Abstract
A comparative phytosociological and habitat analysis of 84 pleustonic phytocoenoses in northern Poland was carried out in order to determine the scale of their floristic distinctiveness and to obtain an answer to the question whether and to what extent the floristic differentiation of pleustonic communities corresponds to the differentiation of their habitat conditions. Based on phytosociological studies, it was shown that the investigated phytocoenoses are not random groups of species, but well differentiated in terms of floristic composition and phytocoenotic structure by communities. It was shown that the studied phytocoenoses represent the following separate associations in phytosociological terms: Salvinio natantis-Spirodeletum polyrhizae, Lemno-Spirodeletum polyrhizae, Riccietum fluitantis and Ricciocarpetum natantis. Hydrochemical studies showed that the floristic distinctiveness of these communities is largely confirmed by the differentiation of properties of the water habitat. Salvinio natantis-Spirodeletum polyrhizae is characterised by the deepest waters (mainly 0.7–1.2 m), alkaline, of a low colour, rich in ions (Mg2+, Na+, Cl−) and therefore characterised by the highest electrolytic conductivity, poor in dissolved organic matter, NO3− and total iron. Lemno-Spirodeletum polyrhizae is characterised by medium deep waters (mainly 0.3–0.8 m), mostly alkaline, of a low colour, less rich in ions (Ca2+, Na+, Cl−) and relatively low in dissolved organic matter and NO3−. Riccietum fluitantis is characterised by shallow waters (mainly 0.2–0.4 m), most often slightly acidic, of a quite high colour, low in cations and Cl−, and relatively rich in total iron, NO3−, and dissolved organic matter. Ricciocarpetum natantis is characterised by the shallowest waters, (mainly < 0.3 m), most often acidic, of the highest colour, low in ions, and rich in total iron, dissolved silica, NO3− and dissolved organic matter. By demonstrating habitat distinctiveness, the studied pleustonic communities may have significance as indicators of various types of aquatic environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Europe, pleustonic plant communities are a characteristic element of aquatic vegetation. They are created by a number of species of the Acoraceae family (Lemna minor, L. trisulca, L. gibba, Spirodela polyrhiza and Wolffia arrhiza, recently also L. turionifera), water fern Salvinia natans and liverworts – Ricciocarpos natans, Riccia fluitans and, less frequently, R. rhenana (e.g. Passarge 1978; Scoppola 1982; Landolt 1986; Doll 1991; Wołek 2006; Šumberová 2011). In southern Europe, pleustonic communities are also formed by the fern Azolla filiculoides (Scoppola 1982; Sanda et al. 1987; Papastergiadou and Babalonas 1993a, b; Šumberová 2011; Cvijanović et al. 2018). Pleustonic communities have already received a lot of attention in Europe, including Poland. Thanks to the data contained in the above-mentioned works, as well as many others (e.g. Rivas-Martinez 1982; Wołek 1997; Hrivnák 2002; Spałek 2005; Wójciak and Urban 2009; Laducci et al. 2011), the floristic composition, structure, range of pleustonic phytocoenoses and types of water ecosystems in which they are most commonly found have been elaborated. A great deal of work has been devoted to syntaxonomic issues, and to distinguishing and classifying communities that were included in a separate Lemnetea class (e.g. Schaminée and Stortelder 1995; Rivas-Martinez et al. 2001; Tzonew et al. 2009; Matuszkiewicz 2014; Mucina et al. 2016; Spałek and Stebel 2020). These phytosociological works brought about, depending on the syntaxonomical approach, a distinction within the Lemnetea class of about 20 communities (associations) occurring in European waters.
A review of the above works shows that the pleustonic communities are simply built, floristically poor, sensitive to water movement and, due to the lack of connection to the ground, mostly unstable. Hence, they are associated primarily with a wide range of small ecosystems with standing or very slow flowing water, shielded from the wind, which are characterised by high variability of physical and chemical properties and water depth. These are mainly oxbow lakes, ponds, drainage ditches, small astatic and peat reservoirs in depressions of the area. The low stability of pleustonic communities has led to suggestions that the occurrence and floristic composition of such phytocoenoses within aquatic ecosystems depend primarily on chance (Wołek 1997, 2006; Wołek and Walanus 2000), i.e. they are not assemblies in the sense of Braun-Blanquet, but clusters of individualistic species in the sense of Gleason (Wołek 2006). The low stability of the pleustonic communities and the relatively wide range of diverse aquatic ecosystems in which they occur, which are characterised by widely known significant variability of the aquatic environment, were largely the reason why the habitat conditions of pleustonic communities have most often been discussed on the basis of field observations and the characteristics of the types of reservoirs. The sometimes added measurements of water properties most often concerned single or several factors (e.g. Scoppola 1982; Van Katwijk and Roelofs 1988; Doll 1991; Landolt 1999). Such data were therefore an extension of the description of research sites and were not the subject of a statistical, comparative analysis that could show the actual habitat differences between communities in terms of water chemistry. The issue of habitat differences between individual pleustonic communities can be found only in a few works (e.g. Wiegleb 1978; Papastergiadou and Babalonas 1993a, b; Kłosowski 1994; Kocić et al. 2008). However, these works do not focus on the pleustonic communities, but present an analysis of a wider range of aquatic plant communities. There is also some habitat data on individual pleustonic species. They are scattered throughout various works on various groups of aquatic plants (e.g. De Lyon and Roelofs 1986a, b; Landolt 1986 and references therein; Gilgen 1989; Stojanović et al. 2004; Michalska-Hejduk et al. 2009). These data were presented in a descriptive manner and were not subject to comparative analyses.
