the clinical symptoms of patients and have provided some evidence for the effectiveness of a classic θ↓β↑ NF protocol in this regard (Bluschke et al., 2018, 2020, 2016a, 2016b; Enriquez-Geppert et al., 2019; Gevensleben et al., 2010, 2009a, 2009b; Lambez et al., 2020; Lofthouse et al., 2012; Riesco-Matías et al., 2021; Van Doren et al., 2019; Yan et al., 2019). In the current study, clinical data (see Table 3) revealed reduced ADHD symptoms across all groups. Since this was the case in both the NF and the control groups, it is thus unclear whether these results reflect practice or training effects. Importantly, however, improvements were comparable across all NF groups, demonstrating that neither of the applied NF protocols carries any disadvantages in terms of symptom development. Therefore, the data suggest that a change in clinical practice of frequency-based NF protocols does not only provide a benefit at the level of cognitive control processes. Rather, clinically relevant ADHD symptom dimensions are still positively affected. Nevertheless, it is important to keep in mind that all effects concerning symptom severity were main effects, indicating similar levels of improvement across all groups. Such somewhat unspecific effects of NF have been reported previously (Ros et al., 2020), demonstrating the need for further differential investigations of influential factors. Altogether, our results indicate that symptom severity and cognitive control processes represent rather distinct, maybe even independent, levels of alterations in ADHD. Further specifically designed studies are required in order to examine whether different NF-related changes on these two levels may occur due to different temporal dynamics, different patterns of variability or different plasticity patterns.
A limitation of the study is that no strictly or systematically randomized or blinded study design was used to avoid any possible systematic biases of group allocation. However, due to the naturalistic setting, group allocation took place in a quasi-random fashion with the allocation not carrying any meaning to participants. Further, assessment was blinded since experimenters conducting testings at T1 and T2 were unaware of group membership of the participants. Since our goal was to systematically analyse differential effects of variations in the frequency bands on cognitive control parameters, no qualitative rating (e.g. ADHD symptoms) took place and the study provides no conclusions concerning the effectiveness on clinical parameters. Instead, our study focused on experimental measures, for which the definition of clinical meaningfulness is difficult due to the lack of absolute or relative cut-off scores. We did not examine the precise neurophysiological processes underlying the observed behavioural changes and do not report brain activity recorded during neurofeedback. Future studies should include session-by-session data on the extent to which the desired up- or downregulation of the respective frequency bands was achieved in order to be able to derive more direct causal inferences concerning the connection between NF training and any changes in assessed cognitive control parameters, since such effects on outcomes have been demonstrated previously (Baumeister et al., 2019). This is important as it is necessary to examine the data in a more fine-grained fashion than it is possible by considering mean outcomes only (Heinrich et al., 2007). In addition, the effects of the number of conducted NF sessions also need to be examined in more detail, with the 16 sessions conducted within this study ranging on the lower end of the spectrum.
Conclusions
In summary, including the enhancement of beta band activity in frequency band neurofeedback protocols is key when aiming to train patients with ADHD to increase cognitive control functions. These consistent enhancing effects on both response inhibition and conflict control were observed both without any concurrent theta regulation and in combination with theta upregulation. The simultaneous downregulation of theta power in addition to beta upregulation (i.e. the “traditional” NF protocol), however, resulted in much weaker effects that did not reach statistical significance. The data call for a change in the clinical usage of NF protocols and show that protocols different to the clinical standard are most effective to in enhancing important facets of cognitive control alongside clinical symptoms in ADHD.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code Availability
The codes from the experiments used in the current study are available from the corresponding author on reasonable request.
Abbreviations
- ADHD:
-
Attention deficit/hyperactivity disorder
- NF:
-
Neurofeedback
- TD:
-
Typically developing controls
References
Arns, M., de Ridder, S., Strehl, U., Breteler, M., & Coenen, A. (2009). Efficacy of neurofeedback treatment in ADHD: The effects on inattention, impulsivity and hyperactivity: A meta-analysis. Clinical EEG and Neuroscience, 40(3), 180–189. https://doi.org/10.1177/155005940904000311
Arns, M., & Strehl, U. (2013). Evidence for efficacy of neurofeedback in ADHD? The American Journal of Psychiatry, 170(7), 799–800. https://doi.org/10.1176/appi.ajp.2013.13020208
Baumeister, S., Wolf, I., Hohmann, S., Holz, N., Boecker-Schlier, R., Banaschewski, T., & Brandeis, D. (2019). The impact of successful learning of self-regulation on reward processing in children with ADHD using fMRI. Attention Deficit and Hyperactivity Disorders, 11(1), 31–45. https://doi.org/10.1007/s12402-018-0269-6
Berger, I. (2011). Diagnosis of Attention Deficit Hyperactivity Disorder: Much Ado about Something. Israel Medical Association Journal, 13, 4.
