Abstract
Peanut (Arachis hypogaea L.) holds significant commercial and dietary importance as a major source of edible oil and protein in Turkey. Stem, collar or root rot, caused by several fungal disease agent, are serious soil-borne diseases of peanut. Accurate and precise identification of the disease agent provides fundamental and precise information for integrated plant management. During the period from June to September 2021, symptoms consistent with collar rot disease, including dark-brown stem rot, chlorotic leaves, wilting, and eventual whole plant death, were observed on peanut plants cultivated in the different districts of Osmaniye Province of Turkey. The disease incidence ranged from 8.0 to 45.0% in the inspected fields with an average of 3.4% overall. Twenty-four single-spore representative isolates were obtained from surface-disinfected symptomatic tissues. Morphological characteristics of fungal mycelium, conidial and pycnidial structures on potato sucrose agar (PSA) and water agar (WA) closely resembled those described for Lasiodiplodia spp. All isolates caused typical collar rot symptoms upon artificial inoculation of peanut seedlings. Morphological identification of Lasiodiplodia spp. isolates was corroborated by MALDI-TOF and molecular analyses utilizing sequences from the internal transcribed spacer (ITS), β-tubulin 2 (tub2) and translation elongation factor-1 alpha (TEF1-α) loci. Phylogenetic analysis confirmed that the representative fungal isolates (MKUBK-B1 and MKUBK-K22) belong to Lasiodiplodia pseudotheobromae. To the best of our knowledge, this is the first report of L. pseudotheobromae infecting peanut plants in Turkey. This work is expected to contribute to previously limited knowledge regarding the host range, incidence and prevalence of L. pseudotheobromae as a soilborne pathogen of peanuts. Due to the potential destructiveness and broad host range of this pathogen, it is essential to develop new strategies to establish more reliable, environmentally sustainable, and cost-effective management approaches for this disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peanut or groundnut (Arachis hypogaea L.) is a vital legume plant of immense agricultural, economic, and nutritional significance. Peanut holds significant commercial and dietary importance as a major source of edible oil and protein on a global scale (Suchoszek-Lukaniuk et al. 2011). Globally, peanuts represent a major agricultural crop, with significant production occurring in countries such as China, India, Nigeria and the United States (FAO 2021). Over the past few decades, there has been a notable 30% increase in global peanut production due to burgeoning demand. Turkey holds a prominent position in worldwide peanut cultivation, with a substantial production of 468,334 tonnes covering 115,838 hectares of cultivated land in 2021 (TUİK 2021). In particularly, in the Eastern Mediterranean Region of Turkey, peanut cultivation is widespread and contributes significantly to the national economy (TUİK 2021). Osmaniye stands out as one of the key peanut-growing provinces in the region, producing 55,146 tonnes on 14,461 hectares of cultivated area in 2021 (TUİK 2021). Although peanut acreage increased in Turkey during last 10 year growing season, total peanut production and yield is much lower than other pioneering peanut-growing countries, such as China, Egypt, Indonesia and USA (FAO 2021). This low yield is believed mainly due to several abiotic (moisture stress, lack of improved cultivars, low soil fertility content and poor crop management practices) and biotic factors such as fungal, bacterial and viral plant diseases of the plants (Debele et al. 2023).
Plant diseases occurring during various stages of cultivation, harvesting, processing, and transportation represent significant biotic constraints on global peanut cultivation worldwide (Minarni and Yuhendra 2019). Given their close interaction with soil, particularly subsoil organs and seed pods, peanut plants are highly susceptible to soilborne fungal agents. Soilborne fungal diseases in peanuts cause significant losses in both plant health and yield. Depending on factors such as crop variety, climate, and environmental conditions, the total yield losses attributed to soilborne diseases can reach 50% (Pal et al. 2014). Owing to their genetic diversity, broad host range, and resistant fungal structures within the soil, the commonly used fungicides in peanut crops have demonstrated limited or no efficacy in disease control (Ahmad et al. 2019). Fungal disease agents belonging to genera such as Aspergillus, Sclerotium, Sclerotinia, Fusarium, Rhizoctonia, Macrophomina, Pythium, Verticillium, Rhizopus, Lasiodiplodia, and Mucor have been identified as major causal agents of peanut diseases in various countries (Akgul et al. 2011; Thiessen and Woodward 2012; Liu et al. 2016; Sun et al. 2021; Debele et al. 2023; Wang et al. 2023a).
The similarity in symptoms manifested on roots and stems poses challenges in accurately identifying soilborne fungal disease agents. The occurrence of new fungal disease species is contingent on environmental factors and the cultivar used. Thus, the precise identification of causal disease agents is of paramount importance. Accurate identification of disease agents provides fundamental and precise information for integrated plant management. Among the soilborne fungal disease agents, Aspergillus niger, Sclerotium rolfsii, and Sclerotinia sclerotiorum are the primary causal agents of root, collar, and stem rot in peanuts, worldwide (Akgul et al. 2011; Liu et al. 2016; Jogi et al. 2018; Jacob et al. 2018; Gudu et al. 2020). Additionally, stem or crown rot caused by Lasiodiplodia spp. has been reported to occur in peanut plants cultivated in various countries (Phipps and Porter 1988; Wang et al. 2017, 2023a; Zhang et al. 2022).
Recent disease surveys conducted in the Osmaniye Province have noted the presence of different fungal species causing collar or stem rot in peanut plants, in addition to common root rot disease agents. The aim of this study was to isolate and identify potential causal agent(s) of peanut collar rot disease in infested peanut fields located in the Osmaniye Province of Turkey.
