Abstract
Using nematode resistant varieties is one of effective and environmental sound strategies being adopted in the management of economically important Meloidogyne species. Wild cucumber (Cucumis africanus) has been reported to possess resistance to Meloidogyne species. Two mechanism of nematode resistance, pre- and post-penetration resistance, had been identified, with post-penetration mechanism being used in plant breeding programs and crop rotation systems. The objective of this study was to determine the mechanism of nematode resistance in C. africanus to M. incognita and M. javanica. 6 weeks old C. africanus seedlings were separately inoculated with 100 s-stage juveniles (J2) of M. incognita and M. javanica. For 30 days, five seedlings were harvested from both M. incognita and M. javanica experiments every other day. Seedlings’ roots were examined for necrotic spots, rootlet interferences, giant cells and root gall numbers as indicators of successful or unsuccessful nematode penetration. Harvesting times were highly significant (P ≤ 0.01) on necrotic spot, rootlet interference and root gall numbers in both C. africanus—M. incognita and—M. javanica relations, but were not significant for giant cell number in C. africanus—M. incognita. The results suggested that C. africanus have post-penetration nematode resistance to both Meloidogyne species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global withdrawal of the highly effective synthetic chemical fumigant nematicides, which had been relied upon for over a century in the management of plant-parasitic nematodes (PPN), has had severe economic ramifications in crop production systems (Caboni et al. 2015). Parasitism by root-knot nematodes (RKN), Meloidogyne species is considered one of the main biotic factors responsible for reduced productivity in various agricultural crops (Mhatre et al. 2019). RKN results in up to 30% yield decline by direct infestation and indirect losses owing to predisposing or breakdown of resistance to other root diseases, such as bacterial wilt, attributing to quantity and quality losses (Muimba-Kankolongo 2018). Meloidogyne genus is a worldwide economically significant pest, comprising over 100 species, (Karuri et al. 2017) including approximately 22 described from Africa (Onkendi et al. 2014) widely distributed on leguminous and flowering plants.
Two Meloidogyne species, M. incognita and M. javanica, have been declared economically important to roughly 4000 host plants, including field crops, ornamentals, medicinal, aromatics plants, and even weeds (Jones et al. 2013; Onkendi et al. 2014). Second-stage juveniles (J2) penetrate roots to establish a feeding site, called giant cell, usually within the pericycle and vascular tissues and form root galls soon after their infection (Mashela et al. 2015). In nematode-susceptible hosts, infection by Meloidogyne species induces the formation of severe root galls, stunted growth, decreased water uptake, imbalances of essential nutrient elements, low evapotranspiration and increased root exudation of amino acids, which reduces soil pH (Saikia et al. 2013).
Up-to-date cultural management procedures are insufficient to fully manage RKN (Trudgill and Blok 2001), even more so with continued restrictions on synthetic chemical use (Desaeger et al. 2017). As a result, it has become critical to develop additional PPN management strategies that are environmentally friendly. Currently, there are numerous studies conducted on the subject all around the world (Baum et al. 2015; Brito et al. 2020; Damasceno et al. 2015; Gupta et al. 2017; Hussain et al. 2018; Laquale et al. 2015; Seo et al. 2019), including screening nematode-resistant genotypes (Chiamolera et al. 2018; Da Silva-Mattos et al. 2019; Hajihassani et al. 2019). These studies are proving to be beneficial, providing additional insights that can lead to increased profits for farmers.
Nematode-resistant hosts may exhibit pre- or post-penetration resistance (Thurau et al. 2010). Pre-penetration nematode resistance is the form of resistance that occurs prior to nematodes coming into contact with the root systems (Ferraz and Brown 2002). This form of resistance prevents penetration of nematode J2 and is characterized by pre-existing morphological factors or the production of root exudates that either attract or repel J2 (Trudgill 2003). Root penetration by RKN has also been attributed to the lack of metabolites required for host identification, repellent host exudates, or the existence of a physical barrier over which the nematode cannot pass (Lee et al. 2017).
