Abstract
Horticultural production systems have to move forwards to an environment-friendly and sustainable plant production. Basic substances can be added to reduce the use of synthetic plant protectant in conventional plant protection strategies. Regarding the protection of ornamental plants, less is known about the usage and behaviour of these alternative strategies and components. In our study, an infection of Podosphaera pannosa and Erysiphe polygoni in two different cultivars of cutting roses and one cultivar of French hydrangea was investigated. We studied the effect of a weekly foliar treatment of the basic substance chitosan (0.05 g/m2) compared to a water control and potassium hydrogen carbonate (VitiSan, Biofa GmbH, Münsing, Germany) in both production systems. As a result, in cutting roses as well as in French hydrangea, we found a significantly decreased infected leaf area following the use of chitosan and VitiSan compared to the water control. In both treatments, we found a stronger effect in French hydrangea, compared to roses. Results also indicate that the basic substance chitosan has to be used at a low pathogen level. We present a promising approach to reduce conventional plant protectants in ornamental plant production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Horticultural production systems have to move forwards to an environment-friendly and sustainable plant production. For the improvement of environment-friendly plant production, different fields of action are in accordance. This includes for instance plant selection, diversification and avoidance of side effects on non-target organism or environment-friendly pest management (Feldmann and Vogler 2020). This is also required according to the integrated pest management and the directive 2009/128/EC of the European Union (EU) (EU 2009a). Therefore, plant protection is in focus, and new methods to control plant diseases are required. Growers have to deal with these new approaches and face uncertainties when implementing them in their system. Often, there is a lack of knowledge regarding the practical application and procedures developed under controlled research conditions are often not directly transferable to their practical use in fields, greenhouses or orchards. Even less is known about this new field of alternative strategies in the protection of ornamental plants. Biological approaches to control plant pathogens are promising tools for modern plant protection. As Lahlali et al. (2022) reviews, different alternative strategies are available. Biocontrol agents can have a direct effect on plant pathogens, for instance on the parasite Trichoderma sp. or on Bacillus sp., a producer of antimicrobial enzymes. Indirect effects including competition for resources or space, as well as the induction of resistances and changes in phytohormones, are potential approaches for alternative plant protection strategies using biocontrol agents (Lahlali et al. 2022).
Basic substances are common products such as vinegar, lecithin or fructose, which are used as food ingredients, pharmaceuticals, biocides or fertilisers (Orconneau et al. 2022). Predominantly, they are not used as plant protectants. In order to achieve a sustainable plant protection, they can be implemented in a plant protection strategy as an additional component (Marchand 2015). But in the authorization process in the EU, no detailed analysis of efficacy of basic substances is demanded (Feldmann and Carstensen 2018). For instance, the basic substances Equisetum arvense or lecithin are suitable for implementing in a plant protection strategy to manage powdery mildews (Jolly et al. 2018; Wulf et al. 2022). Basic substances are not characterised as harmful, and they have no direct or cumulative effects on either human or animal health, or the environment (EU 2009b). Within plant protection strategies, they can replace synthetic plant protectants and support sustainable and organic horticulture (Đurić et al. 2019; Marchand 2017). In the EU pesticide database, they are officially listed with further report reviews describe the kind of authorised use (EU 2022).
Chitosan hydrochloride (following named as chitosan) is well investigated and ‘generally recognised as safe’ (Raafat and Sahl 2009; Žabka and Pavela 2021). For its benefits in plant protection, the polymer has been authorised by the Commission Implementing Regulation (EU) 2021/1165 of 15 July 2021 in combination with the final review report (EU 2021a; b), as a basic substance for use in both conventional and organic farming. Chitosan is a natural occurring polymer which can be synthesised by the deacetylation of chitin (Sharif et al. 2018). The term chitosan stands for a heterogeneous group of polymers with different degrees of acetylation and molecular weights (Costantini and La Torre 2022; Raafat and Sahl 2009). In vitro, these properties have been directly linked to antimicrobial effects (Badawy and Rabea 2011; Goy et al. 2009; Ke et al. 2021; Romanazzi et al. 2017). Chitosan interacts in many different ways with the gene expression and signalling pathways in plants. Regarding plant defence systems, the polymer can act as priming stimulus and thus, protect plants against biotic and abiotic stress (Chakraborty et al. 2020; Sharif et al. 2018). Additionally, the polymer improves growth and can be used as a biostimulator for plant strengthening as well as for improved storage behaviour of fruits and legumes (Ait Barka et al. 2004; Maurya et al. 2019; Katiyar et al. 2015; Zakiullah et al. 2019). Basic substances can be used as seed coatings, foliar sprays or soil supplements, and they represent an eco-friendly approach in modern plant protection (Costantini and La Torre 2022; El Hadrami et al. 2010; Faqir et al. 2021; Romanazzi et al. 2022). In this study, we investigate the effect of a weekly foliar sprayed chitosan suspension on infections of powdery mildew: Podosphaera pannosa in two cultivars of cutting roses and Erysiphe polygoni on French hydrangea. Thereby, the basic substance chitosan was tested in comparison with a water control and a plant protectant commonly used in ornamental plant production systems.
