Abstract
As the world’s population is aging rapidly, cognitive training is an extensively used approach to attempt improvement of age-related cognitive functioning. With increasing numbers of older adults required to remain in the workforce, it is important to be able to reliably predict future functional decline, as well as the individual advantages of cognitive training. Given the correlation between age-related decline and striatal dopaminergic function, we investigated whether eye blink rate (EBR), a non-invasive, indirect indicator of dopaminergic activity, could predict executive functioning (response inhibition, switching and working memory updating) as well as trainability of executive functioning in older adults. EBR was collected before and after a cognitive flexibility training, cognitive training without flexibility, or a mock training. EBR predicted working memory updating performance on two measures of updating, as well as trainability of working memory updating, whereas performance and trainability in inhibition and switching tasks could not be predicted by EBR. Our findings tentatively indicate that EBR permits prediction of working memory performance in older adults. To fully interpret the relationship with executive functioning, we suggest future research should assess both EBR and dopamine receptor availability among seniors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Years of research into neurocognitive aging have demonstrated that with older age, cognitive performance tends to decline, specifically in functions such as episodic memory, processing speed, and cognitive control, which are considered essential for unaffected daily activities. Cognitive control, according to Miyake et al. (2000), can be divided in the three dimensions of updating, shifting, and inhibition, each of which shows impairment with age (Fisk & Sharp, 2004). However, there is strong evidence that age per se is not the best predictor of this decline in functioning. A large literature documents the role of the availability of a variety of neurotransmitters, most prominently among which (in the context of cognitive control) is dopamine (DA).
In a meta-analysis of studies using positron emission tomography (PET) and single-photon emission-computed tomography, Karrer et al. (2017) found that DA receptors in striatal and frontal cortical regions declined by 4–14% per decade. Although these authors could not corroborate age effects on DA synthesis, DA synthesis is known to be associated with considerable individual differences among seniors, and these individual differences do correspond to performance in executive functioning (Berry et al., 2016; Klostermann et al., 2012), perhaps through its effects on frontoparietal (Berry et al., 2016) and frontostriatal (Klostermann et al., 2012) functional connectivity.
The role of DA in executive functioning involves two families of DA receptors, D1 and D2, that express differentially in prefrontal and striatal areas of the brain. D1 is relatively more prevalent in prefrontal areas whereas D1 and D2 both play a prominent role in striatal functions. Here, we focus specifically on striatal D2 receptor binding in the caudate and putamen, which decreases with age (Rinne et al., 1993). The reason for this focus is twofold. First, performance on tasks of episodic memory, processing speed, working memory, and fluency is found to be more strongly associated with striatal D2 receptor binding than with age (Bäckman et al., 2000; Erixon-Lindroth et al., 2005). Although age is generally negatively related to cognitive performance on various tasks, these results suggest that the individual rate of D2 binding in the striatum is able to better predict the degree to which these functions deteriorate. And second, there is some evidence to suggest that stratal D2 binding is expressed in an easy-to-register, overt, and noninvasive proxy measure: spontaneous eye blink rate, as will be discussed in detail below.
As the world’s aging population is growing, with increasing numbers of older adults expected to remain productive in the workforce, it is of great importance to have an affordable and reliable predictor of future decline in cognitive control abilities. Assessment of striatal D2 activity could help in analyzing this relationship. One possibility is to use positron emission tomography (PET) as an indirect assessment of dopamine components. PET relies on radioactive ligands that are inserted into the body and bind to dopamine receptors, which can then be localized and imaged. However, ligand PET is an invasive and very costly procedure. One simple, non-invasive, and cheap method to reliably measure the connection between D2 dopaminergic function and cognitive control in older adults is spontaneous eye blink rate (EBR; Karson, 1983). EBR can be measured using various methods such as electrooculography (Colzato, van den Wildenberg, et al., 2008), eye tracking (Dang et al., 2017), or video recording (Tharp & Pickering, 2011) and has been demonstrated to be a reliable predictor of D2 dopaminergic receptor density in the striatum (Jongkees & Colzato, 2016). Important evidence of this relationship is shown, first of all, in pathologies that involve striatal D2 dopaminergic dysfunction. For instance, EBR is reduced in patients suffering from Parkinson’s disease, which is characterized by a depletion of dopaminergic nigrostriatal neurons (Deuschl & Goddemeier, 1998). Similarly, striatal D2 is significantly reduced in cocaine users (Volkow et al., 1999) who also show a significant decrease in EBR (Colzato, Slagter, et al., 2008; Colzato, van den Wildenberg, et al., 2008). On the contrary, individuals suffering from schizophrenia, which is associated with elevated dopamine release at striatal D2 receptors (Brunelin et al., 2013), demonstrate increased EBR (Mackert et al., 1990). In addition, dopaminergic medication can be seen to influence EBR. Patients provided with dopaminergic D2 antagonists demonstrate a reversal of EBR to (near) normal levels (Bologna et al., 2012; Mackert et al., 1990), and in healthy humans, administration of dopamine D2 agonists increases EBR (Blin et al., 1990). Genetic evidence of the relationship between EBR and striatal dopamine comes from studies linking EBR to the DRD4/7 genotype, which is associated with the control of dopamine in the striatum (Dreisbach et al., 2005).
Moreover, EBR has been used to demonstrate functional differences in various tasks relying on dopamine activity, such as the attentional blink (Colzato, Slagter, et al., 2008; Colzato, van den Wildenberg, et al., 2008), creative thinking (Akbari Chermahini & Hommel, 2010), probabilistic learning (Slagter et al., 2015), and cognitive control. For instance, in task switching, high blink rates are seen to correlate with improved accuracy (Kleinsorge & Scheil, 2017; Zhang et al., 2015) and increased flexibility (Dreisbach et al., 2005). Using a switch task with multiple conditions, the latter authors showed that in individuals with high EBR, switch costs are decreased when responding to novel targets, but increased when novel items posed as distractors, suggesting that increased flexibility comes at a cost of reduced stability, or distractibility. Further support for this claim comes from Müller et al. (2007) and Tharp and Pickering (2011).
By comparison, the relationship between EBR and inhibition is more ambiguous. For instance, increased EBR was shown to be correlated with longer stop-signal reaction time (SSRT), a measure of response inhibition (Colzato, van den Wildenberg, et al., 2009). This association between higher dopamine levels and increased SSRT is backed up by a genetic study of the DRD4/7 genotype (Congdon et al., 2008). Yet, in regular cocaine users, known to exhibit a notably low EBR, SSRT is found to be impaired (Colzato et al., 2007). It is likely that the relationship between inhibition and striatal dopamine follows an inverted U-curve, representing optimal response inhibition with average amounts of dopamine, and is therefore highly dependent on the range of dopamine of the specific sample.
