Abstract
The goal of this investigation was to assess the role of a number of environmental variables on the traits of a peracarid population inhabiting turfs of Ellisolandia elongata in a Mediterranean disturbed location. Samples were collected from a vertical and a horizontal surface to assess the role of orientation, and several structural traits of the turf (number of fronds, their height and width, available phytal area for colonization, and dry weight) were measured. The samples were characterized by their species composition, relative abundance of constant species, total abundance of individuals, and α-diversity (species richness, Shannon index and Pielou’s evenness). Generalized linear models were used to relate environmental variables with ecological descriptors, whereas a cluster plot and a principal correspondence analysis, both based on the Bray-Curtis coefficient, were conducted to detect the presence of distinct assemblages. Results showed orientation to be a key factor governing the assemblages, since samples from vertical and horizontal surfaces formed definite groups based on Bray-Curtis similarities. Moreover, GLMs pointed out that orientation influenced relative abundance of the majority of constant species, as well as species richness and Shannon index. Phytal height had a positive effect on the relative abundances of Hexapleomera bultidactyla, Coxischyrocerus inexpectatus and Hyale stebbingi, and a negative one on Shannon index and evenness, whereas available phytal area influenced positively evenness and negatively the relative abundance of Elasmopus pocillimanus. Thus, it can be inferred that although species composition depended on surface orientation, the spatial structure of the turf was the main driver for assemblage complexity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human altered shorelines are widespread all over the world (Bacchiocchi and Airoldi 2003; Airoldi and Bulleri 2011), and the impacted area is expected to increase considerably in response to human population growth and events related to climate change, such as sea-level rise (Bulleri and Chapman 2010), as well as due to tourism occupation (Gössling et al. 2018). Because of this, artificial infrastructures occupy nearly half of the shorelines in Mediterranean European countries (Airoldi and Bulleri 2011). The replacement of natural surfaces by man-made or human altered ones modify the ecological roles and relationships among species (Bustamante et al. 2014) and fragment or even eliminate natural habitats (Bacchiocchi and Airoldi 2003). The understanding of the drivers regulating function of the resulting new communities is thus of a major interest.
Founder communities of organisms colonize recently altered hard bottoms, and some authors suggested that they might reach a structure similar to that of natural hard-bottom habitats over time (Pister 2009). However, other investigations demonstrated permanent physical features of artificial structures, such as orientation or complexity, to play a more important role than age (Perkol-Finkel et al. 2006). Although similarities between communities inhabiting artificial and natural structures might be observed, several detailed surveys pointed out that they harboured less species (see Bulleri and Chapman 2010). In some cases, effects were less evident and the occurring species presented altered population structure and lower reproductive success (Moreira et al. 2006) or reduced genetic diversity (Fauvelot et al. 2012). The low biodiversity seems related to the lack of a number of key microhabitats on the nearly homogenous artificial surfaces (Firth et al. 2014), but the real extent of the rest of changes in shore communities and the drivers of the alterations are not yet fully understood (Aguilera et al. 2014). In urban environments, seawalls are a most common type of coastal human-made hard structure. Seawalls are vertical or very steep, contrasting the commonly gently sloping natural rocky shores. Orientation is a paramount factor for the structure of littoral communities in temperate zones, where the high levels of Sun exposure on horizontal substrata allow an array of macrophytes to thrive, whereas epibenthic suspension feeders tend to dominate shadowed vertical surfaces (Pacheco et al. 2010). Given the same tidal range, a steeper slope greatly reduces the available littoral area for individuals to settle and thus modifies species-area relationships (Knott et al. 2004). In urbanised areas, even natural littoral substrates are not free of anthropogenic disturbances, as trampling or recreational exploitation can be major drivers of benthic littoral communities, irrespective of the artificial or natural origin of the surface (Airoldi et al. 2016). Thus, the detailed knowledge of littoral ecosystems under human pressure and their drivers becomes of the greatest importance in order to implement management procedures.
Photophilic algae provide and array of micro- and macro-habitats and allow the presence of rich epibiont communities in most temperate rocky littoral habitats (Urra et al. 2013). In case of moderate human disturbance, the large algae forming the canopy of the phytal assemblages in temperate regions tend to disappear, while the small species of the understory remain and constitute characteristic turfs (Mangialajo et al. 2007; Bonifazi et al. 2017). These turfs usually consist of a dense net of thalli belonging to one or more species of Corallinales, Ceramiales and other filamentous algae (Connell et al. 2014). Usually, a diverse and populous community of small vagile invertebrates thrives within the turfs (Izquierdo and Guerra-García 2011; Bertolini 2019), so they are a suitable ground for research on the biological processes that control macrofaunal populations (Guerra-García et al. 2012). The importance of the spatial structure of the phytal substratum as a driver of the organization of the communities is twofold. Evidently, the quality of refuge provided from wave action, desiccation and predators relies heavily on frond height and density but other more subtle drivers, such as the available area for epiphytic algae to grow and feed herbivorous invertebrates, depend on spatial structure (Berthelsen et al. 2015). Taking this into account, the integrity of algal coverage becomes a key factor in the maintenance of benthic communities suffering human impact.