Materials and methods
Field surveys and plant sampling
Peanut collar rot symptoms were observed, and diseased peanut plant samples (n = 65) were collected from a total of 95 peanut fields in Kadirli (37°21′17.5"N 36°02′05.6"E), Merkez (37°07′12.8"N 36°11′21.0"E), Sumbas (37°26′38.2"N 36°00′31.4"E), Bahçe (37°12′03.3"N 36°35′54.6"E), Düziçi (37°14′55.1"N 36°24′51.6"E), and Toprakkale (37°04′36.8"N 36°09′21.4"E) districts of Osmaniye Province, Turkey, from th June to September 2021. Disease prevalence was recorded as the percentage of crown rot affected groundnut fields observed relative to the total number of field examined, whereas disease incidence was determined by calculating the proportion of diseased plants observed relative to the total number of plants examined in each field.
Morphological characterisation of fungal isolates
To isolate the fungal agents from the affected groundnut plants, the stems and roots were thoroughly washed under running tap water. Plant parts containing necrotic and intact tissues (5 mm2) were excised from the lesion edges. These tissue samples were surface-disinfected in 2% NaClO for 2 min, followed by two rinses in sterile distilled water. Subsequently, the samples were air-dried on sterile blotting papers. Disinfected plant tissue pieces from each plant sample were plated on PSA (potato sucrose agar) supplemented with streptomycin sulfate (50 µg ml−1). The plates were incubated at 25 ± 1 °C for 5 days in the dark. Fungal colonies that were morphologically similar were subcultured onto new PSA plates to obtain pure cultures. Further purification was achieved by transferring hyphal tips from 5 day-old cultures to obtain a single-spore culture. These isolates were preserved on PSA plates at 4 ± 1 °C for subsequent morphological and molecular analyses (Soylu et al. 2023).
To induce sporulation, pure cultures were transferred onto 2% water agar (WA) to promote the development of morphological structures such as pycnidia and conidia on the media. Thin sections of sterilized Cogon grass leaves (Imperata cylindrica) were placed on the surfaces of WAs adjacent to the mycelial discs to facilitate pycnidial and conidial formation (Pipattanapuckdee et al. 2019). Following the formation of fungal structures on WA, the morphology of the conidiogenous cells, paraphyses, and conidia (mature and immature) were observed and their dimensions (n = 50) were measured under a light microscope equipped with a Nomarski DIC attachment (Olympus BX51, Japan).
Identification of fungal isolates by molecular and MALDI-TOF analysis
The fungal isolates were identified using a MALDI-TOF Biotyper (Matrix-assisted Laser Desorption Ionisation-Time of Flight Mass Spectrometry, Bruker Daltonics, Bremen, Germany) The fungal isolates were cultured in sterile PSB (potato sucrose broth) supplemented with streptomycin sulfate (50 µg ml−1) for 72 h. Representative isolates in the PSB were suspended in 900 µl of absolute ethanol and centrifuged at 13,000g for 2 min after which mycelial masses (300 µl) were obtained. The resulting supernatant was discarded, and the pellet was left to air-dry. The remaining pellet, approximately 20 mg in weight, was suspended in 50 μl of 70% formic acid. Then, 50 μl of acetonitrile was added. The resulting mixture underwent brief vortexing and was subsequently centrifuged at 13,000 g for 2 min. A 1 μl volume of the supernatant was deposited onto a steel target plate (Bruker Daltonics) and dried at room temperature. The samples were overlaid with 2 μl of matrix solution and air-dried at room temperature. Biotyper Real-Time Classification (RTC) software (Biotyper 3.0; Microflex LT; Bruker Daltonics) was used to identify all the isolates at the species level (Ferreira et al. 2011).
The fungal isolates were identified molecularly by sequencing three genetic loci: the internal transcribed spacer (ITS) rDNA, the translation elongation factor-1 alpha (TEF1-α), and the β-tubulin 2 (tub2) gene. Genomic DNA of representative isolates was extracted from 7-day-old aerial mycelium of pure cultures using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany). The ITS, tub2, and TEF1-α loci of fungal isolates were amplified and sequenced using the primer sets ITS4/ITS5, Bt2a/Bt2b, and EF1-688F/EF1-1251R, respectively (White et al. 1990; Glass and Donaldson 1995; Alves et al. 2008). The consensus sequences obtained for the ITS, tub2 and TEF1-α loci of the MKUBK-B1 and MKUBK-K22 isolates were deposited in the NCBI GenBank database. Taxonomic classification and analysis were conducted using various species obtained from the NCBI GenBank database. Molecular Evolutionary Genetic Analysis (MEGA 11) software was used to construct the phylogenetic tree. A phylogenetic tree was constructed utilizing the maximum likelihood method, and bootstrap testing was performed with 1,000 replicates following the method described by Tamura et al. (2021).
Pathogenicity tests
All fungal isolates (n = 24) were subjected to pathogenicity tests by inoculating stems of 3-week-old peanut plants (cv. NC7) via artificial inoculation. The inoculation sites were initially surface sterilized by wiping with 70% ethyl alcohol. Mycelial plugs, cut from the margin of fresh colonies, were applied onto wounds at the base of healthy peanut seedling stems and wrapped with Parafilm (Zhang et al. 2022). For each isolate, four healthy seedlings were used. Control seedlings (n = 4) were inoculated with sterile PSA plugs. All inoculated plants were incubated in a growth chamber at a temperature of 26 ± 2 °C, with a 12-h photoperiod and 80% humidity up to 14 days. For each isolate, daily inspections were carried out on the inoculated seedlings to record disease symptoms at the inoculation sites. The pathogenicity test was repeated, and fungal isolates were subsequently re-isolated from the inoculated seedlings.