From the presented literature, it can be clearly seen that in the case of pleustonic phytocoenoses, there is no comparative research on community-habitat relations. Conducting such studies may show to what extent the floristical distinctiveness of pleustonic phytocoenoses is permanent and dependent on the abiotic environment. It can also explain which of the physical and chemical features of water are most important in the differentiation of communities and to what extent pleustonic communities differ from one another. As a result, such research may also provide a basis for determining the bioindicative significance of the distinguished communities.
In Poland, as part of research on the ecology of aquatic plants and their communities which has been conducted for several decades, the habitat conditions of several groups of pleustonic plants have also been analysed, especially phytocoenoses of Lemna minor, Spirodela polyrhiza and Riccia fluitans (Kłosowski 1994; Kłosowski and Jabłońska 2009).
These studies, however, did not focus on pleustonic phytocoenoses, but covered the entire complex of aquatic communities or aquatic and rush communities.
Extensive research on the ecology of the pleustonic communities was initiated in 2009. Its effect was an assessment of the habitat conditions of phytocoenoses with a dominance of Salvinia natans in the Oder and Vistula valleys (Kłosowski et al. 2020). This work is a continuation of that research. It presents a comparative phytosociological and habitat analysis of pleustonic communities occurring in northern Poland. Its main goal is to obtain an answer to the question of what is the relationship between the floristic and phytocoenotic diversity of the types of pleustonic communities more frequently occurring in this area and the abiotic environment, mainly in terms of water chemistry and depth.
The following research hypotheses were formulated:
-
1.
Pleustonic phytocoenoses are not random groups of species, but communities with clearly marked repeatability of species composition, which is largely dependent on the abiotic environment, including water chemistry.
-
2.
Although pleustonic communities occur in a wide range of habitat variability, they exhibit a significant degree of distinctiveness in terms of the physical and chemical properties of water and, therefore, at least some of them may have bioindicative significance.
Material and methods
Field sampling
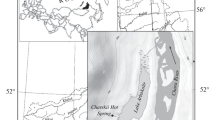
The data for the study was collected in the growing season (from July to the end of September) in the years 2009–2017. In the case of the area of north-eastern Poland, data collected earlier were also taken into account (Kłosowski and Jabłońska 2009). When conducting the research, the type of aquatic ecosystem was not the main determinant, but rather the occurrence (presence) of plant phytocoenoses (patches) dominated by pleustonic species. In patches dominated by pleustonic species, phytosociological relevés were recorded using the Braun-Blanquet method, generally accepted in Poland and Europe (Dzwonko 2007). In each relevè, a list of plant species was made and the abundance of each species was estimated according to the Braun-Blanquet six-point cover-abundance scale: + < 5%, 1 = 5%, 2 = 5–25%, 3 = 25–50%, 4 = 50–75%, 5 = 75–100%. In total, 84 stands with pleustonic phytocoenoses were examined and 84 phytosociological relevés of an area of several m2 (about 5 m2) were made. Each relevé was located in the central part of a given patch. The phytosociological relevés were collected from oxbow lakes, ponds, canals, drainage ditches, astatic reservoirs, clay pits and small peat reservoirs. The study area with groups of pleustonic phytocoenoses stands is shown in Fig. 1.
In each relevé the average water depth was measured using a scaling oar and a surface water sample was collected for physical and chemical analysis (84 water samples were taken in total). Samples of surface water were taken in plastic containers.