In post-penetration nematode resistance J2 are allowed to penetrate the root systems (Desmedt et al. 2020), with passive chemicals previously called elicitors, activated to form the phytoalexins, that have nematicidal properties (Desmedt et al. 2020, 2022). Some of the phytoalexins induce hypersensitive response (HR), that appear as necrotic spots, where cells around the nematode wither (Huysmans et al. 2017), thereby preventing feeding, development of J2 and reproduction. According to Lopez-Gomez and Verdejo-Lucas (2017), post-penetration incompatibility in resistant crops is associated with failure of giant cells to develop further into root galls. Rootlet interference and small underdeveloped root galls are also characteristics of post-penetration nematode resistance (Benková and Bielach 2010). In sedentary RKN, this type of resistance is further subdivided into early and late resistance, wherein early resistance that occurs during migration or early site establishment, and late resistance that occurs after the establishment of a feeding site (Fuller et al. 2007).
Between the two mechanisms of resistance, only post-penetration nematode resistance can be introgressed (Thurau et al. 2010), dictating the need to establish the mechanism of nematode resistance in any nematode resistant plant species in order for it to serve as a candidate of introgression. Among the available alternative techniques to methyl bromide, plant resistance is one of the most investigated techniques in PPN management (Onkendi et al. 2014). Most crops lack resistant genotypes to Meloidogyne species as observed in four commercial genera of Cucumis, Citrullus, Cucurbita and Lagenaria within the Cucurbitaceae family (Liu et al. 2016; Thies et al. 2016; Verdejo-Lucas and Talavera 2019; Singh and Patel 2015). Cucumis africanus is highly resistant to Meloidogyne species (Pofu et al. 2012); however, the mechanism of nematode resistance in this crop has not been established.
Therefore, the objective of this study was to determine the mechanism of nematode resistance in C. africanus to M. incognita and M. javanica.
Materials and methods
Experimental procedures
Two separate experiments were conducted under greenhouse conditions at North-West University, South Africa. Greenhouse temperature were set at 25 ± 2 °C, with temperatures and humidity controlled using thermostatically activated fans and wet-wall at opposite ends. Seeds of C. africanus were sown in seedling trays filled with pasteurized (300 ℃ for 1 hour) fine sand and raised for 6 weeks. Uniform seedlings were transplanted into 250 ml polystyrene cups, filled with 200 ml pasteurized fine sand and placed at 10-cm inter- and intra-row spacing. In each experiment, the treatments comprised of 15 harvesting times, experimentally done in a randomized complete block design (RCBD), with five replications. Isolates of M. incognita and M. javanica were each raised on nematode-susceptible tomato (Solanum lycopersicum) cv. ‘Floradade’ seedlings and roots collected for egg masses when needed. Egg masses were hand-picked using a tooth pick and hatched in distilled water for 72 h (Powers et al. 1991). A day after transplanting, Cucumis seedlings were each inoculated by dispensing approximately 100 J2 of M. incognita or M. javanica using a 20 ml plastic syringe into 5-cm-deep furrow around the seedling stem and covered with growing medium. Harvesting was done every other day, for a period of 30 days starting from 2-days after inoculation. Seedlings were fertilized once with Super Phosphate (Efekto Care, Bryanston, South Africa) and NPK (2:3:2) and irrigated with 30 ml tap water every other day.
Data collection
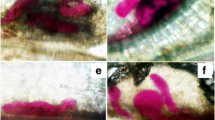
At each harvest, seedling roots were severed and the shoots discarded. Roots were rinsed in tap water to remove soil particles, blotted dry using paper towel and stained (Bybd et al. 1983). The whole root system was soaked in 1.5% NaOCl solution for four minutes to remove any associated microbe, rinsed in tap water, followed by a 15 min immersion in tap water to remove excess NaOCl. Root samples were each stained by covering with 30 ml tap water mixed with 1 ml acid fuchsin and boiled for 30 s. The solution was cooled to room temperature and roots distained by putting in acidified glycerin with a few drops of 5 N HCl, which were heated to boiling, followed by cooling to room temperature. Root samples were each placed in a petri dish and closed with the top lid for assessment under the stereomicroscope at 45 × magnification for necrotic spots, rootlet interference, giant cells and root gall number.
