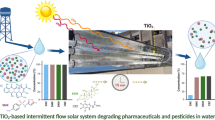
Abstract
Solar energy, along with other renewable resources, has the potential to be a major contributor to solving environmental issues in the future, as illustrated by the most recent advancements in solar photocatalytic technology. Indeed, wastewater treatment using a parabolic solar collector for industrial processes is gaining ground owing to improved system performance and economic benefits. The fabricated parabolic trough collector (PTC) incorporates reflective, parabolic panels that focus solar energy onto a transparent tube positioned along the parabolic focal line, where solar-powered photochemical reactions occur. This study investigated the design, implementation, and effectiveness of a concentrated sunlight system for removing industrial dyes and emerging large-use pharmaceutical contaminants in the presence of H2O2 at a small demonstrator scale (10 L/h). A spectrophotometric assessment revealed that subjecting Remazol Brilliant Blue (RBB, 60 ppm) and ciprofloxacin (CIP, 10 ppm) to irradiation in the presence of 0.1 M H2O2 (RBB) or 0.01 M H2O2 (CIP) for 3 h resulted in a degradation rate exceeding 60% and 80%, respectively. Furthermore, the total organic content (TOC) analysis indicates a very high total removal yield for RBB. On these bases, a techno-economic analysis is produced, and economic viability is discussed. The data reveal that the annual costs for water treatment, considering investment, electricity, and catalyst expenses over a 12-month period are significantly lower for our PTC-based prototype than for a comparable artificial UV-based equipment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The over-dependence on fossil fuel as a primary energy source has adverse effects on health, the environment, and climate. To address these problems, researchers are progressively finding ways towards the exploitation of energy from renewable sources. Numerous companies on a global scale manufacture solar technology and provide innovative solutions for cleaner and reduced-cost energy. The implementation of solar power is extensively employed across various domains including the provision of water heating and electricity generation. However, while a great deal of lab-scale research has been carried out on solar photocatalysis for water purification, prototype level studies are still relatively rare (Suresh et al. 2014; Krüger et al. 2008; Fernández-García et al. 2010; Pitz-Paal and Second 2014; Ydrissi et al. 2020; Esteban García et al. 2021).
Considering the application to wastewater purification, the use of PTCs in solar concentrating technology is prevalent, as an eco-friendly and sustainable strategy (Tanveer and Tezcanli Guyer 2013; Bhagwati et al. 2023; Saini et al. 2023). Over the long term, this system has the potential to be economically efficient. Following the establishment of the initial setup, operational expenses typically prove to be less than those associated with traditional, energy-intensive methods of wastewater treatment. Moreover, solar energy concentration systems can be structured in a modular manner, facilitating scalability and adaptability to various industrial environments (Saini et al. 2023; Fredriksson et al. 2021).
The substantial load of pollutants in industrial wastewater, along with its possible repercussions on water quality and public health, designates it as a significant environmental issue (Singh et al. 2023). Antibiotics stand out as emerging contaminants (Michael et al. 2013; Hou et al. 2020; Ferro et al. 2015). This problem is made urgent by the recent pandemic situation that has led to a massive increase in the use of especially antibacterial drugs for which conventional water treatment methods have limited removal capacity. The release of these pollutants into the environment involves risks to the ecosystem and health, due to both direct exposure and the generation of resistant bacteria (Harrington et al. 2022; Wang et al. 2022; Huang et al. 2022; Al-Riyami et al. 2018). Beyond the immediate environmental and health considerations, there exist concomitant economic implications. The emergence of antibiotic-resistant bacterial strains has the potential to engender heightened healthcare expenditures, precipitated by the imperative for more intricate and specialized treatments (Saeed et al. 2023).
Also, the discharge of textile industry waste products, specifically dyes, is a significant contributor to water pollution (Islam and Mostafa 2019; Ben et al. 2021; Lellis et al. 2019; Hossain et al. 2018). Notably, vinyl sulfone dyes—a type of reactive dye commonly applied to cotton, silk, and wool known as Remazol dyes, after their original trademark—are commonly employed (Lewis 2011). Despite their effectiveness for dyeing, vinyl sulfone dyes are highly toxic and pose a significant threat to both aquatic and human life. Once released into water bodies, vinyl sulfone dyes can persist for long periods of time and cause skin irritation, damage to internal organs, as well as lasting damage to ecosystems. Some dyes are recognized as potential cancer-causing agents, suggesting a risk of cancer development upon exposure. (Lellis et al. 2019). Additionally, certain dye compounds can disrupt the endocrine system, affecting hormonal balance in both humans and wildlife. This interference can result in various negative effects on reproductive and developmental processes (Kumar et al. 2008). Moreover, due to their specific chemical composition, the degradation of these dyes presents a particular challenge for conventional physical and chemical treatment methods (Gouvêa et al. 2000; Niebisch et al. 2010; Ara et al. 2013; Sanmuga Priya et al. 2015).