However, regarding the link between EBR and working memory, evidence points to an absence of association. For instance, no relationship is found between EBR and performance on the operation span task (Tharp & Pickering, 2011) or a mental counters task (Zhang et al., 2015). This is supported by earlier evidence from a study in cocaine users (Colzato, Huizinga, et al., 2009; Colzato, van den Wildenberg, et al., 2009) who are shown to perform equally to non-users on several different tasks of working memory. As inhibition is driven mainly by the nigrostriatal D2 pathway and working memory updating by the mesocortical D1 pathway (Colzato, Huizinga, et al., 2009; Colzato, van den Wildenberg, et al., 2009), most likely EBR is not a reliable predictor of functioning of the latter. Nonetheless, correlational results from a 3-back task (Zhang et al., 2015) reveal a negative relationship, suggesting decreased updating ability with higher EBR.
In sum, as the association between EBR and separate dimensions of cognitive control seems to vary with different tasks and samples, the relationship (be it linear or nonlinear) still remains somewhat controversial, demonstrating the need for further research with multiple tests in each domain.
Considering that dopaminergic systems (and especially striatal D2 receptor binding) decline with increasing age, it seems natural to assume that this would manifest itself as a decrease in EBR. Though one study indeed reports a significant decrease after age 40, which continues to decline with each decade of life (Chen et al., 2003), the majority finds no difference between EBR in young and older ages (Bentivoglio et al., 1997; Kruis et al., 2016; Zaman et al., 1998). A number of studies (Deuschl & Goddemeier, 1998; Sforza et al., 2008; Sun et al., 1997) even report a notable increase in EBR in 70 to 79-year-olds in comparison to middle-aged adults, though most samples are small and the differences are not significant. As EBR and striatal D2 binding evolve differently with age, it is possible that the beforementioned association between EBR and striatal D2 binding is no longer valid in older ages. Still, a direct relationship would need to be determined more robustly in future studies measuring both EBR and striatal D2 function in older adults, before firm conclusions can be drawn.
Furthermore, not much is known about the relationship between EBR and cognitive functioning in healthy older populations. One recent study in older adults suggests that EBR is negatively related to cognitive performance, as measured with a general clinical screening (Ladas et al., 2014). However, this sample consisted of both healthy older adults and individuals suffering from mild cognitive impairment, the latter of which displayed a significantly higher EBR, thus confounding the correlation. Given the lack of knowledge in this field, it remains important to examine the connection between EBR and cognitive functioning in older age. Due to the inter-individual variability within the older population (Christensen et al., 1999; Kanai & Rees, 2011; Raz et al., 2010), it is important to also study the individual differences between older adults rather than focus on the differences between age groups. For this reason, we initiated a study in healthy older adults in which we measured EBR along with several assessments of cognitive control to gain more knowledge on this subject.
Recent attempts have been made to counteract the age-related decline using cognitive training (e.g., Karbach & Verhaeghen, 2014). Improvements have been reported in various domains, such as working memory (Buschkuehl et al., 2008; Zinke et al., 2014), task switching (Basak & O’Connell, 2016; Karbach & Kray, 2009), and multitasking (Cassavaugh & Kramer, 2009). In young adults, increased dopamine release and functional activation in the striatum have been noted after training (Bäckman et al., 2017; Kühn et al., 2013) though this effect was not replicated in older adults (Dahlin et al., 2008). Multiple studies also show that dopamine activity in the striatum is associated with improvement in working memory after training. For example, Dahlin et al. (2008) show that in young adults, generalization of updating training to untrained tasks of working memory (known as transfer) is dependent on striatal activity. Using individual differences in dopaminergic D2 levels to predict training success could add to our knowledge on who benefit from different types of interventions. In young adults, baseline striatal gray-matter volume has been shown to be associated with later training improvement on a strategic video game (Erickson et al., 2010), with dorsal striatum specifically predicting performance on a game engaging cognitive flexibility. This suggests that the striatum plays a key role in learning from flexibility training and that individual differences in volume or activation can predict later training success. Yet, this still leaves open the question whether this extends to transfer from training. Also, so far such a paradigm has not yet been investigated in older adults. If EBR could serve as a predictor of future decline as well as potential training advantages in older ages, this would offer a substantial benefit to society.
In our own longitudinal intervention project (Buitenweg et al., 2017), we assessed the effectiveness of a cognitive flexibility training on cognitive control functions in a large group of healthy elderly adults. Flexibility was induced by continuous switching between various games. Though results indicated transfer on multiple cognitive functions, these effects were visible in all three conditions of the training, which we interpreted as having been caused by practice and expectancy effects. However, we also noted large individual differences in cognitive test scores and the degree of change after training. For this reason, training success should not only be assessed at the group level, but also tested for individual variables that can predict training benefit (Buitenweg et al., 2012). We previously found that age, baseline mental condition, education level, or the number of training sessions did not predict training success (Buitenweg et al., 2017). However, as stated above, it is likely that individual striatal D2 function would prove to be a more reliable predictor. Therefore, we will additionally study the question whether susceptibility for training benefit is dependent on individual differences in striatal D2, as proxied by EBR.
There is evidence that the link between individual differences in executive functions on the one hand, and EBR on the other hand, can be modulated by polymorphisms, such as Val158Met (Colzato et al., 2010) affecting dopamine regulation in different areas of the brain. What’s more, a training study by Colzato et al. (2014) showed that genotypes linked to differential PFC dopamine transmission differed in their benefit from a flexibility game training. Nonetheless, although this suggests that dopamine function seems to determine the degree to which transfer takes place after a training, this genetic relationship concerns prefrontal D1, rather than striatal D2. Knowledge on this topic is still scarce, and a direct connection with striatal D2 or EBR and individual training benefit has not been confirmed. Future research combining EBR measurement with genotyping and functional differences in executive control or benefit from cognitive training could clarify this relationship.
The primary purpose of this study was to investigate the relationship between striatal D2 and cognitive control functions in healthy elderly adults using EBR, focusing on individual difference among the older adults. We collected EBR before and after a cognitive intervention, consisting of a frequent and an infrequent switching condition, and a mock training condition. Based on previous literature, we hypothesized that high EBR (indicating high striatal D2 receptor binding) should predict lower switching costs and increased SSRTs at baseline, but not baseline working memory updating as measured with the operation span task. Considering the negative relationship with EBR found on an N-Back task (Zhang et al., 2015), we also studied whether the same result on this task would be found in older adults. Additionally, we wanted to know whether dopamine modulates training improvement and benefit of a cognitive flexibility training in this population. We tested the hypothesis that the association between flexibility training and its benefits was different for varying levels of striatal dopamine availability. One possibility is that higher striatal dopamine availability is related to higher training success and transfer after flexibility training, but not after other interventions. Such a result would have meaningful implications for the state of cognitive training in aging, as it implies being able to predict which individuals profit more from this type of intervention. However, it is also possible that EBR is not sensitive enough to predict differences in training success, in which case other possibilities to investigate this relationship will have to be considered.
Materials and Methods
This study is part of the overarching TAPASS training project, a randomized controlled double-blind design intended to test effectiveness of a cognitive flexibility training, full results of which are published elsewhere (Buitenweg et al., 2017, 2018.
