Abstract
The study centers on the valorization of Beta macrocarpa Guss., an endangered Mediterranean wild plant that grows in Tunisia. This plant is disappearing due to a reduction in marginal areas and a lack of awareness of this important crop wild relative (CWR). This prompted us to carry out work to assess the nutritional and functional value of its plant shoots in relation to physicochemical soil properties at three different Tunisian sites covering the north (Sijoumi), the center (Enfidha) and the south (Kerkennah) of the country. All soil samples showed an alkaline pH and high salinity. Sijoumi, Enfidha and Kerkennah soils were classified as loamy, silty clay loamy and sandy, respectively. Chemical analysis revealed that all soils, especially the sandy one, were low in total nitrogen, organic matter and microelements. Plant analysis showed that shoots harvested from the loamy soil presented the highest levels of carbohydrate (19.1 g/100 g FW) and fiber (6.1 g/100 g FW) and the greatest energetic value (94 kcal/100 g FW), whereas shoots collected from the sandy soil showed the highest contents of protein (4.1 g/100 g FW), ash (5.2 g/100 g FW), total polyphenols and flavonoids (39.01 mg GAE/g DW; 27.8 mg CE/g DW), and the greatest DPPH scavenging capacity (IC50 = 0.74 mg/ml). The results suggest that Beta macrocarpa, which naturally grows in poor and salt-affected soils, could play a crucial role in maintaining the biodiversity and sustainability of agro-ecosystems, particularly in marginal areas, and could also provide an alternative source of food with significant nutritional value and health benefits.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Today, while scientists have different opinions on the origin of climate change, all of them agree that soil consumption, soil degradation and the loss of biodiversity along with the reduction in food production are due mainly to the development of a bad farming paradigm and, to some extent, the increase in the global human population. In any case, some scientists state that climate change is exerting considerable pressure upon agriculture and food production (Parry et al. 2004; Ray et al. 2015) at a time when an increase in food production of about 60% is needed to supply the increasing global population, which will reach 9.7 billion by 2050 (United Nations 2019). Warm temperatures and frequent periods of drought cause decreases in crop production (Long and Ort 2010) and quality (Dwivedi et al. 2013), and the scientific community from some countries in the Mediterranean Basin is focusing on this issue: it is attempting to identify the associated problems in order to find sustainable solutions, as in the project REstoration ACTions for the MEDiterranean (REACT4MED) (https://react4med.eu). Moreover, climate change also affects the distribution and virulence of crop pests and diseases, which constitute an additional threat to agricultural production (Patz and Kovats 2002; McMichael et al. 2006). This will have a considerable impact on agricultural sustainability and food security, which is especially important given that around 800 million people are already suffering from food insecurity and malnutrition (FAO 2014). To cope with these negative impacts of climate change which our world is facing nowadays, we need to develop a more resilient agriculture system that is capable of ensuring food security over the coming decades (Gasparini et al. 2021). Thus, the exploration of new plant resources with significant nutrition potential and a high tolerance to climatic change should be considered.
One alternative source of food is wild edible plants (WEPs), which are naturally adapted to a wide range of soils and climates and can survive in hard environmental conditions (Zait and Schwartz 2018). They constitute a valuable reservoir of nutritious and healthy foods: they are rich in minerals, vitamins and fiber (Baldermann et al. 2016) as well as in plant secondary metabolites, i.e., alkaloids, polyphenolic compounds and essential fatty acids, and they also possess some medicinal properties, including anti-bacterial, hepatoprotective and anti-carcinogenic activities (Heywood 1999). For millennia, WEPs have supported food security (Addis et al. 2005) and have alleviated malnutrition and poverty in rural communities of the Mediterranean Basin (Maxted and Vincent 2021), where farming is the main source of food and income (Baldermann et al. 2016; Harisha et al. 2021). Even today, they are used as additional sources of healthy food, bioactive compounds and medicine (Keller et al. 2005; Termote et al. 2011; Ulian et al. 2020; Casella et al. 2023). Among WEPs, crop wild relatives (CWRs) constitute an important category of wild edible plants (Maxted and Kell 2009; Perrino et al. 2014; Perrino and Calabrese 2014; Maxted et al. 2006; Pinela et al. 2017; Perrino and Perrino 2020; Perrino and Wagensommer 2021; Accogli et al. 2023) that are closely related to cultivated species of socio-economic value (Maxted et al. 2006) and can be used to improve tolerance and/or resistance to biotic and abiotic stresses and to enhance production in terms of quantity and quality (Redden 2015).
Beta macrocarpa is an annual CWR species that belongs to the Chenopodiceae family (Pottier Alapetite 1979) and is closely related to sugar beet crops (El-Mokni et al. 2022). It is widespread in inland or coastal habitats in western and eastern Mediterranean areas (Hessini et al. 2020). In Tunisia, one of the most important hotspots of CWR diversity in the Mediterranean Basin, this species is exclusively found in salty ecosystems, precisely at the edge of sebkhas, among halophyte tufts (Abdelly 1997). It is a salt-tolerant plant that can withstand high soil salinity through morphological, structural and functional adaptations (Hamouda et al. 2016), and so it could potentially be used to valorize the salt-affected soils in marginal areas (Abdelly 1997; Barreira et al. 2017). In some rural regions of Tunisia, this wild beet is considered part of the local food tradition and consumed as cultivated vegetables (Dop et al. 2020).
Despite the significant economic, social and ecological benefits that B. macrocarpa can offer, and the fact that is still traditionally consumed by local communities, it remains underexploited. This is due to a lack of knowledge of its nutritional value and health benefits (Ratnayake et al. 2021; Nirmala et al. 2022). An analysis of the plant's proximate and mineral composition and its functional properties could enable it to be recommended as part of a balanced modern diet, as a food supplement or as a functional ingredient for large-scale human consumption.
The present study aimed to (i) evaluate the proximate and mineral composition as well as the functional properties of Beta macrocarpa growing in different pedo-climatic areas in relation to soil characteristics and (ii) highlight its potential as a new healthy and resilient food source with high nutritional value. To the best of our knowledge, this is the first study to assess the nutritional profile and functional properties of Beta macrocarpa shoots growing in different regions of Tunisia in relation to edaphic factors.