Beste, C., Ness, V., Falkenstein, M., & Saft, C. (2011). On the role of fronto-striatal neural synchronization processes for response inhibition—Evidence from ERP phase-synchronization analyses in pre-manifest Huntington’s disease gene mutation carriers. Neuropsychologia, 49(12), 3484–3493. https://doi.org/10.1016/j.neuropsychologia.2011.08.024
Bluschke, A., Broschwitz, F., Kohl, S., Roessner, V., & Beste, C. (2016a). The neuronal mechanisms underlying improvement of impulsivity in ADHD by theta/beta neurofeedback. Scientific Reports, 6, 31178. https://doi.org/10.1038/srep31178
Bluschke, A., Friedrich, J., Schreiter, M. L., Roessner, V., & Beste, C. (2018). A comparative study on the neurophysiological mechanisms underlying effects of methylphenidate and neurofeedback on inhibitory control in attention deficit hyperactivity disorder. NeuroImage. Clinical, 20, 1191–1203. https://doi.org/10.1016/j.nicl.2018.10.027
Bluschke, A., Roessner, V., & Beste, C. (2016b). Editorial perspective: How to optimise frequency band neurofeedback for ADHD. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 57(4), 457–461. https://doi.org/10.1111/jcpp.12521
Bluschke, A., Schreiter, M. L., Friedrich, J., Adelhöfer, N., Roessner, V., & Beste, C. (2020). Neurofeedback trains a superordinate system relevant for seemingly opposing behavioral control deficits depending on ADHD subtype. Developmental Science, 23(6), e12956. https://doi.org/10.1111/desc.12956
Buzsáki, G. (2006). Rhythms of the brain. Oxford Univ.
Catalá-López, F., Hutton, B., Núñez-Beltrán, A., Page, M. J., Ridao, M., Macías Saint-Gerons, D., Catalá, M. A., Tabarés-Seisdedos, R., & Moher, D. (2017). The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: A systematic review with network meta-analyses of randomised trials. PLoS ONE, 12(7), e0180355. https://doi.org/10.1371/journal.pone.0180355
Cavanagh, J. F., & Frank, M. J. (2014). Frontal theta as a mechanism for cognitive control. Trends in Cognitive Sciences, 18(8), 414–421. https://doi.org/10.1016/j.tics.2014.04.012
Chmielewski, W. X., Mückschel, M., Dippel, G., & Beste, C. (2016). Concurrent information affects response inhibition processes via the modulation of theta oscillations in cognitive control networks. Brain Structure & Function, 221(8), 3949–3961. https://doi.org/10.1007/s00429-015-1137-1
Chmielewski, W. X., Mückschel, M., Stock, A.-K., & Beste, C. (2015). The impact of mental workload on inhibitory control subprocesses. NeuroImage, 112, 96–104. https://doi.org/10.1016/j.neuroimage.2015.02.060
Cohen, M. X. (2014). A neural microcircuit for cognitive conflict detection and signaling. Trends in Neurosciences, 37(9), 480–490. https://doi.org/10.1016/j.tins.2014.06.004
Cortese, S., Ferrin, M., Brandeis, D., Holtmann, M., Aggensteiner, P., Daley, D., Santosh, P., Simonoff, E., Stevenson, J., Stringaris, A., Sonuga-Barke, E. J. S., Asherson, P., Banaschewski, T., Brandeis, D., Buitelaar, J., Coghill, D., Cortese, S., Daley, D., Danckaerts, M., … Zuddas, A. (2016). Neurofeedback for attention-deficit/hyperactivity disorder: Meta-analysis of clinical and neuropsychological outcomes from randomized controlled trials. Journal of the American Academy of Child & Adolescent Psychiatry, 55(6), 444–455. https://doi.org/10.1016/j.jaac.2016.03.007
Dahan, A., Ryder, C. H., & Reiner, M. (2018). Components of motor deficiencies in ADHD and possible interventions. Neuroscience, 378, 34–53. https://doi.org/10.1016/j.neuroscience.2016.05.040
Dekkers, T. J., Agelink van Rentergem, J. A., Koole, A., van den Wildenberg, W. P. M., Popma, A., Bexkens, A., Stoffelsen, R., Diekmann, A., & Huizenga, H. M. (2017). Time-on-task effects in children with and without ADHD: Depletion of executive resources or depletion of motivation? European Child & Adolescent Psychiatry, 26(12), 1471–1481. https://doi.org/10.1007/s00787-017-1006-y
Diamond, A. (2013). Executive functions. Annual Review of Psychology, 64, 135–168. https://doi.org/10.1146/annurev-psych-113011-143750
Döpfner, M., Görtz-Dorten, A., & Lehmkuhl, G. (2008). Diagnostik-System für Psychische Störungen im Kindes- und Jugendalter nach ICD-10 und DSM-IV. Huber.