Material and methods
The study was carried out in a greenhouse under controlled environmental conditions. The experiment was located at longitude 10° 05′ 0.02’’, latitude 53° 30′ 33.5’’ and an altitude 3 m above sea level. Two trials were conducted. The first experiment with cutting roses (Rosa ssp.) took place from March to April 2022. The second, with French hydrangea (Hydrangea macrophylla cultivar ‘Bouquet rose’), was performed simultaneously from March to May 2022. Both trials were conducted at the same experimental site.
Plant material, growth conditions, experimental setup and data acquisition
The cutting roses (Rosa ssp.) were planted in spring 2021 in growbags (size 1.2 × 0.2 m) filled with 70% of coconut fibre and 30% of perlite. In a plot, 20 plants of roses were placed. The cultivars Beluga and Susan were set alternated with 10 plants per cultivar. The plots were 1 × 2 m in size. The temperature was regulated between 20 and 16 °C, and the water supply occurred via drip irrigation, and above a light intensity of 70 kLux, plants were shaded. No artificial light was given during the trial periods.
The Hydrangea macrophylla cultivar ‘Bouquet rose’ was planted in spring 2020 in soil. Plots were created with 1 × 2 m and seven plants per plot. Temperature, light and irrigation were managed as for the roses.
The experiment was set up as a randomised block design, with four replicates in each treatment. An infection of powdery mildew occurred naturally, and no artificial infection was performed. During the experiment, the infestation with P. pannosa and E. polygoni was recorded randomly by checking propagules via light microscope (Fig. 1). The disease severity was estimated on eight roses and seven French hydrangea plants per plot. In the case of the roses, the first and last plant of each plot was neglected to exclude border effects. To ensure an adequate sample size, all French hydrangea plants were included in the data acquisition. In both trials, data collection started prior to the first application (day 0) and was continued weekly until day 35 in cutting roses and day 49 in French hydrangea. During the experiment, plant height was measured at day 1 and 28 in cutting roses and at day 7, 22 and 42 in French hydrangea.
Treatments with potassium hydrogen carbonate and chitosan
In both trails, a water control and a practice-standard treatment were also tested, and all treatments were carried out weekly. The amount of sprayed mixture was adjusted to the plant height. Plants under 50 cm were treated with 100 mL/m2 mixture, and plants higher than 50 cm were treated with 150 mL/m2 mixture. All applications were carried out with a 3 L backpack sprayer (Profi Star 3, Birchmeier Sprühtechnik AG, Stetten, Switzerland).
The plant protectant VitiSan (Biofa GmbH, Münsingen Germany, Reg. no. 007593-00) served as reference commonly used in practice. Its active ingredient, potassium hydrogen carbonate (994.9 g/kg), is a fungicide with both a protective and curative effect. Depending on the plant height, the foliage was treated with 0.25 g/m2 (plants under 50 cm) or 0.375 g/m2 (plants above 50 cm).
The basic substance chitosan hydrochloride was obtained as the commercially available product ‘ChiPro plant’ (ChiPro GmbH, Bremen, Germany). The treatments were conducted with 0.05 g/m2 diluted in 100 mL/m2 or 150 mL/m2, depending on the plant height.
Statistical analyses
The statistical analysis was performed using R in combination with RStudio (R Version 4.2.1, RStudio Version 1.3.1093). The randomised block design was included in the statistical model. After a one-way analysis of variances (ANOVA), mean comparisons were carried out via the Dunnett-test (R package emmeans, Version 1.7.5). Thus, all significant differences were with reference to the water control.
Results
Growth of P. pannosa on rose and E. polygoni on French hydrangea
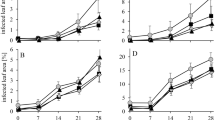
When comparing the growth of P. pannosa on the rose cultivars Beluga and Susan, it differs not significantly. In the water-treated plants, the powdery mildew showed a continuous growth on both cultivars. At the beginning of the experiment, the infected leaf area was moderate (Beluga 29.68 ± 2.24%; Susan 21.61 ± 1.80%) and rose up to a high infection severity at the end of the experiment (Beluga 58.39 ± 2.94%; Susan 62.42 ± 3.45%, Fig. 2).
