Abstract
The tomato russet mite Aculops lycopersici has become a challenging pest in tomato production in the EU. The number of available acaricides is low, and the efficacy of biological control is limited. With this study, we aim to understand better the unhindered dispersal dynamics and develop a method to reduce dispersal on plants.
To better understand the dynamics of A. lycopersici dispersal in layered tomato cultivation under practical conditions, a first trial was carried out. The trial confirmed that first A. lycopersici symptoms in practical cultivation usually occur in the lower or the middle third of tomato plants and then move upwards on plants. It was observed that plants, for a limited period of time often are able to grow new healthy leaves in the same pace as existing leaves, mostly in the lower and middle part of the plant are damaged by A. lycopersici. This is possible due to the fast growth rate of hybrid tomato varieties in layer cultivation. To test if the observed effect can be supported by further slowing down the upwards movement of the pest, a second trial was conducted. Here, the stems of inoculated tomato plants were blocked weekly for A. lycopersici by applying a ring of insect glue 15 cm below the tip of the plants. This stem blockage severely impaired the only active dispersal mode of A. lycopersici: walking. The growth of new plant material, when the method is applied, is able to exceed the speed with which A. lycopersici destroys plant material in layered tomato cultivation. This resulted in significantly less plant damage and prevented fruit damage on all treated plants. The approach of manipulating the plant stem and thereby restricting the movement of the mite on tomato plants could potentially be exploited for plant protection purposes under practical conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The tomato russet mite Aculops lycopersici (Tryon; Acari: Eriophyoidea), is a vagrant eriophyoid mite that is considered a pest in different Solanaceae crops (Perring and Farrar 1986). A. lycopersici is currently found throughout the world in both tropical and temperate regions. Before 1999, there were only minor incidents of A. lycopersici in Germany. However, in recent years, the mite has occurred more frequently in tomato production in Germany (Merz 2020) and other parts of the EU. This observation is in line with reports of an increased economic impact of eriophyoid mites in general in several regions of the world (Duso et al. 2010). A. lycopersici causes bronzing and a russeted appearance of leaves and stem as it feeds on surface cells (Royalty and Perring 1988), damaging the upper and lower epidermis of the tomato plants (Royalty and Perring 1988). The feeding can result in curling of leaf edges, followed by the dropping of leaves (Capinera 2001), and in severe cases, it leads to the death of the whole plant (Keifer et al. 1982).
A. lycopersici is a problematic pest due to its high reproduction rate, relatively small body size with a maximum length of 0.2 mm (Haque and Kawai 2003), and the fact that a limited number of plant protection agents are authorised for this pest in Germany (BVL 2023). Despite studies that show a certain effect of predatory mites against A. lycopersici (Brodeur et al. 1997; Park et al. 2010; Park et al. 2011), the efficacy of these natural enemies in practical conditions is small (van Houten et al. 2013). More recent studies show very promising results for different predatory mites under semi-practical conditions but have yet to be confirmed with trials in practical cultivation (Pijnakker et al. 2022a, b; Vervaet et al. 2022; Castañé et al. 2022). In practise, recognition of A. lycopersici infestation coincides with recognition of symptoms on plants which first usually appear in the lower part of plants (Bailey and Keifer 1943; Capinera 2001). Especially early symptoms, such as light chlorosis on leaves, or light grey and brown shading on the stem, are easily overlooked. Later developing and more obvious symptoms can be misdiagnosed as a fungal pathogen by unexperienced farmers or workers.
So far, there is not one ideal strategy to combat A. lycopersici outbreaks in tomato cultivation. One reason for this is that too little is known about A. lycopersici dispersal patterns in modern layered tomato cultivation. As a pest that is unable to fly and that walks only at a low speed, A. lycopersici makes use of further techniques to disperse and reach new host plants (Sabelis and Bruin 1996; Michalska et al. 2010). Michalska et al. (2010) categorised the dispersal modes of eriophyoid mites into active dispersal, such as walking to uncolonized plant surfaces or to secondary plants in direct contact to the already colonized plant, and passive dispersal such as transferral via air currents, phoresy (i.e. using moving vectors as transport vehicle) or raindrop splashes (Jeppson et al. 1975). The described dispersal modes have been studied in different eriophyoid mites, but not yet specifically in A. lycopersici. However, they are assumed to apply for A. lycopersici too (Capinera 2001). According to Sabelis and Bruin (1996), aerial dispersal is considered the most important mode under natural conditions.
In the artificial greenhouse environment, A. lycopersici faces distinct conditions for dispersal that are very different to those they encounter under natural conditions. While dispersal via raindrops can be disregarded entirely, active dispersal via walking short distances in relative comparison is likely to be of higher importance to reach new host plants, as all neighboured plants are hosts, or uncolonized plant surfaces within one plant. In addition, dispersal via phoresy is likely with several different airborne tomato pests (Michalska et al. 2010) or beneficials, but also via humans working on the crop with clothes or tools that can function as a carrier medium (Capinera 2001). Dispersal via air currents will also occur in greenhouses, as ventilation is often used to reduce air humidity (Kittas and Bartzanas 2007). Whether dispersal can be triggered via droplets or airstreams produced in the application process of pesticides has not been looked into until today.