Advanced oxidation processes (AOPs) (Deng and Zhao 2015), particularly via photocatalysis, have been proposed as an alternative for the treatment of recalcitrant wastewater. These processes are characterized by the production of radical species, typically the ·OH radical, on semiconductor materials in contact with water, following the absorption of photons (Sirés et al. 2014; Rodríguez et al. 2004). Alternatively, radicals can be produced by a sacrificial source such as H2O2 either by simple photochemical dissociation or in photo-Fenton reactions (Lewis 2011). In particular, the light-induced homolytic dissociation of H2O2 can provide an efficient way to generate hydroxyl radicals (Liu et al. 2017) in water. These radicals then proceed to react with most organic pollutants resulting, in principle, into their complete mineralization (Venkatadri and Peters 1993). This procedure was adopted in our study to assess the effectiveness of a concentrated sunlight system in removing harmful industrial contaminants.
Two general forms of concentrating solar collectors exist, namely point-focus systems—encompassing parabolic dish collectors and central receiver towers—and line-focus systems, including linear Fresnel and PTC (Ydrissi et al. 2020; Xie et al. 2011; Coelho et al. 2014; Golli et al. 2021). Parabolic trough systems are the most deployed and advanced ones (Ydrissi et al. 2020; Alamr and Gomaa 2022; Cavallaro 2009). The fundamental concept underlying this technology entails the focusing of solar radiation onto a tubular receptor in which, wastewater may circulate so that the solar radiation energy will be an input for photochemical reactions. Indeed, the elementary parts of a PTC are the parabolic trough shape, which has a symmetrical section of a parabola around its vertex, and a reflective surface that concentrates the radiation on the tubular receiver located in the focus line of the parabola (Braham and Harris 2009).
This article outlines a straightforward design and its implementation for producing a solar concentrator with the aim of assessing the technical performance and economic viability of a PTC for solar wastewater treatment of two case-study compounds: a large-use antibiotic (Ciprofloxacin) and a vinyl sulfone industrial dye (Remazol Brilliant Blue). The first part describes the design of the adopted parabola configuration together with a detailed description of the main components. The second part presents the characterization of the mirror by means of reflectivity measurements and the results of tests including the evaluation of the manufactured PTC efficiency for the degradation of the two different types of pollutants (dyes, and pharmaceuticals) using H2O2 as a sacrificial hydroxyl radicals source. Finally, based on these data, a techno-economic assessment discussing the viability of this technology is presented.
Experimental and methodology
Reactor
Given that this work concerns the use of solar concentration for the purification of contaminated water, and that the size of the final apparatus must be on the scale of a small industrial prototype, we start with the description of the receiver hosting the treatment processes. Once the geometry of this component has been defined, the technical aspects of the concentrator will be presented, which must be suitable for the type of reactor.
The amount of circulating water is established to be 5 L moving from a tank to the solar focus, where a quartz tube (Helios quartz, quality class NHI®-1100) is positioned with a volumetric capacity of 2.85 L and acting as a reactor. The quartz cylinder is closed at both ends by two Teflon lids, with the one at the bottom containing an inlet port for liquid circulation in polyamide pipes (external diameter 8 mm, internal diameter 6 mm). The top lid is upheld by a T-junction, with one of the ports housing a thermocouple for temperature monitoring, and another port being the liquid outlet. Solution circulation is achieved using a dosing peristaltic pump (Seko, model KRFF0210) sustaining a flow rate of 10 L/h (Fig. 1).
The reactor is fixed on the mechanical structure of the solar concentrator. The structure is made of a ground-supported galvanized steel column, with a flat metal frame installed on top, where the parabolic mirror is mounted. The frame is moved by a two-axis automatic tracker motor system. The motors are guided by the signal of two sensors that constantly measure the maximum average irradiance and allow the motors to adjust to the best illumination throughout the day.
Solar parabolic trough concentrator design
Geometric parameters of PTC
A preliminary investigation of geometry is necessary to attain optimal effectiveness (Fig. 2) and capture the maximal radiative intensity obtained at the collector aperture (Yılmaz and Mwesigye 2018; Izweik et al. 2016; Waghmare and Gulhane 2017).
The collector's profile is established using the spatial coordinates (x and y) as follows:
In Eq. (1), \({\text{f}}\) is the focal length, which defines the position of the solar receiver part relative to the parabola vertex and may be calculated as:
φr denotes the rim angle which is obtained as follows:
Rr is the radius of the rim. The local mirror radius may be calculated at any point along the collector
The geometric characteristics of the designed collector are depicted in Table 1.