Most peracarids are infaunal or epifaunal bottom-dwelling animals with a wide variety of feeding habits (Guerra-García et al. 2014; De la Ossa-Carretero et al. 2016). They are ecologically and trophically important (Carvalho et al. 2018), show narrow ecological ranges (De la Ossa-Carretero et al. 2012) and have relatively poor dispersion abilities (Kelly et al. 2006). Although most species are sensitive to pollutants, other are able to accumulate toxic substances ant to tolerate ample fluctuations in physico-chemical parameters of water and sediment. For these reasons, they are often studied as indicators of environmental disturbance (Dauvin and Ruellet 2007; Guerra-García et al. 2009).
The goal of this investigation was to assess the role of a number of environmental variables on abundance of individuals and species composition of a low-shore peracarid population on a Mediterranean disturbed location. To achieve it, samples were collected from a vertical and a horizontal surface in order to assess the role of orientation, and several structural traits of the phytal component of each sample were measured, aiming to detect other potential sources of variation.
Materials and methods
Study area
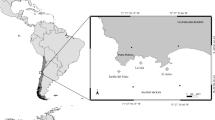
The study area is a located within the urban area of Villaricos, a village belonging to the municipality of Cuevas de Almanzora (Almería) in south-eastern Spain (Fig. 1). The coast in this region has suffered little urban transformation and consists of a series of gently stepping rocky areas alternating with little sandy beaches. The studied cove (34º14’53.67’’N, 1º46’11.33’’W) is limited in its northern side by a group of flat outcrops of schist rock that are close to the beach. In the southern side, a wharf was built of conglomerate rock extracted from nearby quarries, so chemical composition of both hard surfaces is equivalent. Due to old age, many blocks of stone fall along time, giving the wall a spatial heterogeneity similar to that of the natural shore, as in other localities (Moschella et al. 2005). The sea wall faces the northeast, whereas the horizontal surface faces the southeast. The SIMAR buoy net provides data on wind, sea level and wave parameters from 1958 to present time (de Swart et al. 2020). Attending to data measured by wave buoy SIMAR 2,063,087 moored just off Villaricos harbour, prevailing winds blew from NE and to a slightly lesser extent from SW, so the sea wall was more exposed to wave action than the horizontal surface. Dense turfs of Ellisolandia elongata (J. Ellis and Solander) K. R. Hind and G. W. Saunders, 2103 lay on both types of surface.
Sampling methods
Sampling was conducted in August 2015, coinciding with highest phytal development and faunal population densities in the western Mediterranean (Urra et al. 2013) and aiming to minimize the influence of seasonal trends (Bueno et al. 2016). Ten samples were collected by scraping off a 100 cm2 square area of rocky bottom immediately below water surface; similarly sized sample areas has proved to be representative for studies on peracarid populations from other locations (Bueno et al. 2016). In order to evaluate the role of orientation on peracarid population, half of the samples (site 1: samples Vill-1 to Vill-5) were collected from the sea wall located on the southern side of the cove and the rest (site 2: samples Vill-6 to Vill-10) from the flat rocky surface on the northern side; samples were haphazardly selected within each type of surface. The scraped material was preserved in 70% ethanol and stored in hermetic plastic bags. This material was examined under a dissecting microscope in the laboratory.
The structural traits of the turf were characterized for each sample though a number of parameters. In each sample, the fronds were counted (NF) and fifty were randomly selected. For each frond, length from the holdfast to the distal end and maximum width were estimated in mm. Assuming that fronds were approximately triangular, the area of each selected one was calculate in mm2 using the formula area = (heigth x width)/2. For every sample, mean values for the three parameters were computed; the first two were used to characterize the turf (He and Wi, respectively). By multiplying the number of fronds by the mean area, the total available phytal area (APA) in the sample was obtained and expressed in cm2. Subsequently, dry weight (DW) in g was measured after drying for 48 h at 84 °C (Urra et al. 2013).
All the collected peracarids were identified to species level and counted in order to create a species/abundance matrix (abundance expressed as number of individuals per 100 g of algae).
Data analyses
A number statistical analyses were conducted to evaluate the role of orientation and the structural traits of the turf in the peracarid community. Based on species/abundance matrix, each sample was also characterized by the total abundance of peracarids and by its α-diversity, computing the species richness and Shannon and Pielou’s evenness indexes. The software used to compute ecological indexes was PAST 4 (Hammer et al. 2001). Relative abundance (ratio species abundance/total peracarid abundance) was calculated for all the species in each sample and its value for the constant species –those present in all the samples– was treated as ecological descriptors of the former.