Water sample and data analysis
The pH and electrical conductivity (EC) were determined directly in the field using a Hach senSION 156 portable meter. Samples of surface water for analysis of PO43−, NH4+, NO3− and total iron were fixed by adding 1 ml of concentrated sulphuric acid. All samples were transported to the laboratory and analysed immediately. Before analysis, the samples were filtered (hard cellulose filters type 390-Filtrak-Munktell) and kept refrigerated at 4 °C until all determinations were completed. Fourteen physical and chemical properties of the water and water depth were taken into account. PO43−, NO3−, NH4+, total iron, dissolved silica and colour were determined by standard colorimetric methods using a Spekol 11 spectrophotometer; Ca2+, Na+ and K+ were determined on a YENWAY PFP7 flame spectrophotometer; Mg2+ on a UNICAM 939 atomic absorption spectrophotometer; Cl− by the Mohr argentometric method and chemical oxygen demand (COD) as consumption of KMnO4 in an acidic environment. All analyses were carried out according to the procedures described in the works of Hermanowicz et al. (1999) and the Hach Company (1992).
In order to organise the material, in which there are a lot of relevés with co-domination of species, correspondence analysis (CA) was applied using CANOCO for Windows Version 5 (Šmilauer and Lepš 2014), giving the possibility of grouping the relevés according to the abundance of each species in space. In this analysis, a modification of the Braun-Blanquet coverage scale was applied, where + = 1%, 1 = 10%, 2 = 20%, 3 = 37.5%, 4 = 62.5%, 5 = 87.5% (Dzwonko 2007).
The separate groups of relevés were compiled in a synthetic phytosociological table taking into account the abundance, constancy and cover coefficient of all species. Constancy was estimated according to the following scale of species occurring in the relevés of the association: I–1–20% of relevés, II–21–40% of relevés, III–41–60% of relevés, IV–61–80% of relevés, V–81–100% of relevés. The coefficient of cover was estimated using the formula:
Coefficient of cover = total of species mean coverage/number of relevés of the association × 100.
Taxonomic nomenclature was adopted from The Plant List (2013), and the names of plant associations studied after Šumberová (2011). Syntaxonomic nomenclature of higher vegetation units was adopted after Mucina et al. (2016). The assignment of plant associations and diagnostic species to these units was given according to Matuszkiewicz (2014).
The habitats of the surveyed pleustonic communities were compared in terms of individual properties of water and its depth. The ranges of features (minimum and maximum values), medians, and interquartile ranges are presented. Due to significant deviations from the normal distribution of the measured properties, a nonparametric Wilcoxon rank sum test was used to demonstrate the significance of the differences with the correction for Benjamini and Hochberg (1995) for multiple comparisons to demonstrate the significance of the differences. The test was performed in the R program (R Core Team 2016). All statements of statistical significance refer to the 0.05 probability level (p ≤ 0.05). The relationships between the analysed habitat factors in the pleustonic communities were determined on the basis of principal components analysis (PCA) using CANOCO for Windows Version 5 (Šmilauer and Lepš 2014).
Results
Phytosociological and habitat characteristics of the investigated types of phytocoenoses
According to the dominance and co-dominance of pleustonic species, there were four groups among the examined phytocoenoses (Fig. 2).
Correspondence analysis (CA) for all data points and species; symbols represent points categorised in terms of phytosociological status: RN–Ricciocarpetum natantis, RF–Riccietum fluitantis, SN–Salvinio natantis-Spirodeletum polyrhizae, LS–Lemno-Spirodeletum polyrhizae; eigenvalues: 1st axis–0.6133, 2nd axis–0.3379, 3rd axis–0.2870, 4th axis–0.2058
In three of them, the dominance of one species is definitely seen in most patches. These are phytocoenoses of Salvinia natans, of Ricciocarpos natans and of those dominated by Riccia fluitans. As can be seen from the graph (Fig. 2), these three groups do not overlap and are clearly distinct from each other. A fourth, poorly differentiated group consists of phytocoenoses with a dominance or co-dominance of duckweed and spirodela species, namely Lemna minor, L. trisulca and Spirodela polyrhiza. This group occupies an intermediate position on the graph (Fig. 2) between the group of Riccia fluitans and Salvinia natans phytocoenoses and slightly overlaps them due to the co-dominance of duckweed with these species in some phytocoenoses. Most phytocoenoses of duckweeds and spirodela, however, retain clear distinctiveness.