Material and methods
Sampling sites
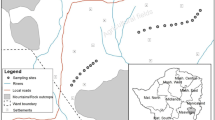
The sampling of soil and plants was carried out in three different sites (Fig. 1a, b). The first site was the Sebkha of Sijoumi (36° 75′ 69.51″ N, 10° 12′ 22.26″ E), located in the north of Tunisia (Tunis Governorate), which is characterized by a semi-arid climate in its temperate winter (P = 450 mm, annual T = 18.6 °C); the second one was situated in the center of the country, in Sebkha of Enfidha (36° 05′ 31.5″ N, 10° 27′ 23.5″ E) (Sousse Governorate), which is also characterized by a semi-arid climate in its temperate winter (P = 339 mm, annual T = 19.5 °C); and the third site was located in the south, more exactly in the Kerkennah Islands (34° 70′ 21.02″ N, 11° 14′ 84.65″ E) (Sfax Governorate), which are characterized by an arid climate in their warm winter (P = 195 mm, annual T = 25 °C).
Soil analysis
For soil analysis, 1 kg of soil was taken at 20–30 cm depth at each site and then dried in the air at ambient temperature, ground, sieved and stored until analysis in the laboratory. The soil texture was determined through its particle size distribution determined via the pipette method (Piper 1947; El-Amier et al. 1995), and the textural class was attributed according to the USDA classification. The pH of the soil was measured using the filtrate obtained from a soil suspension in distilled water (1:2.5) by using a pH meter. The electrical conductivity (EC) was determined on an aqueous soil extract (1:2 w/v) using a conductometer and expressed in dS/m. Among the chemical properties, the determination of the total carbonate CaCO3 percentage (%) was achieved using the gas volumetric method and a Dietrich–Fruhling calcimeter to quantify the CO2 evolved in a closed system after HCl treatment (Leogrande et al. 2021). The total nitrogen was determined through the modified Kjeldahl method proposed by Bremner (1996). In order to evaluate the available phosphorus in the soil, the method of Olsen and Sommers (1982) was used. The organic carbon was determined by the Walkley–Black method described by Nelson and Sommers (1996). The amount of organic matter in the soil was calculated according to the following formula: organic matter (%) = organic carbon (%) × 1.724. The exchangeable cations (Na+, Ca2+, Mg2+ and K+) were determined by using BaCl2 (1 M) and triethanolamine buffered at pH 8.1 as an exchange solution. The assessment of cations was achieved using inductively coupled plasma atomic emission spectroscopy (ICP-OES). The microminerals (Fe, Mn, Cu, Zn and Ni) were extracted by DTPA (5 mM), CaCl2 (10 mM) and triethanolamine buffered at pH 7.3. The determination of available microminerals was carried out by using ICP-OES.
Plant material
The Beta macrocarpa (Guss.) plant specimens (Fig. 2) from three populations belonging to the same sites (Sijoumi, Enfidha and Kerkennah) in which soil sampling was performed as described above were collected at the flowering stage (March–April 2020). The harvested plants were authenticated in situ according to the morphological characters reported by Pottier Alapetite (1979) in Flore de la Tunisie, and the voucher specimens were deposited in the herbarium of the Tunisian National Institute of Agronomic Research (INRAT). For plant analysis, the roots were removed from the freshly collected plants, and the aerial parts (stems, leaves and flowers) were washed with tap water, rinsed with distilled water and dried in a ventilated oven at 50 °C for 24 h. After that, the dried samples were ground into powder using a grinder and kept at room temperature until further use (Piscitelli et al. 2015; Perrino et al. 2021).
Plant analysis
Proximate composition
The proximate composition, including moisture, crude proteins, carbohydrates, fat, fiber, ash and energy, was determined using the official Association of Official Agricultural Chemists (AOAC 1990) methods. The moisture, which corresponds to the weight loss upon drying the plants in the oven, was determined from the fresh (FW) and dry weights according to the following formula:
Total protein content was estimated from the total N content measured following the micro Kjeldahl method. The ash content was obtained through sample incineration at 550 °C. The crude fat was extracted with petroleum ether using a Soxhlet apparatus and determined according to the method of Señoráns and Luna (2012). The crude fiber was measured by treatment of the sample with 1.25% H2SO4 and 1.25% NaOH, with filtering and washing with hot water performed after each step. After disgestion, the residue obtained was dried in an oven at 105 °C ± 2 °C for 2 h and ashed at 550 °C in a furnace. The loss of weight on ignition was expressed as the content of crude fiber. The total carbohydrate value was given by 100% (percentage of ash + percentage of fat + percentage of protein). All results were expressed in g/100 g (DW). The energetic value was calculated by multiplying the total carbohydrate, lipid and protein values by factors of 4, 9 and 4, respectively, summing the products, and expressing the result in kilocalories (kcal) (Guil-Guerrero et al. 1998).
Mineral composition
The mineralization of the samples was carried out using a microwave mineralizer. For mineral element analysis, about 0.3 g of each sample was digested with 6 ml of concentrated HNO3 and 1 ml of 30% H2O2, and this mixture was left to react overnight. After digestion, the analysis of macroelements (Ca, Mg, K and Na) and microelements (Zn, Cu, Mn, Fe and Ni) was carried out by ICP-OES. The colorimetric method of Murphy and Riley (1962) was used for the determination of phosphorus in mineralized samples. Results were expressed in mg/100 g (FW).
Total phenolic compounds and total flavonoid content
The total polyphenol contents of the methanolic extracts of all samples were determined by the Folin–Ciocalteu method adapted by Dewanto et al. (2002). Briefly, 125 ml of each extract was diluted with 500 ml of distilled water and 125 ml of the Folin–Ciocalteu reagent was added. This mixture was allowed to stand for 3 min before 1250 ml of Na2CO3 (7%) was added. The solution was then incubated in the dark for 90 min before measuring the absorbance at 760 nm in a UV–visible spectrophotometer (T60). Total polyphenol contents of extracts were expressed in mg gallic acid equivalent per gram (mg GAE/g DW) using a calibration curve with gallic acid (0–500 mg/ml). The total flavonoid contents of the extracts were determined using the aluminum chloride colorometric method (Dewanto et al. 2002). In this, 250 ml of extract was mixed with 75 ml of NaNO2 (5%). Next, 150 ml of AlCl3 (10%) and 500 ml of NaOH (1 M) were added. The absorbance was measured at 510 nm. The total flavonoid content was calculated using the calibration curve for catechin (0–500 mg/ml) and was expressed in mg of catechin equivalent per g of dry weight (mg CE/g DW).