Infected leaf area of P. pannosa on rose cultivars Beluga (A) and Susan (B) after weekly foliar application of the plant protectant VitiSan (potassium hydrogen carbonate) and the basic substance chitosan (mean ± standard error; nBeluga, VitiSan = 29, nBeluga other = 31, nSusan, VitiSan = 28 and nSusan, other = 31)
In the first weeks, until day 21, the infected leaf area of the fungal pathogen E. polygoni on water-treated French hydrangea plants was low (day: 0 2.33 ± 0.60%; Day 21: 4.48 ± 0.95%, Fig. 3). After day 21, the infection on the water-treated plants increased and reached a high level at the end of the experiment (day 49: 37.41 ± 3.51%).
Reduction in powdery mildew after foliar application of potassium hydrogen carbonate
Following the weekly foliar treatment of the plant protectant VitiSan, the growth of P. pannosa decreased significantly in comparison with the water control. Still, in both rose cultivars, the infected leaf area increased over time and ended with a high infection severity (day 35: Beluga 50.0 ± 2.86%; Susan 54.6 ± 2.41%). The treatment significantly reduced the infected leaf area at day 7 (29.3 ± 1.96%, P < 0.01), 14 (31.0 ± 1.80%, P = 0.03) and 35 (P = 0.02) on the cultivar Beluga. The infected leaf area of the treated cultivar Susan remained significantly below that of the water control continuously from day 7 onwards (22.68 ± 0.91%, P < 0.25).
The foliar treatment of the plant protectant potassium hydrogen carbonate performed better in French hydrangea than in roses. Due to the low growth rate of E. polygoni in French hydrangea in the first weeks, there was no significant difference between the water control and the treatment with potassium hydrogen carbonate in the infected leaf area until day 28 (6.0 ± 1.15%). After day 35 (15.37 ± 1.73%, P < 0.01), the treatment resulted in a significantly reduced infected leaf area until the end of the experimental time (day 49: 37.41 ± 3.51%, P < 0.01).
Reduction in powdery mildew after foliar application of chitosan
After the foliar applications of the basic substance chitosan, the infection of P. pannosa differed between the rose cultivars. On the cultivar Beluga, a significant reduction in the infected leaf area compared to the water control was determined only once (day 21: 38.2 ± 1.88%, P = 0.03). On the cultivar, Susan, the growth of the fungal pathogen was significantly reduced from day 7 until day 28 (29.52 ± 1.68%, P = 0.02; 45.0 ± 2.78%, P > 0.01). On the last day, there was no significant difference (day 35: 53.87 ± 3.12%).
In French hydrangea, the effect of the chitosan application was more pronounced than in roses. In comparison with the water control, a significant decrease in growth of E. polygoni was observed from day 35 (6.79 ± 1.23%, P < 0.01) until the end of the experiment at day 49 (22.71 ± 3.39%, P < 0.01). However, a reduction in the infected leaf area due to the use of chitosan was not as pronounced as after the application of the plant protectant VitiSan.
Plant height of roses and French hydrangea after foliar treatments with VitiSan and chitosan
The plant height of the water-treated roses at day 1 after the first application was 32.33 ± 1.35 cm (Beluga) and 33.46 ± 2.16 cm (Susan, Fig. 4). On day 28, the plants had reached a height of 55.25 ± 2.30 cm (Susan) and 52.75 ± 2.99 cm (Beluga).
Plant height of rose cultivars Susan and Beluga (A) and French hydrangea (B) after weekly foliar treatment of potassium hydrogen carbonate (VitiSan) and the basic substance chitosan (ChiPro Plant) (mean ± standard error; nrose, VitiSan = 23, nrose, other = 24, nhydrangea, control = 27 and nhydrangea other = 28). The letters indicate no differences of the treatments compared to the water control in rose cultivars or French hydrangea
In the control plots, the French hydrangea had a height of 25.74 ± 0.16 cm on day 7 after the first application. The growth of the plants continued to 32.33 ± 0.22 cm on day 22 and 38.33 ± 0.29 cm on day 42.
On each data collection day, there was no significant differences in the heights of the roses or French hydrangea plants of the VitiSan or chitosan treated plants in comparison with the water control.