For within-plant dispersal, A. lycopersici individuals tend to move upwards and aggregate on the highest tips of different plant organs such as leaves or fruits. The logical explanation for this is that, since plants grow upwards, younger uncolonized plant tissue is available at higher locations. This behaviour is also assumed to facilitate dispersal via air currents (Sabelis and Bruin 1996).
Tomatoes are commonly cultivated in a system called layer cultivation. This cultivation method produces a high dynamic in the tomato crop and makes the recording of pest dispersal challenging. Nevertheless, layer cultivation currently is the most common cultivation method.
That almost none of the relevant studies cited in the latest A. lycopersici review by Vervaet et al. (2021) were conducted under practical growing conditions underlines the relevance of the trials conducted for this study: In a first trial, the unhindered movement of A. lycopersici was assessed by closely monitoring the corresponding symptom development throughout a tomato crop and within plants growing in layer cultivation in the typical double row arrangement (dispersal trial). Based on the observations from this first trial, in a second trial tomato plants were grown in layer cultivation, and it was investigated whether interfering with A. lycopersici within-plant dispersal by blocking the stem using insect glue would reduce pest damage (glue ring trial). The glue ring trial was based upon the hypothesis that walking is the most important within-plant dispersal mode; the glue ring at the stem aimed to interfere with this mode of dispersal.
Materials and methods
Dispersal trial: setup, greenhouse conditions and cultivation work
For the dispersal trial, 160 plants of the cultivar Baylee F1 (Enza Zaaden) were sown on the 14th of March in 2017 and singled into pots with a 10 cm diameter on the 22nd of March. The plants were planted into four adjacent soil-greenhouses on the 19th of April. Each greenhouse contained 40 plants divided into two double rows (a and b), each consisting of 2 × 10 plants (Fig. 1 left). This setup resulted in 8 separate double rows. The in-row distance between plants was 50 cm, the distance between the two single rows of a double row was 100 cm, the distance between the centre of the double rows was 200 cm. The minimum temperature of the greenhouses was set to 18.0 °C and whenever the temperature fell below this level the heating system was activated. Roof windows were opened at temperatures above 22.0 °C and side windows opened above 24.0 °C. There was no active cooling or humidity control in the greenhouses.
Left: birds-eye-view of the dispersal trial showing all eight double rows in the four consecutive greenhouses. Each number displays one plant, each double row consists of 2 × 10 plants. The squares indicate plants that were inoculated with A. lycopersici. Arrows indicate the direction in which the plants were layered and thereby in which direction the vertical leafy part of the plants were moving as the plants grew. Right: schematic display of tomato plants in layer cultivation viewed from the side. To allow better visualisation, plant stems are shown without leaves. The illustration shows a double row with 2 × 5 plants
When provided with good growing conditions, modern hybrid tomato varieties can reach a stem length far beyond 10 m within one growing season. Since greenhouses are not high enough to allow for a vertical stem of 10 m, and to keep the fruit zone at a workable height, tomato plants are usually grown in “layer cultivation”. To resemble practical conditions, layer cultivation was also chosen for the two trials of this study. A detailed schematic of layer cultivation is depicted in Fig. 1. As the tomato plants grow at the top, additional growing thread is unwound from the metal hooks depicted in Fig. 1 (right), and the plants are lowered to give room for more growth while the vertical leafy part of the plants, together with the hooks, move along the horizontal wire at the top. Before the lower part of the plant is aligned horizontally, it is defoliated and fruits are harvested. At the end of the double row, plants are simply hung on the other wire and from that point on they move in the opposite direction as they grow further. This switch explains why the stems curve at the bottom, at the end of each double row. In the results section for the dispersal trial, the single row closer to the viewer is termed the “front row” and the one behind is termed the “back row”.
On the 19th of June, two plants in each double row on one side of the greenhouse (Fig. 1-Left) were inoculated with 30 adult A. lycopersici individuals of unidentified sex at the stem in the middle height of the leaf area, roughly 125 cm above ground. The inoculation was repeated on the 26th of June, 7 days past (first) inoculation (dpi), and again on the 6th of July, 17 dpi, to ensure inoculation success and sufficiently high pest pressure. The A. lycopersici individuals used in this trial had been reared on tomato plants. The mites in the rearing originated from a private garden near Brunswick (Germany). Cultivation work such as winding the tomato plants around the twines, removing side shoots, defoliation and harvest was always started at the inoculated plant 1 in each double row. Between each double row, gloves were disinfected to avoid that A. lycopersici individuals were carried from one double row to another. Cultivation works (removing side shoots, unwinding growing thread, removing leaves at the bottom, harvesting fruits) were conducted twice per week.