DPPH scavenging assay
The capacity of the extracts to scavenge DPPH (2,2-diphenyl-1-picrylhydrazyl) was investigated via the procedure formerly described by Ben Mahmoud et al. (2022). For that, 50 ml of a particular concentration of ascorbic acid (five concentrations in the range 0.5–5 µg/ml were tested) or extract (five concentrations in the range 0.5–4 mg/ml were tested) was mixed with 250 ml of an ethanolic solution of DPPH (8.66 × 10–5 M), and these were allowed to react in the dark for 30 min. Then, the absorbance of the resulting solution was read at 517 nm. The antiradical activity was determined using the following formula:
where Abscontrol is the absorbance of the control (DPPH solution without the extract) and Abssample is the absorbance of DPPH solution with the extract. The antiradical activity was expressed as the IC50 value (mg/ml)—the extract dose required to cause a 50% decrease in the absorbance at 517 nm. Ascorbic acid was used as a positive control for comparison.
Statistical analysis
All measured data were reported as the mean ± standard deviation of triplicate measurements. Significant differences (P < 0.05) between means were analyzed by ANOVA and the Duncan test using the program package Statgraphics Centurion version 18.1.01. The Duncan test, at a 5% threshold, helps to group the individuals whose means for a particular parameter are not significantly different (Dagnelie 1975); it is slightly more conservative than the Fisher LSD test. The analysis of variance was performed for a 95% confidence interval (or 0.05) using the estimated means.
Results
In order to mitigate the impacts of climate change, to preserve ecosystems and to support the food security of the growing global population, we have to develop a sustainable agriculture system based on more resilient crops. The exploration of non-conventional crops, such as CWRs, with significant quality and high adaptability to climate change constaints is therefore highly recommended. In this context, the present work attempted to explore an edible CWR, Beta macrocarpa, as an alternative food source by analyzing its proximate and mineral composition and assessing its functional properties. Soil physicochemical parameters were also analyzed.
Taxonomy and nomenclature
The genus Beta L. belongs to the subfamily Betoideae and the family Chenopodiaceae. Currently, two sections are recognized in the Beta genus: Beta and Corollinae; the latter now includes the former section Nanae (Hohmann et al. 2006). The ten species of the section Beta are wind pollinators that are mainly distributed along European coasts. B. adenensis Pamukç. grows in some eastern Mediterranean areas in Greece and Turkey, while B. corolliflora Zosimovic ex Buttler, B. lomatogona Fisch. & C.A. Mey. and B. macrorhiza Steven are reported in Iran, the Transcaucasus and Turkey, B. lomatogona is found in Cyprus, and B. macrorhiza is found in the North Caucasus. B. nana Boiss. & Heldr., B. patula Aiton and B. trojana Pamukç. ex Aellen are endemic to Greece, two small islets of the Madeira Archipelago and Turkey, respectively. B. trigyna Waldst. & Kit. is reported in many countries, ranging from the former Yugoslavia to Turkmenistan and Ukraine. B. palonga R.K. Basu & K.K. Mukh. is native to Bangladesh, India and Pakistan (Powo 2023, https://powo.science.kew.org/). Finally, B. macrocarpa Guss., which is a primary wild relative of cultivated beets, is native to Algeria, the Balearic Islands, the Canary Islands, Cyprus, Greece, Italy, Crete, Libya, Morocco, Palestine, Portugal, Sardinia, Sicily, Spain, Tunisia and Turkey, while it was introduced to France and Northwest Mexico (Tutin et al. 1993; Dobignard and Chatelain 2011; Rebman et al. 2016).
B. macrocarpa is distinguished from B. vulgaris subsp. maritima (L.) Arcang. by some morphological characteristics (Letschert 1993). B. vulgaris subsp. maritima is characterized by a stem which is up to 80 cm long and procumbent to erect; its leaves are up to 10 cm long; its cymes have about 1–3 flowers; and its roots are usually not swollen. On the other hand, B. macrocarpa is characterized by basal leaves that are oblong-spathulate to triangular-ovate; its cymes have about 2–3 flowers; it has a pelviform receptacle and hard fruits, and the segments are up to 5 mm in size, erect, and often incurved at the apex (Tutin et al. 1993). Both diploid and tetraploid forms of the Beta section are found within B. macrocarpa, which is exclusively distributed in the Canary Islands. The tetraploid type is believed to be a natural amphidiploid hybrid between diploid B. macrocarpa and an unknown diploid of the B. vulgaris complex (Abe and Tsuda 1987). The diploid type of B. macrocarpa is distributed in the Mediterranean Basin, while the tetraploid type is endemic to the Canary Islands (2n = 36) (Lange and De Bock 1989).
Homotypic synonyms. Beta vulgaris var. macrocarpa (Guss.) Moq. in Chenop. Monogr. Enum. 14 (1840); Beta vulgaris subsp. macrocarpa (Guss.) Thell. in Mém. Soc. Sci. Nat. Math. Cherbourg 38:190 (1912).
Heterotypic synonyms. Beta bourgaei Coss. in Notes Pl. Crit. 44 (1849).
Conservation status. B. macrocarpa represents an area of occupancy (AOO) of less than 500 km2 in Europe, with a discontinued distribution. It is therefore regionally assessed as being endangered (EN) (Bilz et al. 2011).
Soil properties
The results of the physicochemical analysis of the soils at the Sijoumi, Enfidha and Kerkennah sites are presented in Table 1.
The soil texture analysis revealed that Sijoumi soil consisted of clay (21%), silt (35%) and sand (43%); Enfidha soil was made up of clay (38%) and silt (50%); whereas Kerkennah soil was mainly sand (96%). According to the USDA classification, Sijoumi soil is classified as loamy, Enfidha soil as silty clay loamy and Kerkennah soil as sandy. With regards to pH and electrical conductivity (EC), statistical analysis (P < 0.05) showed that there was no significant variation; all soils displayed an alkaline pH (> 8), and high electrical conductivities of around 12, 25 and 32 dS/m were observed for the Kerkennah, Enfidha and Sijoumi soils, respectively (Table 1).