In order to evaluate the environmental drivers influencing the peracarid community, the descriptors of the phytal turf were evaluated as predictor continuous variables, whereas orientation was a categorical predictor (vertical vs. horizontal). The normality of residuals was checked for each descriptor of the phytal turf by means of a Shapiro-Wilks W test and variables were log (x + 1) transformed where appropriated. When normality criterion was achieved, continuous predictor variables were standardized to zero mean and divided by their standard deviation (z-scored) to allow a comparison of the estimators within the analysis (Greenacre and Primicerio 2013). Collinearity among standardized values of descriptors of the phytal turf (Pearson |r| > 0.6) was checked and variables were excluded where appropriate. The relationship between descriptors of the phytal turf and orientation was assessed by comparing mean values of the former between orientation categories. Significant differences were determined through a one-way ANOVA test, setting significance value at p < 0.05, when both normality and homoscedasticity criteria were achieved; otherwise, a non-parametric Kruskal-Wallis test for equal medians between orientation categories was performed on untransformed data (significance value set at p < 0.05). Descriptors of the phytal turf that showed significant differences between categories were considered correlated to orientation and excluded from further analyses. The ecological descriptors –α-diversity indexes and relative abundance of constant species– were considered response values and treated in the same way as predictors variables in terms of normality and standardization. To relate predictor with response variables, Generalized Linear Model (GLM) with normal distribution and identity link analyses were conducted. Validation of the models was done using Shapiro-Wilks W test to check the normality of residuals for each model. Results were considered significant at p < 0.05. SPSS 23.0 for Windows (SPSSInc, Chicago, IL, USA) was employed to perform GLMs.
Aiming to detect the presence of different assemblages in the location, the values of the Bray-Curtiss coefficient as measure of the similarity between samples were calculated from the abundance matrix after all data were log (x + 1) transformed to avoid excessive influence of most abundant species (Clarke and Warwick 2001). The results of the obtained similarity matrix were represented in an UPGMA cluster plot and a principal correspondence analysis (PCoA). In case that more than one assemblage were detected in both analyses, a one-way PERMANOVA test was run in Bray-Curtis similarity matrix to test significance of the detected differences in the composition of assemblages. For this analysis, p-values were obtained from 9999 permutations of residuals under a reduced model and significance was set at p < 0.05. These statistical analyses were made using the software package PAST 4 (Hammer et al. 2001). To identify the taxa responsible for differences between assemblages, SIMPER analyses based on the same distance matrix was conducted through PRIMER 6 (Clarke and Warwick 2001).
Results
The structural features of Ellisolandia elongata turfs from each sample are given in Table 1. The number of fronds ranged from 1583 in sample Vill-2 to 262 in Vill-6 (mean number 716.40, standard deviation 341.80), height ranged from 26.34 mm in Vill-6 to 20.80 mm in Vill-2 (23.35 ± 1.72), width ranged from 19.80 mm in Vill-6 to 10.30 mm in Vill-2 (14.79 ± 3.07), available phytal area ranged from 1864.86 cm2 in Vill-9 to 703.81 cm2 in Vill-6 (1200. 96 ± 385.69), and dry weight ranged from 38.29 g in Vill-2 to 23.22 g in Vill-10 (29.88 ± 4.79).
A total of 3451 specimens of peracarids, belonging to 18 species were collected (Table 1). Six species were collected from all the samples, namely the tanaidacean Hexapleomera bultidactyla (represented by 1115 specimens, mean abundance 426.94 ± 7.02 ind./100 g of algae), the amphipods Jassa marmorata (676 specimens, mean abundance 220.07 ± 169.57), Elasmopus pocillimanus (524 specimens, mean abundance 196.54% ± 219.23), Hyale stebbingi (185 specimens, mean abundance 65.49 ± 81.43), and Coxischyrocerus inexpectatus (37 specimens, mean abundance 12.84 ± 6.84), and the isopod Ischyromene lacazei (300 specimens, mean abundance 95.88 ± 63.98). Although absent from some samples, the isopod Joeropsis brevicornis (158 specimens, mean abundance 59.71 ± 66.72) and the amphipods Ampithoe riedli (157 specimens, mean abundance 59.94 ± 71.81) and Stenothoe tergestina (152 specimens, mean abundance 46.82 ± 43.99) also achieved large abundances.