Participants
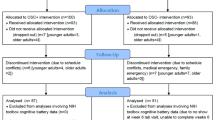
Participants were healthy older adults (60–85 years old) interested in cognitive training. All participants owned a computer with internet and scored 26 or above on the Telephone Interview Cognitive Status (TICS; Brandt et al., 1988). None used dopaminergic medication, or suffered from disorders associated with dopaminergic abnormalities (schizophrenia, Parkinson’s disease, ADHD), severe visual impairment or colorblindness, or a history of substance abuse or stroke. The final sample included 158 participants, who were randomly assigned to one of three conditions (described below). Full written informed consent was given by all participants prior to participation. The study was approved by the local Ethics Committee of the University of Amsterdam and registered under number 2012-BC-2566. All procedures were conducted in compliance with the Declaration of Helsinki, relevant laws, and institutional guidelines.
Procedure
The study consisted of a pre-training (T0) and a post-training (T2) test session, approximately 13 weeks apart, with a 12-week training in between. During both sessions, a large testing battery was administered to assess transfer of this intervention, results of which are mentioned elsewhere (Buitenweg et al., 2017). Subjects were asked to sleep sufficiently and avoid alcohol the night before a test session. As EBR is found to be stable during the daytime hours, but increases in the evening (Barbato et al., 2000), testing was always done between 9 am and 6 pm.
At the start of the testing session, participants were asked for additional permission to make a recording of their face. They were not told the reason for the recording, so as not to draw conscious attention to eye blinks. Participants were seated about 80-cm distance from a white wall with a fixation cross (following Deuschl & Goddemeier, 1998) and were asked to fixate on the target in a relaxed state without speaking or keeping their eyes closed. Although some suggest that 1 min is enough to get reliable values of EBR (Deuschl & Goddemeier, 1998), most studies record for a period of 4 to 6 min (Colzato, Huizinga, et al., 2009; Colzato, van den Wildenberg, et al., 2009; Slagter et al., 2015), which is followed in the current study. We recorded participants’ faces for 5 min using a video camera (Canon Legria FS2000) that was visible to the participant, positioned at the height of their chin, approximately 60 cm in front of them.
Intervention
We modified a selection of games from the existing website www.braingymmer.com to construct three training programs, based on our experience with cognitive tasks in older adult participants and our reading of the literature. The order of these games was pre-programmed to prevent participants from exclusively selecting the games of their choice. Each session consisted of approximately 30 min of game play.
Cognitive Training
Within the cognitive training, we discerned two groups: frequent switching and infrequent switching. In the frequent switching condition, training sessions consisted of 10 games of 3 min each, forcing participants to frequently switch between different tasks and functions, thereby maximizing flexibility. In the infrequent switching condition, flexibility was minimized by allowing games in one session to be played for 10 min each. Both groups included nine games, divided over the cognitive domains working memory, reasoning, and attention. In the frequent switching condition, two consecutive games were always from different domains, to increase variability and flexibility. Each game consisted of 20 difficulty levels. Feedback on game performance was given with zero to three stars on the screen after each game. Adaptiveness was maintained by encouraging participants to train at a higher level each time two or three stars had been attained. To enable everyone to familiarize themselves equally with the games, the infrequent switching schedule was enforced for both groups in the first training week. After completion of the 12-week training, total time spent per game was the same for both groups.
Mock Training
The games in the mock training were selected to be predominantly visually stimulating. To reduce variability, fewer, less cognitively stimulating games were chosen compared to the experimental conditions. To minimize the demand on flexibility, games in one session were played for 10 min each. Although stars were attained in the same manner as in the cognitive training, we instructed participants to stay on a specific level for a week, thus reducing adaptiveness.
Materials
As we expected to find a relationship between striatal D2 and cognitive control functions, we focused our analysis on the three main executive functions shifting, inhibition, and updating, following the classification model by Miyake et al. (2000). To this end, we selected tasks similar to those used in previous literature linking EBR to cognitive control functions (Colzato, Huizinga, et al., 2009; Colzato, van den Wildenberg, et al., 2009; Tharp & Pickering, 2011; Zhang et al., 2015).
Switch Task
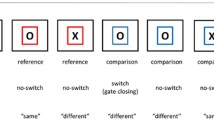
We used a switch task based on Rogers and Monsell (1995) which is known to assess most pure switching cost (van Holstein et al., 2011). In the switch task, stimuli were random combinations of a letter and a digit, appearing in one of four quadrants. Depending on which quadrant the stimulus appeared in, participants either responded to the digit or the letter. Digits were categorized as above or below 5 and letters as capitals or lowercase. To correct for possible eye movement effects, a horizontal and a vertical version of the task were created. In the horizontal version, stimuli in the top two quadrants required a response to the letter task and in the lower two quadrants a response to the digit task. In the vertical version, a response to the letter task was required in the right quadrants and to the digit task in the left quadrants. The task version was counterbalanced between participants, but was kept equal between time points within the same participant. The stimulus was presented in clockwise order, such that stimulus location on each trial was predictable, and a switch to the other task was required every other trial. Participants were encouraged to respond as fast and as accurately as possible. In between task blocks, participants were instructed to respond more accurately if accuracy fell below 91% and to respond faster if accuracy rose above 97%, to maintain a balance between speed and accuracy. Presentation of the stimulus occurred for 5000 ms, with an interstimulus interval (ISI) of 200 ms. Participants practiced the letter and the number task in separate blocks of 24 trials each. Subsequently, the switch task was practiced in a block of 32 trials. The actual task included four blocks of 48 trials. Switch cost was calculated by subtracting accuracy and reaction time on non-switch trials from switch trials, such that higher scores signified higher cognitive flexibility.
Stop-signal Task
In the stop-signal task (Logan et al., 1984), participants are required to make a speeded response on go trials and inhibit a response on stop trials. In this case, participants indicated whether a green arrow, presented on the screen, pointed to the left or to the right (go trial). In 20% of trials, the arrow turned red after a stop-signal delay (SSD), in which case participants had to withhold a response (stop trial). To attain successful inhibition on 50% of stop trials (Verbruggen & Logan, 2009), SSD before a stop trial started at 300 ms and was adjusted dynamically to individual performance, decreasing 50 ms after a correctly inhibited response and increasing 50 ms after a failure to inhibit. On go trials, participants were instructed to respond as fast as possible and not to wait in anticipation of a possible stop cue. To ensure that our data did not include participants who waited on most stop trials, we employed a cut-off of 10 to 90% correct inhibition and a minimum of 60% correct on go trials (van Muijden et al., 2012). Stop-signal reaction time (SSRT) was calculated by sorting all correct go trial reaction times, taking the time corresponding to the percentage of correct stop trials and subtracting the mean SSD from this number (Logan et al., 1984).
Operation Span Task
For the operation span task (Unsworth et al., 2005), participants are asked to remember letters while simultaneously solving simple math problems. The operation span task therefore is thought to be a measure of updating or working memory. On each trial, single letters and equations were alternately presented on screen. Participants thus memorized a constantly updated string of letters, with a set size of 3–7 letters per trial. Following a complete set, participants were asked to recall the letters in the correct order by selecting them from a list on the screen. After entering the recalled string of letters, participants received feedback on performance on math operations and letter reproduction. There were 15 trials in total, with each set size presented 3 times in random order so participants could not predict how many items would be presented. Letters were randomly selected from a list (F, H, J, N, R, Y, K, L, Q, T, P, or S). Regarding the math operations, participants were asked to evaluate an equation as being true or false (e.g., 4 + 4 = 7). Both tasks were first practiced separately. The letter practice task consisted of 4 trials of 2 to 3 letters and the math task of 15 separate math operations. To prevent a tradeoff between solving equations and remembering letters, an 85% accuracy on equations was required. The final score on the operation span task is the total number of correctly recalled letters.