dosReis, S., Park, A., Ng, X., Frosch, E., Reeves, G., Cunningham, C., Janssen, E. M., & Bridges, J. F. P. (2017). Caregiver treatment preferences for children with a new versus existing attention-deficit/hyperactivity disorder diagnosis. Journal of Child and Adolescent Psychopharmacology, 27(3), 234–242. https://doi.org/10.1089/cap.2016.0157
Emser, T. S., Johnston, B. A., Steele, J. D., Kooij, S., Thorell, L., & Christiansen, H. (2018). Assessing ADHD symptoms in children and adults: Evaluating the role of objective measures. Behavioral and Brain Functions, 14(1), 11. https://doi.org/10.1186/s12993-018-0143-x
Engel, A. K., & Fries, P. (2010). Beta-band oscillations—Signalling the status quo? Current Opinion in Neurobiology, 20(2), 156–165. https://doi.org/10.1016/j.conb.2010.02.015
Enriquez-Geppert, S., Smit, D., Pimenta, M. G., & Arns, M. (2019). Neurofeedback as a treatment intervention in ADHD: Current evidence and practice. Current Psychiatry Reports, 21(6), 46. https://doi.org/10.1007/s11920-019-1021-4
Erhart, M., Döpfner, M., Ravens-Sieberer, U., The BELLA study group. (2008). Psychometric properties of two ADHD questionnaires: Comparing the Conners’ scale and the FBB-HKS in the general population of German children and adolescents – Results of the BELLA study. European Child & Adolescent Psychiatry, 17(1), 106–115. https://doi.org/10.1007/s00787-008-1012-1
Faedda, N., Romani, M., Rossetti, S., Vigliante, M., Pezzuti, L., Cardona, F., & Guidetti, V. (2019). Intellectual functioning and executive functions in children and adolescents with attention deficit hyperactivity disorder (ADHD) and specific learning disorder (SLD). Scandinavian Journal of Psychology, 60(5), 440–446. https://doi.org/10.1111/sjop.12562
Fetz, E. E. (2013). Volitional control of cortical oscillations and synchrony. Neuron, 77(2), 216–218. https://doi.org/10.1016/j.neuron.2013.01.003
Gevensleben, H., Holl, B., Albrecht, B., Schlamp, D., Kratz, O., Studer, P., Rothenberger, A., Moll, G. H., & Heinrich, H. (2010). Neurofeedback training in children with ADHD: 6-month follow-up of a randomised controlled trial. European Child & Adolescent Psychiatry, 19(9), 715–724. https://doi.org/10.1007/s00787-010-0109-5
Gevensleben, H., Holl, B., Albrecht, B., Schlamp, D., Kratz, O., Studer, P., Wangler, S., Rothenberger, A., Moll, G. H., & Heinrich, H. (2009a). Distinct EEG effects related to neurofeedback training in children with ADHD: A randomized controlled trial. International Journal of Psychophysiology, 74(2), 149–157. https://doi.org/10.1016/j.ijpsycho.2009.08.005
Gevensleben, H., Holl, B., Albrecht, B., Vogel, C., Schlamp, D., Kratz, O., Studer, P., Rothenberger, A., Moll, G. H., & Heinrich, H. (2009b). Is neurofeedback an efficacious treatment for ADHD? A randomised controlled clinical trial. Journal of Child Psychology and Psychiatry, 50(7), 780–789. https://doi.org/10.1111/j.1469-7610.2008.02033.x
Hammond, D. C. (2011). What is neurofeedback: An update. Journal of Neurotherapy, 15(4), 305–336. https://doi.org/10.1080/10874208.2011.623090
Heinrich, H., Busch, K., Studer, P., Erbe, K., Moll, G. H., & Kratz, O. (2014). EEG spectral analysis of attention in ADHD: Implications for neurofeedback training? Frontiers in Human Neuroscience, 8, 611. https://doi.org/10.3389/fnhum.2014.00611
Heinrich, H., Gevensleben, H., & Strehl, U. (2007). Annotation: Neurofeedback – Train your brain to train behaviour. Journal of Child Psychology and Psychiatry, 48(1), 3–16. https://doi.org/10.1111/j.1469-7610.2006.01665.x