Dispersal trial: symptom assessment
A. lycopersici-typical symptoms were assessed two times per week for each plant and in total 33 times over a period of 16.5 consecutive weeks. The first assessment was conducted on June 19th directly before the first inoculation. The vertically aligned and leafy part of each plant was divided into three different heights (low, mid and high) and scored separately into classes 1: healthy; 2: light symptoms (stem showing light grey/rust brown discoloration, leaves showing light discoloration/light chlorosis); and 3: strong symptoms (stem showing strong, rust brown, coloration and strongly reduced trichome coverage, leaf browning, necrosis and clear leaf deformation). To make sure that the symptoms did not derive from other causes, symptoms were investigated closely with magnification lense where necessary and presence of A. lycopersici on symptomatic plant organs was checked frequently on a sample basis with sticky tape imprints. Due to the size of the plants, the plant numbers and the practical growing conditions, the dispersal data were derived solely from symptom development. Detailed and comprehensive probing for A. lycopersici presence or counting individuals would have been too time intensive. This is especially due to the heterogenic distribution of A. lycopersici within plants and within single plant organs. To ensure that symptoms were assessed independently, the results of the previous assessment were not on hand to the person conducting the assessment.
Glue ring trial: setup, greenhouse conditions and cultivation work
For the glue ring trial, 48 plants of the cultivar Baylee F1 (Enza Zaaden) were sown on the 11th of March in 2019 and singled into pots with a 10 cm diameter on the 21 of March. The plants were planted into two adjacent soil-greenhouses on the 24th of April. Each greenhouse contained 24 plants in total divided into 6 plots. Each plot consisted of 2 × 2 plants in a double row as shown in Fig. 2. The climate control measures in this trial were identical to those implemented in the dispersal trial.
The plants were grown in layer cultivation. Defoliation and harvest were conducted once per week, prior to the sampling of leaves and after winding tomatoes around the twines and removing of side shoots. Winding and shoot removal were done twice per week and separately from defoliation and harvest. Gloves were always changed after work was finished at each plot. To quickly produce symptoms, all plants were inoculated, each with (equally) highly infested tomato leaves (estimated as having at least 2000 A. lycopersici individuals per leaf) on the 19th of June. The infested leaves were placed on a leaf between the middle and the upper third of the plants. To test whether the inoculation had been successful, all plants were checked for A. lycopersici individuals seven days post inoculation, with sticky tape imprints taken from the stem as described in Pfaff et al. (2020). On the 22nd of July, 33 dpi, inoculation with A. lycopersici was repeated but this time the inoculation site was 10 cm below the tip of each plant, on the youngest fully unfolded leaf so as to simulate A. lycopersici reaching the highest parts of tomato plants. The applied A. lycopersici individuals were taken from the same rearing as those used for the dispersal trial. The trial consisted of two treatments, each with six plots of four plants. The treatments were (1) positive control without countermeasures against A. lycopersici, and (2) treatment with an insect glue ring applied weekly to the stem of the tomato plant at the same day the sampling was carried out. As soon as the first symptoms of A. lycopersici were visible, 5 glue rings were applied evenly over the height of every plant to the stem of the plants, followed by a weekly glue ring application 15 cm below the tip of the plants. The applied insect glue was “Temmen Insektenleim” (Temmen GmbH, Hattersheim, Germany). On average the glue covered a height of 2 cm on the plant stem. This glue is the adhesive that is usually used on coloured sticky traps. It was specifically chosen for this trial as it does not change viscosity when exposed to heat, and it maintains a high level of adhesiveness over time. Macrolophus pygmaeus, a predatory plant bug used for biological control of several insect pests, was introduced into the greenhouses on the 29th of April to mimic practical growing conditions and enable potential phoresy such that A. lycopersici could overcome the glue barrier.
Glue ring trial: symptom assessment
The total number of leaves and the number of leaves showing A. lycopersici-typical symptoms were counted weekly over a period of 84 days, between the 17th of June and the 9th of September. With these data, it was possible to calculate the proportion of symptomatic leaves on every plant. In this trial, in contrast to the dispersal trial, the severity of the observed symptoms was not noted. The seven-day growth of 12 plants in the glue ring trial (one plant per plot in both treatments) was measured over a period of four weeks.
Statistical analysis
Statistical analysis was performed with the Software ‘R’ (R Core Team 2021; version 4.1.0).
To test whether the working direction in the dispersal trial had an effect on time until symptom development, a Kaplan–Meyer curve was fitted, followed by a log-rank test conducted using the ‘survival’ R-package (Therneau 2021). For comparison, plants 3, 4 and 5 (in working direction) were compared with the plants 18, 17 and 16 (not in working direction). The plants were selected for their equal “in-row” distance to the inoculated plants. The direct neighbours to the inoculation plants, 2 and 19, were left out as the chance that these plants would have been quickly colonised by walking A. lycopersici individuals was assumed to be high. It was not distinguished between different plant heights, only the timestamp of the first symptom on a plant was considered in the analysis. The survival analysis was chosen because not all plants developed symptoms. The plants that did not develop symptoms were still included as censored data points in the analysis.
