Abstract
Current analysis methods for obtaining mean shear modulus of skeletal muscles with ultrasound shear-wave elastography are limited by contamination with non-contractile tissues and manual operation of video processing. In this work, we develop new ultrasound image processing methods to assess muscle activities. We build upon previous research by using a 6-DOF robotic manipulator system and indirectly quantifying extensor carpi ulnaris (ECU) and triceps brachii longus (TRI) muscle elasticity during loading using ultrasound shear-wave shear modulus elastography. The purposes of this study were to (1) develop an automatic image-processing algorithm for removing non-contractile tissues from muscle elastography videos and (2) understand the effect of the removal on comparison of mean shear modulus of muscles across static motor tasks with variable muscle loadings in healthy humans. The developed algorithm with optimized clustering and thresholding identified and removed non-contractile tissues from muscle elastography videos with > 90% accuracy in arm muscles, causing reductions in the spatial variability of shear modulus data within the region of interest in healthy young adults. Removal of non-contractile tissues can alter the mean shear modulus of the muscles and influenced task comparisons by substantially altering the ranking of tasks by mean shear modulus.







Similar content being viewed by others
References
Akagi, R., Tanaka, J., Shikiba, T., Takahashi, H.: Muscle hardness of the triceps brachii before and after a resistance exercise session: a shear wave ultrasound elastography study. Acta Radiol. 56(12), 1487–1493 (2015)
Beck, T.W., Housh, T.J., Johnson, G.O., Weir, J.P., Cramer, J.T., Coburn, J.W., Malek, M.H.: Mechanomyographic amplitude and mean power frequency versus torque relationships during isokinetic and isometric muscle actions of the biceps brachii. J. Electromyogr. Kinesiol. 14(5), 555–564 (2004)
Bercoff, J., Chaffai, S., Tanter, M., Sandrin, L., Catheline, S., Fink, M., Gennisson, J., Meunier, M.: In vivo breast tumor detection using transient elastography. Ultrasound Med. Biol. 29(10), 1387–1396 (2003)
Bercoff, J., Tanter, M., Fink, M.: Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans. Ultrason. Ferroelect. req. Control 51(4), 396–409 (2004)
Bernstein, N.A.: The co-ordination and regulation of movements. (1967)
Bouillard, K., Hug, F., Guével, A., Nordez, A.: Shear elastic modulus can be used to estimate an index of individual muscle force during a submaximal isometric fatiguing contraction. J. Appl. Physiol. 113(9), 1353–1361 (2012)
Bouillard, K., Nordez, A., Hug, F.: Estimation of individual muscle force using elastography. PLoS One 6(12), e29261 (2011)
Brosnan, T., Sun, D.-W.: Improving quality inspection of food products by computer vision—a review. J. Food Eng. 61(1), 3–16 (2004)
Clark, D.J., Ting, L.H., Zajac, F.E., Neptune, R.R., Kautz, S.A.: Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J. Neurophysiol. 103(2), 844–857 (2010)
Crowninshield, R.D., Brand, R.A.: A physiologically based criterion of muscle force prediction in locomotion. J. Biomech. 14(11), 793–801 (1981)
Darby, J., Li, B., Costen, N., Loram, I., Hodson-Tole, E.: Estimating skeletal muscle fascicle curvature from b-mode ultrasound image sequences. IEEE Trans. Biomed. Eng. 60(7), 1935–1945 (2013)
Du, C.-J., Sun, D.-W.: Recent developments in the applications of image processing techniques for food quality evaluation. Trends Food Sci. Technol. 15(5), 230–249 (2004)
Gallagher, W., Ding, M., Ueda, J.: Relaxed individual control of skeletal muscle forces via physical human–robot interaction. Multibody Syst. Dyn. 30(1), 77–99 (2013)
Graf, S., Gariepy, J., Massonneau, M., Armentano, R.L., Mansour, S., Barra, J.G., Simon, A., Levenson, J.: Experimental and clinical validation of arterial diameter waveform and intimal media thickness obtained from B-mode ultrasound image processing. Ultrasound Med. Biol. 25(9), 1353–1363 (1999)
Jacob, S.W., Francone, C.A., Lossow, W.J.: Structure and function in man. In: Unknown Host Publication Title. (1978)