Discussion
To control fungal pathogens, we have to consider alternative strategies such as the use of natural ingredients and basic substances, not only in agriculture and orchards, but also in ornamental horticultural (Brzozowski and Mazourek 2018). Still, there is a lack of knowledge in the use and behaviour of theses natural ingredients, in particular in ornamental cropping systems. This is even more challenging when considering the huge variety of plant families and species of ornamentals (Kisvarga et al. 2022; Richter et al. 2021). Within ornamental plant production systems, powdery mildew species are amongst the most severe fungal pathogens. In Germany, over 80 plant protectants (including sales extensions) are authorised to control these pathogens (BVL 2022).
In this study, we show the potential of the basic substance chitosan to control P. pannosa on roses and E. polygoni on French hydrangea. The reduction in the plant pathogens appears to depend on the fungus species and on the particular stage of infection. Thus, an early application is important when using chitosan for plant protection purposes under normal growing conditions.
El Hadrami et al. (2010) describe three different effects of chitosan with respect to plant protection. First, the polymers build a layer on the leaf surface that reduces infection by forming a mechanical barrier. Particularly, with regard to the postharvest metabolism of fruits and vegetables, this has an important effect on the shelf life and improves storage properties (Romanazzi et al. 2017; 2018; Romero et al. 2022).
Second, chitosan has a direct antifungal effect on different plant pathogens (Maurya et al. 2019). Several studies show a reduced mycelium growth of Pyricularia grisea, Botrytis cinerea, Fusarium oxysporum, Sclerotinia sclerotiorum, Rhizoctonia solani and Alternaria solani in vitro and in planta following the use of chitosan (DeGenring et al. 2022; Rabea et al. 2005; Reglinski et al. 2010; Sathiyabama et al. 2014; Vega et al. 2021; Žabka and Pavela 2021; Zhang et al. 2003). An inhibited spore germination and hyphal growth after chitosan application in vitro have already been demonstrated (Palma-Guerrero et al. 2008). Lopez-Moya et al. (2019) link the antifungal effect to a disruption of the synthesis of cell wall components, membranes and membrane-associated proteins. In another study, it was found that a concentration of 1 mg/ml of chitosan appears seems to efficiently reduce the growth of several fungi in vitro (El Hadrami et al. 2010). In our study, we used a concentration of 0.33–0.5 mg/mL of chitosan (depending on the plant height) for the foliar treatments. However, this concentration is below the threshold determined by El Hadrami et al. (2010).
The third effect has been attributed to an induced resistance of the plant against fungal pathogens (El Hadrami et al. 2010). Previous studies have already described a systemic induced resistance in vivo. Faoro et al. (2008) and Sathiyabama et al. (2014) could induce a resistance in non-treated leaves of Hordeum vulgare and Solanum lycopersicum against Blumeria graminis and A. solani, respectively. Vega et al. (2021) show similar results with B. cinerea on S. lycopersicum, S. melongena, Arabidopsis thaliana and Nicotiana benthamiana. The authors also discuss a concentration threshold in the effect of chitosan. Likewise, Deshaies et al. (2022) describe an induced resistance of Triticum aestivum against F. graminearum after using chitosan as an inducer. An effect on different foliar diseases in ornamental blooming flowers and shrubs is also mentioned by Wojdyła (2004). A detailed investigation on the defence signalling pathways and the metabolic response of the plants and fungi after a chitosan treatment is described by Deshaies et al. (2022), Lopez-Moya et al. (2019), Pongprayoon et al. (2022) and Stasińska-Jakubas and Hawrylak-Nowak (2022).
For the efficacy and applicability of chitosan as a foliar spray, the polymer length and the associated molecular weight are important factors. Due to the better solubility of smaller molecules, a lower molecular weight is more suitable for spray application in horticulture (Korbecka-Glinka et al. 2022). Polymer length plays an important role with regard to the influence of chitosan on biological systems. In terms of a direct antimicrobial effect, Ke et al. (2021) associated smaller molecules with a better penetration through the cell surface into the intracellular space of pathogens. This aspect is supported by the results of Attjioui et al. (2021), showing a higher inhibition of F. graminearum after the use of low-molecular weight chitosan compared to medium-molecular weight chitosan. In addition, reviews have already discussed the higher efficacy of low-molecular weight chitosan in relation to plant responses (Badawy and Rabea 2011; Korbecka-Glinka et al. 2022). To date, most plant responses studies have been conducted on seedlings and less is known about the effect of the molecular weight when chitosan is applied as a foliar spray on established plants.