Chemical analysis results indicated that the total carbonate (TC), total nitrogen (TN), organic carbon (OC), organic matter (OM) and available phosphorus (P) contents were higher in Sijoumi and Enfidha soils than in Kerkennah soil. The TC level was about 15% and 12% in Sijoumi and Enfidha soils, respectively, against only 5% in Kerkennah. TN was found at levels of 1.1, 0.9 and 0.5 g/kg at the Sijoumi, Enfidha and Kerkennah sites, respectively. OC and OM contents differed significantly (P = 0.01) among the soil samples; the highest values (18.6 g/kg and 32 g/kg) were registered in Sijoumi soil. Available phosphorus (P) levels were about 21.5 and 25.4 mg/kg, respectively, in Enfidha and Sijoumi versus 6.4 mg/kg in Kerkennah. The data concerning exchangeable cations revealed that Mg2+ was the most abundant cation in all soils, particularly in Enfidha (5187 mg/kg); Ca2+ was more abundant in Sijoumi soil (753 mg/kg) than in Enfidha and Kerkennah soils. K+ (57–63 mg/kg) and Na+ (5–89 mg/kg) were present at the lowest levels. For microelements, an ANOVA test revealed a significant variation (P < 0.01) among the three analyzed soils (Table 1). Fe was the most abundant element in all soils (515–743 mg/kg), followed by Mn (25–142 mg/kg). Cu, Ni and Zn were detected in significantly smaller amounts. The lowest levels of all microelements were found in Kerkennah soil.
Plant analysis
Proximate composition
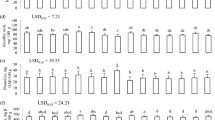
The proximate composition, including the moisture, protein, fat, carbohydrate, crude fiber and ash contents as well as the energetic value, was expressed on a fresh weight (FW) basis (Table 2). According to ANOVA, all of the nutrient contents varied significantly (P < 0.05) among the plant samples. The moisture content was generally high in all shoot samples, especially in Enfidha (85.4 g/100 g FW) and Kerkennah (81.3 g/100 g FW) shoots. The protein levels were higher in Kerkennah (4.1) and Sijoumi (3.1 g/100 g FW) than in Enfidha (2.4 g/100 g FW) shoots. The highest rate of ash (5.2 g/100 g FW) was found in shoots from the Kerkennah site. Concerning crude fat and crude fiber, the greatest amounts were registered in Sijoumi shoots. Carbohydrates constituted the most abundant nutrient in all populations (P < 0.01); the highest content (19.1 g/100 g FW) was obtained in Sijoumi shoots, with a significantly lower rate (9.1 g/100 g FW) found in both Enfidha and Kerkennah shoots. The highest energetic value (94 kcal/100 g FW) was also recorded in Sijoumi shoots.
Mineral composition
As shown in Table 2, the results concerning the composition of the B. macrocarpa shoots in terms of macrominerals showed significant differences (P < 0.05) in the levels of Ca, Mg and Na. Na was the most abundant element found in B. macrocarpa shoots, especially at the Kerkennah site, with a concentration of about 1056 mg/100 g DW. Ca and Mg were found at their highest levels in shoots at the Sijoumi and Kerkennah sites. P was the least abundant macromineral in B. macrocarpa shoots (38–53 mg/100 g FW). The micromineral composition showed that Fe was the most abundant (4.6–9.6 mg/100 g FW), followed by Mn, Zn and Cu. Ni was detected in the smallest quantities, ranging from 0.06 to 0.09 mg/100 g FW (Table 2).
Functional properties
Functional properties of B. macrocarpa shoots were evaluated, including the total phenolic compounds (TPC), total flavonoid (TF) content and DPPH scavenging ability. Statistical analysis showed highly significant variation (P < 0.01) among shoot extracts. As shown in Table 4, TPC and TF recorded the highest values (of 39.01 mg GAE/g DW and 27.8 mg CE/g DW, respectively) in methanolic extracts of shoots from the Kerkennah site. Significantly lower values were found in shoots from the Enfidha (11.26 mg GAE/g DW; 5.86 mg CE/g DW) and Sijoumi (9.07 mg GAE/g DW; 4.61 mg CE/g DW) sites. Regarding DPPH scavenging activity, the results revealed that the antioxidant activity (IC50 = 0.74 mg/ml) of methanolic extracts of Kerkennah shoots was stronger than that of extracts from shoots from the Enfidha (IC50 = 1.36 mg/ml) and Sijoumi (IC50 = 2.64 mg/ml) sites.
Discussion
Soil properties
The analysis of physical and chemical parameters of the soil is of interest since those parameters strongly affect the growth and development of plants (Mehalaine and Chenchouni 2020).
In our study, the analysis of physical parameters revealed different soil textures according to the USDA classification: Sijoumi soil is classified as loamy, Enfidha soil as silty clay loamy and Kerkennah soil as sandy. Regardless of their different textures, all soils displayed an alkaline pH and high electrical conductivity (12–32 dS/m), indicating very high salinity (Nguyen et al. 2020). In arid and semi-arid regions such as our study area, the soils are generally alkaline and highly saline (Bello et al. 2021), which can be attributed to environmental factors like low precipitation, a high temperature and evaporation.
Regarding chemical properties, the results show that the soils contain different levels of total carbonate. According to Marschner (1995), these soils can be considered to be calcareous soils because they contain a certain amount of total carbonate. Total carbonate is an inorganic source of carbon that is mainly present in soils in arid and semi-arid areas in the form of calcium carbonate (CaCO3) (Leogrande et al. 2021), which affects both the physical and chemical properties of the soil (Leogrande et al. 2021) as well as its fertility (by reducing the availability of nutrients; Matar et al. 1992).