Data analysis
Prior to analysis of variance (ANOVA), all data were transformed through log10(x + 1) to normalize the variances. Data were subjected to ANOVA through the 2008 SAS software. The mean sum of squares were partitioned to provide the contribution of sources of variation in the total treatment variation (TTV) of variables (Gomez and Gomez 1984). Treatment means were separated using Waller-Duncan Multiple Range test at 5% level of probability. Unless stated otherwise, all treatment effects were discussed at 5% level of probability.
Results
Harvesting times were highly significant (P ≤ 0.01) on necrotic spot number, rootlet interference number and root gall number contributing 59, 64 and 50% in TTV of the respective variables, but were not significant on giant cell number (Table 1). In the first 18 days after inoculation, necrotic spots, rootlet interference and root galls were not noticeable. The first necrotic spots and root galls were observed after 20 days, whereas rootlets after 22 days (Table 2).
Harvesting time had highly significant effects on necrotic spot number, giant cell number, rootlet interference number and root gall number, contributing 55, 71, 63 and 59% in TTV of the respective variables (Table 1). HR was noticeable 26 days after inoculation, giant cells and root galls after 18 days, whereas, rootlets were observed after 16 days (Table 2).
Discussion
According to Chitambar and Raski (1984), M. incognita is more pathogenic and becomes aggressive with time in comparison with M. javanica, which could explain why necrotic spots for C. africanus—M. javanica relations were observed 6 days after C. africanus—M. incognita relations. HR is known to be a common response to RKN infection in resistant crops (Chitambar and Raski 1984; Das et al. 2008; Freire et al. 2010; Lee et al. 2021), resulting in cell death and prevention of nematode feeding site formation and nematode development, leading to subsequent nematode death (Postnikova et al. 2015). HR in nematode-infected cells representatives of hyperactive responses in nematode resistant plants (Mashela et al. 2016). According to Nicholson and Hammerschmidt (2003), HR indicates the presence of phenols that play a role in plant defense. When Abifarin et al. (2019) investigated phytochemical and antioxidant activities of C. africanus, they found that the plant has phenolic compounds in fruits, leaves and roots.
The presence of such phytochemicals could be responsible for pathogen-associated molecular patterns (PAMP) in C. africanus. PAMP result in incompatible nematode-host interactions that triggers the up-regulation of a network of host genes and corresponding proteins involved in an innate response known as pathogen-triggered immunity (PTI) (Melillo et al. 2006). Coffee (Coffea canephora cv. ‘Apoata’), resistant to M. exigua exhibited HR, which further inhibited formation of feeding site (Vieira et al. 2013). Moon et al. (2010) also observed necrotic spots in resistant C. annuum cultivars exposed to M. incognita. Two resistant alyce clover (Alysicarpus ovalifolium hybrids, FL-1 and FL-3), showed attributes of HR to M. arenaria (Powers et al. 1991). Marini et al. (2016) observed similar results for resistant oats (Avena sativa cv. ‘IPR Afrodite’) when exposed to M. incognita at 15 days after inoculation.
Delay in giant cells response had been previously reported in nematode resistant trials using molecular approaches (Escobar and Fenoll 2021). The giant cells appeared as deeper stained spots with multiple nuclei that failed to develop beyond the zygote-like size. In nematode-susceptible plant species, giant cells are formed as multinucleate structures formed when the feeding cell and those around it responds to RKN infection by undergoing repeated mitosis without cytokinesis (Huang et al. 2003). The successful establishment of giant cells is essential for nematode development. Meloidogyne species evolved strategies that enable them to induce giant cell formation on thousands of plant species by manipulating important factors of plant cell development (Caillaud et al. 2008). This group of notorious pathogens secrete chemical compounds called gene products through the sub-ventral and dorsal gland cells during migration and sedentary phases, respectively (Gheysen and Fenoll 2002; Tripathi et al. 2015). The release of gene products is important during RKN migration and feeding site establishment because it enables nematode growth to subsequent stages (Curtis 2008; Siddique et al. 2022). Anti-plant gene, on the other hand, is a strategy by host plants when plant genes that respond to nematode feeding and secretions to allow for successful partnerships between PPN and plants are silenced (Mashela et al. 2016). Thus, the phytotoxic chemical compounds that destroy the feeding structures, giant cells, are upregulated.