N-Back Task
In the N-back task (de Vries & Geurts, 2014), a series of stimuli was presented, and participants were asked to indicate whether the stimulus shown is equal to the one presented n trials earlier. Stimuli consisted of black and white drawings of objects. Working memory demand was varied using three different difficulty levels: 0-back, 1-back, and 2-back. The 0-back condition was used as a control and required participants to respond with “yes” when a picture of a car was presented and “no” for all other stimuli. In the 1-back condition, participants responded with “yes” when the current picture matched the previous one and “no” if it did not. In the 2-back condition, participants responded with “yes” when the current picture was identical to the one shown two trials previously and “no” if it was not. Participants were encouraged to respond as fast and as accurately as possible. The task was first explained on screen with oral instructions from the experimenter. Subsequently, participants practiced all three levels of the task, using a paper-version practice block of 15 trials and an on-screen practice block of 24 trials. The experimental task consisted of four blocks of each level, with 24 trials per level. We implemented a minimum of 50% correct on the 2-back task, to prevent scores under chance level. Performance on this task was calculated as the difference between the percentage correct on 2-back and percentage correct on 0-back items (Kirchner, 1958).
Questionnaires
To assess fatigue, we used the Fatigue subscale of the Checklist Individual Strength (Vercoulen et al., 1997). Anxiety was measured using the Hospital Anxiety Depression Scale (HADS; Zigmond & Snaith, 1983). For more detailed descriptions of these scales, see Buitenweg et al. (2018).
Training Performance
We computed overall training Z-scores and gain scores between the first and last training session. To acquire Z-scores, we calculated the percentage of each level’s maximum possible score and added up level scores to a total game score and scores within each domain to a domain score. For each of the three conditions, we subsequently calculated the mean training score and transformed them to Z-scores.
EBR
The first 10 s of each recording were discarded, to prevent the instruction from interfering with EBR. The recording was viewed frame by frame across a 5-min interval and scored for each blink by a researcher blind to the training condition. Movement of the eyelids was scored as a blink whenever the upper eyelid fully covered the pupil. EBR was defined as the mean number of blinks per minute.
Analysis
We investigated the ability to use EBR as a predictor of cognitive control functions and training benefit in healthy elderly adults. For the first hypothesis, a series of linear regression analyses was carried out, with performance on switch cost, stop-signal reaction time, operation span task, and N-back task as dependent variables and pre-training (baseline) EBR as the independent variable. A series of ANCOVAs was run for the second hypothesis, using difference scores for the N-back task, SSRT and switch cost, and training success (gain score and training z-score) as dependent variables, group as the independent variable, and baseline EBR as the covariate. We included the interaction term group ∗ EBR to establish whether benefit of one training was higher for different levels of EBR. All participants who completed the post-training cognitive testing session were included in the main analyses. Pearson’s correlation coefficient was used for all correlations. Assumptions of normality and linearity were checked by inspecting the P-P plots and scatter plots of EBR with task scores. Homoscedasticity was checked by examining the scatterplots of the standardized residuals versus standardized predicted values. Correlations between all predictors were run to check for multicollinearity. Outliers were detected using Grubbs’ Extreme Studentized Deviation test (Grubbs, 1950). We ran analyses with and without outliers. Reported results are without outliers. IBM SPSS Statistics for Windows, version 22 (IBM Corp., Armonk, N.Y., USA) was used for all statistical analyses. A p-value of 0.05 (two-tailed if not mentioned otherwise) was considered significant. Bonferroni corrections for multiple testing were used for all analyses.
Results
Participants
We invited 158 participants to the lab at T0, 107 of whom agreed to a short recording of their face. Participants who did not give permission for the recording were slightly older, t(151) = 2.02, p = 0.046, and more educated, t(151) = 2.17, p = 0.032, than those who did, but otherwise did not differ on baseline variables.
The video quality of two videos was too poor to calculate a reliable blink rate per minute. Two videos were too short, and one participant fell asleep while recording. One outlier was removed. Five participants were found to wear contact lenses, of which two participants wore only one. The difference in baseline EBR between participants with contacts (M = 9.1, SD = 4.1) and those without (M = 15.2, SD = 12.7) was not significant, t(91) = 1.06, p = 0.29. We therefore chose not to remove these participants from the sample. Data of 101 participants was used for analysis on T0 (age 60–85, M = 67.4, 61.4% female, mean years of education 13.4). Performance on all cognitive control tasks is shown in Table 1. After training, 38 participants agreed to a second EBR recording at T2. One participant did not follow the instructions, which rendered EBR of this person unusable. Thus, post-training EBR data is based on 37 participants, which will solely be used to report possible differences between the two time points.
EBR Results
Mean overall blink rate was 14.9 (SD = 12.1) at baseline and 14.8 (SD = 13.4) post-training. A high intercorrelation appeared between baseline and post-training measurements (r = 0.86, n = 37, p < 0.0005), and there was no effect of time, signifying stability of this measure.
Blink rate was not correlated with gender or age. Higher blink rate at baseline was associated with a lower reported state of fatigue (r = − 0.18, n = 90, p = 0.043), and at post-training this relationship was even stronger (r = − 0.41, n = 36, p = 0.006). Blink rate was not correlated with reported anxiety. Results remained the same when including outliers.
EBR as a Predictor of Cognitive Control Performance
Simple linear regression analysis was used to test whether EBR could be used to predict performance on operation span task, N-back task, switch task, and stop task. For the operation span task, EBR explained 10.7% of the variance (R2 = 0.107, F(1,69) = 8.30, p = 0.005). Participants’ predicted operation span task performance was equal to 56.01 − 0.34 ∗ EBR. Though we expected no relationship, this suggests that increased EBR was related to a decrease in operation span. Additionally, EBR was able to explain 8.7% of the variance of the N-back task (R2 = 0.087, F(1,86) = 8.22, p = 0.005). Participants’ predicted N-back performance equaled − 8.71 − 0.17 ∗ EBR, implying that increased EBR was related to a decrease on N-back task performance, as expected. Blink rate did not explain any variance of the switch task (R2 = 0.033, F(1,96) = 3.27, p = 0.07) or the stop task (R2 < 0.001, F(1,93) = 0.02, p = 0.88).
As both significant results concerned accuracy measures, as opposed to latency, in order to determine whether measurement type played a role, we added an extra analysis of EBR predicting switch cost accuracy and stop-signal accuracy. Neither of these was able to be significantly predicted by EBR (both ps > 0.17).