Kim, N.-D., Amin, V., Wilson, D., Rouse, G., Udpa, S.: Ultrasound image texture analysis for characterizing intramuscular fat content of live beef cattle. Ultrason. Imaging 20(3), 191–205 (1998)
Klamroth-Marganska, V., Blanco, J., Campen, K., Curt, A., Dietz, V., Ettlin, T., Felder, M., Fellinghauer, B., Guidali, M., Kollmar, A.: Three-dimensional, task-specific robot therapy of the arm after stroke: a multicentre, parallel-group randomised trial. Lancet Neurol. 13(2), 159–166 (2014)
Kong, Y.-K., Hallbeck, M.S., Jung, M.-C.: Crosstalk effect on surface electromyogram of the forearm flexors during a static grip task. J. Electromyogr. Kinesiol. 20(6), 1223–1229 (2010)
Koo, T.K., Guo, J.-Y., Cohen, J.H., Parker, K.J.: Relationship between shear elastic modulus and passive muscle force: an ex vivo study. J. Biomech. 46(12), 2053–2059 (2013)
Kot, B.C.W., Zhang, Z.J., Lee, A.W.C., Leung, V.Y.F., Fu, S.N.: Elastic modulus of muscle and tendon with shear wave ultrasound elastography: variations with different technical settings. PLoS One 7(8), e44348 (2012)
Lacourpaille, L., Hug, F., Bouillard, K., Hogrel, J.-Y., Nordez, A.: Supersonic shear imaging provides a reliable measurement of resting muscle shear elastic modulus. Physiol. Meas. 33(3), N19 (2012)
Lapole, T., Tindel, J., Galy, R., Nordez, A.: Contracting biceps brachii elastic properties can be reliably characterized using supersonic shear imaging. Eur. J. Appl. Physiol. 115(3), 497–505 (2015)
Latash, M.L.: The bliss (not the problem) of motor abundance (not redundancy). Exp. Brain Res. 217(1), 1–5 (2012)
Lawrence, J.H., De Luca, C.: Myoelectric signal versus force relationship in different human muscles. J. Appl. Physiol. 54(6), 1653–1659 (1983)
Liao, W.-W., Wu, C.-Y., Hsieh, Y.-W., Lin, K.-C., Chang, W-y: Effects of robot-assisted upper limb rehabilitation on daily function and real-world arm activity in patients with chronic stroke: a randomized controlled trial. Clin. Rehabil. 26(2), 111–120 (2012)
Loram, I.D., Maganaris, C.N., Lakie, M.: Use of ultrasound to make noninvasive in vivo measurement of continuous changes in human muscle contractile length. J. Appl. Physiol. 100(4), 1311–1323 (2006)
Madeleine, P., Bajaj, P., Søgaard, K., Arendt-Nielsen, L.: Mechanomyography and electromyography force relationships during concentric, isometric and eccentric contractions. J. Electromyogr. Kinesiol. 11(2), 113–121 (2001)
Maher, R.M., Hayes, D.M., Shinohara, M.: Quantification of dry needling and posture effects on myofascial trigger points using ultrasound shear-wave elastography. Arch. Phys. Med. Rehabil. 94(11), 2146–2150 (2013)
Martini, F.H., Bartholomew, E.F.: Essentials of Anatomy and Physiology: Pearson New International Edition. Pearson Higher Ed. (2013)
Mogk, J.P., Keir, P.J.: Crosstalk in surface electromyography of the proximal forearm during gripping tasks. J. Electromyogr. Kinesiol. 13(1), 63–71 (2003)
Nordez, A., Hug, F.: Muscle shear elastic modulus measured using supersonic shear imaging is highly related to muscle activity level. J. Appl. Physiol. 108(5), 1389–1394 (2010)
Otsu, N.: A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybernet 9(1), 62–66 (1979)
Peng, Q., Jones, R.C., Constantinou, C.E.: 2D Ultrasound image processing in identifying responses of urogenital structures to pelvic floor muscle activity. Ann. Biomed. Eng. 34(3), 477–493 (2006)
Purslow, P.P.: The structure and functional significance of variations in the connective tissue within muscle. Comp. Biochem. Physiol. A 133(4), 947–966 (2002)
Rodriguez, K.L., Roemmich, R.T., Cam, B., Fregly, B.J., Hass, C.J.: Persons with Parkinson’s disease exhibit decreased neuromuscular complexity during gait. Clin. Neurophysiol. 124(7), 1390–1397 (2013)
Salman, M., Sabra, K.G., Shinohara, M.: Assessment of muscle stiffness using a continuously scanning laser Doppler vibrometer. Muscle Nerve 50(1), 133–135 (2014)
Shinohara, M., Sabra, K., Gennisson, J.L., Fink, M., Tanter, M.: Real-time visualization of muscle stiffness distribution with ultrasound shear wave imaging during muscle contraction. Muscle Nerve 42(3), 438–441 (2010)