The nutritional status of the sandy soil (at the Kerkennah site) was the poorest, especially in terms of organic carbon, organic matter and some minerals like Fe, Mn and Zn. The low fertility of sandy soil has also been reported in previous studies (Papafilippaki et al. 2015; Artikova et al. 2021) and has been attributed to the low nutrient retention capacity of such soils (Cambrollé et al. 2015). While the loamy soil exhibited relatively high fertility, especially in terms of organic matter and organic carbon, it is still poor (Dridi and Arfaoui 2017). In semi-arid regions, calcareous soils with high salinity and alkalinity are deficient in plant nutrients (Akhtar et al. 2019); in particular, organic matter (Aliat et al. 2016), organic carbon (Vimlesh and Giri 2011), organic nitrogen (Schröder 2014) and minerals (Akhtar et al. 2019; Wahba et al. 2019). Organic matter, which is the main reservoir of soil organic carbon and nitrogen (Hoffland et al. 2020), is reduced by high soil salinity (Qadir et al. 2000; Gonçalo Filho et al. 2019), leading to reductions in organic carbon and nitrogen. Moreover, the availability of minerals (P, K, Mg, Fe and Zn) is impaired in alkaline calcareous soils due to a nutritional imbalance between these minerals and calcium (Wahba et al. 2019).
Plant analysis
The analysis of the nutritional value of wild plants is crucial to demonstrate their edibility for humans. In this study, we assessed the nutritional properties of Beta macrocarpa shoots collected from three different sites (Sijoumi, Enfidha and Kerkennah) through their proximate and mineral compositions.
Proximate composition analysis showed a high moisture content in all B. macrocarpa shoots; the moisture content exceeded those found in Beta maritima (Tan et al. 2017), some leafy vegetables (Slavin and Lloyd 2012), and even halophytes such as Arthrocnemum indicum, Halocnemum strobilaceum and Suaeda fruticosa (Maatallah-Zaier et al. 2020). The low moisture content (73.6 g/100 g FW) recorded in Sijoumi shoots could be attributed to the high level of soil salinity. A similar observation has been reported for Limonium algarvense leaves, for which the moisture content level decreases with increasing salinity (Rodriguez et al. 2020).
This parameter is crucial to the plant’s ability to withstand severe climatic conditions, especially salinity and drought (Flowers and Colmer 2008; Hameed and Khan 2011). It allows the elevated salt concentration and the osmotic pressure in the tissues of salt-stressed plants to be reduced (Dajic 2006). It is also a very important parameter for food processing, storage and food quality (Okello et al. 2018). B. macrocarpa shoots also showed higher crude protein levels (2.4–4.1 g/100 g FW) than those reported in the literature for wild (Tan et al. 2017; Pinela et al. 2017) and cultivated (Baião et al. 2017) beets as well as for some leafy vegetables such as green lettuce, red lettuce and spinach (Slavin and Lloyd 2012; Colonna et al. 2016). Proteins constitute about 20% of the human body (Satter et al. 2016), and they are involved in the building of muscles, bones and skin and in the control of many body functions through hormone formation (Mau et al. 1999). The highest protein content was found in shoots growing in the poorest soil (the Kerkennah site). This finding corroborates a previous study which showed that the protein level in leaves of Crithmum maritimum increased as soil fertility decreased (Martins-Noguerol et al. 2023). Protein metabolism is affected by a low availability of minerals, by interactions between them (Sun et al. 2018), and/or by an impoverishment in nitrogen, which is the main component of amino acids (Schlüter et al. 2013). So, the higher protein level obtained in Kerkennah shoots could be linked to the low nitrogen and mineral contents of the soil.
B. macrocarpa shoots can cover between 4.7 to 8.1% of the recommended daily protein intake (Table 3) (Meyers et al. 2006) and can therefore be proposed as a good protein source. Kerkennah shoots also accumulated the highest ash level (5.2 g/100 g FW). The ash content represents the total amount of minerals within the biomass (Satter et al. 2016). It is vital for determining the nutritive value of foods (Agboola and Adejumo 2013). According to our results, the shoots of B. macrocarpa accumulated ash at a higher level than reported for B. maritima (Tan et al. 2017) and could provide a valuable amount of minerals for human consumption. B. macrocarpa shoots were also richer in crude fiber than B. maritima (Tan et al. 2017) and B. vulgaris (Baião et al. 2017) shoots and some leafy vegetables (Slavin and Lloyd 2012), covering around 24% of the recommended daily intake.
B. macrocarpa constitutes the vital source of essential fatty acids and energy (Suh et al. 2015) and is involved in many important cell mechanisms (Satter et al. 2016). Its shoots, which provided less than 1% of the recommended daily intake of fat (Table 3), can be considered a low-fat food (Meyers et al. 2006).
Fiber plays a crucial role in preventing several human diseases, such as cardiovascular pathologies, gastrointestinal disorders and colon cancer (Fuller et al. 2016). Also, it controls cholesterol blood levels and glycemia (Hounsome and Hounsome 2011). According to the American Institute of Medicine, B. macrocarpa shoots constitute a potential source of fiber for nutrition and the food industry (Chau and Huang 2003).
Carbohydrates constitute the most abundant nutrient (9.1–19.1 g/100 g FW) found in shoots from all populations. These values are higher than those previously reported for other species belonging to the same genus, such as Beta maritima (Tan et al. 2017; Pinela et al. 2017) and Beta vulgaris (Baião et al. 2017), and can cover between 3.5 and 7.3% of the total daily recommended intake of carbohydrates (260 g/day for adults) (Table 3) (Meyers et al. 2006). Carbohydrates are the most abundant compounds in living plants and constitute the main source of energy production for our body’s cells and the brain (El-Amier et al. 2022; Ebifa-Othieno et al. 2020). Also, they act as regulators and substrates for several specific biochemical processes (Bokov et al. 2017). The highest level was registered in shoots from the Sijoumi site, which could be related to the fact that those shoots also had the highest fiber and carbohydrate contents (El-Amier et al. 2022) and therefore the highest energetic value. Energy is essential for many functions of the body, such as respiration and protein synthesis (Ebifa-Othieno et al. 2020)..