Results
Disease surveys for fungal isolates
Between June and September 2021, in addition to three major soilborne fungal pathogens S. rolfsii, A. niger and R. solani, the presence of new fungal species causing collar or stem rot in peanut plants was observed in surveyed fields (n = 95) across the Kadirli, Merkez, Sumbas, Bahçe, Düziçi, and Toprakkale districts of Osmaniye Province, Turkey. The initial disease symptoms included the appearance of necrotic spots that developed into discoloured necrotic lesions, primarily at the base of peanut plant stems (collar). As the disease progresses, symptoms such as dark-brown stem rot, chlorotic leaves, wilting, and eventual whole-plant death are frequently observed in affected plants in the field (Fig. 1a). Among the surveyed districts, the highest disease prevalence was recorded for the Kadirli district with 34.38%, followed by Sumbas and Bahçe districts with 33.33%, Toprakkale district with 28.57%, Merkez district with 24.14%, and Düziçi district with 16.67%, respectively (Table 1). The incidence ratio of the disease in the surveyed fields, where the diseased plants were recorded, ranged from 8.0 to 45.0% with an average of 4.19% overall.
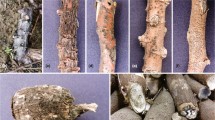
Collar rot caused by Lasiodiplodia pseudotheobromae on peanut plants. a Severely affected plant showing stem rot, chlorotic leaves, wilting, and eventual whole plant death (arrow). b Fungal culture on potato sucrose agar after 7 days of incubation. c and d Sporulation induced on 2%water agar overlaid with sterilized Cogon grass leaves (arrow). Dark brown to black conidiomata were observed on the surface of the grass leaf (arrow)
Isolation, morphological characterisation and pathogenicity of fungal isolates
The fungal pathogen was initially isolated on PSA and incubated at room temperature to observe colony characteristics. All isolates exhibited dense, fluffy, initially grayish-white aerial mycelia that later turned into pale gray colonies on PSA after 10–14 days of incubation (Fig. 1b). Since fungal isolates did not sporulate easily on PSA nutrient media, fungal cultures were transferred onto 2% WA overlaid with sterilized Cogon grass leaves to encourage pycnidial and conidial production on the media (Fig. 1c, d).
The morphological characteristics of fungal structures developed on WA were visualized. Conidiomata, which appeared on the surface of the main veins of the grassy leaves (Fig. 1d), exhibited unilocular characteristics and ranged in color from dark brown to black in colour (Fig. 1d). Aseptate fungal paraphyses, arising among conidiogenous cells, were hyaline, and cylindrical, with rounded ends (Fig. 2a). The conidiogenous cells were hyaline, cylindrical with swollen base. The ellipsoidal immature conidia (n = 50) were hyaline and non-septate, measuring 22.5–30.5 × 13.5–17.5 µm (Fig. 2b, c). Mature conidia (n = 50) were dark brown and one-septate, with longitudinal stripes measuring 22.5–32.0 × 12.5–14.5 µm (Fig. 2b, d).
Pathogenicity tests were conducted using all representative isolates on healthy 3-week-old peanut plant (cv. NC7) (Fig. 3). Typical necrotic lesions, similar to those observed on naturally infected plants, were evident at the inoculation sites 7 days after inoculation. The control plants did not exhibit any symptoms. The morphological characteristics of the original isolate were confirmed after re-isolation, fulfilling Koch's postulates. In the pathogenicity tests, no differences in virulence were observed among the representative isolates tested. Since the twenty four isolates were pathogenic and displayed similar morphological and cultural characteristics, two isolates (MKUBK-B1 and MKUBK-K22) from different cultivated areas were randomly selected for molecular study. Representative single-spore cultures of the fungal isolates MKUBK-B1 and MKUBK-K22 were deposited at the Hatay Mustafa Kemal University BİSAK Microbial Culture Collection Centre in Turkey.
Pathogenicity test on peanut seedling (cv. NC7). a–c Pathogenicity was confirmed by placing mycelial plugs on wounds at the base of peanut seedling stems (arrow) and wrapping them with parafilm (arrow). d Disease lesions and wilting developed at the inoculation sites (arrow) 7 days after inoculation
MALDI-TOF analysis and molecular characterization of fungal isolates
The identities of the morphologically identified isolates, obtained from infested plants with typical disease symptoms and re-isolates, were confirmed by MALDI-TOF analysis. The protein profiles of all the isolates matched those of the reference isolate Lasiodiplodia sp. DSM 832 in the MALDI-TOF/MS library (Fig. 4).
The ITS, tub2 and TEF1-α loci were sequenced to confirm the molecular identification of the representative fungal isolates MKUBK-B1 and MKUBK-K22. The PCR products were purified and sequenced. The resulting consensus sequences of 514 bp (using ITS4/ITS5), 440 bp (using Bt2a/Bt2b), and 500 bp (using EF1-688F/EF1-1251R) were deposited in the NCBI GenBank (Table 2). The ITS, tub2, and TEF1-α loci sequences of the MKUBK-B1 and MKUBK-K22 isolates were compared with those of various Lasiodiplodia spp. isolates from different host plants available in the NCBI GenBank database using the BLAST tool.