EBR as a Predictor of Training Benefit
To test if EBR can predict training benefit, an ANCOVA was conducted between baseline EBR and difference scores on cognitive control tasks and training success, including the interaction between EBR and group to determine if EBR affected benefit of one of the interventions. There was a significant interaction between group and EBR on change in performance on the N-back task, F(2,79) = 6.00, p = 0.004, ɳp2 = 0.132. Plotting of this relationship revealed a negative regression line for the non-flexible intervention group compared to the other two interventions (see Fig. 1). EBR was not related to a change in performance on the switch task, F(2,81) = 0.80, p = 0.45, the stop task, F(2,75) = 0.90, p = 0.41, or the operation span task, F(2,54) = 1.60, p = 0.21, after any specific intervention. Training improvement was not affected by an interaction between group and EBR, both using gain scores, F(2,83) = 0.73, p = 0.49, and Z-scores, F(2,83) = 0.05, p = 0.96.
Discussion
The present study used spontaneous eye blink rate (EBR) as a proxy of striatal D2 receptor binding to relate striatal D2 to cognitive control functioning and trainability in older adults. Our results demonstrate that EBR significantly predicted performance on working memory updating, both as measured with an operation span task and an N-back task. EBR failed to predict switching performance and response inhibition. Furthermore, EBR predicted training benefits on the N-back task for two of the conditions, but not for any of the other tasks.
EBR as a Predictor of Cognitive Control Performance
Both N-back and operation span task scores were found to be negatively correlated to EBR. Although we expected no relationship with the operation span task based on the literature (Tharp & Pickering, 2011), the predicted correlation with N-back task performance has previously been observed in young (Zhang et al., 2015). The latter suggests that it is possible to predict working memory performance in older adults using an indirect indicator of striatal D2 availability. Our other hypotheses regarding a positive relationship with switch cost and a negative correlation with inhibition were not confirmed. This implies that despite previous findings in young adults—demonstrating prediction of these functions using EBR (Colzato, Huizinga, et al., 2009; Colzato, van den Wildenberg, et al., 2009; Dreisbach et al., 2005; Kleinsorge & Scheil, 2017)—a similar relationship was not found in older adults.
There are several possible alternative interpretations for the present findings. One involves the differences in the tasks used in previous studies and the current one. For instance, in Tharp and Pickering (2011), the switch task consisted of only one switch per task block, whereas in ours a switch occurred every other trial. Also, their usage of the two perseveration and learned irrelevance conditions allowed participants with high EBR to respond to a novelty bias. As novelty was not present as an element in our own switch task, perseveration and distractibility were balanced. In Kleinsorge and Scheil (2017), the appearance of a pre-cue or a task cue allowed participants to prepare for a switch on certain trials but not on others, which introduced an extra element into the paradigm which was not present in ours. Accordingly, the tasks used in these studies differed from ours in such a way that they possibly tapped into different subfunctions.
Moreover, something that might have played a larger role in our sample of older adults than it did in the aforementioned samples of young participants concerns the speed-accuracy tradeoff. This phenomenon stems from participants’ reluctance to make errors, thereby inadvertently choosing to spend more time on a task in order to keep the number of errors to a minimum (Forstmann et al., 2011; Starns & Ratcliff, 2010). This is observed in the very high percentage of correct stop trials, which should, by default, have stayed around 50% (Verbruggen & Logan, 2009), and does so in previous studies in young subjects. In our sample, despite removing the data of subjects with correct stop trials above 90%, the average percentage rose above 60%, suggesting that many participants waited in anticipation of the stop cue. We, therefore, cannot rule out that in our sample we were not able to measure inhibition in pure form, but in a form somewhat obfuscated by conservative response strategies.
Our working memory tasks covaried with EBR, while the switching and stopping tasks did not. A notable difference between those that did and did not covary is the focus on speed versus accuracy. Both N-back and operation span tasks emphasized accuracy, with less emphasis on speed, allowing participants to spend more time on each trial. On our switch and stop tasks, by contrast, speed was central to performance, both by emphasizing the importance of speed from the beginning and by giving participants less time per trial, compared with said working memory tasks. Although we did not find any effects on the switch and stop tasks in an additional analysis of accuracy, these measures are still embedded in a task that is greatly speed-related. One option for future research to attend to the speed-accuracy tradeoff is the use of a diffusion model, such as the EZ model (Wagenmakers et al., 2007), to estimate currently unaddressed elements of participant behavior such as drift rate and non-decision time. Due to violated assumptions, this model was not applicable to our current dataset. Nonetheless, we encourage the implementation of such models in further research to circumvent the tradeoff dilemma.
Besides the selection of similar tasks, one additional point to keep in mind when comparing studies that use EBR as a predictor of other functions is that the average blink rate often differs from one study report to the next, depending on the sample (Colzato et al., 2007; Colzato, Huizinga, et al., 2009; Colzato, van den Wildenberg, et al., 2009). If the relationship is not linear, as is thought to be the case at least in the association with working memory and inhibition (Cools & D’Esposito, 2011), then the results are greatly dependent on measurement of the left or right side of that curve.
Despite the many studies stating the possibilities of predicting performance on tasks relying on striatal D2 functioning with EBR, the picture is not entirely consistent. Some studies find that these links pertain primarily to D1 (Colzato et al., 2014; Colzato, Huizinga, et al., 2009; Colzato, van den Wildenberg, et al., 2009), whereas two PET studies found no evidence for a direct link between EBR and dopamine D2 receptor availability (Dang et al., 2017; Sescousse et al., 2018). The latter two studies are important, as they provide direct measures of striatal D2 receptor density. Yet, both studies included only 20 young adult participants; reliable correlations remain to be confirmed in larger samples and should be established also among older adults, among whom individual differences in striatal D2 receptor density may be expected to be much more pronounced. Still, in view of these findings, we may speculate on a meaningful interpretation. While working memory ability relies more on prefrontal D1 (McNab et al., 2009; Takahashi et al., 2008), stopping and switching have been tentatively related more to striatal D2 (Cools & D’Esposito, 2011; Haluk & Floresco, 2009), thus providing a suggestive explanation of why we found EBR to predict performance on working memory but not stop and switch tasks.
Though the average blink rate in our own sample was generally similar to that reported for older participants in previous literature (Deuschl & Goddemeier, 1998; Zaman et al., 1998), the variability was large, with some high and some almost absent blink rates. Though individual differences can be even higher in advanced age (Lindenberger et al., 2008; Mella et al., 2018), external circumstances might have played a role in this. For instance, stress or anxiety can increase blinks (Cruz et al., 2011). Though in the current study anxiety scores were found not to be related to EBR, anxiety was assessed using an online, at-home questionnaire. It is possible that at the time of EBR measurement, a more temporary anxiety affected (i.e., increased) blink rate. Likewise, several participants reported that during EBR recording, while staring at the blank wall, they felt almost hypnotized, much like a meditative trance. Previous research demonstrates that long-time meditators show lower blink rates (Kruis et al., 2016), though this was not the case for short-time practice. As we did not register whether participants were long-time meditators or felt nervous or anxious during the test sessions, it is not clear whether the more extreme blink rates were caused by such circumstances or might just be ascribed to general individual differences.