Geometric concentration ratio (GCR)
The geometric concentration ratio (GCR) for a parabolic trough collector refers to the ratio between the area that intercepts the solar radiation at the collector aperture (Aa) and the area that receives the sunlight (Arec) (Rodríguez et al. 2004; Spasiano et al. 2015). A high GCR indicates that a large proportion of incident light is focused onto a smaller area, providing a more intense energy flux (Fig. 3).
A scheme representing the parabolic trough solar collector (Esmaeili et al. 2023)
The geometrical concentration ratio is given by Izweik et al. (2016); Esmaeili et al. 2023):
a: aperture width, Dao: outer diameter of absorber tube.
The GCR has a minimum value of 6 when considering an outer diameter of 60 mm of the receiver tube and a maximum value of 118 while taking into account the focal spot size in the center of the quartz tube, which is about 3 mm.
Manufacture and assembly of different components
Support structure
Table S1 shows the schematic of subparts of PTC assembly with their respective dimensions. In addition to the base panel, the collector assembly comprises a pair of “supporting arms” to uphold the quartz tube and mirror. The “lateral apertures” are made of 10 mm thick solid aluminum and the reflectors were securely attached to the supporting arms utilizing aluminum C-profile inserts.
Parabolic reflectors
Finding the ideal reflective surface for solar photochemical applications is a challenge, as it requires a balance of UV reflectivity, weather resistance, and cost-effectiveness. A good compromise on these essential criteria is electropolished anodized aluminum (Spasiano et al. 2015; Jorgensen and Govindarajan 1991; Malato et al. 2002), such as the 0.5-mm-thick anodized aluminum MIRO-SUN® sun provided by Alanod, Germany. This mirror features a central core in aluminum with anodized layers on both sides, an epoxy layer is deposited in the back for protective installation and a protective weatherproof coating is present on the top.
The direct and reflected spectra of the sun were acquired on the PTC solar system under ideal atmospheric conditions, as shown by the spectra in Fig. 4a. These spectra were obtained using a mini spectrophotometer TM–UV–VIS (Hamamatsu Photonics K.K. model C10082CA, with minispec EvaluationSoftware), orienting it towards the direct sun rays and toward the reflective mirror. Remarkably, the mirror largely reproduces the solar spectrum without distortions. The mirror reflectance was measured by using a double-beam spectrophotometer (VARIAN Cary 5000), and corresponding reflectance spectra as a function of wavelength is presented in Fig. 4b. The mirror's optical properties feature a reflectivity above 80% in the measured range between 330 and 800 nm.
Receiver tube
The reactor tube is an enclosed medium that hinders the evaporation of volatile substances. Since the photochemical reactor envelops the operative medium, it must be transparent to UV–visible radiation and it must also withstand pressure build-ups for industrial perspectives. Therefore, the tubular-shaped reactor configuration is the most appropriate for handling and pumping water. This configuration is deemed ideal for the photoreactor setup to maintain the required pressure and flow rates needed for circulation systems.
The options for optically transmissive and UV-resistant materials are restricted. In addition, it is essential for the reactor component to be unaffected by the hazardous substances, predominantly OH radicals that may course through it. Consequently, the use of quartz for the tube (60.00 ext. dia. × 2.50 thick × 1200. 00 length mm) is preferred since it boasts remarkable UV transmittance and chemical resilience at high temperatures (Rodríguez et al. 2004; Qu et al. 2007; Olia et al. 2019).
Solar tracking system
To improve the effectiveness of direct solar radiation collection, the platform is equipped with a solar tracking system that keeps the aperture plane perpendicular to the incident sunlight (Spasiano et al. 2015), and its concentration on the focal line (Fig. 5). The collector’s dual-axial tracking system is maneuvered through both azimuthal (pertaining to an axis aligned with the north–south direction) and elevational (pertaining to an axis aligned with the east–west direction) axes. Additionally, the tracking system can be programmed to cope with adverse climatic conditions or precarious circumstances.
Sketch of the parabolic trough collector (Olia et al. 2019)
The tracking technology employed here is supplied by the DEGER company (DEGERtraker 3000NT) and it is originally designed to carry photovoltaic modules. The electronic part is used as provided, while the supporting frame has been adapted in-house to support the PTC instead of solar panels.
Preliminary test of solar wastewater treatment application
The manufactured PTC efficiency for the degradation of two different types of pollutants (dye and pharmaceutical) has been studied. 5 L of 60 mg/L of RBB and 10 mg/L of CIP solutions have been studied. The dyes were provided by a textile plant (ERAK, Istanbul, Turkiye) and the pharmaceutical CIP was obtained from Sigma Aldrich (Standard PHR1044). All irradiation experiments were preceded by a 30-min homogenization stage with the PTC (i.e., not facing direct sunlight on to PTC).