To test whether the weekly application of a glue ring to the stem of tomato plants had an effect on the proportion of leaves damaged by A. lycopersici, generalised linear mixed effects models with beta family were fitted using the ‘glmmTMB’ R-package (Brooks et al. 2017). The interaction between glue ring treatment and sampling date was fitted as a fixed effect, plant ID nested in plot, nested in double row, nested in greenhouse was fitted as random effect to account for the trial structure. To account for the repeated measurements over time, for each plant an (AR1) autocorrelation structure was fitted since this model had a considerably lower AIC (Aikaike information criterion) in comparison to the model without AR1 when fitted with restricted maximum likelihood (REML). The AIC allows a relative comparison of the goodness of fit between models by penalising models with higher numbers of independent variables. Significance of model parameters was assessed using Wald χ2 test and ANOVA type 3 sums of squares using the ‘car’ R-package (Fox and Weisberg 2019).
A Tukey post hoc test for comparison of the estimated marginal means between the symptomatic leaf proportion in the glue ring treatment and in the positive control at each sampling date was conducted using the ‘emmeans’ R-package (Lenth 2021).
Results
Dispersal trial
Time until first symptom development on inoculated plants
Eleven out of 16 plants showed first symptoms on the 27th of July, 38 days post (first) inoculation (dpi). Of the five exceptions, one of the two plants in the double row b in greenhouse-chamber 5.2 showed first symptoms 42 dpi on the 31st of July. The remaining four inoculation plants of the double rows a and b in the greenhouse-chamber 5.1 showed first symptoms between 101 dpi on the 28th of September, and 123 dpi on the 20th of October. The first symptoms developed in an area of 5 cm around the inoculation spot on the stem on each plant, partly on leaves growing in this area.
Height at which first symptoms occurred
Of the 160 tomato plants in the dispersal trial, 12 plants remained free of A. lycopersici symptoms. Of the remaining 148 plants, 143 plants showed first A. lycopersici symptoms either in the lower or the middle third of the plant, or in both of these heights at the same time. Five plants showed first symptoms either at the same time in the middle and the high third of the plant, or in all three heights at the same time, as shown in Fig. 3.
Count of tomato plants on the Y-axis, and the height at which they showed first A. lycopersici symptoms displayed on the X-axis for inoculated plants and plants that were not inoculated. None of the plants showed first symptoms just in the high third or combined in the low and the high third (n = 160, of which 16 were inoculated plants)
Influence of working direction on symptom development
Three out of the 24 plants in working direction did not produce any symptoms and eleven plants did not produce strong symptoms. Two of the 24 plants not in working direction did not produce any symptoms and nine plants did not produce strong symptoms. A Kaplan–Meyer curve followed by a log-rank test revealed that there was no significant difference between the group of plants in working direction (plants 3, 4 and 5) and the group of plants not in working direction (plants 16, 17 and 18) in the time until first symptoms (p = 1) and strong symptoms (p = 0.4) occurred (Fig. S1 & S2 in the supplementary information).
“In-row-dispersal” versus “inter-row-dispersal” to neighbouring plants in double rows
It was observed that in the surroundings of the inoculated plants with the numbers 1 and 20, the in-row neighbouring plants 2, 3, 19 and 20 produced strong and lasting symptoms slightly earlier compared to the neighbouring plants in the corresponding single row within the double row. Since the plants in the single row of the inoculation plants 1 and 20 move in the opposite direction to the corresponding single row, the inter-row neighbouring plants of the inoculation plants changed frequently as opposed to the in-row neighbouring plants 2 and 19, which remained the same. Thus, a valid statistical comparison is difficult. Nevertheless, this phenomenon can be observed more or less prominently in most of the double rows displayed in Fig. 4.
Symptom development in the vertical leafy part of plants, displayed separately for the eight double rows, each labelled as front and back row (and shown above each other). Each tile stack displays a single plant, with its plant number displayed underneath. Each plant is stacked threefold, divided into the heights in which symptoms were sampled (low, mid, high). For a better understanding, refer to Fig. 1 and the respective section on “layer cultivation”. Numbers in the tiles display: The assessment post inoculation at which first symptoms occurred in that height on that particular plant (upper number), the number of times symptom intensity decreased (lower left number) and the number of times symptom intensity increased (lower right number) from one sampling date to the next. Empty tiles indicate that the plant remained free of symptoms. The shading of the tiles corresponds to the assessment date at which first symptoms occurred (dark: early sampling date, light: late sampling date). Plants 1 and 20, marked with a bold black frame, were inoculated with A. lycopersici
Tomato growth versus speed of symptom development
Inspecting the symptom development across plant height levels and time shown in Fig. 4, it appears that the symptoms on multiple plants visible at specific heights either decreased in severity or disappeared entirely in those heights (for instance plant 20 in double row 5.1_a, plants 1–6, 10, 11 and 13–20 in 5.2_a, plants 1, 2, 5, 12–14, 17 and 19 in 5.3_b and plants 1, 3–6, 8, 10, 11 and 16 in 5.4_a). Not visible in the chosen symptom display of Fig. 4 but nevertheless observed: Plants that only showed symptoms in the high or middle third, showed them in the middle and lower third in following sampling dates, whereas the high or middle third of the plants was observed to be symptom free. Also, in some cases the middle or high third of the plants remain symptom free over several sampling dates while in the lower third of the plant's symptoms were apparent.
A. lycopersici upward movement on plants
A. lycopersici populations tended to move upwards on the plants. This behaviour led to A. lycopersici individuals accumulating on the highest points on different plant organs. These accumulations closely resemble pollen or dust debris (Fig. 5). The observed accumulations remained in these high spots until the particular leaves or fruits were removed.