The application of chitosan in combination with other products and techniques, such as nanotechnology or bacterial metabolites, may present an interesting approach for the future. For example, Sathiyabama and Manikandan 2018 reduced the disease severity of P. grisea using chitosan-copper nanoparticles on Eleusine coracan. Furthermore, the use of chitosan in nanotechnology has been recently discussed by Jain et al. (2022) and Mehta et al. (2022). In the case of bacterial metabolites, Ruano-Rosa et al. (2022) showed a decreased viability of E. necator ascospores after a treatment with chitosan in combination with bacterial metabolites. Additionally, chitosan nanoparticles are also known to improve the postharvest durability of cutting flowers (Spricigo et al. 2021).
The active ingredient of VitiSan functions by covering fungal spores and drying out the mycelium due to osmotic pressure. This has a more protective effect than curative (Jamar et al. 2007; Palmer et al. 1997). This is in accordance with the described effect on the infected leaf area observed in our study. The inhibition of pathogen growth and so reduction in infected leaf area was not as large in cutting roses compared to French hydrangea.
In our study, we could show an acceptable effect of chitosan when used in French hydrangea. In cutting roses, a significant reduction was measured; however, the effect was not substantial. Although both treatments have no negative effects on plant growth, nevertheless, the effect on the pathogen in cutting roses might be too small for growers for implementing a plant protection strategy. Similar results were reported by Ferreira et al. (2022), who concluded that the control of Plasmopara viticola and Pseudocercospora vitis on Vitis labrusca using chitosan was insufficient.
As previously mentioned, chitosan can serve as an inducer for a systemic acquired resistance against a pathogen. Our data demonstrate very clearly that an early application with respect to infection stage of the pathogen is required. The induced resistance seems to have the most important effect on the infected leaf area, especially in French hydrangea. It is therefore to consider the time of application when applying chitosan as part of a plant protection strategy in a practical horticultural setting. Comparing the actual costs of the basic substance chitosan and the plant protectant VitiSan, the use of chitosan (60 €/ha) requires a higher monetary input than the use of VitiSan (18.75–28.13 €/ha, depending on the plant height).
In summary, basic substances such as chitosan can contribute to a reduction in the use of chemical plant protectants. It has been shown by several authors that chitosan induces mechanism in plants against various biotic and abiotic stress factors and helps in formation of barriers enhancing plant’s productivity. Growers have to contemplate the specific properties of basic substances and their specific application in each case, making plant protection more challenging in the future (Maurya et al. 2019). This requires more knowledge amongst farmers, which will need to be considered in consulting and education strategies. Finally, the use of alternative plant protection strategies, such as the application of basic substances, may also have higher costs.
References
Ait Barka E, Eullaffroy P, Clément C, Vernet G (2004) Chitosan improves development, and protects Vitis vinifera L. against Botrytis cinerea. Plant Cell Rep 22:608–614. https://doi.org/10.1007/s00299-003-0733-3
Attjioui M, Gillet D, El Gueddari NE, Moerschbacher BM (2021) Synergistic antimicrobial effect of chitosan polymers and oligomers. Mol Plant Microbe Interact 34:770–778. https://doi.org/10.1094/MPMI-07-20-0185-R
Badawy MEI, Rabea EI (2011) A biopolymer chitosan and its derivatives as promising antimicrobial agents against plant pathogens and their applications in crop protection. Int J Carbohydr Chem 2011:1–29. https://doi.org/10.1155/2011/460381
Brzozowski L, Mazourek M (2018) A sustainable agricultural future relies on the transition to organic agroecological pest management. Sustainability 10:2023. https://doi.org/10.3390/su10062023
BVL (2022) Verzeichnis zugelassener Pflanzenschutzmittel. Bundesamt für Verbraucherschutz und Lebensmittelsicherheit. Available on: https://apps2.bvl.bund.de/psm/jsp/index.jsp. (accessed: 12.09.2022)