The temperature in the reactor was monitored by an insulated T-type thermocouple immersed in the studied liquid and kept under 70 °C by pausing the experiment if needed. This was done simply by moving the PTC out of focus and allowing the solution to cool underflow for a few minutes. This was only needed during experiments with the intensely colored RBB, while temperature never reached more than 45 °C with CIP. Solar irradiance values were measured with a pyrheliometer (Kipp&Zonnen, model CHP1) and they ranged between the values of 600 and 700 W/m2. The PTC is installed in Trento, northern Italy (46.0664°N, 11.1507°E) and experiments were carried out between 10:30 and 14:00 local time, during the months of March and July 2023. Every 15 min in the first hour of the experiment, 1 ml of the studied fluid was collected for the acquisition of the absorbance spectrum by a double-beam spectrophotometer (VARIAN Cary 5000 UV–VIS–NIR), to evaluate the reaction kinetically. After that, every 30 min, 1 ml of the solution was analyzed. Note that the degradation can be simulated using a pseudo-first order reaction ln (Ci/C) = k × t, authenticated by Langmuir–Hinshelwood for the decreasing amount of pollutant. The constant rate, k, can be obtained from the graphed linear correlation of ln(C0/C) versus t (Gopalakrishnan et al. 2011). Qualitative measurements of CO2 evolution were done using an IR sensor (COZIR GSS 5% model) and the Gaslab software for data acquisition. To evaluate the contribution of direct photolysis, blank experiments in the absence of H2O2 were also performed.
Textile industry dye
RBB dye degradation was examined in the presence of 0.01 M, 0.1 M, and 1 M H2O2 (130 v/v) under concentrated sunlight, as reported in Fig. 6a. It was observed that hydrogen peroxide was not activated in the absence of irradiation and the concentration of the dye remained unaltered for the duration of 30 min under dark.
At 0.01 M H2O2 concentration, the degradation of the RBB under light achieved only about 20% in 3 h under concentrated sunlight. Increasing the concentration of H2O2 to 1 M increased decolorization efficiencies, which resulted in 53% degradation in 1 h. With 0.1 M H2O2 concentration and 3-h reaction time it reached about 61% degradation. The rate constant k for the tests at H2O2 concentrations of 1 M, 0.1 M, and 0.01 M were 0.01178, 0.00446, and 0.00114 min−1, respectively (Fig. 6b). In the case of direct photolysis, in the absence of radical source, the observed discoloration was negligible.
Additionally, CO2 emissions were measured and reported (Figure S1) to qualitatively confirm the occurrence of mineralization. Indeed, •OH radicals may lead to the total oxidation of organic compounds with CO2 as the final product (Fendrich et al. 2018). Total organic content analysis confirms a mineralization yield of 72% with 0.1 M H2O2.
Ciprofloxacin (CIP)
For the CIP antibiotic, a photolysis test with concentrated sunlight resulted in 34% degradation, while with just 0.01 M of H2O2, the degradation resulted in 88% (Fig. 7a). Moreover, when using only non-concentrated (non-CC) light, without focusing, the degradation of CIP was negligible compared to the results obtained when exposing the solution to concentrated (CC) sunlight. This last test highlights the role of the parabolic solar concentrator. The k for CIP was 0.00965 min−1 (Fig. 7b). In this case, TOC analysis shows only an 8% mineralization yield. It is worth highlighting here that the aim of antibiotic residuals treatment is generally inactivation rather than mineralization, since the ultimate goal is that of preventing the arising of resistance in bacteria. To this end, the Ciprofloxacin structural change demonstrated by the disappearance of the UV–visible absorption might be sufficient. Further in-depth studies on the residual antibiotic activity of the treated water are underway to verify it.
Techno-economic assessment
An economic assessment of the small-scale prototype is presented in this section to elucidate the water treatment costs involved in the operation of the proposed system. This assessment takes into account a comparison between (1) our PTC-based process versus an artificial UV-light apparatus with 1000 W light power, which we consider with equivalent or higher efficiency as the proposed PTC, both in continuous circulation flow rate of 10 L/h and (2) a process using 0.1 M H2O2 as sacrificial agent versus the benchmark colloidal photocatalyst Evonik P25 TiO2 nanoparticles employed with 1 g/L concentration. Specifically, the study provides estimates in such conditions for 3-h treatments achieving 80% removal for RBB-based on the investment cost, electricity usage, and consumable materials pricing (Giménez et al. 2019).