The floristic composition and detailed cover coefficients of all four phytocoenoses groups are presented in Table 1. Based on Fig. 2, it was assumed that these four phytocoenoses groups correspond to the following associations distinguished by many European researchers in phytosociological terms: Salvinio natantis-Spirodeletum polyrhizae, Ricciocarpetum natantis, Riccietum fluitantis and Lemno-Spirodeletum polyrhizae (Šumberová 2011). Such a nomenclature was adopted in the table and in the further part of the work. From Table 1, it can be seen that in the phytocoenoses of liverworts, apart from the dominant ones, other pleustonic species have the largest share, primarily Lemna minor, L. trisulca, and Spirodela polyrhiza. In the Ricciocarpetum natantis patches, Riccia fluitans is quite common, and in Riccietum fluitantis phytocoenoses Ricciocarpos natans is common, but does not achieve a high coverage rate. In both types of liverwort communities, a small share of other aquatic species is noted, and a fairly long list of rushes (Phragmito-Magnocaricetea class) with the largest share being held by the sedges (Carex rostrata and C. vesicaria). In the phytocoenoses of liverworts, especially in Ricciocarpetum natantis also the participation of peat bog species is clear, such as Utricularia minor, Drepanocladus aduncus, Epilobium paustre, Comarum palustre and Calla palustris. Phytocoenoses with a dominance of Salvinia natans are characterised by a high proportion of both species of duckweeds (Lemna minor and L. trisulca) and Spirodela polyrhiza, as well as by the lack of Riccia fluitans and the occurrence in only a few phytocoenoses with a minimum degree of coverage of Ricciocarpos natans. However, the largest share is noted of aquatic species from the Potamogetonetea class, mainly Hydrocharis morsus-ranae, Ceratophyllum demersum, Nuphar lutea and Elodea canadensis and Myriophyllum verticillatum. Among rushes, only Sparganium erectum and Sagittaria sagittifolia are found with greater persistence. In Lemno-Spirodeletum polyrhizae phytocoenoses, both the list of aquatic and rush species is quite long, but only a few aquatic species occur with higher constancy. These are Salvinia natans, Hydrocharis morsus ranae and Ceratophyllum demersum. However, it can be clearly seen that the dominant species achieving the highest degrees of constancy and coverage coefficients in these phytocoenoses are Lemna minor, L. trisulca and Spirodela polyrhiza. It is worth noting that these species also achieve high degrees of constancy in all other types of pleustonic phytocoenoses, but their coverage is much lower than in the phytocoenoses of duckweeds and spirodela (Table 1). However, in general it can be said that species of duckweeds and spirodela are a kind of common thread throughout all types of pleustonic phytocoenoses studied.
In the studied area, the pleustonic phytocoenoses compared develop mostly in ecosystems with standing water. In the case of liverwort patches, these are mainly astatic reservoirs, depressions on the periphery of peat bogs, sometimes also drainage ditches, and rarely canals. In the latter, mainly Ricciocarpos natans was found. Phytocoenoses with a dominance of Salvinia natans occur mainly in oxbow lakes, ponds, canals and, less frequently, in drainage ditches, while patches of duckweeds and spirodela are found in all these types of aquatic ecosystems.
Physical and chemical properties of water in the investigated types of phytocoenoses
The results of the comparative analysis of water habitats of the 4 types of phytocoenoses studied are presented in Figs. 3, 4, 5. Figure 3 clearly shows the dispersion of points of individual types of phytocoenoses. However, it is seen that these habitats can be divided into two main groups. On the right side of the graph, in the area of high and very high values of such features as total iron, COD-KMnO4, NO3−, and low values of other physical and chemical features of water, there are points representing the phytocoenoses of Ricciocarpetum natantis, and in the vast majority also Riccietum fluitantis. The opposite situation is observed in the case of Salvinio natantis-Spirodeletum polyrhizae phytocoenoses. The values of total iron and COD-KMnO4 indicating the content of dissolved organic matter as well as NO3− are low in the habitats of these phytocoenoses, while the values of EC, pH, Na+, Cl−, Mg2+, Ca2+, K+ and depth are higher compared to the habitats of liverworts. In the case of such features as dissolved silica, total iron, NH4+, as well as PO43−, high values were observed in the waters of some phytocoenoses of the liverworts as well as in the patches of Salvinio-Spirodeletum polyrhizae, and low values in others. A special place in the graph is occupied by points representing the phytocoenoses of Lemno-Spirodeletum polyrhizae. They are located at the bottom of the graph and overlap to a large extent with the Salvinio natantis-Spirodeletum polyrhizae points (values of EC, pH, Na+, Cl−, Mg2+, Ca2+, K+ and depth), and to a lesser extent with the points of both types of liverwort phytocoenosis, especially Riccietum fluitantis (values of COD-KMnO4, NO3−, dissolved silica, total iron, NH4+, PO43−). However, this only applies to some of the points.