EBR as a Predictor of Training Benefit
Last, we observed that EBR was more predictive of training success in N-back performance for the non-flexible intervention group compared to the other groups, while EBR was not predictive of performance in any of the other measures, regardless of training condition. Although we acknowledge that the sample size for this comparison was relatively small (due mostly to drop-out in the post-training measurement), the present setup does provide a unique opportunity to study if EBR predicts trainability. The observed pattern appeared to be driven mostly by the negative predictive relationship between EBR and N-back task on baseline. As the relationship between training improvement and activity and volume changes in the striatum have been found in young adults (Dahlin et al., 2008), it would be most relevant to investigate whether EBR is able to predict training success at least in younger populations. Yet, as far as EBR allows for an indirect assessment of striatal D2 activity, it is possible that the relationship between dopamine and training benefit cannot be demonstrated with this measure. Moreover, as the task improvements demonstrated by participants in our sample (Buitenweg et al., 2017) most likely occurred due to practice effects, another feasible explanation is that our training did not offer sufficient stimulation for benefit to take place and therefore did not support adequate prediction by individual elements. Further research should determine whether such an indirect relationship between EBR and training benefit could be found.
In Conclusion
To summarize, we determined predictive validity for working memory performance, but not switching or response inhibition, using EBR. EBR appears to be predictive of training benefits only for working memory performance, at least in the older adult population. The current findings suggest modest possibilities in predicting working memory performance in older adults using EBR. Further research using the measurement of actual dopamine receptor availability along with EBR within this population is essential to determine whether this relationship can be demonstrated in older ages, providing us a necessary background for interpreting the link with cognitive control functions. Furthermore, investigations should focus on whether the currently found predictions hold also under different circumstances or with other tasks of working memory. Meanwhile, it remains essential to continue searching for a reliable predictor of functional cognitive decline and possibilities for improvement, in the older population.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Akbari Chermahini, S., & Hommel, B. (2010). The (b)link between creativity and dopamine: Spontaneous eye blink rates predict and dissociate divergent and convergent thinking. Cognition, 115(3), 458–465.
Bäckman, L., Ginovart, N., Dixon, R. A., Wahlin, T. R., Wahlin, Å., Halldin, C., & Farde, L. (2000). Age-related cognitive deficits mediated by changes in the striatal dopamine system. American Journal of Psychiatry, 157(4), 635–637.
Bäckman, L., Waris, O., Johansson, J., Andersson, M., Rinne, J. O., Alakurtti, K., & Nyberg, L. (2017). Increased dopamine release after working-memory updating training: Neurochemical correlates of transfer. Scientific Reports, 7(1), 7160.
Barbato, G., Ficca, G., Muscettola, G., Fichele, M., Beatrice, M., & Rinaldi, F. (2000). Diurnal variation in spontaneous eye-blink rate. Psychiatry Research, 93(2), 145–151. https://doi.org/10.1016/s0165-1781(00)00108-6
Basak, C., & O’Connell, M. A. (2016). To switch or not to switch: Role of cognitive control in working memory training in older adults. Frontiers in Psychology, 7, 230.
Bentivoglio, A. R., Bressman, S. B., Cassetta, E., Carretta, D., Tonali, P., & Albanese, A. (1997). Analysis of blink rate patterns in normal subjects. Movement Disorders, 12(6), 1028–1034.
Berry, A. S., Shah, V. D., Baker, S. L., Vogel, J. W., O'Neil, J. P., Janabi, M., ... & Jagust, W. J. (2016). Aging affects dopaminergic neural mechanisms of cognitive flexibility. Journal of Neuroscience, 36(50), 12559–12569.
Blin, O., Masson, G., Azulay, J., Fondarai, J., & Serratrice, G. (1990). Apomorphine-induced blinking and yawning in healthy volunteers. British Journal of Clinical Pharmacology, 30(5), 769–773.
Bologna, M., Fasano, A., Modugno, N., Fabbrini, G., & Berardelli, A. (2012). Effects of subthalamic nucleus deep brain stimulation and l-DOPA on blinking in Parkinson’s disease. Experimental Neurology, 235(1), 265–272.
Brandt, J., Spencer, M., & Folstein, M. (1988). The telephone interview for cognitive status. Cognitive and Behavioral Neurology, 1(2), 111–118.
Brunelin, J., Fecteau, S., & Suaud-Chagny, M. (2013). Abnormal striatal dopamine transmission in schizophrenia. Current Medicinal Chemistry, 20(3), 397–404.
Buitenweg, J. I. V., van de Ven, R. M., Prinssen, S., Murre, J. M. J., & Ridderinkhof, K. R. (2017). Cognitive flexibility training: A large-scale multimodal adaptive active-control intervention study in healthy older adults. Frontiers in Human Neuroscience, 11, 529.
Buitenweg, J. I. V., van de Ven, R. M., Ridderinkhof, K. R., & Murre, J. M. J. (2018). Does cognitive flexibility training enhance subjective mental functioning in healthy older adults? Aging, Neuropsychology, and Cognition, 26(5), 688–710.
Buitenweg, J. I. V., Murre, J. M. J., & Ridderinkhof, K. R. (2012). Brain training in progress: A review of trainability in healthy seniors. Frontiers in Human Neuroscience, 6, 183.
Buschkuehl, M., Jaeggi, S. M., Hutchison, S., Perrig-Chiello, P., Däpp, C., Müller, M., & Perrig, W. J. (2008). Impact of working memory training on memory performance in old-old adults. Psychology and Aging, 23(4), 743.
Cassavaugh, N. D., & Kramer, A. F. (2009). Transfer of computer-based training to simulated driving in older adults. Applied Ergonomics, 40(5), 943–952.
Chen, W., Chiang, T., Hsu, M., & Liu, J. (2003). The validity of eye blink rate in Chinese adults for the diagnosis of Parkinson’s disease. Clinical Neurology and Neurosurgery, 105(2), 90–92.
Christensen, H., Mackinnon, A., Korten, A., Jorm, A., Henderson, A., Jacomb, P., & Rodgers, B. (1999). An analysis of diversity in the cognitive performance of elderly community dwellers: Individual differences in change scores as a function of age. Psychology and Aging, 14(3), 365.
Colzato, L. S., van den Wildenberg, W. P. M., & Hommel, B. (2007). Impaired inhibitory control in recreational cocaine users. PLoS ONE, 2(11), e1143.
Colzato, L. S., van den Wildenberg, W. P. M., & Hommel, B. (2008). Reduced spontaneous eye blink rates in recreational cocaine users: Evidence for dopaminergic hypoactivity. PLoS ONE, 3(10), e3461.
Colzato, L. S., van den Wildenberg, W. P. M., & Hommel, B. (2014). Cognitive control and the COMT val 158 met polymorphism: Genetic modulation of videogame training and transfer to task-switching efficiency. Psychological Research Psychologische Forschung, 78(5), 670–678.
Colzato, L. S., van den Wildenberg, W. P. M., van Wouwe, N. C., Pannebakker, M. M., & Hommel, B. (2009). Dopamine and inhibitory action control: Evidence from spontaneous eye blink rates. Experimental Brain Research, 196(3), 467–474.