The ecological indexes obtained are also presented in Table 1. Sample Vill- 10 harboured the highest abundance (3385 ind./100 g of algae) and Vill-2 the lowest (566.73), mean value for abundance was 1239.00 and standard deviation was 830.51. Species richness ranged from 14 in sample Vill-7 to 9 in Vill-2 (11.30 ± 1.57), Shannon index ranged from 1.90 in Vill-7 to 1.36 in Vill-5 (1.62 ± 0.18), and evenness ranged from 0.79 in Vill-8 to 0.54 in Vill-6 (0.68 ± 0.09).
Shapiro-Wilks test confirmed that all the variables regarding phytal structure followed a normal distribution except number of fronds, which was log (x + 1) transformed. Pearson test showed number of fronds to be collinear with the rest of variables describing turf spatial structure, so it was not considered for the analyses. In turn, width and height were collinear and the former was excluded from analyses. None of the one-way ANOVA tests between orientation categories met the homoscedasticity assumption for the rest of descriptors of the phytal turf, so relationship was checked through non-parametric Kruskal-Wallis test. Dry weight showed significant differences between the samples collected from site 1 and those from site 2, indicating a direct relationship with orientation and, consequently it was also excluded from the analyses.
The three α-diversity indexes, as well as the relative abundance of C. inexpectatus, E. pocillimanus, H. bultidactyla and I. lacazei, followed a normal distribution; the relative abundance of H. stebbingi and of J. marmorata did so after the values were log (x + 1) transformed. Distribution of residuals validated all the GLMs except for abundance. Results for the constant species (Table 2) showed that orientation was a main influencing factor for all the constant species except for H. stebbingi and C. inexpectatus. Jassa marmorata was clearly more abundant on site 1 (vertical surface), whereas E. pocillimanus and H. bultidactyla were more abundant on site 2 (horizontal one); I. lacazei was generally more abundant on site 1 but trend was not as clear as in the other species (Table 1). Turf height was a positive factor for relative abundance of C. inexpectatus and H. bultidactyla, and a negative one in the case of H. stebbingi, whereas available phytal area influenced negatively the relative abundance of E. pocillimanus. On the other hand, the relative abundance of I. lacazei showed no relationship with any of the phytal structure aspects. Regarding ecological indexes (Table 3), orientation influenced species richness and Shannon index, but not evenness. Structure of the turf did not condition species richness but had an effect on Shannon index, which was negatively influenced by turf height, and on evenness, negatively influenced by height and positively by available phytal area.
Based on their specific composition, the samples formed two groups located separately in the cluster dendrogram (Fig. 2) and showing about 80% of internal similarity. The groups consisted of the samples collected from vertical and horizontal surfaces, respectively. In PCoA ordination plot (Fig. 3), samples from vertical and horizontal surfaces separated along first axis, being the group of samples from vertical surface more cohesive. These results indicate the existence of two different assemblages depending on orientation. Attending the one-way PERMANOVA analysis, the difference between the two groups of peracarid samples was significant (Pseudo-F = 13.47; p = 0.008). Nine species contributed with more than 5% to the overall dissimilarity between assemblages according the SIMPER analysis (Table 4). Of these, S. tergestina and J. marmorata were more abundant on the vertical surface, whereas the rest did so on the horizontal one and T. dulongii was only present in the latter type of surface.
Discussion
The set of most abundant species in the peracarid community herein described are common ones in turfs of Ellisolandia elongata on natural surfaces from the Mediterranean coast of the Iberian Peninsula and close regions. Large abundances of the amphipods Jassa marmorata, Stenothoe tergestina, Hyale stebbingi and of the isopod Ischyromene lacazei have been previously recorded in the region (Conradi and López-González 2001; Izquierdo and Guerra García 2011; Guerra-García et al. 2012). In these studies, Ampithoe riedli, Coxischyrocerus inexpectatus and Joeropis brevicornis were also reported to occasionally achieve large population densities. However, some species reported as dominant in these works, such as Elasmopus vachoni Mateus & Mateus, 1966, Stenothoe monoculoides (Montagu, 1813) or Jassa cadetta Karpp, Rampin & Libertini, 2008 were not found in the presently studied location. In turn, Elasmopus pocillimanus was reported as dominant in coralline turfs in central Mediterranean (Marchini et al. 2019) and the tanaidacean Hexapleomera bultidactyla has been reported to achieve very high population densities in red algae turfs (Esquete and Fernández-González 2016).
The GLMs pointed out that orientation was a main driver determining the assessed ecological descriptors, since all of them but evenness and relative abundance of H. stebbingi and of C. inexpectatus were influenced. Moreover, results of Bray-Curtis analysis showed that samples collected from vertical and horizontal surfaces significantly differed in terms of species composition. On the other hand, the values of the measured diversity indexes did not always depend on orientation, and Shannon and evenness indexes –which describe better than species richness the community complexity– showed an inversely proportional relationship with the height of the algae forming the turfs.