Principal component analysis (PCA) for data points (2 RN point, 1 SN point and 1 LS point were excluded as outliers) and chemical water properties (excluding colour); symbols represent points categorised in terms of phytosociological status: RN–Ricciocarpetum natantis, RF–Riccietum fluitantis, SN–Salvinio natantis-Spirodeletum polyrhizae, LS–Lemno-Spirodeletum polyrhizae; eigenvalues: 1st axis–0.3624, 2nd axis–0.1577, 3rd axis–0.0884, 4th axis–0.0838
Differentiation of the pleustonic associations studied in relation to water properties. White boxes show 25–75% interquartile ranges of values, the horizontal black lines in boxes show the medians. Whiskers show the amplitudes of values. RN–Ricciocarpetum natantis, RF–Riccietum fluitantis, SN–Salvinio natantis-Spirodeletum polyrhizae, LS–Lemno-Spirodeletum polyrhizae
Statistically significant differences of chemical and physical habitatcharacteristics between pleustonic associations studied. + : significant at p ≤ 0.05; − : not significant at p ≤ 0.05. RN–Ricciocarpetum natantis, RF–Riccietum fluitantis, SN–Salvinio natantis-Spirodeletum polyrhizae, LS–Lemno-Spirodeletum polyrhizae
This picture of the situation is fully confirmed in Figs. 4 and 5. Figure 4 shows the differentiation of water habitats of the compared communities in terms of the amplitudes of the analysed features and typical values (white boxes on the graph), which may indicate optimal habitat conditions. These white boxes shifted towards higher values in waters with liverworts compared to Salvinio natantis-Spirodeletum polyrhizae and Lemno-Spirodeletum polyrhizae for total iron, COD-KMnO4 and NO3−. A similar trend also applies to colour and PO43− and NH4+. For almost all of the other features, the opposite is true. At the same time, the differences between the phytocoenoses of Salvinia natans in relation to the patches of duckweeds and spirodela, already shown in the PCA diagram (Fig. 3), are also visible (for SN, white boxes are shifted towards higher values of Mg2+, Na+, K+, Cl−, electrolytic conductivity and also depth). Figure 4 also shows some differences between the aquatic habitats of both types of liverwort phytocoenoses. These differences concern generally broader ranges of most water features and lower depth and higher values of colour, total iron, dissolved silica and Mg2+ in the case of Ricciocarpetum natantis. The significance of the shown trends in the differentiation of water habitats of the studied pleustonic communities was largely demonstrated statistically (Fig. 5). Significant differences between the liverwort communities and the phytocoenoses of salvinia and duckweeds and spirodela concern 8 characteristics (Ca2+, pH, Na+, NO3−, Cl−, colour, COD-KMnO4, depth). In the case of Mg2+ and electrolytic conductivity, the significance of the differences does not only concern Ricciocarpetum natantis and Lemno-Spirodeletum polyrhizae, and Riccietum fluitantis does not differ significantly from Salvinio natantis-Spirodeletum polyrhizae in terms of total iron. K+ significantly differentiates the communities of liverworts from the phytocoenoses of salvinia. The differences between Lemno-Spirodeletum polyrhizae and Salvinio natantis-Spirodeletum polyrhizae have been highlighted above (Figs. 3 and 4). These include Mg2+, Na+, Cl−, electrolytic conductivity and depth. In the case of liverwort communities, a significant difference between them was shown in terms of only one feature, namely dissolved silica.
Discussion
As mentioned in the introduction, pleustonic communities, due to the lack of connection with the bottom of aquatic ecosystems, are considered by phytosociologists to be extremely unstable compared to other aquatic plant communities and are characterised by high variability of floristic composition (Wołek 1997, 2006; Landolt 1999; Matuszkiewicz 2014). This is confirmed by the fact that a large number of communities covering many combinations of several pleustonic species have been described in Europe (e.g. Doll 1991; Hrivnák 2002; Tzonew et al. 2009; Šumberová 2011). On this basis, can it be concluded that these communities are only random collections of species, as some authors claim? (Wołek 1997; Wołek and Walanus 2000). It can be agreed with the above-mentioned authors, as Landolt (1999) does, that the initial composition of a given pleustonic community forming in newly emerging aquatic ecosystems is difficult to predict and depends on the species available for colonisation, especially depending on the first, main coloniser. However, the situation changes with the development of the community, i.e. an increase in the share of not only pleustonic species, but also other species, e.g. underwater, anchored in the bottom, or rushes, and the creation of a layered structure. The research carried out in this paper indicates the existence of such a situation. In the studied communities, there is a fairly significant share not only of anchored aquatic plant species (Potamogetonetea class), but also of species from the class grouping rush vegetation (Phragmito-Magnocaricetea). These plants undoubtedly participate in stabilising the phytocoenotic structure of the surveyed communities. The fact that the species composition of the pleustonic communities is not a coincidence is also confirmed by the fact that many of the same types occur in different, often distant areas of Europe. This also applies to the pleustonic communities investigated in this study. Outside Poland, they are noted in numerous European countries (see Šumberová 2011 and references therein). Of course, there are supra-regional, geographical floristic differences between these communities, which, however, do not change the main elements of the species composition that determine their structure and physiognomy. The results of this work indicate that the surveyed pleustonic communities are well distinguished both in terms of species composition and phytocoenotic structure.