Marini et al. (2016) also noticed that M. incognita gradually initiated giant cells that failed to develop into root galls in resistant roots of A. sativa 18 days after inoculation. Similarly, Wehner et al. (1991) observed small, poorly formed giant cells in resistant cucumber (C. sativus) and African horned cucumber (C. metuliferus) exposed to M. hapla. Observation of the under-developed giant cells also agreed with observations in resistant soybean (Glycine max cvs. ‘Jackson’ and ‘PI 200,538’) exposed to M. arenaria (Pedrosa et al. 1996) and in resistant cayenne pepper (Capsicum annuum cvs. ‘02G132’ and ‘03G53’) (Moon et al. 2010). Pedrosa et al. (1996) indicated that resistance to M. arenaria was expressed in G. max as small, poorly formed giant cells. In all the cited examples, the cultivars had post-penetration nematode resistance. The giant cell serves as a source of nutrients for the developing nematode (Bartlem et al. 2014). The post-penetration compatibility in susceptible crops is usually associated with optimal development of giant cells that form a large multinucleate structure which, however, fail to develop in nematode resistant crops (Ortiz 2011).
Compensatory rootlet growth was observed originating adjacent to the under-developed giant cells. Mechanisms behind compensatory rootlet development in RKN-infected resistant hosts have not been investigated. However, considering the tactics RKN implement throughout migratory phases, it is possible that resistant hosts develop lateral rootlets to supplement for roots that can no longer transport nutrients and water from soil to aboveground parts. After root penetration, RKN seek for a suitable feeding site and position themselves strategically in the vascular bundles. While host feeder roots absorb nutrients and water, which move in the plant's vascular system to aboveground plant parts, these nutrients are channeled into RKN throughout their development (Bartlem et al. 2014). Villordon and Clark (2018) noted an increase in lateral root growth on sweet potato resistant (cv. ‘Bayou Belle’) compared to susceptible variety (cv. ‘Beauregard’) hosts. The observations supported those in nematode-resistant G. max that was exposed to M. javanica (Doyle and Lambert 2003) and on nematode-resistant Trifolium repens that was exposed to M. trifoliophila (Mercer et al. 2004).
Generally, in nematode-susceptible plant species, when Meloidogyne J2 develop through J3, J4 and adult female stages, the adjacent root cells bulge to form a root gall. Of 39 cultivars of C. annuum screened for nematode resistance, six (‘02G132’, ‘03G62’, ‘04G8’, ‘99G198’, ‘03G53’ and ‘CM334’) were resistant to M. incognita, with few undeveloped root galls (Moon et al. 2010). Pedrosa et al. (1996) and Herman et al. (1991) noticed fewer J2 advancing to subsequent stages of Meloidogyne species.
In a host-parasitic interaction study, tomato host reactions to Meloidogyne species parasitism were initiated during the first 12 h after infection (Siddique et al. 2014). However, in the two Cucumis species against the Meloidogyne species in the current study, there was no evidence of rapid host reactions. Findings by Ramatsitsi and Dube (2020) explained and supported the findings in the current study wherein there were no detectable nematode juveniles in roots at 30 days after inoculation even though they were observed earlier after inoculation. At 30 days after inoculation, Marini et al. (2016) also found a decrease in nematode numbers inside the roots of a resistant A. sativa cv. ‘IPR Afrodite’ that was exposed to M. incognita. At the onset of feeding, the nematode becomes sedentary, going through three molts before becoming a mature adult female, with males migrating out of the plant without playing any role in reproduction (Caillaud et al. 2008).
The results showed similar mechanisms of resistance in the roots of nematode resistant C. africanus for both M. incognita and M. javanica. The discovery of post-penetration nematode resistance to Meloidogyne species would very certainly increase the use of C. africanus in plant breeding and crop rotation systems, hence extending the applications and economic relevance of C. africanus. For future research, efforts could be made to investigate whether C. africanus is predisposed to other soil-borne pathogens through puncture wounds from penetration of the nematodes.