Once the chemical nature of the rocky substratum and spatial heterogeneity were discarded, the causes of the differences between the studied vertical and horizontal hard bottoms are difficult to define, but different studies suggested changes in wave and/or solar exposure as main drivers. Previous research showed the importance of water-flow due to wave action above other factors as driver, not only for littoral communities on the whole (Jensen and Denny 2016; Burel et al. 2019), but also specifically for the perecarid assemblages (Bueno et al. 2016). Drag forces caused by wave action tend to condition the communities dwelling on vertical and on horizontal substrata in a different way, since in the latter wave energy experiences important dissipation (Marshall and Stephenson 2011) and sedimentation rates are usually higher (Balata et al. 2007). Additionally, the surveyed vertical surface was more exposed to prevailing winds and waves in the region than the horizontal surface. Since strong water flows caused by waves have been proposed to select those species able to reduce the risk of displacement by firmly attaching to algae (Berthelsen et al. 2015), dominance of species adapted to stronger hydrodynamics might be expected on the studied vertical surface if wave exposition was the main driver. Nevertheless, previous investigations suggested strong preference for more protected habitats for J. marmorata (Karez and Ludynia 2003), which dominated the sampled vertical surface. Moreover, Esquete and Fernández-González (2016) reported very high abundances of H. bultidactyla, dominant on the studied horizontal surface in site 2, in exposed environments such as the fouling communities of open sea aquaculture facilities. Thus, drag forces or wave exposure seem to have a lesser effect on driving the peracarid assemblages in the studied location.
The effect of Sun radiation exposure is important in the altitudinal distribution of littoral organisms (Prusina et al. 2014). Vertical habitats are usually shaded and solar radiation is less intense than on horizontal ones (Knott et al. 2004; Firth et al. 2014), reducing physical stress for intertidal invertebrates. This is important in the Mediterranean littoral, where thermal stress can be frequent (Sarà et al. 2014; Seabra et al. 2011) reported that organisms inhabiting horizontal Sun-exposed surfaces along Iberian coast often suffered clearly higher temperatures than those dwelling on shaded surfaces. Thus, it can be suspected that differences in Sun exposure might explain the differences between the peracarid assemblages. Marchini et al. (2019) detected that E. pocillimanus, which clearly dominated the horizontal surface in the surveyed area, showed a clear preference for warmer conditions while J. marmorata poorly tolerated high temperatures. Based on these cues, amelioration of temperature and desiccation by orientation related shadowing seems a likely cause of the observed dissimilarities in the studied peracarid assemblages.
The inversely proportional relationship between turf height and the peracarid assemblage complexity, indicated by values of evenness and Shannon index, might seem strange as the habitat complexity concomitant with the larger fronds of canopy forming algae has long been known and associated with high α-diversity values (Stelling-Wood et al. 2019). However, previous studies showed that the situation tends to be the opposite in coralline turfs even under natural condition, and animal abundance and diversity were larger when fronds were shorter (Kelaher 2003). The cause is obscure, but might relate to the interaction between frond length and sediment trapped, which may hinder movement of vagile fauna in higher turfs (Kelaher and Castilla 2005). The fact that higher turfs harbour less complex peracarid assemblages is important because articulated coralline algae tend to grow higher under human impact (Mamo et al. 2020), as in altered urbanised shores (Airoldi et al. 2016), indicating a trend to reduced α-diversity for stronger disturbances.
Conclusions
The samples from vertical and horizontal surface formed two distinct and significantly different groups based on Bray-Curtis similarities on species composition. GLMs pointed out that orientation influenced α-diversity in some degree (species richness and Shannon index, but not evenness) and relative abundance of the majority of constant species. However, GLMs also showed that evenness and Shannon index were negatively influenced by turf height and that available phytal area positively influenced evenness. It can be concluded that species composition of the assemblages chiefly depended on surface orientation in the studied location, but the assemblage complexity was strongly influenced by the spatial structure of the turf.
Data Availability
All the specimens herein studied are deposited at the Biology Department of Universidad Autónoma de Madrid.
