Abstract
In 2013 and 2014, the effects of different products and plant extracts in the control of Aphelenchoides ritzemabosi (Schwartz, 1911) Steiner and Buhrer, 1932 on Anemone hupehensis plants variety Prinz Heinrich were estimated. Experiments were carried out under containerized cultivation. Evaluation of the effectiveness of nematicides was conducted using two common methods based on the number of nematodes per leaf and the percentage of damaged leaves. In both experiments oxamyl and abamectin, with aqueous extract of Allium sativum, were the most effective (efficacy about 40%). The aqueous bulb extracts of A. sativum, solution of extracts of Quillaja saponaria and solution of spirotetramat in combination with aqueous extract of A. sativum were ineffective. In both experimental years, the significant correlation between the number of nematodes in leaves and the sampling date was recorded. Based on Spearman rank correlation test and regression, it was shown that counting of nematodes in leaves is still the most reliable method for diagnosis of the damage caused by foliar nematodes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Foliar nematode (Aphelenchoides ritzemabosi) (Schwarz 1911) Steiner and Buhrer is a common plant-parasite infecting more than 200 plant species. The characteristic symptoms of infection are concerned with lesions bounded by the major veins in leaves. Leaves parasitized by these nematodes loose color, turn brown and fall off (de Waele 2002). Because of the broad spectrum of hosts and the life cycle of these nematodes that takes place inside the leaf, there is a necessity to use suitable strategies to prevent the spread of A. ritzemabosi and to select corresponding nematicide that effectively penetrate the leaf tissue.
Among the active ingredients of pesticides used for plant protection against different nematodes, the most popular are: oxamyl (Philis 1994; Tu et al. 1996), spirotetramat (Smiley et al. 2011, 2012), diazinon (La Mondia 1999; Jagdale and Grewal 2002), carbofuran (Adegbite and Agbaje 2007; Jada et al. 2011). Despite the efficacy of chemical substances, nematicides are being increasingly replaced by various eco-friendly biological products. At present, there are no registered nematicides available for the control of foliar nematodes on ornamental plants, neither in Poland, nor in a number of other countries. This situation forces the search for alternative solutions. Many phytochemicals are known from their nematicidal activity. Plant oils may include some of the long list of compounds but not all of them. Most plant oils studied for nematode control are essential oils containing large proportions of volatile terpenes and other compounds. These compounds involved in plants may act as repellents, attractants, hatching stimulants or inhibitors as well as nematotoxicants (Chitwood 2002). Plant extracts and microorganisms antagonistic to nematodes or producing antibiotics are also successfully used in biological controls in laboratory tests (Nguyen et al. 2009), glasshouse trials (Van Damme et al. 2005) and field experiments (Rao et al. 2004; Okeniyi et al. 2013). Among biological products, the nematicidal properties were revealed by an aqueous bulb extract of Allium sativum L. (Tibugari et al. 2012; El-Nagdi and Youssef 2013), extracts of the soapbark tree—Quillaja saponaria Molina (Insunza et al. 2001; San Martin and Magunacelaya 2005), extracts of Mentha sp. L. (Caboni et al. 2013), oils of Syzygium aromaticum (L.) Merrill and Perry and Thymus vulgaris L. (Elbadri et al. 2008), abamectin—produced by an actinomycete bacterium Streptomyces avermitilis (ex Burg et al. 1979) Kim and Goodfellow 2002 (James et al. 2006; Korayem et al. 2008) and azadirachtin isolated from the neem tree Azadirachta indica A. Juss. (Lynn et al. 2010; Khan et al. 2012). Studies conducted by Jagdale and Grewal (2002) showed that some products are effective only in in vitro tests, whereas they are not suitable in the case of infected leaves. Research conducted by Tibugari et al. (2012), also El-Nagdi and Youssef (2013) with extracts of garlic, as well as by San Martin and Magunacelaya (2005) with extracts of the soapbark tree, showed that these components have nematicidal activity not only in laboratory