Shinohara, M., Søgaard, K.: Mechanomyography for studying force fluctuations and muscle fatigue. Exerc. Sport Sci. Rev. 34(2), 59–64 (2006)
Taniguchi, K., Shinohara, M., Nozaki, S., Katayose, M.: Acute decrease in the stiffness of resting muscle belly due to static stretching. Scand. J. Med. Sci. Sports 25(1), 32–40 (2015)
Ueda, J., Ming, D., Krishnamoorthy, V., Shinohara, M., Ogasawara, T.: Individual muscle control using an exoskeleton robot for muscle function testing. IEEE Trans. Neural Syst. Rehab. Eng. 18(4), 339–350 (2010)
Yang, M.-S., Hu, Y.-J., Lin, K.C.-R., Lin, C.C.-L.: Segmentation techniques for tissue differentiation in MRI of ophthalmology using fuzzy clustering algorithms. Magn. Reson. Imaging 20(2), 173–179 (2002)
Yoshitake, Y., Takai, Y., Kanehisa, H., Shinohara, M.: Muscle shear modulus measured with ultrasound shear-wave elastography across a wide range of contraction intensity. Muscle Nerve 50(1), 103–113 (2014)
Zhou, Y., Zheng, Y.-P.: Estimation of muscle fiber orientation in ultrasound images using revoting hough transform (RVHT). Ultrasound Med. Biol. 34(9), 1474–1481 (2008)
Acknowledgements
Special thanks to Dr. Tsukasa Ogasawara, Dr. Atsutoshi Ikeda, and Kazuya Aomoto for handling all aspects of controlling the robotic manipulator and for assisting with data collection. This study was supported in part by the National Science Foundation (IIS1142438, OISE-1209539) and Nakatomi Foundation.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The computational muscle model used in this research is briefly presented below.
1.1 Inputs and outputs
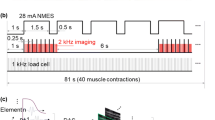
The MATLAB (Mathworks, Natick, MA, USA) model predicts the force generation of 51 muscles across 12 joints of the human right arm and torso. The user specifies the baseline or nominal loading condition, target muscles, desired ratio γd of force change in the target muscles, a vector θ of joint angles, and the available degrees of freedom for the robot to induce γd. The model returns the realizable changes γa in muscle activation, and the experimental robotic loading Fd to induce γa. The chosen target muscles were triceps brachii longus (TRI L), and extensor carpi ulnaris (ECU). Muscles in the forearm, such as ECU, are smaller, closely packed, exhibit greater redundancy, and thus present a greater challenge for control and measurement of muscle activity (Beck et al. 2004; Madeleine et al. 2001). The value γd is the ratio of desired muscle force to nominal case muscle force (e.g. γd = 1.5 means desired muscle force is 1.5 times the nominal case muscle force). The loading conditions and γd for all experimental and nominal tasks performed during the study are listed in Table 1. Predicted nominal loading, as a percentage of maximum voluntary contraction, was 34.0% for TRI L and 22.4% for ECU. All γd values were realizable (i.e. γa = γd) based on the model.
1.2 Inverse solution for nominal endpoint loading
The computational prediction of human muscle force requires a mathematical description of how loads applied at the hand translate to proximal joint torques. For our study, a human subject grasps a robotic manipulator as the manipulator applies forces and torques to the hand. These loads are represented as a vector F. For a human musculoskeletal model of M muscles controlling movement about N joints, the torques at each joint are given by
where τ h ϵ RN is the human joint torque vector resulting from muscle forces. For our model, N = 12 and M = 51. Let θ be the joint angle vector (i.e., the posture of the arm), J be the Jacobian matrix relating the joint torques to end-point loading at the hand, and. g(θ) be the joint torque vector due to gravity. The dynamics of the body and robot were deemed negligible and omitted. The human joint torque vector is described by
where A ϵ RNxM is the moment-arm matrix of the muscles and f = [f 1 …f M ] ϵ RM is the human muscle force vector. Muscle force must be positive and has a physiological upper-bound such that 0 ≤ f j ≤ jMAXj, (j = 1,…,M). A has been obtained from other human musculoskeletal models and published biometric data (Ueda et al. 2010).