References
Aguilera MA, Broitman BR, Thiel M (2014) Spatial variability in community composition on a granite breakwater versus natural rocky shores: lack of microhabitats suppresses intertidal biodiversity. Mar Pollut Bull 87:257–268. https://doi.org/10.1016/j.marpolbul.2014.07.046
Airoldi L, Bulleri F (2011) Anthropogenic disturbance can determine the magnitude of opportunistic species responses on marine urban infrastructures. PLoS ONE 6(8):e22985. https://doi.org/10.1186/s10152-016-0480-9
Airoldi L, Ponti M, Abbiati M (2016) Conservation challenges in human dominated seascapes: the harbour and coast of Ravenna. Reg Stud Mar Sci 8:308–318. https://doi.org/10.1016/j.rsma.2015.11.003
Bacchiocchi F, Airoldi L (2003) Distribution and dynamics of epibiota on hard structures for coastal protection. Estuar Coast Shelf Sci 56:1157–1166. https://doi.org/10.1016/S0272-7714(02)00322-0
Balata D, Piazzi L, Benedetti-Cecchi L (2007) Sediment disturbance and loss of beta diversity on subtidal rocky reefs. Ecology 88:2455–2461. https://doi.org/10.1890/07-0053.1
Berthelsen AK, Hewitt JE, Taylor RB (2015) Coralline turf-associated fauna are affected more by spatial variability than by host species identity. Mar Biodiver 45:689–699. https://doi.org/10.1007/s12526-014-0270-z
Bertolini C (2019) Can secondary species maintain a primary role? Consistent inter-regional effects of understory algae on diversity. Mar Biodiver 49:841–849. https://doi.org/10.1007/s12526-018-0862-0
Bonifazi A, Ventura D, Gravina MF, Lasinio GJ, Belluscio A, Ardizzone GD (2017) Unusual algal turfs associated with the rhodophyta Phyllophora crispa: benthic assemblages along a depth gradient in the Central Mediterranean Sea. Estuar Coast Shelf Sci 185:77–93. https://doi.org/10.1016/j.ecss.2016.12.013
Bueno M, Dena-Silva SA, Flores AAV, Leite FPP (2016) Effects of wave exposure on the abundance and composition of amphipod and tanaidacean assemblages inhabiting intertidal coralline algae. J Mar Biolog Assoc UK 96:761–767. https://doi.org/10.1017/S002531541500123X
Bulleri F, Chapman MG (2010) The introduction of coastal infrastructure as a driver of change in marine environments. J Appl Ecol 47:26–35. https://doi.org/10.1111/j.1365-2664.2009.01751.x
Burel T, Schaal G, Grall J, Le Duff M, Chapalain G, Schmitt B, Gemin M, Boucher O, Gall EA (2019) Small-scale effects of hydrodynamics on the structure of intertidal macroalgal communities: a novel approach. Estuar Coast Shelf Sci 226:106290. https://doi.org/10.1016/j.ecss.2019.106290
Bustamante M, Tajadura J, Gorostiaga JM, Saiz-Salinas JI (2014) Response of rocky invertebrate diversity, structure and function to the vertical layering of vegetation. Estuar Coast Shelf Sci 147:148–155. https://doi.org/10.1016/j.ecss.2014.06.001
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation. PRIMER-E Ltd., Plymouth
Connell SD, Foster MS, Airoldi L (2014) What are algal turfs? Towards a better description of turfs. Mar Ecol Prog Ser 495:299–307. https://doi.org/10.3354/meps10513
Conradi M, López-González PJ (2001) Relationships between environmental variables and the abundance of peracarid fauna in Algeciras Bay (southern Iberian Peninsula). Inv Pesq 27:481–500. https://doi.org/10.7773/cm.v27i4.504
Dauvin JC, Ruellet T (2007) Polychaete/amphipod ratio revisited. Mar Pollut Bull 55:215–224. https://doi.org/10.1016/j.marpolbul.2006.08.045
De la Ossa-Carretero JA, Del Pilar-Ruso Y, Giménez-Casalduero F, Sánchez-Lizaso JL (2016) Amphipoda assemblages in a disturbed area (Alicante, Spain, Western Mediterranean). Mar Ecol 37:503–517. https://doi.org/10.1111/maec.12264
De la Ossa-Carretero JA, Del Pilar-Ruso Y, Giménez-Casalduero F, Sánchez-Lizaso JL, Dauvin JC (2012) Sensitivity of amphipods to sewage pollution. Estuar Coast Shelf Sci 96:129–138. https://doi.org/10.1016/j.ecss.2011.10.020
De Swart RL, Ribas F, Calvete D, Kroon A, Orfila A (2020) Optimal estimations of directional wave conditions for nearshore field studies. Cont Shelf Res 196:104071. https://doi.org/10.1016/j.csr.2020.104071
Esquete P, Fernandez-Gonzalez V (2017) Description, systematics and ecology of a new tanaidacean (Crustacea, Peracarida) species from Mediterranean fish farms. Helgol Mar Res 70:1–13. https://doi.org/10.1186/s10152-016-0480-9
Fauvelot C, Constantini F, Virgilio M, Abbiati M (2012) Do artificial structures alter marine invertebrate genetic makeup? Mar Biol 159:2797–2807. https://doi.org/10.1007/s00227-012-2040-4