tests, but also in greenhouse experiments. As it has been shown, an active compound against plant parasitic nematodes in A. sativum is allicin (Gupta and Sharmaj 1993) and the active compound in Q. saponaria against these pests are saponins (Zasada et al. 2010) or whole extracts containing saponin and non-saponin fractions (San Martin and Magunacelaya 2005). Most of the available information is concerned with the management of the root lesion or root-knot nematodes (Ingham et al. 2000; Agbenin et al. 2005; Zasada et al. 2010; Giannakou 2011). Protection against foliar nematodes is mainly based on chemical products, and only a few articles describe other methods of control (La Mondia 1999; Jagdale and Grewal 2002). From the economic point of view, it is important to find solutions for limiting foliar nematodes in container cultivation of ornamental plants conducted on a commercial scale. Evaluation of the effectiveness of nematicides is most often carried out on the basis of the number of nematodes isolated from soil (El-Nagdi and Youssef 2013; Giannakou, 2011; Ingham et al. 2000; Lynn et al. 2010) or plant material (Cabrera et al. 2009; Rao et al. 2004). In the case of foliar nematodes, extraction of specimens is associated with leaf destruction. Therefore, on each sampling date, the nematodes are isolated from the subsequent leaf, hence the initial population variant. The easiest way to estimate the effectiveness of pesticide is the evaluation of the number or percentage of damaged leaves (Hesling and Wallace 1960; Szczygieł 1969; Kohl et al. 2010). This kind of assessment is a non-invasive method although not completely foolproof. Nematodes could also be extracted from asymptomatic leaf tissue. Riedel and Powell (1974) isolated A. fragariae from leaves without visible symptoms, during the control of foliar nematodes in begonia with oxamyl. Kohl et al. (2010) and Kohl (2011) also found foliar nematodes in asymptomatic lantana leaves. It is crucial to evaluate the usefulness of this method in the estimation of nematicide efficacy in comparison with the standard procedure based on counting nematodes in leaves.
The objectives of the present study were: (i) the estimation of the efficacy of the products in the management of A. ritzemabosi on Anemone hupehensis (Lemoine) Lemoine in conditions of the container cultivation, typical for the production in nurseries, (ii) the comparison of two assessment methods of efficacy of tested pesticides and biocontrol products.
Materials and methods
Experimental procedure
Specimens of A. ritzemabosi were collected from infected plants in commercial nurseries located in Central Poland. Species identification was carried out based on morphology and morphometric parameters according to available identification keys and species descriptions (Siddiqi 1974a, b; Baranovskaya 1981; Andrássy 2007). Healthy plants were infested by spraying a water solution of living nematodes. Two analogical container trials using infected plants were conducted in 2013 and 2014 at the experimental field of the Research Institute of Horticulture in Skierniewice. The first research was established in June, the second one in September. Tests on plants of A. hupehensis (Lemoine) of the variety Prinz Heinrich were carried out for 4 consecutive weeks.
The following treatments were used:
-
1.
Untreated control,
-
2.
Vydate 10 G (oxamyl 100 g/kg, DuPont) at a dose 0.05 g/l of soil; one soil application,
-
3.
2.5% aqueous extract of A. sativum L. (prepared according to Dąbrowski and Seredyńska (2007)); 4 foliar applications at 7-day intervals.
-
4.
10% solution of Quillaja extract (2.2% water-glycolic extract of bark Q. saponaria Molina, Provital S.A.); 4 foliar applications at 7-day intervals.
-
5.
0.15% solution of Movento 100 SC (spirotetramat 100 g/l, Bayer CropScience) + 2.5% aqueous extract of A. sativum (prepared as above); 2 foliar applications of garlic extract at 1-week intervals alternately with 2 applications of Movento at 2-weeks intervals.
-
6.
0.075% solution of Vertimec 018 EC (abamectin 18 g/l, Syngenta Crop Protection) + 2.5% aqueous extract of A. sativum (prepared as above); 2 foliar applications of garlic extract at 1-week interval alternately with 2 applications of Vertimec at 2-weeks intervals.