Chakraborty M, Hasanuzzaman M, Rahman M, Khan AR, Bhowmik P, Mahmud NU, Tanveer M, Islam T (2020) Mechanism of plant growth promotion and disease suppression by chitosan biopolymer. Agriculture 10:624. https://doi.org/10.3390/agriculture10120624
Costantini E, La Torre A (2022) Regulatory framework in the European Union governing the use of basic substances in conventional and organic production. J Plant Dis Prot 129:715–743. https://doi.org/10.1007/s41348-022-00569-9
de Vega D, Holden N, Hedley PE, Morris J, Luna E, Newton A (2021) Chitosan primes plant defence mechanisms against Botrytis cinerea, including expression of Avr9/Cf-9 rapidly elicited genes. Plant Cell Environ 44:290–303. https://doi.org/10.1111/pce.13921
DeGenring LM, Dickson RW, Poleatewich AM (2022) Inhibition of Botrytis cinerea growth and suppression of gray mold on petunia leaves using chitosan. Plant Dis. https://doi.org/10.1094/PDIS-07-22-1628-RE
Deshaies M, Lamari N, Ng CKY, Ward P, Doohan FM (2022) The impact of chitosan on the early metabolomic response of wheat to infection by Fusarium graminearum. BMC Plant Biol 22:73. https://doi.org/10.1186/s12870-022-03451-w
Đurić M, Mladenović J, Bošković-Rakočević L, Šekularac G, Brković D, Pavlović N (2019) Use of different types of extracts as biostimulators in organic agriculture. Acta Agric Serbica 24:27–39. https://doi.org/10.5937/AASer1947027D
El Hadrami A, Adam LR, El Hadrami I, Daayf F (2010) Chitosan in plant protection. Mar Drugs 8:968–987. https://doi.org/10.3390/md8040968
EU (2009a) Directive 2009a/128/EC of the European Parliament and of the Council of 21 October 2009 establishing a framework for community action to achieve the sustainable use of pesticides. https://eur-lex.europa.eu/eli/dir/2009/128/oj. Accessed 18 October 2022
EU (2009b) Commission Regulation No 1107/2009b. OJ L 309/1 of 24.11.2009: 1–50
EU (2021a) Commission Implementing Regulation (EU) 2021a/1165 of 15 July 2021. OJ L 253 of 16.07.2021: 13–48
EU (2021b) Final review report for the basic substance chitosan hydrochloride. SANCO/12388/2013 (rev. 3 of 25.01.2021b)
EU (2022) European pesticide database. Available on: https://ec.europa.eu/food/plants/ pesticides/eu-pesticides-database_en (accessed: 12.09.2022)
Faoro F, Maffi D, Cantu D, Iriti M (2008) Chemical-induced resistance against powdery mildew in barley: the effects of chitosan and benzothiadiazole. Biocontrol 53:387–401. https://doi.org/10.1007/s10526-007-9091-3
Faqir Y, Ma J, Chai Y (2021) Chitosan in modern agriculture production. Plant Soil Environ 67:679–699. https://doi.org/10.17221/332/2021-PSE
Feldmann F, Carstensen C (2018) Efficacy and risks of “biorationals.” J Plant Dis Prot 125:517–521. https://doi.org/10.1007/s41348-018-0186-0
Feldmann F, Vogler U (2020) Towards sustainable performance of urban horticulture: ten challenging fields of action for modern integrated pest management in cities. J Plant Dis Prot 125:517–521. https://doi.org/10.1007/s41348-018-0186-0
Ferreira GM, Moreira RR, Jarek TM, Nesi CN, Biasi LA, May De Mio LL (2022) Alternative control of downy mildew and grapevine leaf spot on Vitis labrusca. Australas Plant Path 51:193–201. https://doi.org/10.1007/s13313-021-00836-7
Goy RC, de Britto D, Assis OBG (2009) A Review of the antimicrobial activity of chitosan. Polímeros Ciência e Tecnologia 19:241–247. https://doi.org/10.1590/S0104-14282009000300013
Jain T, Srivastava K, Kumar S, Dutta PK (2022) Current and future prospects of chitosan-based nanomaterials in plant protection and growth. In: Kumar S, Madihally SV (eds) Role of chitosan and chitosan-based nanomaterials in plant sciences. Elsevier, Amsterdam, pp 143–163
Jamar L, Lefrancq B, Lateur M (2007) Control of apple scab (Venturia inaequalis) with bicarbonate salts under controlled environment. J Plant Dis Prot 114:221–227. https://doi.org/10.1007/BF03356221
Jolly M, Vidal R, Marchand PA (2018) Lecithins: a food additive valuable for antifungal crop protection. Int J Econ Plants 5:104–107. https://doi.org/10.23910/IJEP/2018.5.3.0243
Katiyar D, Hemantaranjan A, Singh B (2015) Chitosan as a promising natural compound to enhance potential physiological responses in plant: a review. Indian J Plant Physiol 20:1–9. https://doi.org/10.1007/s40502-015-0139-6