Data availability
Data sets available at Harvard Data verse https://doi.org/10.7910/DVN/IVQAKM and https://doi.org/10.7910/DVN/D3CI4M
References
Abifarin TO, Afolayan AJ, Otunola GA (2019) Phytochemical and antioxidant activities of Cucumis africanus L.f.: a wild vegetable of South Africa. J Evid Based Integr Med 24:251569019836391
Bartlem DG, Jones MG, Hammes UZ (2014) Vascularization and nutrient delivery at root-knot nematode feeding sites in host roots. J Exp Bot 65:1789–1798. https://doi.org/10.1093/jxb/ert415
Baum C, El-Tohamy W, Gruda N (2015) Increasing the productivity and product quality of vegetable crops using arbuscular mycorrhizal fungi: a review. Sci Hort 187:131–141. https://doi.org/10.1016/j.scienta.2015.03.002
Benková E, Bielach A (2010) Lateral root organogenesis-from cell to organ. Curr Opin Plant Biol 13:677–683. https://doi.org/10.1016/j.pbi.2010.09.006
Brito ODC, Ferreira JCA, Hernandes I, Da Silva EJ, Dias-Arieira CR (2020) Management of Meloidogynejavanica on tomato using agro-industrial wastes. Nematol 22:1–14. https://doi.org/10.1163/15685411-bja10018
Bybd DW, Kirkpatrick T, Barker KR (1983) An improved technique for clearing and staining plant tissues for detection of nematodes. J Nematol 15:142–143
Caboni P, Saba M, Oplos C, Aissani N, Maxia A et al (2015) Nematicidal activity of furanocoumarins from parsley against Meloidogyne spp. Pest Manag Sci 71:1099–1105. https://doi.org/10.1002/ps.3890
Caillaud MC, Dubreuil G, Quentin M, Perfus-Barbeoch L, Lecomte P et al (2008) Root-knot nematodes manipulate plant cell functions during a compatible interaction. J Plant Physiol 165:104–113. https://doi.org/10.1016/j.jplph.2007.05.007
Chiamolera F, Martins A, Soares P, Cunha-Chiamolera T (2018) Reaction of potential guava rootstocks to Meloidogyneenterolobii. Revista Ceres 65:291–295. https://doi.org/10.1590/0034-737X201865030010
Chitambar JJ, Raski DJ (1984) Reactions of grape rootstocks to Pratylenchusvulnus and Meloidogyne spp. J Nematol 16:166–170
Curtis RH (2008) Plant-nematode interactions: environmental signals detected by the nematode’s chemosensory organs control changes in the surface cuticle and behaviour. Parasite 15:310–316. https://doi.org/10.1051/parasite/2008153310
Da Silva-Mattos V, Mulet K, Cares JE, Gomes CB, Fernandez D et al (2019) Development of diagnostic SCAR markers for Meloidogynegraminicola, M. oryzae, and M. salasi associated with irrigated rice fields in Americas. Plant Dis 103:83–88. https://doi.org/10.1094/PDIS-12-17-2015-RE
Damasceno J, Soares A, Jesus F, Sant’Ana R (2015) Sisal leaf decortication liquid residue for controlling Meloidogynejavanica in tomato plants. Hort Bras 33:155–162. https://doi.org/10.1590/S0102-053620150000200004
Das S, DeMason DA, Ehlers JD, Close TJ, Roberts PA (2008) Histological characterization of root-knot nematode resistance in cowpea and its relation to reactive oxygen species modulation. J Exp Bot 59:1305–1313. https://doi.org/10.1093/jxb/ern036
Desaeger J, Dickson DW, Locascio SJ (2017) Methyl bromide alternatives for control of root-knot nematode (Meloidogyne spp) in tomato production in Florida. J Nematol 49:140–149. https://doi.org/10.21307/jofnem-2017-058
Desmedt W, Mangelinckx S, Kyndt T, Vanholme B (2020) A phytochemical perspective on plant defense against nematodes. Front Plant Sci 11:602079. https://doi.org/10.3389/fpls.2020.602079
Desmedt W, Kudjordjie EN, Chavan SN, Zhang J, Li R et al (2022) Rice diterpenoid phytoalexins are involved in defence against parasitic nematodes and shape rhizosphere nematode communities. New Phytol 235:1231–1245. https://doi.org/10.1111/nph.18152