Spraying was carried out at a temperature of 16–20 °C and at a minimum humidity of 63%. In both experiments, 40 plants per combination were used (four repetitions for combination, ten plants in each replication). The estimation of the efficacy of products was based on the number of isolated nematodes and on the percentage of damaged leaves. Nematodes were extracted from two leaves of each plant by the Baermann funnel method and counted under the light microscope at 32× magnification. Nematodes and the percentage of damaged leaves were counted twice: before treatment (PRE-T) and at the end of the experiment (T + 28).
Statistical analysis
The results were statistically analyzed using the analysis of variance (ANOVA) using the transformed number of nematodes per leaf according to function: y = log (x + 1) and % of damaged leaves according to Bliss transformation (y = arcsin√x). The significance of differences between means was evaluated using the multiple Duncan’s test at a level of α = 0.05. The analysis was performed in STATISTICA v. 10 (Stat Soft Inc., 2011). The average number of nematodes counted per leaf at each sampling date for each product was tested for correlation with the percentage of damaged leaves using the Spearman rank correlation test. Data were also elaborated by the regression method according to the linear function (y = bx + a) for the relationship between the number of nematodes per leaf and the percentage of damaged leaves counted in the successive dates of observation (y), and the number of days passing from the date of application to the successive dates of observation (x). Equation y = bx + a determined the regression line, in which parameter “b” in linear relation indicated the sloping angle of line and the means speed of nematode reduction, and parameter “a” meant the initial level of nematode population on the day of treatment. The significance of the difference in parameters was estimated using Student’s t test, at a level α = 0.05. On the basis of the average number of nematodes, the effectiveness of the products was calculated, according to the Henderson–Tilton formula. To evaluate the effectiveness of the product in the control of nematodes, the criteria was used in accordance with the Regulation of the Polish Ministry of Agriculture and Rural Development, on August 4, 2004—Journal of Law No. 183, item 1890, where at least 80% = a good level of control; 60–80% = a medium level of control and 40–60% = a limited level of control.
Results
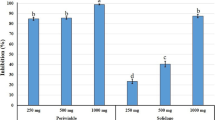
In the experiment conducted in 2013, 4 weeks after the first treatment of products, both the average number of nematodes and the percentage of damaged leaves increased in all tested products. The lowest increase of the nematode population was observed for oxamyl and abamectin with aqueous extract of garlic. The application of oxamyl also resulted in the lowest growth in the percentage of damaged leaves (Table 1). The efficacy of this nematicide was 40%. A similar effect was achieved 28 days after the application of abamectin with aqueous extract of A. sativum, in which the efficiency reached 42.2%. The application of other products was ineffective. The correlation between the number of nematodes in leaves and the percentage of damaged leaves was only significant in the untreated control.
The results of the second experiment revealed the efficiency of oxamyl and abamectin with aqueous extract of A. sativum (Table 2). The reduction in the number of nematodes was also observed in the case of other tested products, but it did not reach the limited level of pest control. The decrease in the number of nematodes was contributed to the reduction in the percentage of damaged leaves with the exception of oxamyl, despite the highest efficacy (44.4%). The Spearman rank correlation test did not prove the above observation.
In both years, in almost all treatments, the correlation between the number of nematodes in leaves and the sampling date was significant and the equation of the regression line with parameters a and b was determined (Tables 3, 4). In contrast, the correlation between the percentage of damaged leaves and the sampling date was not significant.
Discussion
Current studies showed difficulties in the control of foliar nematodes. Only two of the five tested substances (oxamyl and abamectin with aqueous extract of garlic) were effective at a limited level in both experiments. The reduction of the population of A. ritzemabosi in the case of other products was observed only in the second year. However, it did not reach 40%. The reaction of pesticides is different depending on environmental conditions and biological factors. Wheeler et al. (2013) observed the relationship between the irrigation rate, soil moisture and the nematicide performance. The studies of Oka et al. (2013) indicated an impact of such soil environments as soil type and organic matter. It is possible that in favorable conditions, the efficacy may increase; however, it requires further detailed studies.