Ke C-L, Deng F-S, Chuang C-Y, Lin C-H (2021) Antimicrobial actions and applications of chitosan. Polymers 13:904. https://doi.org/10.3390/polym13060904
Kisvarga S, Farkas D, Boronkay G, Neményi A, Orlóci L (2022) Effects of biostimulants in horticulture, with emphasis on ornamental plant production. Agronomy 12:1043. https://doi.org/10.3390/agronomy12051043
Korbecka-Glinka G, Piekarska K, Wiśniewska-Wrona M (2022) The use of carbohydrate biopolymers in plant protection against pathogenic fungi. Polymers 14:2854. https://doi.org/10.3390/polym14142854
Lahlali R, Ezrari S, Radouane N, Kenfaoui J, Esmaeel Q, El Hamss H, Belabess Z, Ait Barka E (2022) Biological control of plant pathogens: a global perspective. Microorganisms 10:596. https://doi.org/10.3390/microorganisms10030596
Lopez-Moya F, Suarez-Fernandez M, Lopez-Llorca LV (2019) Molecular mechanisms of chitosan interactions with fungi and plants. Int J Mol Sci 20:332. https://doi.org/10.3390/ijms20020332
Marchand PA (2015) Basic substances: an opportunity for approval of low-concern substances under EU pesticide regulation. Pest Manag Sci 71:1197–1200. https://doi.org/10.1002/ps.3997
Marchand PA (2017) Basic substances under EU pesticide regulation: an opportunity for organic production? Org Farming 3:16–19. https://doi.org/10.12924/of2017.03010016
Maurya S, Kumar S, Jat KK, Kumari P (2019) Chitosan: a novel bioactive compound for management of plant diseases: a review. J Pharmacogn Phytochem 8:2281–2286
Mehta MR, Biradar SP, Mahajan HP, Bankhele RR, Hivrale AU (2022) Chitosan and chitosan-based nanoparticles in horticulture: past, present and future prospects. In: Kumar S, Madihally SV (eds) Role of chitosan and chitosan-based nanomaterials in plant sciences. Elsevier, Amsterdam, pp 453–474
Orconneau Y, Taylor A, Marchand PA (2022) Basic substances in organic agriculture: current status. Chron Bioresour Manag 6:76–83
Palma-Guerrero J, Jansson H-B, Salinas J, Lopez-Llorca LV (2008) Effect of chitosan on hyphal growth and spore germination of plant pathogenic and biocontrol fungi. J Appl Microbiol 104:541–553. https://doi.org/10.1111/j.1365-2672.2007.03567.x
Palmer CL, Horst RK, Langhans RW (1997) Use of bicarbonates to inhibit in vitro colony growth of Botrytis cinerea. Plant Dis 81:1432–1438. https://doi.org/10.1094/PDIS.1997.81.12.1432
Pongprayoon W, Siringam T, Panya A, Roytrakul S (2022) Application of chitosan in plant defense responses to biotic and abiotic stresses. Appl Sci Eng Prog 15:3865
Raafat D, Sahl H-G (2009) Chitosan and its antimicrobial potential—a critical literature survey. Microb Biotechnol 2:186–201. https://doi.org/10.1111/j.1751-7915.2008.00080.x
Rabea EI, El BM, Rogge TM, Stevens CV, Höfte M, Steurbaut W, Smagghe G (2005) Insecticidal and fungicidal activity of new synthesized chitosan derivatives. Pest Manag Sci 61:951–960. https://doi.org/10.1002/ps.1085
Reglinski T, Elmer PAG, Taylor JT, Wood PN, Hoyte SM (2010) Inhibition of Botrytis cinerea growth and suppression of botrytis bunch rot in grapes using chitosan. Plant Pathol 59:882–890. https://doi.org/10.1111/j.1365-3059.2010.02312.x
Richter E, Götte E, Hack G (2021) Zierpflanzenproduktion in Deutschland- mit alternativen strategien im pflanzenschutz zukunftsfähig? J Kult 73:333–352. https://doi.org/10.5073/JFK.2021.11-12.01
Romanazzi G, Feliziani E, Baños SB, Sivakumar D (2017) Shelf life extension of fresh fruit and vegetables by chitosan treatment. Crit Rev Food Sci Nutr 57:579–601. https://doi.org/10.1080/10408398.2014.900474
Romanazzi G, Feliziani E, Sivakumar D (2018) Chitosan, a biopolymer with triple action on postharvest decay of fruit and vegetables: eliciting antimicrobial and film-forming properties. Front Microbiol 9:2745. https://doi.org/10.3389/fmicb.2018.02745
Romanazzi G, Orçonneau Y, Moumni M, Davillerd Y, Marchand PA (2022) Basic substances, a sustainable tool to complement and eventually replace synthetic pesticides in the management of pre and postharvest diseases: reviewed instructions for users. Molecules 27:3484. https://doi.org/10.3390/molecules27113484
Romero J, Albertos I, Díez-Méndez A, Poveda J (2022) Control of postharvest diseases in berries through edible coatings and bacterial probiotics. Sci Hortic 304:111326. https://doi.org/10.1016/j.scienta.2022.111326