Doyle EA, Lambert KN (2003) Meloidogynejavanica chorismate mutase 1 alters plant cell development. Mol Plant Microbe Interact 16:123–131. https://doi.org/10.1094/mpmi.2003.16.2.123
Escobar C, Fenoll C (2021) Compatible interactions between plants and endoparasitic nematodes-a follow-up of ABR volume 73: plant nematode interactions-a view on compatible interrelationships. Adv Bot Res 100:237–248. https://doi.org/10.1016/bs.abr.2021.03.001
Ferraz L, Brown D (2002) An introduction to nematodes: plant nematology. Pensoft, Bulgaria
Freire EV, Carneiro R, Costa P, Gomes A, Santos M et al (2010) Resistance to Meloidogyne incognita expresses a hypersensitive-like response in Coffeaarabica. Eur J Plant Pathol 127:365–373. https://doi.org/10.1007/s10658-010-9603-3
Fuller VL, Lilley CJ, Atkinson HJ, Urwin PE (2007) Differential gene expression in Arabidopsis following infection by plant-parasitic nematodes Meloidogyne incognita and Heteroderaschachtii. Mol Plant Pathol 8:595–609. https://doi.org/10.1111/j.1364-3703.2007.00416.x
Gheysen G, Fenoll C (2002) Gene expression in nematode feeding sites. Annu Rev Phytopathol 40:191–219. https://doi.org/10.1146/annurev.phyto.40.121201.093719
Gomez KA, Gómez AA (1984) Statistical procedures for agricultural research. John Wiley and Sons, New York
Gupta R, Singh A, Srivastava M, Singh V, Gupta MM et al (2017) Microbial modulation of bacoside A biosynthetic pathway and systemic defense mechanism in Bacopamonnieri under Meloidogyne incognita stress. Sci Rep 7:41867. https://doi.org/10.1038/srep41867
Hajihassani A, Rutter WB, Luo X (2019) Resistant pepper carrying N, Me1, and Me3 have different effects on penetration and reproduction of four major Meloidogyne species. J Nematol 51:1–9
Herman M, Hussey RS, Boerma HR (1991) Penetration and development of Meloidogyne incognita on roots of resistant soybean genotypes. J Nematol 23:155–161
Huang G, Gao B, Maier T, Allen R, Davis EL et al (2003) A profile of putative parasitism genes expressed in the esophageal gland cells of the root-knot nematode Meloidogyne incognita. Mol Plant Microbe Interact 16:376–381. https://doi.org/10.1094/mpmi.2003.16.5.376
Hussain M, Zouhar M, Ryšánek P (2018) Suppression of Meloidogyne incognita by the entomopathogenic fungus Lecanicilliummuscarium. Plant Dis 102:977–982. https://doi.org/10.1094/pdis-09-17-1392-re
Huysmans M, Lema AS, Coll NS, Nowack MK (2017) Dying two deaths-programmed cell death regulation in development and disease. Curr Opin Plant Biol 35:37–44. https://doi.org/10.1016/j.pbi.2016.11.005
Jones JT, Haegeman A, Danchin EG, Gaur HS, Helder J et al (2013) Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol 14:946–961. https://doi.org/10.1111/mpp.12057
Karuri HW, Olago D, Neilson R, Mararo E, Villinger J (2017) A survey of root knot nematodes and resistance to Meloidogyne incognita in sweet potato varieties from Kenyan fields. Crop Prot 92:114–121. https://doi.org/10.1016/j.cropro.2016.10.020
Laquale S, Candido V, Avato P, Argentieri MP, D’Addabbo T (2015) Essential oils as soil biofumigants for the control of the root-knot nematode Meloidogyne incognita on tomato. Ann Appl Biol 167:217–224. https://doi.org/10.1111/aab.12221
Lee HA, Lee HY, Seo E, Lee J, Kim SB et al (2017) Current understandings of plant nonhost resistance. Mol Plant Microbe Interact 30:5–15. https://doi.org/10.1094/MPMI-10-16-0213-CR
Lee IH, Kim HS, Nam KJ, Lee KL, Yang JW et al (2021) The defense response involved in sweetpotato resistance to root-knot nematode Meloidogyne incognita: comparison of root transcriptomes of resistant and susceptible sweetpotato cultivars with respect to induced and constitutive defense responses. Front Plant Sci 12:671677