The ability to use oxamyl in the management of nematodes showed in this study was also verified by other authors. In Jagdale and Grewal (2002) studies, Vydate in formulation GR showed over 70% reduction of A. fragariae population on Hosta spp. Similar results were observed by Zasada et al. (2010). Their experiments concerned with the management of root lesion nematode Pratylenchus penetrans also revealed high nematicidal efficacy of oxamyl.
Present research demonstrated that abamectin, in combination with aqueous extract of A. sativum, may be effective in controlling the population of A. ritzemabosi. Both abamectin and garlic extract were previously tested by other researchers. Cabrera et al. (2009) and Faske and Starr (2007) obtained the efficacy of abamectin in reducing the number of Meloidogyne spp. In turn, James et al. (2006) reported that this substance may be useful in the protection against pine wilt disease caused by Bursaphelenchus xylophilus. Aqueous extracts of A. sativum significantly reduced root-knot infection indices on tomato with respect to Meloidogyne incognita (Agbenin et al. 2005, El-Nagdi and Youssef 2013) and M. javanica (Tibugari et al. 2012). Park et al. (2005) found that garlic oils, whose main compounds were diallyl disulfide, diallyl sulfide and diallyl trisulfide, could be useful as nematicides for the practical use against pine wood nematode. In our study, the separate application of extract of A. sativum did not prove to be effective. The combined use of these products is better in achieving repeated effects in the reduction of foliar nematodes.
Spirotetramat, in combination with an extract of A. sativum, was not effective. Data from the literature also shows that the use of spirotetramat against nematodes varies. Smiley et al. (2011) suggested that applications of this substance reduced the density of Heterodera avenae. In turn, the research of Zasada et al. (2010) and Smiley et al. (2012) showed inefficiency of this product in the controlling of Pratylenchus spp. population. These differences suggest that nematode species are characterized by differential sensitivity to the nematicides. Similar observations were noted by La Mondia (1999).
The application of extract of Q. saponaria was also ineffective. The ability to control nematodes by this extract was reported in several previous publications (Insunza et al. 2001; Giannakou 2011; San Martin and Magunacelaya 2005). In Chałańska et al. (2013), studies on anemone plants showed that the application of soapbark tree extract was highly effective, but the concentration of Q. saponaria extract used in that study was five times higher and caused phytotoxicity symptoms in plants. In this present study, the application of harmless doses of this product did not reduce the population of nematodes. Conclusions drawn by the comparison of literature data and the results of this study are that in future, studies also with different doses should be tested—higher than in the present study, but still not causing phytotoxicity.
The selection of a suitable product in managing foliar nematodes is associated with many difficulties. The efficacy of nematicides depends on various factors. In our study, the impact on results could be altered by atmospheric temperature and humidity. Szczygieł (1966) and Szczygieł and Hasior (1972) drew attention to this. With respect to foliar nematodes, additional difficulties are connected with the effective penetration of nematicides into leaf tissue. Available products for nematode control could protect the plants against visible damage caused by foliar nematodes, without exterminating all specimens (Kohl 2011). The precise estimation of the population of A. ritzemabosi before and after pest control application is necessary in order to make a proper assessment of the effectiveness of the tested pesticide and to determine the further implementation of a plant protection plan, depending on environmental conditions and the number of nematodes. As it was shown in the present research, in spite of the counting of nematodes from another leaf at each sampling date, this method is still the most reliable diagnosis of nematode damage. Moreover, it allows to determine the number of nematodes after the treatments. It is an essential part of foliar nematode management because they are known as a highly reproductive group (La Mondia 1999) requiring constant monitoring.
References
Adegbite, A. A., & Agbaje, G. O. (2007). Efficacy of Furadan (carbofuran) in control of root-knot nematode (Meloidogyne incognita Race 2) in hybrid yam varieties in south-western Nigeria. World Journal of Agricultural Sciences, 3(2), 256–262.