Glue ring trial
A. lycopersici induced symptom development
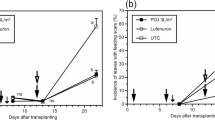
At the first four sampling dates, 2 days prior to inoculation until 19 days post (first) inoculation (dpi) there were no significant difference in terms of the proportion of symptomatic leaves between plants that received a weekly glue ring and plants that received no treatment (positive control) (Fig. 6). From 26 dpi until 82 dpi the proportion of symptomatic leaves in the glue ring treatment was significantly lower compared to the positive control (post hoc test p-values: 26 dpi: 0.00122; 33 dpi: 1.14e-7; 40 dpi: 2.01e-14; 47 dpi: < 2e-16; 54 dpi: < 2e-16; 61 dpi: 9.07e-14; 68 dpi: 3.74e-6; 75 dpi: 1.02e-6; 82 dpi: 2.5e-11). From 40 to 82 dpi, the median proportion of symptomatic leaves in the positive control ranged between 20 and 40%, whereas there were virtually no symptoms in the plants which received the glue ring treatment. At 68 and 75 dpi, symptoms increased temporarily in the glue ring treated plants, with the median ranging from 5 to 10% symptomatic leaves but still remained significantly lower compared to the positive control. There is a slight decreasing trend in the proportion of symptomatic leaves in the positive control and the glue ring treatment at 75 and 82 dpi. Of the 24 plants in the positive control, 18 showed fruit damage. No plant in the glue ring treatment developed fruit damage.
Comparison of the proportion of symptomatic leaves per plant for plants that had a weekly glue ring applied to their stem (n = 24, grey box) and plants that received no treatment (n = 24, white box) over a period of 84 days. Each dot represents one plant at the sampling date. Boxplots display lower and upper quartiles (boxes), and the lowest and highest proportion of symptomatic leaves within 1.5 × the lower and higher quartile range (whisker). The horizontal line in each boxplot displays the median. The black error bars display the 95% confidence intervals obtained from the generalized linear mixed model. The stars at the top indicate significant differences between the treatments at the particular sampling dates according to a post-hoc Tukey test
Figure 7 shows tomato plants that received a glue ring treatment on their stem. A. lycopersici individuals accumulate as they are prevented from reaching the higher parts of the tomato plant. There are clearly visible symptoms below the glue ring.
Growth rate of tomato plants
The seven-day growth was relatively constant throughout the measurement period in July and August. On average the tomato plants grew 25.47 cm every 7 days (Fig. 8).
Weekly plant growth (in cm, n = 12) across five weeks of observation starting with the 18th July (29 dpi). Each dot represents one plant at the sampling date. Boxplots display lower and upper quartiles (boxes) and the lowest and highest proportion of symptomatic leaves within 1.5 × the lower and higher quartile range (whisker). The horizontal line in each boxplot shows the median
Beneficial insects
The glue rings remained free of the introduced M. pygmaeus. M. pygmaeus individuals were found in high numbers on all plants, both on plants that had received the glue ring treatment and on those that had not. No count was performed, so it is not possible to conclude whether M. pygmaeus showed a preference for plants that had or did not have a glue ring.
Discussion
In the dispersal trial under practical conditions, following inoculation with a small number of A. lycopersici individuals (< 30), first symptoms became visible 38 dpi. Other trials conducted under practical conditions with low inoculation numbers at the same trial station also supported the conclusion that it takes at least four to six weeks for first symptoms to develop (data not shown). This finding corroborates observations in other studies, for example, Pijnakker et al. (2022a) reported that first symptoms appeared after five weeks. First symptoms on inoculated plants always developed directly at and surrounding the inoculation sites. This indicates that small numbers of A. lycopersici arriving on a new plant tend to feed and propagate at the arrival site before they move upwards, likely when population density has increased. This assumption and the assumption that a large population of A. lycopersici is required to produce significant plant damage, are the fundamental preconditions upon which the following glue ring trial was developed.
The absence of a significant difference in symptom development between plants in working direction and those not in working direction in this trial indicates that in the close proximity of double rows, transport via gloves and tools (as reported in Capinera (2001)), seems to have played a less important role as compared to the other possible dispersal modes. However, since the total number of plants with 2 × 10-plants double rows was relatively small, it cannot be excluded that A. lycopersici individuals were transported by workers from plant 1 to, for instance, plants 16, 17 and 18 that were classified as “not in working direction”.
From visual inspection of the dispersal trial results, it is clear that in-row neighbouring plants tended to develop strong and lasting symptoms earlier than inter-row neighbouring plants. One likely reason for this is that between in-row neighbouring plants not only leaves but also stems touch as soon as they are aligned horizontally at the bottom (Fig. 1, right) whereas with the plants in the other single row of a double row only leaf contact exists. Additionally, the vertical leafy parts in the two single rows of a double row move in opposite directions, resulting in a much shorter time of plant-to-plant contact as compared to permanent in-row neighbour plants. It can be assumed that this shorter period of contact means less mites were able to move to the inter-row neighbouring plants. Another reason for this effect in this particular trial could be the larger inter-row planting distance (1 m) compared to the in-row planting distance (0.5 m), although especially the in-row distance between the vertically leafy part of plants can vary throughout the season. As there are no stable parameters due to the plant movement in layer cultivation, it was not possible to test this observation in a statistical model.