Liu B, Liu X, Liu Y, Xue S, Cai Y et al (2016) The infection of cucumber (Cucumissativus L.) roots by Meloidogyne incognita alters the expression of actin-depolymerizing factor (adf) genes, particularly in association with giant cell formation. Front Plant Sci 7:1393. https://doi.org/10.3389/fpls.2016.01393
Lopez-Gomez M, Verdejo-Lucas S (2017) Penetration and post-infection development of root-knot nematodes in watermelon. Span J Agric Res 15:e1010. https://doi.org/10.5424/sjar/2017154-11189
Marini PM, Garbuglio DD, Dorigo OF, Machado ACZ (2016) Histological characterization of resistance to Meloidogyne incognita in Avenasativa. Trop Plant Pathol 41:203–209. https://doi.org/10.1007/s40858-016-0088-2
Mashela PW, Dube ZP, Pofu KM (2015) Managing the phytotoxicity and inconsistent nematode suppression in soil amended with phytonematicides. In: Meghvansi MK, Varma A (eds) Organic amendments and soil suppressiveness in plant disease management. Springer International Publishing, Berlin, pp 147–173
Mashela PW, Ndhlala AR, Pofu KM, Dube ZP (2016) Phytochemicals of nematode-resistant transgenic plants. In: Jha S (ed) Transgenesis and secondary metabolism. Springer International Publishing, Berlin, pp 1–16
Melillo MT, Leonetti P, Bongiovanni M, Castagnone-Sereno P, Bleve-Zacheo T (2006) Modulation of reactive oxygen species activities and H2O2 accumulation during compatible and incompatible tomato-root-knot nematode interactions. New Phytol 170:501–512. https://doi.org/10.1111/j.1469-8137.2006.01724.x
Mercer CF, Hussain SW, Moore KK (2004) Resistance reactions to Meloidogynetrifoliophila in Trifoliumrepens and T. semipilosum. J Nematol 36:499–504
Mhatre PH, Karthik C, Kadirvelu K, Divya KL, Venkatasalam EP et al (2019) Plant growth promoting rhizobacteria (PGPR): a potential alternative tool for nematodes bio-control. Biocatal Agric Biotechnol 17:119–128. https://doi.org/10.1016/j.bcab.2018.11.009
Moon HS, Khan Z, Kim SG, Son SH, Kim YH (2010) Biological and structural mechanisms of disease development and resistance in chili pepper infected with the root-knot nematode. Plant Pathol J 26:149–153. https://doi.org/10.5423/PPJ.2010.26.2.149
Muimba-Kankolongo A (2018) Food crop production by smallholder farmers in Southern Africa: challenges and opportunities for improvement. Elsevier, Waltham
Nicholson RL, Hammerschmidt R (2003) Phenolic compounds and their role in disease resistance. Annu Rev Phytopathol 30:369–389. https://doi.org/10.1146/annurev.py.30.090192.002101
Onkendi EM, Kariuki GM, Marais M, Moleleki LN (2014) The threat of root-knot nematodes (Meloidogyne spp.) in Africa: A review. Plant Pathol 63:727–737. https://doi.org/10.1111/ppa.12202
Ortiz R (2011) Agrobiodiversity management for climate change. In: Lenné J, Wood D (eds) Agrobiodiversity management for food security: critical review. CABI, New York, pp 189–211
Pedrosa EM, Hussey RS, Boerma HR (1996) Cellular responses of resistant and susceptible soybean genotypes infected with Meloidogynearenaria races 1 and 2. J Nematol 28:225–232
Pofu M, Mashela P, Shimelis H (2012) Host-status and host-sensitivity of wild Cucumis species to Meloidogyne incognita race 4. Acta Agric Scand B Soil Plant Sci 62:329–334. https://doi.org/10.1080/09064710.2011.614633
Postnikova OA, Hult M, Shao J, Skantar A, Nemchinov LG (2015) Transcriptome analysis of resistant and susceptible alfalfa cultivars infected with root-knot nematode Meloidogyne incognita. PLoS ONE 10:e0118269. https://doi.org/10.1371/journal.pone.0118269