BLAST analysis indicated that the sequences of the ITS (GenBank accession no. OR610719, OR610757), tub2 (GenBank accession no. OR620961, OR620962) and TEF1-α (GenBank accession no. PP066969, PP066970) loci of the representative isolates shared 100% identity with L. pseudotheobromae (for ITS: OK427342, MK368390; for tub2: OK489788, MN794202; for TEF1-α: MK693707, EF622060) derived from various host plant species including peanut, longan, pistachio, cacao, and orange.
Phylogenetic analyses were conducted using the maximum likelihood method of MEGA 11 software on the individual sequences of the ITS, tub2, and TEF1-α loci. To construct the tree, sequences of the ITS, tub2, and TEF1-α loci of reference isolates from different host plants available in the NCBI GenBank database were retrieved (Table 2). The ITS sequences of Lasiodiplodia spp isolates from different host plants available in NCBI GenBank along with the representative L. pseudotheobromae MKUBK-B1 and MKUBK-K22 isolates were used (Table 2). As shown in Fig. 5, the L. pseudotheobromae MKUBK-B1 and MKUBK-K22 isolates were well placed in the L. pseudotheobromae clade. These isolates clustered together with L. pseudotheobromae from peanut (OK427342), a type strain of CBS (KF766193), rubber tree (KJ607141), longan fruit (MK368390), and cashew (KT247480) trees (Fig. 5).
The phylogenetic tree based on maximum likelihood illustrates the relationships between Lasiodiplodia pseudotheobromae MKUBK-B1 and MKUBK-K22, along with reference isolates. The tree was constructed using the partial ITS sequences of 10 Lasiodiplodia isolates from various hosts, with the Fusicoccum stromaticum CMW 13434 sequences serving as the outgroup. The sequence of OK427342 represents Lasiodiplodia pseudotheobromae isolated from peanut (Zhang et al. 2022). The bootstrap values for 1000 replicates are displayed on the branches. The numbers on the branches indicate the level of support
The sequences of the tub2 locus of the peanut isolates MKUBK-B1 and MKUBK-K22 were also compared with 11 nucleotide sequences of L. pseudotheobromae and other Lasiodiplodia species available in GenBank (Table 2). Phylogenetic analysis clearly revealed that the peanut isolates MKUBK-B1 and MKUBK-K22 were L. pseudotheobromae. These isolates clustered together with L. pseudotheobromae isolates from peanut (OK489788), CBS type strain (EU673111), pistachio nut (MN794202), and cacao (MK693702) trees (Fig. 6).
The phylogenetic tree based on maximum likelihood illustrates the relationships between L. pseudotheobromae MKUBK-B1 and MKUBK-K22, along with reference isolates. The tree was constructed using the partial tub2 sequences of 11 Lasiodiplodia isolates from various hosts, with the Spencermartinsia viticola CBS 117009 sequence serving as the outgroup. The sequences of OK489788, EU673111 and MN794202 represent Lasiodiplodia pseudotheobromae isolates from peanut, the CBS type strain, pistachio nut and the cacao tree, respectively. The bootstrap values for 1000 replicates are displayed on the branches. The numbers on the branches indicate the level of support
The phylogenetic tree constructed using the sequences of the TEF1-α locus of the peanut isolates MKUBK-B1 and MKUBK-K22 was also compared with 9 nucleotide sequences of Lasiodiplodia species available in GenBank (Table 2). Phylogenetic analysis clearly revealed that the peanut isolates MKUBK-B1 and MKUBK-K22 clustered together with L. pseudotheobromae isolates from cacao (MK693707), rose (EF622061), and orange (EF622060), trees (Fig. 7). All loci constituted a distinct group separate from the nearest clade of L. theobromae, a reported causative agent of peanut collar rot (Phipps and Porter 1998; Wang et al. 2023a).
The phylogenetic tree based on maximum likelihood illustrates the relationships between Lasiodiplodia pseudotheobromae MKUBK-B1 and MKUBK-K22, along with reference isolates. The tree was constructed using the partial TEF1-α sequences of 11 Lasiodiplodia isolates from various hosts, with the Spencermartinsia viticola CBS 117009 sequence serving as the outgroup. The sequences of OK489788, EU673111 and MN794202 represent Lasiodiplodia pseudotheobromae isolates from peanut, the CBS type strain, pistachio nut and the cacao trees, respectively. The bootstrap values for 1000 replicates are displayed on the branches. The numbers on the branches indicate the level of support
Discussions
Peanut plants face significant threats from various soilborne fungal diseases worldwide (Thiessen and Woodward 2012; Wang et al. 2023a). Southern blight, stem rot and root rot caused by Sclerotium rolfsii, Aspergillus niger, Sclerotinia sclerotiorum, Rhizoctonia solani and Fusarium spp., are three prominent soilborne diseases that result in substantial yield and quality losses in peanut-growing regions globally (Liu et al. 2016; Jogi et al. 2016; Wang et al. 2017; Jacob et al. 2018). As soilborne disease agents manifest similar symptoms, definitive species identification can only be achieved through morphological and molecular methods. Accurate identification of disease agents is crucial for the development of new disease-resistant cultivars/breeding lines (Thirumalaisamy et al. 2019).