In almost all 148 plants, first symptoms were observed in the lower and/or middle third section of the plant. This finding corroborates reports in the literature (Bailey and Keifer 1943; Capinera 2001) that first symptoms usually occur in the lower half of cultivated tomato plants; here proven for layer cultivation. Three hypotheses can explain this phenomenon. I: the leaves in the lower and middle part of tomato plants in layer cultivation are usually completely unfolded—in contrast to the leaves in the upper part of the plant, the leaves at the lower and middle section of the plant have reached their maximum size. This means there is more plant and leaf contact to neighbouring plants in the lower and middle part of the plant. This increased contact makes it easier for A. lycopersici populations to transfer onto the lower and middle thirds of neighbouring plants. II. The time initial infestations require to produce symptoms to tomato plants is slower than plant growth. As it was shown in the dispersal trial, the average time until first symptom development in warm months on hybrid tomato plants after inoculation with small numbers of A. lycopersici individuals (< 30) was at least 38 days. On average plants grew 25 cm every seven days (Fig. 8). Thirty-eight days equates therefore, to approximately 135 cm of growth. In consequence even when a small number of A. lycopersici individuals reach the highest parts of a plant, the symptoms appear 135 cm below the tip. III. A. lycopersici makes use of the fact that the lower parts of the plant stems come into contact as soon as the plants are layered, to infest new plants. In this situation A. lycopersici colonize new plants mainly from the lowest defoliated regions, damaging lower leaves first.
For a limited period of time, it seems there was an equilibrium between the pace at which new symptoms occurred, and the growth of the plant, or even a temporary period of time where the plant was able to “grow free of symptoms”. A. lycopersici individuals that reached the leaves in the lower half of the tomato plants, and populations that built up on these leaves were removed in the frequent defoliation and harvest process in the lower part of plants. This removal happens within a timeframe of four weeks, considering a time window based on the leaf wall height (180 cm divided by two for the lower half) and the growth rate at the trial station (25 cm/7 days). What is happening in layer cultivation is, metaphorically speaking, a race for height between the growing plant and the A. lycopersici population that is damaging the plant. Sooner or later this race is usually won by A. lycopersici, in its pace depending on different environmental factors, such as drought stress (Pfaff et al. 2020), or temperature and humidity (Haque and Kawai 2003). Due to the exponential population growth of A. lycopersici, at some stage the destruction of photosynthetically active leaf-area ultimately progresses faster than new leaves can grow, slowing down growth even further.
Given the limited number of tools that exist to control A. lycopersici, a review of the dispersal modes of A. lycopersici and the distinct behaviour patterns this mite shows, reveals possible weaknesses that can be exploited to better control infestations. As reported in the literature (Michalska et al. 2010), and observed in this study (Fig. 5), A lycopersici has a strong tendency to move upwards on plants. Possible triggers for this could be reaching a certain population density and / or reaching a plant damage threshold that lowers the quality of the feeding site. When looking at upward within-plant dispersal and movement in layered tomato cultivation, the role of walking as the only active dispersal mode, seems to be of high importance in the combined movement of larger populations. The accumulation of A. lycopersici individuals at high points (Fig. 5) indicates that this behaviour probably is due to an instinct, such as non-compass negative gravitaxis as described in Grob et al. (2021), rather than being a cognitive decision to search for less populated surfaces with better feeding conditions. The tendency to move upwards seems to be of such a linear nature that when moving upwards on the stem of tomato plants, A. lycopersici tend to avoid leaves that point downwards. In the conducted trials, downward pointing leaves seemed to show symptoms less often or at a later stage compared to leaves that had a more horizontal or upward orientation. Also, once they arrived at high points, the A. lycopersici accumulations seemed to remain in these locations. As a consequence, the majority of these “stranded” mites did not manage to disperse aerially, or they failed to attach to carrier vectors (phoresy). This observation underlines the importance of the stem layer cultivation as the only plant organ that allows completely unhindered and dead-end-free upward movement on tomato plants.