Colzato, L. S., Huizinga, M., & Hommel, B. (2009). Recreational cocaine polydrug use impairs cognitive flexibility but not working memory. Psychopharmacology (berl), 207(2), 225.
Colzato, L. S., Slagter, H. A., Spapé, M. M., & Hommel, B. (2008). Blinks of the eye predict blinks of the mind. Neuropsychologia, 46(13), 3179–3183.
Colzato, L. S., Waszak, F., Nieuwenhuis, S., Posthuma, D., & Hommel, B. (2010). The flexible mind is associated with the catechol-O-methyltransferase (COMT) Val158Met polymorphism: Evidence for a role of dopamine in the control of task-switching. Neuropsychologia, 48(9), 2764–2768.
Congdon, E., Lesch, K. P., & Canli, T. (2008). Analysis of DRD4 and DAT polymorphisms and behavioral inhibition in healthy adults: Implications for impulsivity. American Journal of Medical Genetics Part b: Neuropsychiatric Genetics, 147(1), 27–32.
Cools, R., & D’Esposito, M. (2011). Inverted-u–shaped dopamine actions on human working memory and cognitive control. Biological Psychiatry, 69(12), e113–e125.
Cruz, A. A., Garcia, D. M., Pinto, C. T., & Cechetti, S. P. (2011). Spontaneous eyeblink activity. The Ocular Surface, 9(1), 29–41.
Dahlin, E., Neely, A. S., Larsson, A., Backman, L., & Nyberg, L. (2008). Transfer of learning after updating training mediated by the striatum. Science (New York, N.Y.), 320(5882), 1510–1512. https://doi.org/10.1126/science.1155466
Dang, L. C., Samanez-Larkin, G. R., Castrellon, J. J., Perkins, S. F., Cowan, R. L., Newhouse, P. A., & Zald, D. H. (2017). Spontaneous eye blink rate (EBR) is uncorrelated with dopamine D2 receptor availability and unmodulated by dopamine agonism in healthy adults. eNeuro, 4(5), ENEURO. 0211–17.2017.
de Vries, M., & Geurts, H. M. (2014). Beyond individual differences: Are working memory and inhibition informative specifiers within ASD? Journal of Neural Transmission, 121(9), 1183–1198.
Deuschl, G., & Goddemeier, C. (1998). Spontaneous and reflex activity of facial muscles in dystonia, Parkinson’s disease, and in normal subjects. Journal of Neurology, Neurosurgery, and Psychiatry, 64(3), 320–324.
Dreisbach, G., Müller, J., Goschke, T., Strobel, A., Schulze, K., Lesch, K., & Brocke, B. (2005). Dopamine and cognitive control: The influence of spontaneous eyeblink rate and dopamine gene polymorphisms on perseveration and distractibility. Behavioral Neuroscience, 119(2), 483.
Erickson, K. I., Boot, W. R., Basak, C., Neider, M. B., Prakash, R. S., Voss, M. W., & Gratton, G. (2010). Striatal volume predicts level of video game skill acquisition. Cerebral Cortex, 20(11), 2522–2530.
Erixon-Lindroth, N., Farde, L., Wahlin, T. B., Sovago, J., Halldin, C., & Backman, L. (2005). The role of the striatal dopamine transporter in cognitive aging. Psychiatry Research, 138(1), 1–12. https://doi.org/10.1016/j.pscychresns.2004.09.005
Fisk, J. E., & Sharp, C. A. (2004). Age-related impairment in executive functioning: Updating, inhibition, shifting, and access. Journal of Clinical and Experimental Neuropsychology, 26(7), 874–890.
Forstmann, B. U., Tittgemeyer, M., Wagenmakers, E. J., Derrfuss, J., Imperati, D., & Brown, S. (2011). The speed-accuracy tradeoff in the elderly brain: A structural model-based approach. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 31(47), 17242–17249. https://doi.org/10.1523/JNEUROSCI.0309-11.2011
Grubbs, F. E. (1950). Sample criteria for testing outlying observations. The Annals of Mathematical Statistics, , 27–58.
Haluk, D. M., & Floresco, S. B. (2009). Ventral striatal dopamine modulation of different forms of behavioral flexibility. Neuropsychopharmacology, 34(8), 2041.
Jongkees, B. J., & Colzato, L. S. (2016). Spontaneous eye blink rate as predictor of dopamine-related cognitive function—A review. Neuroscience & Biobehavioral Reviews, 71, 58–82.
Kanai, R., & Rees, G. (2011). The structural basis of inter-individual differences in human behaviour and cognition. Nature Reviews Neuroscience, 12(4), 231.
Karbach, J., & Kray, J. (2009). How useful is executive control training? Age differences in near and far transfer of task-switching training. Developmental Science, 12(6), 978–990.
Karbach, J., & Verhaeghen, P. (2014). Making working memory work: A meta-analysis of executive-control and working memory training in older adults. Psychological Science, 25(11), 2027–2037.
Karrer, T. M., Josef, A. K., Mata, R., Morris, E. D., & Samanez-Larkin, G. R. (2017). Reduced dopamine receptors and transporters but not synthesis capacity in normal aging adults: a meta-analysis. Neurobiology of Aging, 57, 36–46.
Karson, C. N. (1983). Spontaneous eye-blink rates and dopaminergic systems. Brain, 106(3), 643–653.
Kirchner, W. K. (1958). Age differences in short-term retention of rapidly changing information. Journal of Experimental Psychology, 55(4), 352.
Kleinsorge, T., & Scheil, J. (2017). Integration of advance information about a forthcoming task switch–evidence from eye blink rates. Frontiers in Psychology, 8, 290.
Klostermann, E. C., Braskie, M. N., Landau, S. M., O'Neil, J. P., & Jagust, W. J. (2012). Dopamine and frontostriatal networks in cognitive aging. Neurobiology of Aging, 33(3), 623-e15.
Kruis, A., Slagter, H. A., Bachhuber, D. R., Davidson, R. J., & Lutz, A. (2016). Effects of meditation practice on spontaneous eyeblink rate. Psychophysiology, 53(5), 749–758.
Kühn, S., Schmiedek, F., Noack, H., Wenger, E., Bodammer, N. C., Lindenberger, U., & Lövden, M. (2013). The dynamics of change in striatal activity following updating training. Human Brain Mapping, 34(7), 1530–1541.
Ladas, A., Frantzidis, C., Bamidis, P., & Vivas, A. B. (2014). Eye blink rate as a biological marker of mild cognitive impairment. International Journal of Psychophysiology, 93(1), 12–16.
Lindenberger, U., Nagel, I. E., Chicherio, C., Li, S., Heekeren, H. R., & Bäckman, L. (2008). Age-related decline in brain resources modulates genetic effects on cognitive functioning. Frontiers in Neuroscience, 2, 39.
Logan, G. D., Cowan, W. B., & Davis, K. A. (1984). On the ability to inhibit simple and choice reaction time responses: A model and a method. Journal of Experimental Psychology: Human Perception and Performance, 10(2), 276.
Mackert, A., Woyth, C., Flechtner, K., & Volz, H. (1990). Increased blink rate in drug-naive acute schizophrenic patients. Biological Psychiatry, 27(11), 1197–1202.