In this study, 24 representative fungal isolates exhibiting similar cultural and morphological characteristics were obtained from collar root disease symptoms in Osmaniye Province, the most important peanut growing province in Turkey. Pathogenicity tests confirmed that all tested isolates caused symptoms resembling those observed under field conditions. Fungal isolates were re-isolated from the site of inoculations. The morphological characteristics closely resembled those described for L. pseudotheobromae (Alves et al. 2008). Fungal species belonging to the Botryosphaeriales order are known as soilborne necrotic plant pathogens with a wide host range and widespread distribution (Slippers and Wingfield 2007; Phillips et al. 2013; Batista et al. 2021). Although members of the Botryosphaeriaceae family are generally recognized as opportunistic pathogens, they can cause severe disease symptoms under unfavourable conditions in mostly woody plants (Slippers and Wingfield 2007; Sakalidis et al. 2011; Xie et al. 2019; Castillo 2023; Wang et al. 2023b). Batista et al. (2021) reported that Lasiodiplodia theobromae, Botryosphaeria dothidea, Neofusicoccum parvum, Diplodia sapinea, Diplodia seriata, Dothiorella sarmentorum, and L. pseudotheobromae are the most common species globally (Phillips et al. 2013; Dissanayake et al. 2016; Mehl et al. 2017). Among these species, L. theobromeae had the highest host range with 666 different hosts, followed by Botryosphaeria dothidea with 403 hosts and Neofusicoccum parvum with 223 hosts. L. pseudotheobromae was found to be the causal agent in a relatively lower number of plants with 124 hosts (Batista et al. 2021). L. pseudotheobromae has not been reported as a major fungal pathogen causing significant economic losses in peanut-producing countries. Instead, it occurs as a saprophytes, wound parasite or opportunist on a diverse range of host plants (Phipps and Porter 1998; Phillips et al. 2013; Wang et al. 2023b).
Morphological identification of the representative fungal isolates (MKUBK-B1 and MKUBK-K22) confirmed that they were L. pseudotheobromae through phylogenetic analyses using the sequences of the ITS, tub2 and TEF1-α loci. The TEF-1α locus is widely used in molecular and phylogenetic studies, especially in Botryosphaeriaceae family, because it consists of conserved exonic and variable intronic sequences. Since this locus has excellent phylogenetic resolution, it is suitable for inference of deep phylogenies that capture recent evolutionary and speciation events (Stielow et al. 2015). Phylogenetic analysis based on ITS, TEF-1α, tub2 loci sequences performed with several isolates of Lasiodiplodia spp. confirmed that representative fungal isolates belong to L. pseudotheobromae.
The fungal disease agent L. pseudotheobromae is widely distributed and is a common pathogen in tropical and subtropical countries with a broad host range (Adeniyi and Asogwa 2023; Wang et al. 2024). The pathogen has been reported to affects a wide range of woody perennial and ornamental plants causing root rot, damping-off, leaf spots, twig blight, cankers, stem-end rot, gummosis, branch dieback and pre- and post-harvest fruit rots (Bragard et al. 2023; Guo et al. 2023). This disease agent has also been reported to cause fruit rot in strawberries, mango, citrus, avocado, guava, persimmon and longan fruits in USA (Zhang et al. 2024), Pakistan (Alam et al. 2021), Srilanka (Jayasekara et al. 2022), China (Chen et al. 2021), Brazil (Navarro et al. 2022), Mexico (Bautista-Cruz et al. 2019), and Thailand (Pipattanapuckdee et al. 2019, 2023); dieback in Ormosia pinnata (Li et al. 2020), and mango trees (Ismail et al. 2012), canker and dieback diseases in fruit trees in China (Wang and Song 2021; Wang et al. 2024).
The presence of L. pseudotheobromae causing collar rot in peanuts was previously reported in Nigeria (Ataga et al. 2019) and recently in China (Zhang et al. 2022). In addition to L. pseudotheobromae, the closely related fungal species L. theobromae and L. iranensis were also reported to be associated with peanut seed and collar rot (Phipps and Porter 1998; Ataga et al. 2019; Isalar et al. 2021; Wang et al. 2023a). Although the peanut isolates of L. pseudotheobromae shared morphological characteristics similar to those of other Lasiodiplodia species, they could be differentiated based on the shape and size of their conidia and their parahysis features (Alves et al. 2008; Pipattanapuckdee et al. 2019). It has been reported that the subovoid to ellipsoid-ovoid shaped conidia of L. theobromae, isolated from various hosts are smaller in size than the ellipsoidal conidia of L. pseudotheobromae. Additionally, while the paraphyses of L. theobromae were septate, those of L. pseudotheobromae were mostly aseptate, consistent with the peanut isolates obtained in this study. L pseudotheobromae was previously reported on different host plants in Africa, Asia, North and South America and Autralia and has also been reported from Spain with a restricted distribution (Bragard et al. 2023). Although the presence of L. pseudotheobromae was reported on lemon fruit (Awan et al. 2016), nectarines (Endes et al. 2016), and plum (Endes and Kayım 2022) in Turkey, this study represents the first documented report of L. pseudotheobromae causing collar rot in peanuts in Turkey.
Conclusion
Collar rot disease, caused by Lasiodiplodia spp, typically is manifested as a secondary infection, often triggered by more aggressive plant pathogens or in conditions of plant injury, especially under hot and dry climates (Phipps and Porter 1998). In this investigation, we also observed the coexistence of three highly important soilborne fungal pathogens (S. rolfsii, A. niger, and R. solani) on different plants in the same fields (data not given). This work clearly showed that not only aggressive soilborne fungal pathogens (S. rolfsii, A. niger, and R. solani) but also L. pseudotheobromae causes collar rot in peanuts in Turkey. This reports have highlighted the need to classify the disease as an important emerging pathogen of peanut. This is the first major study to investigate L. pseudotheobromae as an emerging economically important disease of peanut. It is suspected that the occurrence of collar rot in Osmaniye Province could be linked to erratic rainfall and the hot, dry spring and summer months of 2021, coupled with the presence of aggressive major peanut pathogens in the same geographic location and plants from which fungal isolates were obtained. Due to the potential destructiveness and broad host range of this pathogen, it is essential to develop new strategies to establish more reliable, environmentally sustainable, and cost-effective management approaches for this disease.