Holtmann, M., Sonuga-Barke, E., Cortese, S., & Brandeis, D. (2014). Neurofeedback for ADHD: A review of current evidence. Child and Adolescent Psychiatric Clinics of North America, 23(4), 789–806. https://doi.org/10.1016/j.chc.2014.05.006
Khanna, P., & Carmena, J. M. (2015). Neural oscillations: Beta band activity across motor networks. Current Opinion in Neurobiology, 32, 60–67. https://doi.org/10.1016/j.conb.2014.11.010
Krieger, V., & Amador-Campos, J. A. (2018). Assessment of executive function in ADHD adolescents: Contribution of performance tests and rating scales. Child Neuropsychology: A Journal on Normal and Abnormal Development in Childhood and Adolescence, 24(8), 1063–1087. https://doi.org/10.1080/09297049.2017.1386781
Krieger, V., Amador-Campos, J. A., & Gallardo-Pujol, D. (2019). Temperament, executive function, and attention-deficit/hyperactivity disorder (ADHD) in adolescents: The mediating role of effortful control. Journal of Clinical and Experimental Neuropsychology, 41(6), 615–633. https://doi.org/10.1080/13803395.2019.1599824
Lambez, B., Harwood-Gross, A., Golumbic, E. Z., & Rassovsky, Y. (2020). Non-pharmacological interventions for cognitive difficulties in ADHD: A systematic review and meta-analysis. Journal of Psychiatric Research, 120, 40–55. https://doi.org/10.1016/j.jpsychires.2019.10.007
Lofthouse, N., Arnold, L. E., Hersch, S., Hurt, E., & DeBeus, R. (2012). A review of neurofeedback treatment for pediatric ADHD. Journal of Attention Disorders, 16(5), 351–372. https://doi.org/10.1177/1087054711427530
MacKay, W. A. (1997). Synchronized neuronal oscillations and their role in motor processes. Trends in Cognitive Sciences, 1(5), 176–183. https://doi.org/10.1016/S1364-6613(97)01059-0
Micoulaud-Franchi, J.-A., McGonigal, A., Lopez, R., Daudet, C., Kotwas, I., & Bartolomei, F. (2015). Electroencephalographic neurofeedback: Level of evidence in mental and brain disorders and suggestions for good clinical practice. Neurophysiologie Clinique = Clinical Neurophysiology. https://doi.org/10.1016/j.neucli.2015.10.077
Miklós, M., Futó, J., Komáromy, D., & Balázs, J. (2019). Executive function and attention performance in children with ADHD: Effects of medication and comparison with typically developing children. International Journal of Environmental Research and Public Health, 16(20). https://doi.org/10.3390/ijerph16203822
Mückschel, M., Dippel, G., & Beste, C. (2017). Distinguishing stimulus and response codes in theta oscillations in prefrontal areas during inhibitory control of automated responses. Human Brain Mapping, 38(11), 5681–5690. https://doi.org/10.1002/hbm.23757
Ng, X., Bridges, J. F. P., Ross, M. M., Frosch, E., Reeves, G., Cunningham, C. E., & dosReis, S. (2017). A latent class analysis to identify variation in caregivers’ preferences for their child’s attention-deficit/hyperactivity disorder treatment: Do stated preferences match current treatment? The Patient, 10(2), 251–262. https://doi.org/10.1007/s40271-016-0202-z
Nigbur, R., Cohen, M. X., Ridderinkhof, K. R., & Stürmer, B. (2012). Theta dynamics reveal domain-specific control over stimulus and response conflict. Journal of Cognitive Neuroscience, 24(5), 1264–1274. https://doi.org/10.1162/jocn_a_00128
Pscherer, C., Bluschke, A., Mückschel, M., & Beste, C. (2021). The interplay of resting and inhibitory control-related theta-band activity depends on age. Human Brain Mapping, 42(12), 3845–3857. https://doi.org/10.1002/hbm.25469