The mineral compositions of edible vegetables represent one of the most important aspects that influence their use in human nutrition. Minerals (macro- and microminerals) are essential for the proper functioning of the human body (Quintaes and Diez‐Garcia 2015). A deficiency in these minerals is considered a form of malnutrition in many developing countries (Mohanty et al. 2016) and is also considered a health problem, since it leads to many chronic and degenerative disorders (Mzoughi et al. 2019). The results for the macrominerals, which are needed in relatively large amounts to achieve some physiological functions in our body (Mohanty et al. 2016), reveal that Na is the most abundant element in B. macrocarpa shoots. The Na levels greatly surpassed those found in wild beet B. maritima (Tan et al. 2017; Pinela et al. 2017) and some varieties of Swiss chard (B. vulgaris subsp. Cicla) (Pokluda and Kuben 2002). According to Hamouda et al. (2016), B. macrocarpa plants which were exposed to salinity stress accumulated high amounts of Na in their tissues. The same observations were also reported for some halophytes such as Sarcocornia perennis, Salicornia ramosissima, Arthrocnemum macrostachyum (Barreira et al. 2017), Mesembryanthemum nodiflorum, Suaeda maritima and Sarcocornia fruticosa (Castañeda-Loaizaa et al. 2020). So, it seems that B. macrocarpa plants can behave like halophytes by accumulating Na in their tissues without showing any symptoms of toxicity (Papafilippaki et al. 2015). Na is a metabolically toxic ion that is used by halophytes to control cell osmotic adjustment (Flowers 1985). Concerning human consumption, Na is an essential mineral that should be consumed in moderate amounts to avoid cardiovascular diseases (Kotchen et al. 2013). According to the World Health Organization (WHO), the daily intake of sodium should be less than 2000 mg (Castañeda-Loaizaa et al. 2020). With regard to the edibility of B. macracarpa, the shoots harvested from the Sijoumi, Enfidha and Kerkennah sites would, respectively, provide 632, 1056 and 856 mg/100 g FW of Na (Table 2) and cover about 35, 26 and 44% of the daily recommended intake (Table 3) (Meyers et al. 2006).
Regarding potassium, B. macrocarpa can be considered a good source of this nutrient since it can provide appreciable amounts (385–598 mg/100 g FW) (Table 2) and can cover from 19 to 30% of the daily recommanded intake of K (Table 3) (Meyers et al. 2006). So, B. macracarpa can be regarded as a good source of K if consumed in moderation.
Ca is also accumulated to higher levels than in B. maritima (Tan et al. 2017; Pinela et al. 2017). This element is implicated in the salt tolerance process in salt-resistant plants as well as in ion uptake and transport (Yamanouchi et al. 1997). Its consumption is crucial for many body functions, like the blood clotting process, nervous system control, and bone and teeth formation (Mohanty et al. 2016). According to the results illustrated in Table 3, B. macrocarpa shoots can be considered a valuable source of Ca, as they could cover from 12 to 40% of our daily intake (Meyers et al. 2006). The elevated content of Ca in Kerkennah shoots is probably associated with the excess of Na in the soil; the high level of Ca could be used in a defence mechanism exerted by the plant to re-establish homeostatic conditions in the presence of high Na accumulation (Tipirdamaz et al. 2006). Mg is an essential component of bone and cartilage and is a cofactor for many enzymes involved in energy metabolism and the synthesis of protein, RNA and DNA (Mohanty et al. 2016). According to the data obtained, B. macrocarpa can supply significant quantities of Mg: enough to cover almost 70% of the recommended daily intake (Meyers et al. 2006). The least abundant macromineral in B. macrocarpa shoots was P; the shoots can supply only 7% of the body’s daily requirements for this nutrient (Meyers et al. 2006). In fact, the availability of P to the plant for uptake and utilization is impaired in alkaline and calcareous soils due to the formation of poorly soluble calcium phosphate minerals (Hopkins and Ellsworth 2005).
Microminerals (Fe, Mn, Cu and Zn), which are required in trace amounts, are also important for normal functioning of the body and play a major role in redox processes (Mzoughi et al. 2019). Fe was the most abundant element among the microminerals and was found at higher concentrations than in B. maritima (Pinela et al. 2017). This could be linked to its abundance in the soil. Approximately 70% of our daily recommended intake of this element could be provided by B. macrocarpa shoots (Table 3) (Meyers et al. 2006). Fe is a very important element for body functioning, and a deficiency of Fe can cause several diseases, like anemia (Quintaes and Diez‐Garcia 2015) as well as impaired physical and cognitive development and an increased risk of morbidity in children (WHO 2005). Mn is also an important element in several body functions, like fat and carbohydrate metabolism, brain and nerve function, calcium absorption (Mohanty et al. 2016), and blood sugar regulation (Gupta and Gupta 2014). Given the elevated Mn content in B. macrocarpa shoots—especially in shoots at the Sijoumi site (2.3 mg/100 g FW), which could cover more than the daily requirement of Mn (114%) (Table 3) (Meyers et al. 2006)—the consumption of this species in small quantities would meet the adequate daily intake level of Mn. Zn and Cu are also very important minerals; they are required for Fe utilization, glucose metabolism, hemoglobin synthesis (Celik and Oehlenschlaager 2004) and cell division (Gupta and Gupta 2014). In B. macrocarpa shoots, these two minerals were found at similar levels to those in B. maritima (Tan et al. 2017; Pinela et al. 2017). Similarly to some wild edible plants, small amounts of Ni were detected in B. macrocarpa shoots (Shad et al. 2013; Barreira et al. 2017). In alkaline calcareous soils, the interactions of nutrients and their mobilization within the plant play a decisive role in nutrient acquisition (Akhtar et al. 2019). The uptake and use of minerals (P, K, Mg, Fe and Zn) by plants are generally altered due to a nutritional imbalance between these minerals and calcium (Wahba et al. 2019).
Functional properties of B. macrocarpa shoots were assessed, including the total phenolic compounds (TPC), total flavonoid (TF) content and DPPH scavenging ability. Phenolic compounds are secondary metabolites that are synthesized through the shikimic acid and phenylpropanoid pathways in most plant tissues (Table 4). They are important plant constituents which provide many health benefits (de la Rosa et al. 2019), such as antioxidant, anti-inflammatory, anti-tumor and antimicrobial properties (Vermerris and Nicholson 2008). Many previous studies have shown that the consumption of a phenolic- and flavonoid-rich natural diet is linked to improved health and a decreased incidence of neurodegenerative and cardiovascular diseases, cancer and diabetes (Aryal et al. 2019). Moreover, phenolic compounds are known to play a crucial role in the adaptation of plants to their environment (Chowdhary et al. 2021). So, the accumulation of phenolic compounds in methanolic extracts of B. macrocarpa shoots, especially those from the Kerkennah site, could be a response to the stressful conditions to which they were subjected. These plants accumulated more phenolic compounds to prevent oxidative stress and the production of radical oxygen species (Bose et al. 2014), which were generated by the salinity of the soil and the arid climate of the Kerkennah site. The antioxidant acitivity of the shoot extracts was assessed through the DPPH assay, which is the most widely used technique to evaluate radical scavenging potency (Aryal et al. 2019). Our results showed higher antioxidant activity in Kerkennah shoots, which was probably correlated to a high accumulation of phenolic compounds.