Table 2 lists the components for both setups and the experimental conditions. A 12-month period of water treatment was chosen as the time reference with 3 weekly water treatments, meaning nearly 150 runs in a year, each of them with 5 L of 60 ppm concentration of RBB dye present in the batch.
Table 3 details the investment costs for both the PTC and the UV apparatus: the parts acquisition price, and the manufacturing and machined items costs. The 2-axis automatic solar tracking and the UV lighting prices are based on actual market quotations (September/2023). Calculation results show yearly costs of €1230 for the PTC while €3960 for the UV source. Shorter lifetimes (maximum 2 years) of UV lamps influence significantly (88%) the higher investment for the UV system requiring periodical changes, while the PTC tracker's longer-term life motor provides an advantage.
Table 4 details the energy costs for the solar PTC of this work and UV infrastructure for 1 year of water treatments. For this case, it was considered a single unit of treatment (3 h) related to the power consumption of each electrical part from both systems (KWh). The average electricity price of €0.4/KWh was attributed to considering the industrial electricity cost from September 2023 in northern Italy, for this condition energy costs resulted to be €21.60/year and €723.60/year for the PTC and UV respectively. Therefore, PTC energy costs are shown to be a fraction of only 3% in comparison to the UV system, it is important to point out that the PTC advantage is an estimate based on optimal weather conditions and day-light operation, while UV systems can act as backups and substitutes for PTC systems in bad weather conditions and overnight, thus they are complementary.
H2O2 as a sacrificial radical source and a comparison with P25 TiO2 commercial catalyst costs are presented in Table 5. For the case of 5 L water treatment 40 ml of 30% v/v H2O2 is added. In the case of the photocatalyst, a loading of 1 g/L was considered as a comparison due to previously published literature that achieved the same dye equivalent degradations of 80% referenced by TOC analysis (Mattle and Thampi 2013). This analysis does not consider the possible reuse of the TiO2 powder photocatalyst, as it would involve extra processing to recover the particles from the treated solution and this is not a standardized practice yet. However, reuse would in principle reduce the cost per treatment in the long term. The cost for the H2O2 is considered as the average price from three suppliers in tanks of 10 L (sufficient for nearly 18 months) and the P25 is based on updated values for the year 2023 from the original Evonik company. Yearly, the total cost for the acquisition of H2O2 is €120, and for the catalyst is €375. Notably, for a non-reuse case, the cost of using a nanoparticle photocatalyst is 56% superior to using H2O2.
In Table 6, the investment costs, the electricity, and catalyst costs are put together for the water treatment along the 12-month period to represent the total annual cost. Data shows that PTC with H2O2 cost is €1371.60/year, the same PTC with catalyst €1626.60/year. For the UV equipment, they are €4803.60/year with H2O2 and €5058.60/year with catalyst.
Lastly, Table 7 shows the cost per batch treatment of 5 L and an estimate per liter of water treated, PTC with H2O2 cost results in €1.83/L, the same PTC with catalyst €2.17/L. For the UV equipment is €6.40/L with H2O2 and €6.74/L with catalyst.
This assessment reveals that the stronger contributions to cost composition are related to the investment costs, being from 75.6 to 89.7% in the case of PTC, and from 78.3 to 82.4% in the case of a UV system, both considering the use either of H2O2 or TiO2. In specific, the constant feeding of the water treatment by H2O2 or adding in every step TiO2 photocatalyst plays a minor role in the composition of treatment cost per liter.
Conclusions
This investigation focuses on the design and implementation of a solar parabolic trough concentrator, including geometric parameters and optical characteristics, as well as on its efficiency in degrading wastewater containing specific industrial dyes and emerging pharmaceutical contaminants. In the case of Remazol Brilliant Blue dye, the solar PTC application has demonstrated a degradation yield of 61% of which a remarkable 72% is due to mineralization. For the Ciprofloxacin antibiotic case, the degradation resulted in 88%, with only 8% mineralization but likely inactivation. A follow-up work with microbiology investigation is currently underway in our labs. The versatility of this method also holds promising for other pollutants, as evidenced by our ongoing studies involving two different dyes, namely Remazol Yellow and Remazol Red. Finally, our economic evaluation reveals that the solar PTC can significantly reduce treatment costs when compared to UV systems, thereby creating more favourable industrial prospects for mitigating water-related pollution issues.
Availability of data and materials
Data can be made available on request.