The question arises here about the habitat conditions of the pleustonic communities; namely to what extent the floristic composition and phytocoenotic structure of the surveyed communities depend on these conditions? Undoubtedly, the types of aquatic ecosystems in which pleustonic species and communities develop are well recognised. These are oxbow lakes, ponds, drainage ditches, small astatic reservoirs, clay pits, peat bog reservoirs in land depressions, canals, also alluvial pools and flooded sand pits (e.g. Landolt 1986; Wołek 1997; Šumberová 2011 and references therein). The common features of these water habitats highlighted in these works are the mainly stagnant water, the high variability of water level during the growing season, and the enormous variability of water chemistry. Attempts to demonstrate the connection of the occurrence of pleustonic phytocoenoses with the type of body of water (Wołek 1997; Wołek and Kościółek 2012) did not bring a positive result. There is also a lack of detailed research conducted in specific phytosociologically distinguished pleustonic communities, which could show whether and to what extent they are distinct in terms of physical and chemical properties of water. The available hydrochemical data (see introduction) mainly concerned the characteristics of the reservoirs. They allowed certain trends in the occurrence of some pleustonic species in terms of water chemistry to be presented in a descriptive way (e.g. more frequent occurrence of liverworts in more acidic waters in relation to the habitats of salvinia and duckweeds and spirodela). However, these data do not explain whether and to what extent the hydrochemical conditions of individual communities differ. The need for research in this area is indicated by the piecemeal results of Wiegleb (1978) concerning the communities and indicating the relationship of Riccietum fluitantis with waters poorer in Ca2+, Na+, K+ and Cl− and with a lower electrolytic conductivity in relation to algal communities, or by the data of Papastergiadou and Babalonas (1993b) showing that Lemnetum minoris waters are more rich in nutrients in relation to salvinia phytocoenoses. This lack of research on pleustonic habitats and communities is at least partially filled by this work. It presents a comparative, statistical analysis of the physical and chemical properties of water and its depth in the surveyed pleustonic communities without reference to the type of body of water.
Research has shown that despite the generally known relatively low stability of pleustonic communities and the very high variability of their habitats (ecological amplitudes of the studied communities in terms of physical and chemical properties of water clearly overlap), the floristic distinctiveness of these communities is largely confirmed by the differences in properties of the aquatic habitat. This applies in particular to Salvinio natantis-Spirodeletum polyrhizae and Lemno-Spirodeletum polyrhizae, which are distinct from the liverwort communities through the whole complex of aquatic habitat features, but which also differ from each other. On the other hand, separate in phytosociological terms communities of liverworts–Riccietum fluitantis and Ricciocarpetum natantis–occur in waters with similar physicochemical properties, but also in their case, trends differing these habitats can be seen.
Therefore, it seems that the presented direction of research of the community-habitat should be continued. It is important to increase the number of examined phytocoenoses, to take into account other types of phytocoenoses, as well as transitional patches, geographical and seasonal variability of both the floristic composition of communities and habitat factors (e.g. Landolt 1986, 1999; Wołek 1997, 2006).
Currently, taking into account both the statistically significant differences between the surveyed communities and the emerging trends in their occurrence depending on the physical and chemical properties of water and its depth, these communities can be characterised in terms of habitat as follows:
Salvinio natantis-Spirodeletum polyrhizae is characterised by the deepest waters (mainly 0.7–1.2 m), alkaline, of the low colour, rich in ions (Mg2+, Na+, Cl−) and therefore characterised by the highest electrolytic conductivity, poor in dissolved organic matter, NO3− and total iron. Lemno-Spirodeletum polyrhizae is characterised by medium deep waters (mainly 0.3–0.8 m), mostly alkaline, of low colour, less rich in ions (Ca2+, Na+, Cl−) and relatively low in dissolved organic matter and NO3−. Riccietum fluitantis is characterised by shallow waters (mainly 0.2–0.4 m), most often slightly. acidic, of quite high colour, low in cations and Cl−, and relatively rich in total iron, NO3−, and dissolved organic matter. Ricciocarpetum natantis is characterised by the shallowest waters (mainly < 0.3 m), most often acidic, of the highest colour, low in ions, and rich in total iron, dissolved silica, NO3−, and dissolved organic matter.
Conclusions
The obtained results largely confirm the research hypotheses. It can be assumed that the surveyed communities are not random collections of species, they have a specific species composition, which is clearly influenced by the abiotic environment, including water chemistry. Despite the overlapping of ecological amplitudes of the surveyed communities in terms of physical and chemical properties of water, there are habitat differences between them, indicating that these communities may have bioindicative significance as indicators of different types of aquatic habitats.
Data availability
Our manuscript has no associated data.