Pscherer, C., Bluschke, A., Prochnow, A., Eggert, E., Mückschel, M., & Beste, C. (2020). Resting theta activity is associated with specific coding levels in event-related theta activity during conflict monitoring. Human Brain Mapping, 41(18), 5114–5127. https://doi.org/10.1002/hbm.25178
Pscherer, C., Mückschel, M., Bluschke, A., & Beste, C. (2022). Resting-state theta activity is linked to information content-specific coding levels during response inhibition. Scientific Reports, 12(1), 4530. https://doi.org/10.1038/s41598-022-08510-8
Pscherer, C., Mückschel, M., Summerer, L., Bluschke, A., & Beste, C. (2019). On the relevance of EEG resting theta activity for the neurophysiological dynamics underlying motor inhibitory control. Human Brain Mapping, 40(14), 4253–4265. https://doi.org/10.1002/hbm.24699
Razoki, B. (2018). Neurofeedback versus psychostimulants in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: A systematic review. Neuropsychiatric Disease and Treatment, 14, 2905–2913. https://doi.org/10.2147/NDT.S178839
Riesco-Matías, P., Yela-Bernabé, J. R., Crego, A., & Sánchez-Zaballos, E. (2021). What do meta-analyses have to say about the efficacy of neurofeedback applied to children with ADHD? Review of previous meta-analyses and a new meta-analysis. Journal of Attention Disorders, 25(4), 473–485. https://doi.org/10.1177/1087054718821731
Ros, T., Enriquez-Geppert, S., Zotev, V., Young, K. D., Wood, G., Whitfield-Gabrieli, S., Wan, F., Vuilleumier, P., Vialatte, F., Van De Ville, D., Todder, D., Surmeli, T., Sulzer, J. S., Strehl, U., Sterman, M. B., Steiner, N. J., Sorger, B., Soekadar, S. R., Sitaram, R., … Thibault, R. T. (2020). Consensus on the reporting and experimental design of clinical and cognitive-behavioural neurofeedback studies (CRED-nf checklist). Brain: A Journal of Neurology, 143(6), 1674–1685. https://doi.org/10.1093/brain/awaa009
Saad, J. F., & Kohn, M. R. (2015). Is the theta/beta EEG marker for ADHD inherently flawed? Journal of Attention Disorders. https://doi.org/10.1177/1087054715578270
Saleh, M., Reimer, J., Penn, R., Ojakangas, C. L., & Hatsopoulos, N. G. (2010). Fast and slow oscillations in human primary motor cortex predict oncoming behaviorally relevant cues. Neuron, 65(4), 461–471. https://doi.org/10.1016/j.neuron.2010.02.001
Schatz, N. K., Fabiano, G. A., Cunningham, C. E., dosReis, S., Waschbusch, D. A., Jerome, S., Lupas, K., & Morris, K. L. (2015). Systematic review of patients’ and parents’ preferences for ADHD treatment options and processes of care. The Patient, 8(6), 483–497. https://doi.org/10.1007/s40271-015-0112-5
Seki, K., & Fetz, E. E. (2012). Gating of sensory input at spinal and cortical levels during preparation and execution of voluntary movement. Journal of Neuroscience, 32(3), 890–902. https://doi.org/10.1523/JNEUROSCI.4958-11.2012
Sibley, M. H., Graziano, P. A., Ortiz, M., Rodriguez, L., & Coxe, S. (2019). Academic impairment among high school students with ADHD: The role of motivation and goal-directed executive functions. Journal of School Psychology, 77, 67–76. https://doi.org/10.1016/j.jsp.2019.10.005
Skogli, E. W., Andersen, P. N., Hovik, K. T., & Øie, M. (2017). Development of hot and cold executive function in boys and girls with ADHD. Journal of Attention Disorders, 21(4), 305–315. https://doi.org/10.1177/1087054714524984
Studer, P., Kratz, O., Gevensleben, H., Rothenberger, A., Moll, G. H., Hautzinger, M., & Heinrich, H. (2014). Slow cortical potential and theta/beta neurofeedback training in adults: Effects on attentional processes and motor system excitability. Frontiers in Human Neuroscience, 8. https://doi.org/10.3389/fnhum.2014.00555