Agbenin, N. O., Emechebe, A. M., Marley, P. S., & Akpa, A. D. (2005). Evaluation of nematicidal action of some botanicals on Meloidogyne incognita in vivo and in vitro. Journal of Agriculture and Rural Development in the Tropics and Subtropics, 106(1), 29–39.
Andrássy, I. (2007). Free-living nematodes of Hungary (Nematoda errantia), II. In C. Csuzdi & S. Mahunka (Eds.), Pedozoologica Hungarica No. 4. Budapest: Hungarian Natural History Museum.
Baranovskaya, I. A. (1981). Plant and soil nematodes (Aphelenchoididae and Seinuridae). Moskwa: Nauka. (in Russian).
Caboni, P., Saba, M., Tocco, G., Casu, L., Murgia, A., Maxia, A., et al. (2013). Nematicidal activity of mint aqueous extracts against the root-knot nematode Meloidogyne incognita. Journal of Agricultural and Food Chemistry, 61(41), 9784–9788. doi:10.1021/jf403684h.
Cabrera, J. A., Kiewnick, S., Grimm, C., Dababat, A. A., & Sikora, R. A. (2009). Efficacy of abamectin seed treatment on Pratylenchus zeae, Meloidogyne incognita and Heterodera schachtii. Journal of Plant Diseases and Protection, 116(3), 124–128.
Chałańska, A., Łabanowski, G., & Maciorowski, R. (2013). Control efficacy of selected natural products against chrysanthemum foliar nematode—Aphelenchoides ritzemabosi (Schwartz, 1911) Steiner & Buhrer, 1932. Progress in Plant Protection/Postępy w Ochronie Roślin, 53(3), 563–567.
Chitwood, D. J. (2002). Phytochemical based strategies for nematode control. Annual Review of Phytopathology, 40, 221–249. doi:10.1146/annurev.phyto.40.032602.130045.
Dąbrowski, Z. T., & Seredyńska, U. (2007). Characterization of the two-spotted spider mite (Tetranychus urticae Koch, Acari: Tetranychidae) response to aqueous extracts from selected plant species. Journal of Plant Protection Research, 47(2), 113–124.
de Waele, D. (2002). Foliar nematodes: Aphelenchoides species. In J. L. Starr, R. Cook, & J. Bridge (Eds.), Plant resistance to parasitic nematodes (pp. 141–152). New York: CABI Publishing.
Elbadri, G. A. A., Lee, D. W., Park, J. C., Yu, H. Y., Choo, H. Y., Lee, S. M., et al. (2008). Nematocidal screening of essential oils and herbal extracts against Bursaphelenchus xylophilus. The Plant Pathology Journal, 24(2), 178–182. doi:10.5423/PPJ.2008.24.2.178.
El-Nagdi, W. M. A. E., & Youssef, M. M. A. (2013). Comparative efficacy of garlic clove and castor seed aqueous extracts against the root-knot nematode, Meloidogyne incognita infecting tomato plants. Journal of Plant Protection Research, 53(3), 285–288.
Faske, T. R., & Starr, J. L. (2007). Cotton root protection from plant-parasitic nematode by abamectin-treated seed. Journal of Nematology, 39(1), 27–30.
Giannakou, I. O. (2011). Efficacy of a formulated product containing Quillaja saponaria plant extracts for the control of root-knot nematodes. European Journal of Plant Pathology, 130(4), 587–596. doi:10.1007/s10658-011-9780-8.
Gupta, R., & Sharmaj, N. K. (1993). A study of the nematicidal activity of allicin—An active principle in garlic, Allium sativum L., against root‐knot nematode, Meloidogyne incognita (Kofoid and White, 1919) Chitwood, 1949. International Journal of Pest Management, 39(4), 390–392. doi:10.1080/09670879309371828.
Hesling, J. J., & Wallace, H. R. (1960). Susceptibility of varieties of chrysanthemum to infestation by Aphelenchoides ritzema-bosi (Schwartz). Nematologica, 5, 297–302.