References
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B 57:289–300
Hach Company (1992) Hach water analysis Handbook. 2nd (ed.) Loveland, Colorado, U.S.A, p. 831
Cvijanović LD, Lakišić DV, Živković MM, Novković MZ, Andelković AA, Pavlović M, Vikov DM, Raduković SB (2018) An overview of aquatic vegetation in Serbia. Tuexenia 38:269–286
Doll R (1991) Die Pflanzengesellschaften der stehenden Gewässer in Mecklenburg-Vorpommern. Teil. I102:2. Lemnetea-Wasserlinsengesellschaften. Feddes Repertorium 199–216
Dzwonko Z (2007) Przewodnik do badań fitosocjologicznych. Sorus, Poznań-Kraków, p. 307
Gilgen R (1989) Beziehung zwischen Wasserqualität und Vorkommen von Lemnaceae im Hänsiried (Zürich). Berichte Geobotanisches Institut ETH, Stiftung Rübel Zürich 55:89–130
Hermanowicz W, Dożańska W, Dojlido J, Koziorowski B (1999) Fizyczno-chemiczne badanie wody i ścieków. Arkady, Warszawa, p. 847
Hrivnák R (2002) Aquatic plant communities in the catchment area of theIpel river in Slovakia and Hungary. part I. classes Lemnetea and Charetea fragilis. Thaiszia J Botany 12:25–50
Katwijk MM, Roelofs JGM (1988) Vegetaties van waterplanten in relatie tot het milieu afdeling aquatische oecologie. Faculteit der Viskunde en Natuurwwetenschappen, Katholieke Universiteit Nijmegen, Nijmegen, p 133
Kłosowski S (1994) Untersuchungen über Ökologie und Indikatorwert der Wasserpflanzengesellschaften in naturnahen Stillgewässern Polens. Tuexenia 14:297–334
Kłosowski S, Jabłońska E (2009) Aquatic and swamp plant communities as indicators of habitat properties of astatic water bodies in north-eastern Poland. Limnologica 39:115–127
Kłosowski S, Pawlikowski P, Jabłońska E, Podgórska M (2020) Habitat conditions of the Salvinia natans phytocoenoses in the Vistula and Odra river valleys in Poland. Tuexenia 40:327–344
Kocić A, Hengl T, Horvatić J (2008) Water nutrient concentrations in channels in relation to occurrence of aquatic plants: a case study in eastern Croatia. Hydrobiologia 603:253–266
Laducci F, Gigante D, Venanzoni R (2011) An application of the cocktail method for the classification of the hydrophytic vegetation at lake Tresimeno (Central Italy). Fitosociologia 48:3–22
Landolt E (1986) The family of Lemnaceae-a monographic study. Vol. 1. Veröffentlichungen Des Geobotanischen Institutes ETH, Stiftung Rübel Zürich 71:1–566
Landolt E (1999) Pleustonic communities with Lemnaceae in South America. Appl Veg Sci 2:7–16
De Lyon MJH, Roelofs JGM (1986a) Waterplanten in relatie tot waterqwaliteit en bodemgesteldheid. Deel 1. Laboratorium voor Aquatische Oecologie. Katholieke Universiteit Nijmegen, Nijmegen, p. 106
De Lyon MJH, Roelofs JGM (1986b) Waterplanten in relatie tot waterqwaliteit en bodemgesteldheid. Deel 2. Laboratorium voor Aquatische Oecologie. Katholieke Universiteit Nijmegen, Nijmegen, p. 126
Matuszkiewicz W (2014) Handbook on determination of plant communities of Poland—In: Faliński JB (Ed.) Vademecum Geobotanicum 3, Wydawnictwo Naukowe PWN, Warszawa, p. 537
Michalska-Hejduk D, Kopeć D, Drobniewska A, Sumorok B (2009) Comparison of physical and chemical properties of water and floristic diversity of oxbow lakes under different levels of human pressure: a case study of the lower San River (Poland). Ecohydrol Hydrobiol 9:183–191
Mucina L, Bülmann H, Dierßen K, Tichý L (2016) Vegetation of Europe. hierarchical floristic classification system of vascular plant, bryophyte, lichen and algal communities. Appl Veg Sci 19:3–264
Papastergiadou E, Babalonas D (1993a) The relationships between hydrochemical environmental factors and the aquatic macrophytic vegetation in stagnant and slow flowing waters. I. Water quality and distribution of aquatic associations. Archiv Für Hydrobiologie, Supplementband 90:475–491
Papastergiadou E, Babalonas D (1993b) The relationships between hydrochemical environmental factors and the aquatic macrophytic vegetation in stagnant and slow flowing waters. II Evaluation of plant associations indicative value. Archiv Für Hydrobiologie, Supplementband 90:493–506
Passarge H (1978) Zur Syntaksonomie mitteleuropäischer Lemnetea-Gesellschaften. Folia Geobotanica Phytotaxonomica 13:1–16