Data availability statement
The data that support the findings of this study are openly available in GenBank at https://www.ncbi.nlm.nih.gov/genbank/, Reference Numbers OR610719, OR610757, OR620961, OR620962, PP066969 and PP066970.
References
Adeniyi DO, Asogwa, EU (2023) Dynamics of diseases and insect pests of cashew tree. In: Forest microbiology, tree diseases and pest. Academic Press, New York, vol 3, 265–284. https://doi.org/10.1016/B978-0-443-18694-3.00019-5
Ahmad A, Attia AG, Mohamed MS, Elsayed HE (2019) Fermentation, formulation and evaluation of PGPR Bacillus subtilis isolate as a bioagent for reducing occurrence of peanut soilborne diseases. J Integr Agric 18(9):2080–2092. https://doi.org/10.1016/S2095-3119(19)62578-5
Akgul DS, Ozgonen H, Erkılıc A (2011) The effects of seed treatments with fungicides on stem rot caused by Sclerotium rolfsii Sacc., in peanut. Pak J Bot 43:2991–2996
Alam MW, Malik A, Rehman A, Sarwar M, Shafeeq T, Hameed A, Rajput NA, Atiq M (2021) First report of Lasiodiplodia pseudotheobromae causing stem end rot of mango fruit in Pakistan. Plant Dis 105:2249. https://doi.org/10.1094/PDIS-01-21-0099-PDN
Alves A, Crous PW, Correia A, Phillips AJL (2008) Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Diver 28:1–13
Ataga AE, Ikechi-Nwogu CG, Iyanyi NG, Ovbije AR (2019) Molecular characterization of fungi from Arachis hypogaea. Niger J Bot 32(2):1–9
Awan QN, Akgul DS, Unal G (2016) First report of Lasiodiplodia pseudotheobromae causing postharvest fruit rot of lemon in Turkey. Plant Dis 100:2327. https://doi.org/10.1094/PDIS-04-16-0512-PDN
Batista E, Lopes A, Alves A (2021) What do we know about botryosphaeriaceae? An overview of a worldwide cured dataset. Forests 12(3):313. https://doi.org/10.3390/f12030313
Bautista-Cruz MA, Almaguer-Vargas G, Leyva-Mir SG, Colinas-Leon MT, Correia KC, Camacho-Tapia M, Robles-Yerena L, Michereff SJ, Tovar-Pedraza JM (2019) Phylogeny, distribution, and pathogenicity of Lasiodiplodia species associated with cankers and dieback symptoms of persian lime in Mexico. Plant Dis 103:1156–1165. https://doi.org/10.1094/PDIS-06-18-1036-RE
Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Gonthier P, Miret JAJ, Justesen AF et al (2023) Pest categorisation of Lasiodiplodia pseudotheobromae. EFSA J 21:e07737. https://doi.org/10.2903/j.efsa.2023.7737
Castillo SRM (2023) A review of Botryosphaeriales in Venezuela with special reference to woody plants. Ann For Res 66:35–62. https://doi.org/10.15287/afr.2023.2492.
Chen J, Zhu Z, Fu Y, Cheng J, Xie J, Lin Y (2021) Identification of Lasiodiplodia pseudotheobromae causing fruit rot of citrus in China. Plants (Basel) 10(2):202. https://doi.org/10.3390/plants10020202
Debele S, Fininsa C, Dejene M, Tana T (2023) Distribution of groundnut (Arachis hypogaea L.) root rot complex and associated pathogens in eastern Ethiopia. Afr J Plant Sci 17(3):18–29. https://doi.org/10.5897/AJPS2022.2272.