McNab, F., Varrone, A., Farde, L., Jucaite, A., Bystritsky, P., Forssberg, H., & Klingberg, T. (2009). Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science (New York, N.Y.), 323(5915), 800–802. https://doi.org/10.1126/science.1166102
Mella, N., Fagot, D., Renaud, O., Kliegel, M., & De Ribaupierre, A. (2018). Individual differences in developmental change: Quantifying the amplitude and heterogeneity in cognitive change across old age. Journal of Intelligence, 6(1), 10.
Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., Howerter, A., & Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41(1), 49–100.
Müller, J., Dreisbach, G., Brocke, B., Lesch, K., Strobel, A., & Goschke, T. (2007). Dopamine and cognitive control: The influence of spontaneous eyeblink rate, DRD4 exon III polymorphism and gender on flexibility in set-shifting. Brain Research, 1131, 155–162.
Raz, N., Ghisletta, P., Rodrigue, K. M., Kennedy, K. M., & Lindenberger, U. (2010). Trajectories of brain aging in middle-aged and older adults: Regional and individual differences. NeuroImage, 51(2), 501–511.
Rinne, J. O., Hietala, J., Ruotsalainen, U., Säkö, E., Laihinen, A., Någren, K., & Syvälahti, E. (1993). Decrease in human striatal dopamine D2 receptor density with age: A PET study with [11C] raclopride. Journal of Cerebral Blood Flow & Metabolism, 13(2), 310–314.
Rogers, R. D., & Monsell, S. (1995). Costs of a predictible switch between simple cognitive tasks. Journal of Experimental Psychology: General, 124(2), 207.
Sescousse, G., Ligneul, R., van Holst, R. J., Janssen, L. K., de Boer, F., Janssen, M., & Cools, R. (2018). Spontaneous eye blink rate and dopamine synthesis capacity: Preliminary evidence for an absence of positive correlation. European Journal of Neuroscience, 47(9), 1081–1086.
Sforza, C., Rango, M., Galante, D., Bresolin, N., & Ferrario, V. F. (2008). Spontaneous blinking in healthy persons: An optoelectronic study of eyelid motion. Ophthalmic and Physiological Optics, 28(4), 345–353.
Slagter, H. A., Georgopoulou, K., & Frank, M. J. (2015). Spontaneous eye blink rate predicts learning from negative, but not positive, outcomes. Neuropsychologia, 71, 126–132.
Starns, J. J., & Ratcliff, R. (2010). The effects of aging on the speed–accuracy compromise: Boundary optimality in the diffusion model. Psychology and Aging, 25(2), 377.
Sun, W. S., Baker, R. S., Chuke, J. C., Rouholiman, B. R., Hasan, S. A., Gaza, W., & Porter, J. D. (1997). Age-related changes in human blinks. Passive and active changes in eyelid kinematics. Investigative Ophthalmology & Visual Science, 38(1), 92–99.
Takahashi, H., Kato, M., Takano, H., Arakawa, R., Okumura, M., Otsuka, T., & Suhara, T. (2008). Differential contributions of prefrontal and hippocampal dopamine D(1) and D(2) receptors in human cognitive functions. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 28(46), 12032–12038. https://doi.org/10.1523/JNEUROSCI.3446-08.2008
Tharp, I. J., & Pickering, A. D. (2011). Individual differences in cognitive-flexibility: The influence of spontaneous eyeblink rate, trait psychoticism and working memory on attentional set-shifting. Brain and Cognition, 75(2), 119–125.
Unsworth, N., Heitz, R. P., Schrock, J. C., & Engle, R. W. (2005). An automated version of the operation span task. Behavior Research Methods, 37(3), 498–505.
van Holstein, M., Aarts, E., van der Schaaf, M. E., Geurts, D. E., Verkes, R. J., Franke, B., & Cools, R. (2011). Human cognitive flexibility depends on dopamine D2 receptor signaling. Psychopharmacology (berl), 218(3), 567–578.
van Muijden, J., Band, G. P., & Hommel, B. (2012). Online games training aging brains: Limited transfer to cognitive control functions. Frontiers in Human Neuroscience, 6, 221. https://doi.org/10.3389/fnhum.2012.00221
Verbruggen, F., & Logan, G. D. (2009). Models of response inhibition in the stop-signal and stop-change paradigms. Neuroscience & Biobehavioral Reviews, 33(5), 647–661.
Vercoulen, J., Bazelmans, E., Swanink, C., Fennis, J., Galama, J., Jongen, P., & Bleijenberg, G. (1997). Physical activity in chronic fatigue syndrome: Assessment and its role in fatigue. Journal of Psychiatric Research, 31(6), 661–673.
Volkow, N. D., Fowler, J. S., & Wang, G. (1999). Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. Journal of Psychopharmacology, 13(4), 337–345.
Wagenmakers, E., Der Maas, V., Han, L. J., & Grasman, R. P. P. P. (2007). An EZ-diffusion model for response time and accuracy. Psychonomic Bulletin & Review, 14(1), 3–22.
Wagenmakers, E. J., Van Der Maas, H. L., & Grasman, R. P. (2007). An EZ-diffusion model for response time and accuracy. Psychonomic Bulletin & Review, 14(1), 3–22.
Zaman, M., Doughty, M., & Button, N. (1998). The exposed ocular surface and its relationship to spontaneous eyeblink rate in elderly Caucasians. Experimental Eye Research, 67(6), 681–686.
Zhang, T., Mou, D., Wang, C., Tan, F., Jiang, Y., Lijun, Z., & Li, H. (2015). Dopamine and executive function: Increased spontaneous eye blink rates correlate with better set-shifting and inhibition, but poorer updating. International Journal of Psychophysiology, 96(3), 155–161.
Zigmond, A. S., & Snaith, R. P. (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67(6), 361–370.
Zinke, K., Zeintl, M., Rose, N. S., Putzmann, J., Pydde, A., & Kliegel, M. (2014). Working memory training and transfer in older adults: Effects of age, baseline performance, and training gains. Developmental Psychology, 50(1), 304.
Acknowledgements
The authors would like to thank all participants and their relatives for participating in the study.
Funding
This project was part of the research program “Evidence-based adaptive brain training in seniors: Effects of brain structure and dopaminergic system on individual differences in trainability” funded by the National Initiative Brain and Cognition, a part of the Organization for Scientific Research (NWO), under grant number 056–12-010.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
This study was approved by the local Ethics Committee of the University of Amsterdam and registered under number 2012-BC-2566. All procedures were conducted in compliance with the Declaration of Helsinki, relevant laws, and institutional guidelines.
Consent to Participate
The authors affirm that all participants provided full written informed consent prior to participation.
Consent for Publication
All participants provided informed consent for publication of their anonymized data in a scientific journal.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Buitenweg, J.I.V., Murre, J.M.J. & Ridderinkhof, K.R. Spontaneous Eye Blinks Predict Executive Functioning in Seniors. J Cogn Enhanc 5, 468–479 (2021). https://doi.org/10.1007/s41465-021-00217-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41465-021-00217-4