References
Alamr MA, Gomaa MR (2022) A review of parabolic trough collector (PTC): application and performance comparison. Int J Appl Sci Dev 1:24–34. https://doi.org/10.37394/232029.2022.1.4
Al-Riyami IM, Ahmed M, Al-Busaidi A, Choudri BS (2018) Antibiotics in wastewaters: a review with focus on Oman. Appl Water Sci 8:1–10. https://doi.org/10.1007/s13201-018-0846-z
Ara NJ, Hasan MA, Rahman MA et al (2013) Removal of remazol red from textile waste water using treated sawdust—an effective way of effluent treatment. Bangladesh Pharm J 16:93–98. https://doi.org/10.3329/bpj.v16i1.14501
Ben SH, Bouket AC, Pourhassan Z et al (2021) Diversity of synthetic dyes from textile industries, discharge impacts and treatment methods. Appl Sci 11:1–21. https://doi.org/10.3390/app11146255
Bhagwati A, Shah M, Prajapati M (2023) Emerging technologies to sustainability: a comprehensive study on solar desalination for sustainable development. Sustain Manuf Serv Econ 2:100007. https://doi.org/10.1016/j.smse.2022.100007
Braham RJ, Harris AT (2009) Review of major design and scale-up considerations for solar photocatalytic reactors. Ind Eng Chem Res 48:8890–8905. https://doi.org/10.1021/ie900859z
Cavallaro F (2009) Multi-criteria decision aid to assess concentrated solar thermal technologies. Renew Energy 34:1678–1685. https://doi.org/10.1016/j.renene.2008.12.034
Coelho B, Varga S, Oliveira A, Mendes A (2014) Optimization of an atmospheric air volumetric central receiver system: Impact of solar multiple, storage capacity and control strategy. Renew Energy 63:392–401. https://doi.org/10.1016/j.renene.2013.09.026
Deng Y, Zhao R (2015) Advanced oxidation processes (AOPs) in wastewater treatment. Curr Pollut Rep 1:167–176. https://doi.org/10.1007/s40726-015-0015-z
El Ydrissi M, Ghennioui H, Bennouna EG, Farid A (2020) Techno-economic study of the impact of mirror slope errors on the overall optical and thermal efficiencies-case study: solar parabolic trough concentrator evaluation under semi-arid climate. Renew Energy 161:293–308. https://doi.org/10.1016/j.renene.2020.07.015
ElGolli A, Fendrich M, Bazzanella N et al (2021) Wastewater remediation with ZnO photocatalysts: green synthesis and solar concentration as an economically and environmentally viable route to application. J Environ Manag 286:112226. https://doi.org/10.1016/j.jenvman.2021.112226
Esmaeili Z, Valipour MS, Rashidi S, Akbarzadeh S (2023) Performance analysis of a parabolic trough collector using partial metal foam inside an absorber tube: an experimental study. Environ Sci Pollut Res 30:89794–89804. https://doi.org/10.1007/s11356-023-28732-1
Esteban García AB, Szymański K, Mozia S, Sánchez Pérez JA (2021) Treatment of laundry wastewater by solar photo-Fenton process at pilot plant scale. Environ Sci Pollut Res 28:8576–8584. https://doi.org/10.1007/s11356-020-11151-x
Fendrich M, Quaranta A, Orlandi M et al (2018) Solar concentration for wastewaters remediation: a review of materials and technologies. Appl Sci 9:118. https://doi.org/10.3390/app9010118
Fernández-García A, Zarza E, Valenzuela L, Pérez M (2010) Parabolic-trough solar collectors and their applications. Renew Sustain Energy Rev 14:1695–1721. https://doi.org/10.1016/j.rser.2010.03.012
Ferro G, Fiorentino A, Alferez MC et al (2015) Urban wastewater disinfection for agricultural reuse: effect of solar driven AOPs in the inactivation of a multidrug resistant E. coli strain. Appl Catal B Environ 178:65–73. https://doi.org/10.1016/j.apcatb.2014.10.043
Fredriksson J, Eickhoff M, Giese L, Herzog M (2021) A comparison and evaluation of innovative parabolic trough collector concepts for large-scale application. Sol Energy 215:266–310. https://doi.org/10.1016/j.solener.2020.12.017
Giménez J, Esplugas S, Malato S, Peral J (2019) Economic assessment and possible industrial application of a (photo)catalytic process: a case study. In: Marcì G, Palmisano LBT-HP (eds) Heterogeneous photocatalysis: relationships with heterogeneous catalysis and perspectives. Elsevier, pp 235–267
Gopalakrishnan K, Joshi HM, Kumar P et al (2011) Selectivity in the photocatalytic properties of the composites of TiO2 nanoparticles with B- and N-doped graphenes. Chem Phys Lett 511:304–308. https://doi.org/10.1016/j.cplett.2011.06.033