The obtained results indicate that Beta macrocarpa shoots constitute a potential source of valuable nutrients (proteins, fiber, carbohydrates and minerals) and phenolic compounds with high scavenging potency. Thus, their consumption could provide health benefits and improve modern diets (Romojaro et al. 2013). Due to its tolerance to high salinity, low nutrient availability and an arid climate on the one hand and its nutritional value and functional properties on the other, Beta macrocarpa could be considered as an alternative source of high-quality food as a part of a sustainable agriculture in marginal environments.
Conclusions
The results show that Tunisian Beta macrocarpa, which is one of the most important halophytes and CWR species (especially in the Mediterranean Basin, where it is ranked as an endangered (EN) taxon), deserves to be valorized for its nutritional value and to be preserved ex situ in a gene bank. Chemical analysis showed that B. macrocarpa has high nutritional value due to its proximate composition and its mineral contents. The proximate composition of B. macrocarpa shoots revealed substantial protein, a low fat content, fiber and a high carbohydrate content, which confirm the possibility of using this vegetable in salads (sautéed or cooked) and/or in feed, as it has high levels of energy-storage compounds. In addition, B. macrocarpa shoots accumulated high mineral element contents—essentially Na, K, Ca, Mg and Fe. Na and K play an important role as they are components of active osmotic adjustment, which confirms that the species is perfectly salt tolerant. The Tunisian B. macrocarpa is a halophyte species which plays a considerable role in the Mediterranean diet, as it can be used universally in different traditional dishes because of its high nutritional value and mineral content. The large-fruited beet presents high functional properties as it accumulates secondary metabolites (polyphenols and flavonoids) that are synthesized during stress conditions, such as salt stress. These metabolites act as antioxidants, participating in plant growth, reproduction and resistance to pathogens. Other nutritional aspects of this species, such as its vitamin composition, anti-nutritional factors and compounds that are potentially toxic to humans or animals, should be further investigated.
According to our results, the consumption of leaves from B. macrocapra can contribute to a balanced diet and may be used in modern diets, as a dietary supplement or as a functional ingredient in the design of novel organic food products. It can be used as food and/or as a feed plant as it is rich in important components with high nutritional value. It can be grown and cultivated according to the organic farming system, which is based on maintaining biodiversity and ecological services and principles. B. macrocarpa can be cultivated in marginal areas in order to limit fodder deficits and to improve the pastoral value of these regions while conserving their natural resources, and therefore it could be selected as a useful species in many restoration environmental projects in the Mediterranean area, such as REACT4MED (https://react4med.eu/) and EcoplantMed (http://www.ecoplantmed.eu/project/).
References
Abdelly C (1997) Mechanisms of an association between spontaneous medics and perennial halophytes in the edge of Sebkha. PhD dissertation. Faculté des Sciences de Tunis, University of Tunisia, Tunis
Abe J, Tsuda C (1987) Genetic analysis for isozyme variation in the section Vulgares, genus Beta. Jpn J Breed 37:253–261
Accogli R, Tomaselli V, Direnzo P, Perrino EV, Albanese G, Urbano M, Laghetti G (2023) Edible halophytes and halo-tolerant species in Apulia Region (Southeastern Italy): biogeography, traditional food use and potential sustainable crops. Plants 12:549. https://doi.org/10.3390/plants12030549
Addis G, Urga K, Dikasso D (2005) Ethnobotanical study of edible wild plants in some selected districts of Ethiopia. Hum Ecol 33:83–118
Agboola OS, Adejumo AL (2013) Nutritional composition of the fruit of the Nigerian wild date palm, Phoenix dactylifera. World J Dairy Food Sci 8(2):196–200
Akhtar M, Yousaf S, Sarwar N (2019) Hussain S (2019) Zinc biofortification of cereals—role of phosphorus and other impediments in alkaline calcareous soils. Environ Geochem Health 41:2365–2379. https://doi.org/10.1007/s10653-019-00279-6
Aliat T, Kaabeche M, Khomri H, Nouri L, Neffar S, Chenchouni H (2016) A pedological characterization of some inland wetlands and Ramsar sites in Algeria. Land Degrad Dev 27(3):693–705. https://doi.org/10.1002/ldr.2467
AOAC (1990) Official methods of analysis, 15th edn. Association of Official Analytical Chemists, Washington, DC
Artikova HT, Sattorova MM, Jumaev JJO (2021) Prevent salinization and increase the fertility of irrigated sandy and loamy soils. Am J Agric Biol Sci 3:1–6. https://doi.org/10.37547/tajabe/Volume03Issue03-01
Aryal S, Baniya MK, Danekhu K, Kunwar P, Gurung R, Koirala N (2019) Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 8:96. https://doi.org/10.3390/plants8040096
Baião DS, da Silva DVT, Aguila EMD, Paschoalin VMF (2017) Nutritional, bioactive and physicochemical characteristics of different beetroot formulations. In: Karunaratne DN, Pamunuwa G (eds) Food additives. IntechOpen, London. https://doi.org/10.5772/intechopen.69301
Baldermann S, Blagojević L, Frede K, Klopsch R, Neugart S, Neumann A, Ngwene B, Norkeweit J, Schröter D, Schröter A, Schweigert FJ, Wiesner M, Schreiner M (2016) Are neglected plants the food for the future? Crit Rev Plant Sci 35(2):106–119. https://doi.org/10.1080/07352689.2016.1201399