In the human body, the number of muscles exceeds the number of joints (Martini et al. 2013; Jacob et al. 1978), i.e., \( M \gg N \). This muscle redundancy makes mapping of joint torques to muscle force an ill-problem. In order to describe the contribution of each muscle to each joint torque, an empirical optimization of Crowninshield’s cost function was used:
where u (f) is a physiology-based cost function comprising the sum of muscle stress raised to a power. The summed muscle stress is minimized for a given task. c j is a weighting factor of the j-th muscle, indirectly proportional to its physiological cross sectional area (PCSA). For our model, muscle stress is squared, i.e. r = 2, in accordance with (Crowninshield and Brand 1981). When τh is given, calculation of f to predict muscle force is straightforward.
1.3 Forward solution: Force changes and feasibility
In order to control muscle force, one must solve the problem of muscle force prediction given a desired f. The desired f is created by first selecting a nominal load F and predicting f. Vector f includes three categories of muscles: target muscles, non-target muscles (active during the nominal case but not be targeted for muscle control) and inactive muscles (inactive during the nominal case). This f is then modified such that the activity of target muscles are controlled (e.g. increased, decreased, or kept constant) based on γd.
Let f0 be the nominal force vector when a subject is performing the nominal task. The desired forces are given by explicitly specifying the change ratios for each of the target muscles: f td = diag[γ1…γMt]T ft0. The subscript “d” denotes desired forces, and “0” denotes nominal forces. For a perfect control, the closed-form solution is as follows:
where w(*) is a function defined as \( w(f_{i} ) = \partial u({\mathbf{f}})/\partial f = rc_{i} f_{i}^{r - 1} \). \( {\varvec{\upbeta}} \) is a parameter vector that determines the influences on non-target muscles. The following feasibility conditions (FC) must hold:
-
FC 1: The target muscles forces must be exactly realized
-
FC 2: Inactive muscles stay inactive
-
FC 3: Non-target muscles remain active, exert positive force
-
FC 4: The external joint loading, τ ex , is ideal, such that all joint torques τ h are fully controllable by τ ex , where τ ex = J(θ)TF.
According to FC1, the model must exactly realize the target muscle forces such that \( \left| {f_{\text{td}}^{\text{T}} f_{\text{nd}}^{\text{T}} 0^{\text{T}} } \right|^{\text{T}} \, = \,{\text{arg min}}\;u(\varvec{f}) \) with minimum changes in non-target muscles, i.e., \( \left| {f_{\text{nd}} {-}f_{{{\text{n}}0}} } \right| \to { \hbox{min} } . \) The solution is not trivial since ftd must be physiologically feasible, as described in Eq. (3).
Our previous studies with exoskeletons showed that the physical interaction between the exoskeleton and the anatomy of the subject presented limitations in joint control such that the system was inherently non-ideal. This violation of FC4 prompted a switch toward a non-wearable endpoint loading system that trades greater but still imperfect control for greater adjustability and simplicity. The computational model was then modified for relaxed control and the use of a non-wearable robotic manipulator.
Under relaxed control, the initial formulation as shown in Eqs. 1 thru 3 remains the same. However, the aforementioned limitations prompted the use of a tolerance term and generalization of the solution to make the control scheme more widely applicable. This relaxed method therefore reframed the feasibility conditions as optimization criteria to minimize errors in the controlled muscle forces. For FC 1, it is assumed that requiring f = fd, is necessary and possible. In reality, neither is true. Therefore, we introduce a tolerance term e such that f = fd + e. FC 2 and 3 stipulate that inactive muscles must stay inactive, and non-target active muscles must stay active. For this reason, the revised model allows initially active non-target muscles to become inactive and initially inactive muscles to become minimally active. This is achieved by introducing new Lagrange multipliers in the KKT condition:
where the following apply :\( h_{i} \left( f \right) = \tau_{i} - a_{i}^{T} f,\;g_{ji} \left( f \right) = - f_{j} ,\;g_{ji} \left( f \right) = f_{j} - f_{\hbox{max} j} . \)