Gouvêa CAK, Wypych F, Moraes SG et al (2000) Semiconductor-assisted photocatalytic degradation of reactive dyes in aqueous solution. Chemosphere 40:433–440. https://doi.org/10.1016/S0045-6535(99)00313-6
Harrington A, Vo V, Papp K et al (2022) Urban monitoring of antimicrobial resistance during a COVID-19 surge through wastewater surveillance. Sci Total Environ 853:158577. https://doi.org/10.1016/j.scitotenv.2022.158577
He YL, Xiao J, Cheng ZD, Tao YB (2011) A MCRT and FVM coupled simulation method for energy conversion process in parabolic trough solar collector. Renew Energy 36:976–985. https://doi.org/10.1016/j.renene.2010.07.017
Hossain L, Sarker SK, Khan MS (2018) Evaluation of present and future wastewater impacts of textile dyeing industries in Bangladesh. Environ Dev 26:23–33. https://doi.org/10.1016/j.envdev.2018.03.005
Hou W, Yang J, Xu H et al (2020) Syntheses ofNymphaea-like BiOCl with oxygen vacancies for effective removal of tetracycline hydrochloride. CrystEngComm 22:3956–3964. https://doi.org/10.1039/d0ce00417k
Huang F, Hong Y, Mo C et al (2022) Removal of antibiotic resistance genes during livestock wastewater treatment processes: Review and prospects. Front Vet Sci. https://doi.org/10.3389/fvets.2022.1054316
Islam M, Mostafa M (2019) Textile dyeing effluents and environment concerns—a review. J Environ Sci Nat Resour 11:131–144. https://doi.org/10.3329/jesnr.v11i1-2.43380
Izweik TH, Ahmed MA, Albusefi AA (2016) Design, construction, and experimental testing of a parabolic trough collector for process heat applications. Int J Innov Res Sci Eng Technol 5:15890–15900. https://doi.org/10.15680/IJIRSET.2016.0509065
Jorgensen GJ, Govindarajan R (1991) Ultraviolet reflector materials for solar detoxification of hazardous waste. Opt Mater Technol Energy Effic Sol Energy Convers X 1536:194. https://doi.org/10.1117/12.49224
Krüger D, Pandian Y, Hennecke K, Schmitz M (2008) Parabolic trough collector testing in the frame of the REACt project. Desalination 220:612–618. https://doi.org/10.1016/j.desal.2007.04.062
Kumar V, Majumdar C, Roy P (2008) Effects of endocrine disrupting chemicals from leather industry effluents on male reproductive system. J Steroid Biochem Mol Biol 111:208–216. https://doi.org/10.1016/j.jsbmb.2008.06.005
Lellis B, Fávaro-Polonio CZ, Pamphile JA, Polonio JC (2019) Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol Res Innov 3:275–290. https://doi.org/10.1016/j.biori.2019.09.001
Lewis DM (2011) The chemistry of reactive dyes and their application processes. In: Clark M (ed) Handbook of textile and industrial dyeing. Elsevier, pp 303–364. https://doi.org/10.1533/9780857093974.2.301
Liu G, Ji J, Huang H et al (2017) UV/H2O2: an efficient aqueous advanced oxidation process for VOCs removal. Chem Eng J 324:44–50. https://doi.org/10.1016/j.cej.2017.04.105
Malato S, Blanco J, Vidal A, Richter C (2002) Photocatalysis with solar energy at a pilot-plant scale: an overview. Appl Catal B Environ 37:1–15. https://doi.org/10.1016/S0926-3373(01)00315-0
Mattle MJ, Thampi KR (2013) Photocatalytic degradation of Remazol Brilliant Blue® by sol-gel derived carbon-doped TiO2. Appl Catal B Environ 140–141:348–355. https://doi.org/10.1016/j.apcatb.2013.04.020
Michael I, Rizzo L, McArdell CS et al (2013) Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: a review. Water Res 47:957–995. https://doi.org/10.1016/j.watres.2012.11.027
Niebisch CH, Malinowski AK, Schadeck R et al (2010) Decolorization and biodegradation of reactive blue 220 textile dye by Lentinus crinitus extracellular extract. J Hazard Mater 180:316–322. https://doi.org/10.1016/j.jhazmat.2010.04.033
Olia H, Torabi M, Bahiraei M et al (2019) Application of nanofluids in thermal performance enhancement of parabolic trough solar collector: state-of-the-art. Appl Sci. https://doi.org/10.3390/app9030463
Pitz-Paal R (2014) Solar energy—concentrating solar power. In: Letcher TM (ed) Future energy, 2nd edn. Elsevier, Boston, pp 405–431
Qu M, Archer DH, Yin H (2007) A linear parabolic trough solar collector performance model. Proc Energy Sustain Conf 2007:663–670. https://doi.org/10.1115/ES2007-36052
Rodríguez SM, Gálvez JB, Rubio MIM et al (2004) Engineering of solar photocatalytic collectors. Sol Energy 77:513–524. https://doi.org/10.1016/j.solener.2004.03.020