Dissanayake AJ, Phillips AJL, Li XH, Hyde KD (2016) Botryosphaeriaceae: current status of genera and species. Mycosphere 7:1001–1073. https://doi.org/10.5943/mycosphere/si/1b/13
Endes A, Kayim M, Eskalen A (2016) First report of Lasiodiplodia theobromae, L. pseudotheobromae, and Diplodia seriata causing bot canker and gummosis of nectarines in Turkey. Plant Dis 100:2321. https://doi.org/10.1094/PDIS-01-16-0036-PDN
Endes A, Kayim M (2022) Morphological and molecular characterization of Botryosphaeriaceae species associated with dieback and gummosis on plum trees in Turkey. C R Acad Bulg Sci 75:295–302. https://doi.org/10.7546/CRABS.2022.02.16
FAO (2021) FAOSTAT, Word Production data. https://www.fao.org/faostat/en/#data/QCL/visualize. Accessed 10 Oct 2023
Ferreira L, Sánchez-Juanes F, García-Fraile P, Rivas R, Mateos PF, Martínez-Molina E, González-Buitrago JM, Velázquez E (2011) MALDI-TOF mass spectrometry is a fast and reliable platform for identification and ecological studies of species from family Rhizobiaceae. PLoS ONE 6(5):e20223. https://doi.org/10.1371/journal.pone.0020223
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61:1323–1330. https://doi.org/10.1128/aem.61.4.1323-1330.1995
Gudu V, Aydogdu M, Basak M, Kizil S, Uzun B, Yol E (2020) Characterization of a groundnut collection to stem rot disease caused by Sclerotium rolfsii. Australas Plant Pathol 49:691–700. https://doi.org/10.1007/s13313-020-00748-y
Guo MT, Zhang N, Wei SY, Yang CX, Cui CY (2023) First report of Lasiodiplodia pseudotheobromae causing postharvest fruit rot on Indian jujube in China. Plant Dis 107:3307
Isalar OF, Ogbuji NG, Okungbowa FI, Ataga AE (2021) Fungal contaminants associated with groundnut (Arachis hypogaea) seeds. J Bioinform Syst Biol 4:182–193. https://doi.org/10.26502/jbsb.5107029
Ismail AM, Cirvilleri G, Polizzi G, Crous PW, Groenewald JZ, Lombard LL (2012) Lasiodiplodia species associated with dieback disease of mango (Mangifera indica) in Egypt. Australas Plant Pathol 41:649–660. https://doi.org/10.1007/s13313-012-0163-1
Jacob S, Sajjalaguddam RR, Sudini HK (2018) Streptomyces sp. RP1A-12 mediated control of peanut stem rot caused by Sclerotium rolfsii. J Integr Agric 17(04):892–900. https://doi.org/10.1016/S2095-3119(17)61816-1
Jayasekara A, Daranagama A, Kodituwakku TD, Abeywickrama K (2022) Morphological and molecular identification of fungi for their association with postharvest fruit rots in some selected citrus species. J Agric Sci 17:79–93. https://doi.org/10.4038/jas.v17i1.9612
Jogi A, Kerry JW, Brenneman TB, Leebens-Mack JH, Gold SE (2016) Identification of genes differentially expressed during early interactions between the stem rot fungus (Sclerotium rolfsii) and peanut (Arachis hypogaea) cultivars with increasing disease resistance levels. Microbiol Res 184:1–12. https://doi.org/10.1016/j.micres.2015.11.003
Li L, Lei M, Wang H, Yang X, Andargie M, Huang S (2020) First report of dieback caused by Lasiodiplodia pseudotheobromae on Ormosia pinnata in China. Plant Dis 104(10):2551–2555. https://doi.org/10.1094/PDIS-03-20-0647-RE
Liu J, Li X, Jia Z, Zhang T, Wang X (2016) Effect of benzoic acid on soil microbial communities associated with soilborne peanut diseases. Appl Soil Ecol 110:34–42. https://doi.org/10.1016/j.apsoil.2016.11.001
Mehl J, Wingfield MJ, Roux J, Slippers B (2017) Invasive everywhere? Phylogeographic analysis of the globally distributed tree pathogen Lasiodiplodia theobromae. Forests 8:145. https://doi.org/10.3390/f8050145
Minarni WI, Yuhendra (2019) Implementation of case-based reasoning and nearest neighbor similarity for peanut disease diagnosis. J Physics Conf Ser 1196:012053. https://doi.org/10.1088/1742-6596/1196/1/012053
Navarro BL, Molina JPE, Nogueira AF (2022) Penetration by Botryosphaeriaceae species in avocado, guava and persimmon fruit during postharvest. J Phytopathol 170:57–68. https://doi.org/10.1111/jph.13055
Pal KK, Dey R, Tilak KVBR (2014). Fungal diseases of groundnut: control and future challenges. In: Goyal A, Manoharachary C (eds) Future challenges in crop protection against fungal pathogens. Fungal biology. Springer, New York. https://doi.org/10.1007/978-1-4939-1188-2_1
Phillips AJL, Alves A, Abdollahzadeh J, Slipper B, Wingfield MJ, Groenewald JZ, Crous PW (2013) The Botryospheriaceae: genera and species known from culture. Stud Mycol 76:51–167. https://doi.org/10.3114/sim0021
Phipps PM, Porter DM (1998) Collar rot of peanut caused by Lasiodiplodia theobromae. Plant Dis 82:1205–1209. https://doi.org/10.1094/PDIS.1998.82.11.1205
Pipattanapuckdee A, Boonyakait D, Tiyayon C, Seehanam P, Ruangwong OU (2019) Lasiodiplodia pseudotheobromae causes postharvest fruit rot of longan in Thailand. Australas Plant Dis Notes 14:21. https://doi.org/10.1007/s13314-019-0350-9
Pipattanapuckdee A, Seehanam P, Tiyayon C, Boonyakait D, Kunasakdakul K, Supakitthanakorn S, Ruangwong OU (2023) Inhibition of Lasiodiplodia pseudotheobromae causing fruit rot disease of longan by using antagonistic Bacillus siamensis RFCD306. Chiang Mai J Sci 50:e2023004. https://doi.org/10.12982/CMJS.2023.004.