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rivas-Martinez S (1982) Vegetatio Matritensis, I. Datos sobre la vegetación flotante dulceacuícola de la clase Lemnetea minoris. Lazaroa 4:149–154
Rivas-Martinez S, Fernández-Gonzáles F, Loidi J, Lousã M, Penas A (2001) Syntaxonomical checklist of vascular plant communities of Spain and Portugal to associations level. Interia Geobotanica 14:5–341
Sanda V, Popescu A, Peicea I (1987) Cenotaxonomy of vegetal groups in the class Lemnetea W. Koch et Tx. 1952 in Romania. Feddes Repertorium 98:441–446
Schaminée JHJ, Stortelder AHF (1995) Lemnetea minoris (Eensekroos-klasse). In: JHJ Schaminée, EJ Weeda, V Westhoff. Die vegetatie van Niederland. Deel 2. Plantengemeenschappen van vateren, moerassen en natte heiden. Opulus Press, Uppsala, Leiden: 13–28
Scoppola A (1982) Considérations nouvelles sur les végétations des Lemnetea monoris (R. Tx. 1955) em. A. Schwabe et R. Tx. 1981 et contribution a l’etude de cette classe en Italie central. Documents Phytosociologiques 6:1–130
Šmilauer P, Lepš L (2014) Multivariate analysis of ecological data using CANOCO 5, 2nd edn. Cambridge University Press, Cambridge, p 376
Spałek K (2005) Rzadkie I ginące zbiorowiska z klas Lemnetea minori, I Potametea na Równinie Opolskiej. Fragmenta Floristica Et Geobotanica Polonica 12:123–133
Spałek K, Stebel A (2020) Zespół Riccietum fluitantis Slavinić 1956 w starorzeczach południowo-zachodniej Polski. Fragmenta Floristica Et Geobotanica Polonica 27:269–276
Stojanović S, Škorić M, Nikolić L, Lazić D, Knežević A (2004) Salvinia natans (L.) All. (Pteridophyta) in the main canal network of HS DTD. Acta Herbologica 13:89–94
Šumberová K (2011) Vegetace volně plovoucich vodnich rostlin. In: M Chytrý (ed.): Vegetace Česke republiky 3.Vodni mokřadni vegetace: 43–96. Academia, Praha
The Plant List (2013): The plant list, version 1.1. – URL: http://www.theplantlist.org [Accessed 2022–06–30]
Tzonew RT, Dimitrov MA, Roussakova VH (2009) Syntaxa according to the Braun-Blanquet approach in Bulgaria. Phytologia Balcanica 15:209–233
Wiegleb G (1978) Untersuchungen über den Zusammenhang zwischen hydrochemischen Umweltfaktoren und Makrophytenvegetation in stehenden Gewässer. Arch Hydrobiol 83:443–484
Wójciak H, Urban D (2009) Rzęsowate (Lemnaceae) Bugu na odcinku Kryłów–Kostomłoty. Woda-Środowisko-Obszary Wiejskie 9:215–225
Wołek J (1997) Species co-occurrence patterns in pleustonic plant communities (class Lemnetea): are there assembly rules governing pleustonic community assembly? Fragmenta Floristica et Geobotanica. Supplementum 5:3–100
Wołek J, Kościółek A (2012) Występowanie, struktura i ekologia zbiorowisk pleustonowych (klasa Lemnetea) w województwie małopolskim (Polska). Fragmenta Floristica et Geobotanica Polonica 19:99–115
Wołek J, Walanus A (2000) Co-occurrence of lemnids in Argentina: a null model analysis. Fragmenta Floristica et Geobotanica 45:179–192
Wołek J (2006) Metody badań pleustofitów i ich zbiorowisk. In: J. Szmeja: Przewodnik do badań roślinności wodnej. Wydawnictwo Uniwersytetu Gdańskiego, Gdańsk, p. 467
Funding
This work was supported by the Polish Ministry of Science and Higher Education (Research Projects no. SUPB.RN.21.245 and no. SUPB.RN.23.245).
Author information
Authors and Affiliations
Contributions
SK conceptualized the study. SK, PP collected data in the field. SK, PP, EJ and MP performed the data analysis and results visualization. SK wrote the article, with help from MP, EJ. All authors gave final approval for publication and agree to be held accountable for the work performed therein.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest or competing interest.
Additional information
Communicated by Asaeda Takashi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kłosowski, S., Pawlikowski, P., Jabłońska, E. et al. The relationships between the physical and chemical properties of an aquatic environment and the floristic specificity of pleustonic communities in northern Poland. Aquat Ecol 57, 383–395 (2023). https://doi.org/10.1007/s10452-023-10016-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-023-10016-y