Van-Doren, J., Arns, M., Heinrich, H., Vollebregt, M. A., Strehl, U., & Loo, S. K. (2019). Sustained effects of neurofeedback in ADHD: A systematic review and meta-analysis. European Child & Adolescent Psychiatry, 28(3), 293–305. https://doi.org/10.1007/s00787-018-1121-4
Wascher, E., Schneider, D., Hoffmann, S., Beste, C., & Sänger, J. (2012). When compensation fails: Attentional deficits in healthy ageing caused by visual distraction. Neuropsychologia, 50(14), 3185–3192. https://doi.org/10.1016/j.neuropsychologia.2012.09.033
Willcutt, E. G., Doyle, A. E., Nigg, J. T., Faraone, S. V., & Pennington, B. F. (2005). Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biological Psychiatry, 57(11), 1336–1346. https://doi.org/10.1016/j.biopsych.2005.02.006
Womelsdorf, T., & Everling, S. (2015). Long-range attention networks: Circuit motifs underlying endogenously controlled stimulus selection. Trends in Neurosciences, 38(11), 682–700. https://doi.org/10.1016/j.tins.2015.08.009
Yan, L., Wang, S., Yuan, Y., & Zhang, J. (2019). Effects of neurofeedback versus methylphenidate for the treatment of ADHD: Systematic review and meta-analysis of head-to-head trials. Evidence-Based Mental Health, 22(3), 111–117. https://doi.org/10.1136/ebmental-2019-300088
Yordanova, J., Kolev, V., & Rothenberger, A. (2013). Event-related oscillations reflect functional asymmetry in children with attention deficit/hyperactivity disorder. Supplements to Clinical Neurophysiology, 62, 289–301. https://doi.org/10.1016/b978-0-7020-5307-8.00018-1
Zhang, D.-W., Roodenrys, S., Li, H., Barry, R. J., Clarke, A. R., Wu, Z., Zhao, Q., Song, Y., Liu, L., Qian, Q., Wang, Y., Johnstone, S. J., & Sun, L. (2017). Atypical interference control in children with AD/HD with elevated theta/beta ratio. Biological Psychology, 128, 82–88. https://doi.org/10.1016/j.biopsycho.2017.07.009
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by a grant from the Else Kröner-Fresenius Stiftung (2016_A94) to AB and by a grant from the Friede Springer Stiftung (033/2017) to CB. The funders played no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
The study was conducted according to the Declaration of Helsinki and approved by the ethics committee of the Medical Faculty of the TU Dresden.
Consent to Participate
Written informed consent was obtained from all study participants and their legal guardians.
Consent for Publication
Written informed consent was obtained from all study participants and their legal guardians. The ethics committee approved publication in anonymized form.
Conflict of Interest
A.B., E.E., J.F., R.J., A.P., C.P., M.L.S. and B.T. declare no conflicts of interest. V.R. has received payment for consulting and writing activities from Lilly, Novartis and Shire Pharmaceuticals; lecture honoraria from Lilly, Novartis, Shire Pharmaceuticals and Medice Pharma; and support for research from Shire and Novartis. He has carried out (and is currently carrying out) clinical trials in cooperation with the Novartis, Shire and Otsuka companies. C.B. has received payment for consulting from GlaxoSmithKline, Novartis, Genzyme and Teva.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bluschke, A., Eggert, E., Friedrich, J. et al. The Effects of Different Theta and Beta Neurofeedback Training Protocols on Cognitive Control in ADHD. J Cogn Enhanc 6, 463–477 (2022). https://doi.org/10.1007/s41465-022-00255-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41465-022-00255-6