Sakalidis ML, Ray JD, Lanoiselet V, Hardy GES, Burgess TI (2011) Pathogenic Botryosphaeriaceae associated with Mangifera indica in the Kimberley region of Western Australia. Eur J Plant Pathol 130:379–391. https://doi.org/10.1007/s10658-011-9760-z
Slippers B, Wingfield MJ (2007) Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and their impact. Fungal Biol Rev 21:90–106. https://doi.org/10.1016/j.fbr.2007.06.002
Soylu S, Atay M, Kara M, Uysal A, Soylu EM, Kurt Ş (2023) Morphological and molecular characterization of pepper fruit rot disease agent Fusarium incarnatum. J Phytopathol 171:688–699. https://doi.org/10.1111/jph.13228
Stielow JB, Lévesque CA, Seifert KA, Meyer W, Iriny L, Smits D, Renfurm R et al (2015) One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia 35:242–263. https://doi.org/10.3767/003158515X689135
Suchoszek-Lukaniuk K, Jaromin A, Korycińska M, Kozubek A (2011) Nuts and seeds in health and disease prevention. Elsevier, Amsterdam
Sun K, Xie XG, Lu F, Zhang FM, Dai CC (2021) Peanut preinoculation with a root endophyte induces plant resistance to soilborne pathogen Fusarium oxysporum via activation of salicylic acid-dependent signaling. Plant Soil 460:297–312. https://doi.org/10.1007/s11104-020-04807-7
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38:3022–3027. https://doi.org/10.1093/molbev/msab120
Thiessen LD, Woodward JE (2012) Diseases of peanut caused by soilborne pathogens in the Southwestern United States. Int Sch Res Notices 517905. https://doi.org/10.5402/2012/517905
Thirumalaisamy P, Dutta R, Jadon KS, Nataraja M, Padvi RD, Rajyaguru R, Yusufzai S (2019) Association and characterization of the Fusarium incarnatum-F. equiseti species complex with leaf blight and wilt of peanut in India. J Gen Plant Pathol 85(2):83–89. https://doi.org/10.1007/s10327-018-0827-y
TUİK (2021) TUİK Bitkisel Üretim İstatistikleri. https://biruni.tuik.gov.tr/medas/?kn=92&locale=en. Accessed 10 Oct 2023
Wang M, Chen M, Yang Z, Chen N, Chi X, Pan L, Wang T, Yu S, Guo X (2017) Influence of peanut cultivars and environmental conditions on the diversity and community composition of pod rot soil fungi in China. Mycobiol 45(4):392–400. https://doi.org/10.5941/MYCO.2017.45.4.392
Wang J, Li X, Sun X, Huo X, Li M, Han C, Liu A (2023a) Establishment and application of a multiplex PCR assay for detection of Sclerotium rolfsii, Lasiodiplodia theobromae, and Fusarium oxysporum in peanut. Mol Biotechnol 65:1369–1377. https://doi.org/10.1007/s12033-022-00647-1
Wang FH, Zeng Q, Liu C, Zhou YJ, Chen XH, Liu F, Xu XL, Liu YG, Yang CL (2023b) Trunk canker of Juglans sigillata caused by Lasiodiplodia pseudotheobromae in China. Plant Dis 107:1228. https://doi.org/10.1094/PDIS-06-22-1320-PDN
Wang YF, Song XZ, Xie SP, Geng YH, Xu C, Yin XM, Zang R, Guo LH, Zhang M, Guo YS (2024) Diversity of Lasiodiplodia species associated with canker and dieback in fruit trees in the Henan and Shandong Provinces of China. Plant Dis (in Press). https://doi.org/10.1094/PDIS-07-23-1260-SR
Wang W, Song X (2021) First report of Lasiodiplodia theobromae and L. pseudotheobromae causing canker disease of Sacha inchi in Hainan, China. Plant Dis 105:3757. https://doi.org/10.1094/PDIS-11-20-2507-PDN
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) In: PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Xie X, Zhang F, Wang X, Li X, Dai C (2019) Phomopsis liquidambari colonization promotes continuous cropping peanut growth by improving the rhizosphere micro environment, nutrient uptake and disease incidence. J Sci Food Agric 99:1898e1907. https://doi.org/10.1002/jsfa.9385
Zhang X, Li Y, Xu M, Guo Z, Yu J, Song X, He K, Zhang Z, Chi Y (2022) First report of Lasiodiplodia pseudotheobromae causing collar rot of peanut in Shandong Province. China Plant Dis 106:1982. https://doi.org/10.1094/PDIS-10-21-2309-PDN
Zhang JX, Yan JQ, Bai JH, Hu CF, Pan TF, Carrillo YJ, Cardenas DE, Cano LM, Ritenour MA (2024) First report of Lasiodiplodia pseudotheobromae causing postharvest decay of strawberries in Florida. Plant Dis 108:519. https://doi.org/10.1094/PDIS-07-23-1376-PDN
Acknowledgements
This study was financially supported by the Ministry of Agriculture and Forestry General Directorate of Agricultural Research and Policies of the Republic of Türkiye (TAGEM/BSAD/B/21/A2/P1/2560).
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Visualization, investigation, methodology and writing of the original draft were provided by Soner Soylu; Disease surveys, plant sampling and observations of disease prevalence and incidence were conducted by İbrahim Teke, Oktay Burak Özcan, Deniz Sevilmiş, Yaşar Ahu Ölmez, İsa Bilaloğlu, Işılay Lavkor; Senem Özkaya, Merve Kara, Yusuf Gümüş performed the methodology including isolation, morphological characterization and pathogenicity assays; Senem Özkaya and Merve Kara were responsible for the molecular identification, phylogenetic analysis, and sequence analysis of the representative isolates; Emine Mine Soylu contributed to the methodology, morphological identification, purification of the single-spore culture of isolates, and reviewed and edited the manuscript. All authors critically reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there are no conflicts of interest related to this submission.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Özkaya, S., Soylu, S., Kara, M. et al. Disease prevalence, incidence, morphological and molecular characterisation of Lasiodiplodia pseudotheobromae causing collar rot disease on peanut plants in Turkey. J Plant Dis Prot (2024). https://doi.org/10.1007/s41348-024-00933-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41348-024-00933-x