We then defined \( {\mathbf{q}} = \left[ {\begin{array}{*{20}c} {{\mathbf{q}}_{t} } & {{\mathbf{q}}_{n} } & {{\mathbf{q}}_{v} } \\ \end{array} } \right]^{T} = w(\left[ {\begin{array}{*{20}c} {{\mathbf{f}}_{t} } & {{\mathbf{f}}_{n} } & {f_{v} } \\ \end{array} } \right]^{T} ) \). Equation (5) is then represented by:
In Eq. (6), μ was a transformed torque vector used when the transformed force, q, was used instead of f. λ consisted of Lagrange multipliers. With the introduced error tolerance, where \( {\varvec{\upvarepsilon}} \) was a transformed error, e, when q was used instead of f, the conditions could be given by:
Nominal case:
Desired case:
In perfect control, \( {\varvec{\upvarepsilon}}_{t} = {\varvec{\upvarepsilon}}_{v} = \lambda_{nl} = \lambda_{nu} = \lambda_{tu} = 0 \) so that the desired forces were exactly achieved. This relaxed representation allowed a broader range of muscle activation. Furthermore, the relaxed muscle control never failed and was always robust and feasible if an appropriate error tolerance was assigned. The relaxed version of Eq. 4 was
To summarize, the relaxed conditions were as follows:
-
RC 1: Target muscle forces were perfectly realized if:
$$ {\text{rank}}[{\mathbf{A}}_{t} ] = {\text{rank}}[\begin{array}{*{20}c} {{\mathbf{A}}_{t} } & {w({\mathbf{f}}_{td} ) - w({\mathbf{f}}_{t0} ) + {\varvec{\upvarepsilon}}_{t} - {\varvec{\uplambda}}_{tu0} } \\ \end{array} ] $$ -
RC 2: Inactive muscles were kept inactive if:
$$ {\mathbf{A}}_{v} {\varvec{\upalpha}} + {\varvec{\uplambda}}_{vl0} > - \varepsilon_{v} ,\exists {\varvec{\upalpha}} $$ -
RC 3: Non-target muscle forces remained positive if:
$$ w({\mathbf{f}}_{\hbox{max} } ) > {\mathbf{A}}_{n} {\varvec{\upalpha}} + w({\mathbf{f}}_{n0} ) > 0,\exists {\varvec{\upalpha}} $$
In addition to implementing relaxed control, we also considered the limitations of the robotic system. The preceding treatment still assumes the robot is able to apply loads to each of the human’s joints and to the hand. With a robotic manipulator, the system can only apply torques to the hand and all other torques are set to 0. Therefore, an adequate robot loading F may not be available even if ftd is exactly realizable and a solution of τex exists mathematically, the accompanying. This non-ideal robotic system may result in errors in target muscle forces. To overcome this issue, the realizability of τex must be analyzed based on necessary rank conditions. The total torque may be rewritten as:
where \( \varvec{\tau}_{ac} \) is a vector that only contains the torque components controllable by the exoskeleton. E is an expansion matrix that maps these components onto the joint space. While previous studies have left r unspecified, this study explicitly used a cost function with r = 2 (Crowninshield and Brand 1981) to obtain a closed-form solution. The cost function is now quadratic: \( u\left( \varvec{f} \right) = \varvec{f}^{T} \varvec{Sf} \) where \( \varvec{S} = {\text{diag}}[c_{1} , \cdots c_{N} ] \), and \( \varvec{q} = \varvec{w}\left( \varvec{f} \right) = 2\varvec{S}^{ - 1} \varvec{f} \). Substituting Eq. (6) for Eq. (4) yields the solution of \( \varvec{ F}_{\varvec{e}} \), for relaxed control (Gallagher et al. 2013):
where \( {\mathbf{G}} = \left( {2\left[ {\begin{array}{*{20}c} {{\mathbf{A}}_{t}^{T} } & {{\mathbf{A}}_{n}^{T} } \\ \end{array} } \right]\,{\mathbf{S}}\,\left[ {\begin{array}{*{20}c} {{\mathbf{A}}_{t} } \\ {{\mathbf{A}}_{n} } \\ \end{array} } \right]} \right)^{ - 1} {\mathbf{J}}_{e}^{T} \) and \( \psi \) is a free vector parameter mapped onto the null space of G. This solution does not guarantee perfect realization, but minimizes errors in the target muscles in the sense of least mean square. If \( {\text{rank}}[\varvec{G}] = {\text{rank}}[\varvec{G w}\left( {\varvec{f}_{td} } \right) - \varvec{w}(\varvec{f}_{t0} \varvec{ })] \) (rewritten FC1), perfect control is realized. Merging results given in Eqs. (9, 11) will directly determine a motor task from desired target muscles forces with the relaxed conditions. Investigating the association between the range of allowable error tolerance and induced muscle forces will provide quantitative assessment of the approach.
Rights and permissions
About this article
Cite this article
Brown, E., Yoshitake, Y., Shinohara, M. et al. Automatic analysis of ultrasound shear-wave elastography in skeletal muscle without non-contractile tissue contamination. Int J Intell Robot Appl 2, 209–225 (2018). https://doi.org/10.1007/s41315-018-0050-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41315-018-0050-1