Ingham, R. E., Hamm, P. B., Williams, R. E., & Swanson, W. H. (2000). Control of Meloidogyne chitwoodi in potato with fumigant and nonfumigant nematicides. Journal of Nematology, 32(4S), 556–565.
Insunza, V., Aballay, E., & Macaya, J. (2001). Nematicidal activity of aqueous plant extracts on Xiphinema index. Nematologia Mediterranea, 29, 35–40.
Jada, M. Y., Gungula, D. T., & Jacob, I. (2011). Efficacy of carbofuran in controlling root-knot nematode (Meloidogyne javanica Whitehead, 1949) on cultivars of bambara groundnut (Vigna subterranean (L.) Verdc.) in Yola, Nigeria. International Journal of Agronomy, 2011, 1–5. doi:10.1155/2011/358213.
Jagdale, G. B., & Grewal, P. S. (2002). Identification of alternatives for the management of foliar nematodes in floriculture. Pest Management Science, 58(5), 451–458. doi:10.1002/ps.472.
James, R., Tisserat, N., & Todd, T. (2006). Prevention of pine wilt of scots pine (Pinus sylvestris) with systemic abamectin injections. Arboriculture & Urban Forestry, 32(5), 195–201.
Khan, M. R., Solanki, R. D., Bohra, B., & Vyas, B. N. (2012). Evaluation of Achoon (Azadirachtin 1500 ppm) against root knot nematode (Meloidogyne incognita) infecting okra. South Asian Journal of Experimental Biology, 2(4), 149–156.
Kohl, L. M. (2011). Foliar nematodes: A summary of biology and control with a compilation of host range. Plant Health Progress. doi:10.1094/PHP-2011-1129-01-RV.
Kohl, L. M., Warfield, C. Y., & Benson, D. B. (2010). Population dynamics and dispersal of Aphelenchoides fragariae in nursery-grown lantana. Journal of Nematology, 42(4), 332–341.
Korayem, A. M., Youssef, M. M. A., & Mohamed, M. M. M. (2008). Effect of chitin and abamectin in Meloidogyne incognita infesting rapeseed. Journal of Plant Protection Research, 48(3), 365–370. doi:10.2478/v10045-008-0046-1.
La Mondia, J. A. (1999). Efficacy of insecticides for control of Aphelenchoides fragariae and Ditylenchus dipsaci in flowering perennial ornamentals. Supplement to the Journal of Nematology, 31(4S), 644–649.
Lynn, O. M., Song, W. G., Shim, J. K., Kim, J. E., & Lee, K. Y. (2010). Effects of azadirachtin and neem-based formulations for the control of sweetpotato whitefly and root-knot nematode. Journal of the Korean Society for Applied Biological Chemistry, 53(5), 598–604.
Nguyen, D. M. C., Nguyen, V. N., Seo, D. J., Park, R. D., & Jung, W. J. (2009). Nematicidal activity of compounds extracted from medicinal plants against the pine wood nematode Bursaphelenchus xylophilus. Nematology, 11(6), 835–845. doi:10.1163/156854109X424353.
Oka, Y., Shuker, S., & Tkachi, N. (2013). Influence of soil environments on nematicidal activity of fluensulfone against Meloidogyne javanica. Pest Management Science, 69, 1225–1234.
Okeniyi, M. O., Afolami, S. O., Fademi, O. A., & Oduwaye, O. F. (2013). Effect of botanical extracts on root-knot nematode (Meloidogyne incognita) infection and growth of cashew (Anacardium occidentale) seedlings. Academia Journal of Biotechnology, 1(6), 81–86.
Park, I. K., Park, J. Y., Kim, K. H., Choi, K. S., Choi, I. H., Kim, C. S., et al. (2005). Nematicidal activity of plant essential oils and components from garlic (Allium sativum) and cinnamon (Cinnamomum verum) oils against the pine wood nematode (Bursaphelenchus xylophilus). Nematology, 7, 767–774.