Powers LE, Dunn RA, McSorley R (1991) Size differences among root-knot nematodes on resistant and susceptible alyceclover genotypes. J Nematol 23:243–248
Ramatsitsi MN, Dube ZP (2020) Post-infectional resistance in traditional leafy vegetable infected with root-knot nematodes. S Afr J Bot 131:169–173. https://doi.org/10.1016/j.sajb.2020.01.023
Saikia SK, Tiwari S, Pandey R (2013) Rhizospheric biological weapons for growth enhancement and Meloidogyne incognita management in Withaniasomnifera cv. Poshita Biol Control 65:225–234. https://doi.org/10.1016/j.biocontrol.2013.01.014
Seo HJ, Park AR, Kim S, Yeon J, Yu NH et al (2019) Biological control of root-knot nematodes by organic acid-producing lactobacillus brevis wikim0069 isolated from kimchi. Plant Pathol J 35:662–673
Siddique S, Matera C, Radakovic ZS, Hasan MS, Gutbrod P et al (2014) Parasitic worms stimulate host NADPH oxidases to produce reactive oxygen species that limit plant cell death and promote infection. Sci Signal 7(320):ra33. https://doi.org/10.1126/scisignal.2004777
Siddique S, Coomer A, Baum T, Williamson VM (2022) Recognition and response in plant-nematode interactions. Annu Rev Phytopathol 60:143–162. https://doi.org/10.1146/annurev-phyto-020620-102355
Singh T, Patel BA (2015) Management of root-knot nematode (Meloidogyne incognita) in bottle gourd using different botanicals in pots. J Parasit Dis 39:441–445
Thies JA, Ariss JJ, Kousik CS, Hassell RL, Levi A (2016) Resistance to southern root-knot nematode (Meloidogyne incognita) in wild Watermelon (Citrulluslanatus var. citroides). J Nematol 48:14–19
Thurau T, Ye W, Cai D (2010) Insect and nematode resistance. In: Kempken F, Jung C (eds) Genetic modification of plants: agriculture, horticulture and forestry. Springer, Berlin, pp 177–197
Tripathi L, Babirye A, Roderick H, Tripathi JN, Changa C et al (2015) Field resistance of transgenic plantain to nematodes has potential for future African food security. Sci Rep 5:8127. https://doi.org/10.1038/srep08127
Trudgill D (2003) Resistance to and tolerance of plant parasitic nematodes in plants. Annu Rev Phytopathol. https://doi.org/10.1146/annurev.py.29.090191.001123
Trudgill DL, Blok VC (2001) Apomictic, polyphagous root-knot nematodes: exceptionally successful and damaging biotrophic root pathogens. Annu Rev Phytopathol 39:53–77. https://doi.org/10.1146/annurev.phyto.39.1.53
Verdejo-Lucas S, Talavera M (2019) Root-knot nematodes on zucchini (Cucurbita pepo subsp. pepo): pathogenicity and management. Crop Prot 126:104943. https://doi.org/10.1016/j.cropro.2019.104943
Vieira R, Oliveira R, Ferreira P, Ferreira A, Rodrigues F (2013) Defense responses to Meloidogyneexigua in resistant coffee cultivar and non-host plant. Trop Plant Pathol 38:114–121. https://doi.org/10.1590/S1982-56762013000200004
Villordon A, Clark C (2018) Variation in root architecture attributes at the onset of storage root formation among resistant and susceptible sweetpotato cultivars infected with Meloidogyne incognita. Hort Sci 53:1924–1929
Wehner TC, Walters SA, Barker KR (1991) Resistance to root-knot nematodes in cucumber and horned cucumber. J Nematol 23:611–614
Acknowledgements
This study was funded by Potatoes South Africa.
Funding
Open access funding provided by North-West University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report there are no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramatsitsi, N., Ramachela, K. Histological characterization of wild cucumber resistance to Meloidogyne species. J Plant Dis Prot 130, 883–889 (2023). https://doi.org/10.1007/s41348-023-00733-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-023-00733-9