Ruano-Rosa D, Sánchez-Hernández E, Baquero-Foz R, Martín-Ramos P, Martín-Gil J, Torres-Sánchez S, Casanova-Gascón J (2022) Chitosan-Based bioactive formulations for the control of powdery mildew in viticulture. Agronomy 12:495. https://doi.org/10.3390/agronomy12020495
Sathiyabama M, Manikandan A (2018) Application of copper-chitosan nanoparticles stimulate growth and induce resistance in finger millet (Eleusine coracana Gaertn.) plants against blast disease. J Agric Food Chem 66:1784–1790. https://doi.org/10.1021/acs.jafc.7b05921
Sathiyabama M, Akila G, Einstein Charles R (2014) Chitosan-induced defence responses in tomato plants against early blight disease caused by Alternaria solani (Ellis and Martin) Sorauer. Arch Phytopathol Plant Protec 47:1777–1787. https://doi.org/10.1080/03235408.2013.858423
Sharif R, Mujtaba M, Ur Rahman M, Shalmani A, Ahmad H, Anwar T, Tianchan D, Wang X (2018) The multifunctional role of chitosan in horticultural crops; a review. Molecules. https://doi.org/10.3390/molecules23040872
Spricigo PC, Pilon L, Trento JP, Moura MR, Bonfim KS, Mitsuyuki MC, Mattoso LHC, Ferreira MD (2021) Nano-chitosan as an antimicrobial agent in preservative solutions for cut flowers. J Chem Technol Biotechnol 96:2168–2175. https://doi.org/10.1002/jctb.6766
Stasińska-Jakubas M, Hawrylak-Nowak B (2022) Protective, biostimulating, and eliciting effects of chitosan and its derivatives on crop plants. Molecules 27:2801. https://doi.org/10.3390/molecules27092801
Taylor A, Bonafos R, Chovelon M, Parvaud CE, Furet A, Aveline N, Bertrand C, Marchand PA (2022) Equisetum arvense (horsetail) extract: the first approved basic substance allowed for EU crop protection. Int J Bio-Resour Stress Manag 13:566–577. https://doi.org/10.23910/1.2022.2757
Wojdyła AT (2004) Chitosan (biochikol 020 PC) in the control of some ornamental foliage diseases. Commun Agric Appl Biol Sci 69:705–715
Wulf F, Podhorna J, Bandte M, Rybak M, Büttner C (2022) Potential of basic substances in plant protection to reduce Podosphaera pannosa in cut roses. J Plant Dis Prot. https://doi.org/10.1007/s41348-022-00658-9
Žabka M, Pavela R (2021) The dominance of chitosan hydrochloride over modern natural agents or basic substances in efficacy against Phytophthora infestans, and its safety for the non-target model species Eisenia fetida. Horticulturae 7:366. https://doi.org/10.3390/horticulturae7100366
Zakiullah KMF, Irfanullah N, Khaliq A, Riaz M, Razzaq T, Urooj M, Kakar H, Fahemullah SS (2019) Effect of chitosan (growth enhancer) on the growth and yield of different pea (Pisum sativum L.) varieties under arid condition of Bannu Pakistan. Int J Agron Agri R 15:10–20
Zhang M, Tan T, Yuan H, Rui C (2003) Insecticidal and fungicidal activities of chitosan and oligo-chitosan. J Bioact Compat Polym 18:391–400. https://doi.org/10.1177/088391103039019
Acknowledgements
We thank Thomas Gaskin for English proofreading. This research was founded by the Ministry of Economy and Innovation, Free and Hanseatic City of Hamburg, Germany (project AZ 734.650.004/014B).
Author information
Authors and Affiliations
Contributions
Conceptualization was contributed by FW, MR and MB; methodology and investigation were contributed by FW, JP; formal analysis, data curation and visualisation were contributed by FW; validation was contributed by FW, MB; writing—original draft preparation, was contributed by FW; writing—review and editing, was contributed by FW, JP, MB, MR and CB; funding acquisition and supervision were contributed by CB; project administration and resources were contributed by MR, CB.
Corresponding author
Ethics declarations
Conflict of interest
The article is the original work of the authors; it is not published or in consideration elsewhere. All authors agreed in the submission of the manuscript to the Journal of Plant Diseases and Protection. The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wulf, F., Podhorna, J., Rybak, M. et al. Studies on the potential of the basic substance chitosan in managing Podosphaera pannosa on cutting roses and Erysiphe polygoni on French hydrangea. J Plant Dis Prot 130, 579–586 (2023). https://doi.org/10.1007/s41348-023-00714-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-023-00714-y