Philis, J. (1994). Use of dazomet and oxamyl for controlling the root-knot nematode Meloidogyne javanica in glass houses. Nematologia Mediterranea, 22, 241–243.
Rao, M. S., Shylaja, M., & Reddy, P. P. (2004). Bio-management of Meloidogyne incognita on tuberose using a formulation of Pochonia chlamydosporia. Nematologia Mediterranea, 32, 165–167.
Riedel, R. M., & Powell, C. C. (1974). Control of Aphelenchoides fragariae on Rieger begonia with oxamyl. Plant Disease Report, 58(10), 911–913.
San Martin, R., & Magunacelaya, J. C. (2005). Control of plant-parasitic nematodes with extracts of Quillaja saponaria. Nematology, 7(4), 577–585. doi:10.1163/156854105774384732.
Siddiqi, M. R. (1974a). Aphelenchoides fragariae. Descriptions of plant-parasitic nematodes, Set 5, No. 74. St. Albans: CAB International.
Siddiqi, M. R. (1974b). Aphelenchoides ritzemabosi. Descriptions of plant-parasitic nematodes, Set 3, No. 32. St. Albans: CAB International.
Smiley, R. W., Gourlie, J. A., Rhinhart, K. E. L., Marshall, J. M., Anderson, M. D., & Yan, G. P. (2012). Influence of nematicides and fungicides on spring wheat in fields infested with soilborne pathogens. Plant Disease, 96(10), 1537–1547. doi:10.1094/PDIS-02-12-0165-RE.
Smiley, R. W., Marshall, J. M., & Yan, G. P. (2011). Effect of foliar applied spirotetramat on reproduction of Heterodera avenae on wheat roots. Plant Disease, 95(8), 983–989. doi:10.1094/PDIS-01-11-0017.
Szczygieł, A. (1966). Studies on the fauna and population dynamics of nematodes occurring on strawberry plantations. Ekologia Polska, 14, 651–709.
Szczygieł, A. (1969). Ocena skuteczności odkażania sadzonek truskawek ciepłą wodą w zwalczaniu nicieni z rodzaju Aphelenchoides. Prace Instytutu Sadownictwa, 13, 133–145.
Szczygieł, A., & Hasior, H. (1972). Seasonal variations in population of plant parasitic nematodes in strawberry plantations. Ekologia Polska, 20, 507–522.
Tibugari, H., Mombeshora, D., Mandumbu, R., Karavina, C., & Parwada, C. (2012). A comparison of the effectiveness of the aqueous extracts of garlic, castor beans and marigold in the biocontrol of root-knot nematode in tomato. Journal of Agricultural Technology, 8(2), 479–492.
Tu, C. M., Marks, C. F., & Elliot, J. M. (1996). Effects of nematicides on Pratylenchus penetrans, soil nitrification, and growth of flue-cured tobacco. Bulletin of Environmental Contamination and Toxicology, 57, 924–931.
Van Damme, V., Hoedekie, A., & Viaene, N. (2005). Long-term efficacy of Pochonia chlamydosporia for management of Meloidogyne javanica in glasshouse crops. Nematology, 7(5), 727–736. doi:10.1163/156854105775142973.
Wheeler, T. A., Lawrance, K. S., Porter, D. O., Keeling, W., & Mullinix, B. G., Jr. (2013). The relationship between environmental variables and response of cotton to nematicides. Journal of Nematology, 45(1), 8–16.
Zasada, I. A., Walters, T. W., & Pinkerton, J. N. (2010). Post-plant nematicides for the control of root lesion nematode in red raspberry. HortTechnology, 20(5), 856–862.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Chałańska, A., Bogumił, A. & Łabanowski, G. Management of foliar nematode Aphelenchoides ritzemabosi on Anemone hupehensis using plant extracts and pesticides. J Plant Dis Prot 124, 437–443 (2017). https://doi.org/10.1007/s41348-017-0106-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-017-0106-8