Considering the described “race” between plant growth and A. lycopersici population growth, and with the assumed importance of the plant stem in mind, it was decided to test the effect of blocking the stem of tomato plants against mites’ upward movement. This way the hypothesis of walking being the most important within-plant dispersal mode in layered tomato cultivation was put to the test. Despite there being plant contact via leaves within and between plants, in the glue ring trial the blockage of the stems strongly limited the upward-movement of the A. lycopersici populations. Only small numbers of individuals managed to reach the higher sites on the plants, they were of a magnitude that was incapable of creating considerable plant damage. As the glue rings were applied weekly 15 cm below the tip of the plants, the rings were on average 25 cm away from each other. For every new stage above a certain glue ring the A. lycopersici population size was reset to the low number of individuals that reached this particular height. This prevented unlimited population growth, and consequently severe damage to the plants and the fruit in the glue ring treatment compared to the plants in the positive control. Cultivation work in the glue ring trial was done either on the lower part of the plant (defoliation and harvest) or on the higher part (winding of tomato plants and removal of side shoots) to avoid the uncontrolled transport of mites via gloves or tools from lower to higher parts of the plants. To test under more controlled conditions how the scenario of a small number of mites (< 30) reaching higher parts of the plants would influence symptom development, all plants were inoculated 10 cm below the tip on the 22nd of July 33 dpi for a second time. The temporary increase in the proportion of symptomatic leaves in the glue ring trial observed at 68 dpi and 75 dpi before it decreased again at 82 dpi is most likely the result of this secondary inoculation. The effectiveness of glue rings around tomato stems under greenhouse conditions (where there was air movement due to window ventilation and with M. pygmaeus presence as a potential travel vector) against A. lycopersici can be considered additional evidence for the relatively higher importance of walking for the pest’s dispersal and population build up as compared to passive dispersal modes.
Despite significant differences in symptom development, there was no collapse or death of complete plants in the positive control of the glue ring trial. On the contrary, this did occur in the dispersal trial. Even though there was severe damage in the positive control of the glue ring trial, the absence of plants completely dying off might indicate that pest pressure could have been higher. Towards the end of the sampling period the outside temperatures were lower and the humidity higher than the usual in this time of the year (data not shown), which might have influenced the development of the A. lycopersici populations negatively. A repetition of the glue ring trial under dry conditions should be performed. Comparable trials should be conducted in greenhouses with different environmental and climatic conditions, and with different tomato cultivars and A. lycopersici populations to allow for an estimation of efficacy under a broader range of practical conditions.
Despite the fact that the glue ring treatment successfully prevented A. lycopersici damage, there are some downsides to this method. The insect glue that was applied to the stems was chosen for its durable stickiness–a high level of adhesiveness remains even when the glue is exposed to changes in temperature. This stickiness becomes a problem however, when the glue ends up on fruit. In addition to this, the applied insect glue is not selective for A. lycopersici but would also trap beneficial arthropods such as predatory mites that are applied against A. lycopersici. That said, it is known that A. lycopersici benefits from trichomes as these hinder the movement of predatory arthropods, but not A. lycopersici itself (van Houten et al. 2013). For this reason, the use of predatory mites in tomato production has been limited to date. Fortunately, in this particular trial, the introduced M. pygmaeus seemed to be unaffected by the glue rings. In any case, non-sticky alternatives that are semi-permeable for beneficial arthropods should be investigated. Potential alternatives could be substances containing acaricidal compounds, for example Sulphur. Sulphur for instance, is somewhat effective against A. lycopersici (Royalty and Perring 1987) and is sprayed in practice against A. lycopersici. Therefore, a ring at the stem containing or consisting of concentrated sulphur might be feasible although negative effects on predatory mites might occur with this compound as well.
Conclusion
Small numbers of A. lycopersici cause first damage at the initial infestation site on plants and apparently begin to move upwards when a certain amount of plant damage or population size is reached. This typical behavioural pattern can be used as a starting point for a control strategy, particularly in tomato layer cultivation. A good example of how this strategy can be realised under practical growing conditions is the use of physical barriers, as demonstrated with glue rings around tomato stems. The results suggest a closer look at A. lycopersici dispersal interference and the stem as a major route for within-plant movement of A. lycopersici. If this method provides equally good results across a wide range of environmental conditions and with a workable substance, this method will be a useful addition to integrated plant protection in tomato crops against this challenging pest.
References