Saeed U, Insaf RA, Piracha ZZ et al (2023) Crisis averted: a world united against the menace of multiple drug-resistant superbugs -pioneering anti-AMR vaccines, RNA interference, nanomedicine, CRISPR-based antimicrobials, bacteriophage therapies, and clinical artificial intelligence strategies to. Front Microbiol. https://doi.org/10.3389/fmicb.2023.1270018
Saini P, Singh S, Kajal P et al (2023) A review of the techno-economic potential and environmental impact analysis through life cycle assessment of parabolic trough collector towards the contribution of sustainable energy. Heliyon 9:e17626. https://doi.org/10.1016/j.heliyon.2023.e17626
Sanmuga Priya E, Senthamil Selvan P, Umayal AN (2015) Biodegradation studies on dye effluent and selective remazol dyes by indigenous bacterial species through spectral characterisation. Desalin Water Treat 55:241–251. https://doi.org/10.1080/19443994.2014.913999
Singh BJ, Chakraborty A, Sehgal R (2023) A systematic review of industrial wastewater management: evaluating challenges and enablers. J Environ Manag 348:119230. https://doi.org/10.1016/j.jenvman.2023.119230
Sirés I, Brillas E, Oturan MA et al (2014) Electrochemical advanced oxidation processes: today and tomorrow. A Review Environ Sci Pollut Res 21:8336–8367. https://doi.org/10.1007/s11356-014-2783-1
Spasiano D, Marotta R, Malato S et al (2015) Solar photocatalysis: materials, reactors, some commercial, and pre-industrialized applications. A comprehensive approach. Appl Catal B Environ 170–171:90–123. https://doi.org/10.1016/j.apcatb.2014.12.050
Suresh NS, Thirumalai NC, Rao BS, Ramaswamy MA (2014) Methodology for sizing the solar field for parabolic trough technology with thermal storage and hybridization. Sol Energy 110:247–259. https://doi.org/10.1016/j.solener.2014.09.020
Tanveer M, Tezcanli Guyer G (2013) Solar assisted photo degradation of wastewater by compound parabolic collectors: review of design and operational parameters. Renew Sustain Energy Rev 24:534–543
Venkatadri R, Peters RW (1993) Chemical oxidation technologies: ultraviolet light/hydrogen peroxide, Fenton’s reagent, and titanium dioxide-assisted photocatalysis. Hazard Waste Hazard Mater. https://doi.org/10.1089/hwm.1993.10.107
Waghmare SA, Gulhane NP (2017) Optical evaluation of compound parabolic collector with low acceptance angle. Optik (Stuttg) 149:359–371. https://doi.org/10.1016/j.ijleo.2017.09.039
Wang C, Mantilla-Calderon D, Xiong Y et al (2022) Investigation of antibiotic resistome in hospital wastewater during the COVID-19 pandemic: is the initial phase of the pandemic contributing to antimicrobial resistance? Environ Sci Technol 56:15007–15018. https://doi.org/10.1021/acs.est.2c01834
Xie WT, Dai YJ, Wang RZ, Sumathy K (2011) Concentrated solar energy applications using Fresnel lenses: a review. Renew Sustain Energy Rev 15:2588–2606. https://doi.org/10.1016/j.rser.2011.03.031
Yılmaz İH, Mwesigye A (2018) Modeling, simulation and performance analysis of parabolic trough solar collectors: a comprehensive review. Appl Energy 225:135–174. https://doi.org/10.1016/j.apenergy.2018.05.014
Acknowledgements
This work was supported by project Waste2Fresh, funded by the European Union under the Horizon 2020 research and innovation program, grant agreement no. 958491 and project FarSol, funded by the Caritro Foundation (Research & Development 2020 Grant). We also received support from project “Produrre Idrogeno in Trentino—H2@TN” (PAT-Trento). We thank EcoTop srl (Italy) for useful discussions.
Funding
Open access funding provided by Università degli Studi di Trento within the CRUI-CARE Agreement. Funding was provided by: Fondazione Cassa Di Risparmio Di Trento E Rovereto (FarSol), Horizon 2020 Framework Programme (No 958491) and PAT-Trento (H2@TN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Responsible Editor: Mohamed Ksibi.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El Golli, A., Fendrich, M., Bajpai, O.P. et al. Parabolic trough concentrator design, characterization, and application: solar wastewater purification targeting textile industry dyes and pharmaceuticals—techno-economic study. Euro-Mediterr J Environ Integr (2024). https://doi.org/10.1007/s41207-024-00531-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41207-024-00531-1