Firth LB, Thompson RC, Bohn K, Abbiati M, Airoldi L, Bouma TJ, Bozzeda F, Ceccherelli VU, Colangelo MA, Evans A, Ferrario F, Hanley ME, Hinz H, Hoggart SPG, Jackson JE, Moore P, Morgan EH, Perkol-Finkel S, Skov MW, Strain EM, van Belzen J, Hawkins SJ (2014) Between a rock and a hard place: environmental and engineering considerations when designing coastal defence structures. Coast Engin 87:122–135. https://doi.org/10.1016/j.coastaleng.2013.10.015
Gössling S, Hall CM, Scott D (2018) Coastal and Ocean Tourism. In: Salomon M, Markus T (eds) Handbook on Marine Environment Protection. Springer, Cham. https://doi.org/10.1007/978-3-319-60156-4_40
Greenacre M, Primicerio R (2013) Multivariate analysis of ecological data. Fundación BBVA, Bilbao
Guerra-García JM, Baeza-Rojano E, Cabezas MP, Díaz-Pavón JJ, Pacios I, García-Gómez JC (2009) The amphipods Caprella penantis and Hyale schmidtii as biomonitors of trace metal contamination in intertidal ecosystems of Algeciras Bay, Southern Spain. Mar Pollut Bull 58:765–786. https://doi.org/10.1016/j.marpolbul.2009.02.018
Guerra-García JM, Ros M, Izquierdo D, Soler-Hurtado MM (2012) The invasive Asparagopsis armata versus the native Corallina elongata: differences in associated peracarid assemblages. J Exp Mar Biol Ecol 416/417:121–128. https://doi.org/10.1016/j.jembe.2012.02.018
Guerra-García JM, Tierno de Figueroa JM, Navarro-Barranco C, Ros M, Sánchez-Moyano JE, Moreira J (2014) Dietary analysis of the marine Amphipoda (Crustacea: Peracarida) from the Iberian Peninsula. J Sea Res 85:508–517. https://doi.org/10.1016/j.seares.2013.08.006
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological Statistics software package for education and data analysis. Palaeont Electronica 9:4
Izquierdo D, Guerra-García JM (2011) Distribution patterns of the peracarid crustaceans associated with the alga Corallina elongata along the intertidal rocky shores of the Iberian Peninsula. Helgol Mar Res 65:233–243. https://doi.org/10.1007/s10152-010-0219-y
Jensen MM, Denny MW (2016) Life in an extreme environment: characterizing wave-imposed forces in the rocky intertidal zone using temporal resolution hydrodynamic measurement. Limnol Oceanogr 61:1750–1761. https://doi.org/10.1002/lno.10327
Karez R, Ludynia K (2003) Niche differentiation in habitat and current preference corroborates taxonomic distinction of Jassa falcata and Jassa marmorata. Estuar Coast Shelf Sci 58:279–292. https://doi.org/10.1016/S0272-7714(03)00080-5. Amphipoda, Crustacea
Kelaher BP (2003) Changes in habitat complexity negatively affect diverse gastropod assemblages in coralline algal turf. Oecologia 135:431–441. https://doi.org/10.1007/s00442-003-1196-5
Kelaher BP, Castilla JC (2005) Habitat characteristics influence macrofaunal communities in coralline turf more than mesoscale coastal upwelling on the coast of Northern Chile. Estuar Coast Shelf Sci 63:155–165. Estuar Coast Shelf Sci 58:279–292. https://doi.org/10.1016/j.ecss.2004.10.017
Kelly DW, MacIsaac HJ, Heath DD (2006) Vicariance and dispersal effects on phylogeographic structure and speciation in a widespread estuarine invertebrate. Evolution 60:257–267. https://doi.org/10.1111/j.0014-3820.2006.tb01104.x
Knott NA, Underwood AJ, Chapman MG, Glasby TM (2004) Epibiota on vertical and on horizontal surfaces on natural reefs and on artificial structures. J Mar Biolog Assoc UK 84:1117–1130. https://doi.org/10.1017/S0025315404010550h
Mamo LT, Porter AG, Tagliafico A, Coleman MA, Smith SDA, Figueira WF, Kelaher BP (2020) Upgrades of coastal protective infrastructure affect benthic communities. J Appl Ecol 58:295–303. https://doi.org/10.1111/1365-2664.13736
Mangialajo L, Ruggieri N, Asnaghi V, Chiantore M, Povero P, Cattaneo-Vietti R (2007) Ecological status in the Ligurian Sea: the effect of coastline urbanisation and the importance of proper reference sites. Mar Pollut Bull 55:30–41. https://doi.org/10.1016/j.marpolbul.2006.08.022
Marchini A, Ragazzola F, Vasapollo C, Castelli A, Cerrati G, Gazzola F, Jiang C, Langeneck J, Manauzzi MC, Musco L, Nannini M, Zekonyte J, Lombardi C (2019) Intertidal Mediterranean coralline algae habitat is expecting a shift toward a reduced growth and a simplified associated fauna under climate change. Front Mar Sci 6:106. https://doi.org/10.3389/fmars.2019.00106