Bailey SF, Keifer HH (1943) The tomato russet mite, Phyllocoptes destructor keifer: its present status. J Econ Entomol 36:706–712. https://doi.org/10.1093/jee/36.5.706
Brodeur J, Bouchard A, Turcotte G (1997) Potential of four species of predatory mites as biological control agents of the tomato russet mite, Aculops lycopersici (Massee) (Eriophyidae). Can Entomol 129:1–6
Brooks ME, Kristensen K, van Benthem KJ et al (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400
BVL (2023) BVL. https://apps2.bvl.bund.de/psm/jsp/ListeMain.jsp?page=1&ts=1623661260092. Accessed 23 Jun 2022
Capinera J (2001) Handbook of vegetable pests. Academic Press, San Diego
Castañé C, Alomar O, Rocha A et al (2022) Control of Aculops lycopersici with the predatory mite transeius montdorensis. Insects 13:1116. https://doi.org/10.3390/insects13121116
Duso C, Castagnoli M, Simoni S, Angeli G (2010) The impact of eriophyoids on crops: recent issues on Aculus schlechtendali, Calepitrimerus vitis and Aculops lycopersici. Exp Appl Acarol 51:151–168. https://doi.org/10.1007/s10493-009-9300-0
Fox J, Weisberg S (2019) An (R) companion to applied regression, 3rd edn. Sage, Thousand Oaks
Grob R, el Jundi B, Fleischmann PN (2021) Towards a common terminology for arthropod spatial orientation. Ethol Ecol Evol 00:1–21. https://doi.org/10.1080/03949370.2021.1905075
Haque MM, Kawai A (2003) Effect of temperature on development and reproduction of the tomato russet mite, Aculops lycopersici (Massee) (Acari: Eriophyidae). Appl Entomol Zool 38:97–101
Jeppson LR, Keifer HH, Baker EW (1975) Mites injurious to economic plants. University of California Press, Berkeley, Los Angeles, London
Keifer HH, Baker EW, Kono T, et al (1982) An Illustrated Guide to Plant Abnormalities Caused by Eriophyoid Mites in North America, Handbook No. 573. United States Department of Agriculture
Kittas C, Bartzanas T (2007) Greenhouse microclimate and dehumidification effectiveness under different ventilator configurations. Build Environ 42:3774–3784. https://doi.org/10.1016/j.buildenv.2006.06.020
Lenth RV (2021) emmeans: estimated marginal means, aka Least-Squares Means
Merz F (2020) Tomatenrostmilbe. https://ltz.landwirtschaft-bw.de/pb/site/pbs-bw-new/get/documents/MLR.LEL/PB5Documents/ltz_ka/Service/Schriftenreihen/Hinweise zur Pflanzengesundheit/Tomatenrostmilbe_DL/Pflanzengesundheit_Tomatenrostmilbe.pdf
Michalska K, Skoracka A, Navia D, Amrine JW (2010) Behavioural studies on eriophyoid mites: an overview. Eriophyoid Mites Prog Progn. https://doi.org/10.1007/978-90-481-9562-6_3
Park H-H, Shipp L, Buitenhuis R (2010) Predation, development, and oviposition by the predatory mite Amblyseius swirskii (Acari: Phytoseiidae) on tomato russet mite (Acari: Eriophyidae). J Econ Entomol 103:563–569. https://doi.org/10.1603/EC09161
Park H-H, Shipp L, Buitenhuis R, Ahn JJ (2011) Life history parameters of a commercially available Amblyseius swirskii (Acari: Phytoseiidae) fed on cattail (Typha latifolia) pollen and tomato russet mite (Aculops lycopersici). J Asia Pac Entomol 14:497–501. https://doi.org/10.1016/j.aspen.2011.07.010
Perring TM, Farrar CA (1986) Historical perspective and current world status of the tomato russet mite (Acari: Eriophydae). Entomological Society of America
Pfaff A, Gabriel D, Böckmann E (2020) Mitespotting: approaches for Aculops lycopersici monitoring in tomato cultivation. Exp Appl Acarol 80:1–15. https://doi.org/10.1007/s10493-019-00448-3
Pijnakker J, Hürriyet A, Petit C et al (2022a) Evaluation of phytoseiid and Iolinid mites for biological control of the tomato russet mite Aculops lycopersici (Acari: Eriophyidae). Insects 13:1146. https://doi.org/10.3390/insects13121146
Pijnakker J, Moerkens R, Vangansbeke D et al (2022b) Dual protection: a tydeoid mite effectively controls both a problem pest and a key pathogen in tomato. Pest Manag Sci 78:355–361. https://doi.org/10.1002/ps.6647
R Core Team (2021) R: a language and environment for statistical computing
Royalty RN, Perring TM (1987) Comparative toxicity of acaricides to Aculops lycopersici and Homeopronematus anconai (Acari: Eriophyidae, Tydeidae). J Econ Entomol 80:348–351. https://doi.org/10.1093/jee/80.2.348
Royalty RN, Perring TM (1988) Morphological analysis of damage to tomato leaflets by tomato russet mite (Acari: Eriophyidae). J Econ Entomol 81:816–820
Sabelis MW, Bruin J (1996) 1.5.3. Evolutionary ecology: life history patterns, food plant choice and dispersal. World Crop Pests 6:329–366. https://doi.org/10.1016/S1572-4379(96)80020-0
Therneau TM (2021) “Survival” a package for survival analysis in R
van Houten YM, Glas JJ, Hoogerbrugge H et al (2013) Herbivory-associated degradation of tomato trichomes and its impact on biological control of Aculops lycopersici. Exp Appl Acarol 60:127–138. https://doi.org/10.1007/s10493-012-9638-6
Vervaet L, de Vis R, de Clercq P, van Leeuwen T (2021) Is the emerging mite pest Aculops lycopersici controllable? Global and genome-based insights in its biology and management. Pest Manag Sci 77:2635–2644. https://doi.org/10.1002/ps.6265
Vervaet L, Parapurath G, De Vis R et al (2022) Potential of two omnivorous iolinid mites as predators of the tomato russet mite. Aculops Lycopersici J Pest Sci 95:1671–1680. https://doi.org/10.1007/s10340-022-01544-x
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was financially supported by the German Federal Ministry of Food and Agriculture (BMEL) through the Federal Office for Agriculture and Food (BLE), grant number 2816ERA01L.
Author information
Authors and Affiliations
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pfaff, A., Gabriel, D. & Böckmann, E. Observation and restriction of Aculops lycopersici dispersal in tomato layer cultivation. J Plant Dis Prot 131, 155–166 (2024). https://doi.org/10.1007/s41348-023-00817-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-023-00817-6