Marshall RJE, Stephenson WJ (2011) The morphodynamics of shore platforms in a micro-tidal setting: interactions between waves and morphology. Mar Geol 288:18–31. https://doi.org/10.1016/j.margeo.2011.06.007
Moreira J, Chapman MG, Underwood AJ (2006) Seawalls do not sustain viable populations of limpets. Mar Ecol Prog Ser 322:179–188. https://doi.org/meps322179
Moschella PS, Abbiati M, Åberg P, Airoldi L, Anderson JM, Bacchiocchi F, Bulleri F, Dinesen GE, Frost M, Gacia E, Granhag L, Jonsson PR, Satta MP, Sundelo A, Thompson RC, Hawkins SJ (2005) Low-crested coastal defence structures as artificial habitats for marine life: using ecological criteria in design. Coast Eng 52:1053–1071. https://doi.org/10.1016/j.coastaleng.2005.09.014
Pacheco AS, Laudien J, Thiel M, Heilmayer O, Oliva M (2010) Hard-bottom succession of subtidal epibenthic communities colonizing hidden and exposed surfaces off northern Chile. Sci Mar 74:147–154. https://doi.org/10.3989/scimar.2010.74n1147
Perkol-Finkel S, Shashar N, Benayahu Y (2006) Can artificial reefs mimic natural reef communities? The roles of structural features and age. Mar Environ Res 61:21–135. https://doi.org/10.1016/j.marenvres.2005.08.001
Pister B (2009) Urban marine ecology in southern California: the ability of riprap structures to serve as rocky intertidal habitat. Mar Biol 156:861–873. https://doi.org/10.1007/s00227-009-1130-4
Sarà G, Milanese M, Prusina I, Sarà A, Angel DL, Glamuzina B, Nitzan T, Freeman S, Rinaldi A, Palmeri V, Montalto V, Lo Martire M, Gianguzza P, Arizza V, Lo Brutto S, De Pirro M, Helmuth B, Murray J, De Cantis S, Williams GA (2014) The impact of climate change on Mediterranean intertidal communities: losses in coastal ecosystem integrity and services. Reg Environ Change 14(Suppl 1):5–17. https://doi.org/10.1007/s10113-012-0360-z
Seabra R, Wethey DS, Santos AM, Lima FP (2011) Side matters: microhabitat influence on intertidal heat stress over a large geographical scale. J Exp Mar Biol Ecol 400:200–208. https://doi.org/10.1016/j.jembe.2011.02.010
Stelling-Wood TP, Gribben PE, Poore AGB (2019) Habitat variability in an underwater forest: using a trait-based approach to predict associated communities. Funct Ecol 34:888–898. https://doi.org/10.1111/1365-2435.13523
Urra J, Rueda JL, Mateo Ramírez Á, Marina P, Tirado C, Salas C, Gofas S (2013) Seasonal variation of molluscan assemblages in different strata of photophilous algae in the Alboran Sea (western Mediterranean). J Sea Res 83:83–93. https://doi.org/10.1016/j.seares.2013.05.016
Acknowledgements
The author would like to express his great gratitude to Juan Moreira, colleague from the Biology Department of the Universidad Autónoma de Madrid, for his valuable help in the statistical treatment of the data and its interpretation, and to Carlos Navarro-Barranco, from Universidad de Sevilla, for his advice in species identification. The Board of Environmental Affairs of the Junta de Andalucía granted permission to conduct the sampling in Villaricos and the surrounding area. The useful comments and suggestions by three anonymous reviewers greatly improved the scientific quality of the present work, and deserved the most sincere acknowledgement.
Funding
Partial financial support was received from the Spanish Ministry of Science and Innovation [Research Project “Polychaeta VI: Palpata-Canalipalpata I (CGL2014-53332-C5-3-P)”].
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares that he has no competing interests.
Ethics approval
Since neither live animals were used for experimentation nor biological material (be it genetically modified or not) was employed for the current study, approval from the “Cómite de Ética en la Investigación” (Ethics Committee for Research) of the Universidad Autónoma de Madrid was not required, as established in the University, regional and national normative.
Consent to participate
In no case human participants whose consent to participate was needed were involved.
Consent for publication
In this study, human participants whose consent for publication was needed were not involved.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
López, E. Peracarid Assemblages in a Human-Disturbed Location from South-Western Mediterranean Sea: Role of Surface Orientation and Phytal Structure of the Habitat. Thalassas 38, 1175–1186 (2022). https://doi.org/10.1007/s41208-022-00455-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41208-022-00455-z