Barreira L, Resek E, Rodrigues MJ, Rocha MI, Pereira H, Bandarra N, da Silva MM, Varela J, Custódio L (2017) Halophytes: gourmet food with nutritional health benefits? J Food Compost Anal 59:35–42. https://doi.org/10.1016/j.jfca.2017.02.003
Bello SK, Alayafi AH, Al-Solaimani SG, Abo-Elyousr KAM (2021) Mitigating soil salinity stress with gypsum and bio-organic amendments: a review. Agronomy 11:1735. https://doi.org/10.3390/agronomy11091735
Ben Mahmoud K, Wasli H, Ben Mansour R, Jemai N, Selmi S, Jemmali A, Ksouri R (2022) Antidiabetic, antioxidant and chemical functionalities of Ziziphus jujuba (Mill.) and Moringa oleifera (Lam.) plants using multivariate data treatment. S Afr J Bot 144:219–228. https://doi.org/10.1016/j.sajb.2021.08.017
Bilz M, Kell SP, Maxted N, Lansdown RV (2011) European red list of vascular plants. Publications Office of the European Union, Luxembourg. https://doi.org/10.2779/8515
Bokov DO, Samylina IA, Nikolov SD (2017) HPLC/RI and HPLC/PDA phytochemical analysis of free and bound carbohydrates in Galanthus woronowii Losinsk. and G. nivalis L. homeopathic matrix tinctures. Pharm Chem J 50(12):810–813
Bose J, Rodrigo-Moreno A, Shabala S (2014) ROS homeostasis in halophytes in the context of salinity stress tolerance. J Exp Bot 65(5):1241–1257. https://doi.org/10.1093/jxb/ert430
Bremner JM (1996) Nitrogen—total. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner ME (eds) Methods of soil analysis. Part 3. Chemical methods. American Society of Agronomy, Madison, pp 1085–1122. https://doi.org/10.2136/sssabookser5.3.c37
Cambrollé J, Muñoz-Vallés S, Mancilla-Leytón JM, Andrades-Moreno L, Luque T, Figuera ME (2015) Effects of soil physicochemical properties on plant performance of Glaucium flavum Crantz. Plant Soil 386(1):185–193. https://doi.org/10.1007/s11104-014-2258-7
Casella F, Vurro M, Valerio F, Perrino EV, Mezzapesa GN, Boari A (2023) Phytotoxic effects of essential oils from six lamiaceae species. Agronomy 13:257. https://doi.org/10.3390/agronomy13010257
Castañeda-Loaizaa V, Oliveiraa M, Santosa T, Schülera L, Alexandre R, Limad AR, Gamad F, Salazarb M, Nengc NR, Nogueirac JMF, Varelaa J, Barreira L (2020) Wild vs cultivated halophytes: nutritional and functional differences. Food Chem 333:127536. https://doi.org/10.1016/j.foodchem.2020.127536
Celik U, Oehlenschlaager J (2004) Determination of zinc and copper in fish samples collected from Northeast Atlantic by DPSAV. Food Chem 87:343–347
Chau CF, Huang YL (2003) Comparison of the chemical composition and physicochemical properties of different fibers prepared from the peel of Citrus sinensis L. Cv. Liucheng. J Agric Food Chem 51:2615–2618
Chowdhary V, Alooparampil S, Pandya R, Tank J (2021) Physiological function of phenolic compounds in plant defense system. In: Badria FA (eds) Phenolic compounds—chemistry, synthesis, diversity, non-conventional industrial, pharmaceutical and therapeutic applications, vol 26, no 10. IntechOpen, London, pp 185–205. https://doi.org/10.5772/intechopen.101131
Colonna E, Rouphael Y, Barbieri G, Stefania De Pascale S (2016) Nutritional quality of ten leafy vegetables harvested at two light intensities. Food Chem 199:702–710. https://doi.org/10.1016/j.foodchem.2015.12.068
Dagnelie P (1975) Analyse statistique à plusieurs variables. Presses Agronomiques de Gembloux, Gembloux, pp 339–351
Dajic Z (2006) Salt stress. In: Madhava Rao KV, Raghavendra AS, Janardhan Reddy K (eds) Physiology and molecular biology of stress tolerance in plants. Springer, Dordrecht, pp 41–99
de la Rosa LA, Moreno-Escamilla JO, Rodrigo-García J, Alvarez-Parrilla E (2019) Phenolic compounds. In: Elhadi MY (eds) Postharvest physiology and biochemistry of fruits and vegetables. Woodhead, Duxford, pp 253–271. https://doi.org/10.1016/B978-0-12-813278-4.00012-9
Dewanto V, Wu X, Adom KK, Liu RH (2002) Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem 50:3010–3014. https://doi.org/10.1021/jf0115589
Dobignard A, Chatelain C (2011) Index synonymique de la flore d’Afrique du nord 2:1–429. Éditions des conservatoire et jardin botaniques, Genève
Dop MC, Kefi F, Karous O, Verger EO, Bahrini A, Ghrabi Z, El Ati J, Kennedy G, Termote C (2020) Identification and frequency of consumption of wild edible plants over a year in central Tunisia: a mixed-methods approach. Public Health Nutr 23:782–794. https://doi.org/10.1017/S1368980019003409
Dridi I, Arfaoui A (2017) Organic nitrogen distribution in seven Tunisian soil types under contrasting pedogenetic conditions. Environ Earth Sci 76:205. https://doi.org/10.1007/s12665-017-6525-9
Dwivedi S, Sahrawat K, Upadhyaya H, Ortiz R (2013) Chapter 1—Food, nutrition and agrobiodiversity under global climate change. Adv Agron 120:1–128. https://doi.org/10.1016/B978-0-12-407686-0.00001-4
Ebifa-Othieno E, Kabasa JD, Nyeko P, Nakimbugwe D, Mugisha A (2020) Nutritional potential of tamarind (Tamarindus indica L.) from semi-arid and subhumid zones of Uganda. J Food Meas Charact 14(2):1125–1134
El-Amier YA, Zahran MAEK, El-Mamory SH (1995) Évaluation des caractéristiques physico-chimiques de l'eau et des sédiments dans la branche de Rosette, Égypte. J Water Resource Prot 7:1075–1086. http://www.scirp.org/journal/as
El-Amier YA, Soufan W, Almutairi KF, Zaghloul NS, Abd-ElGawad AM (2022) Proximate composition, bioactive compounds, and antioxidant potential of wild halophytes grown in coastal salt marsh habitats. Molecules 27:28. https://doi.org/10.3390/molecules27010028
El-Mokni R, Barone G, Maxted N, Kell S, Domina G (2022) A prioritised inventory of crop wild relatives and wild harvested plants of Tunisia. Genet Resour Crop Evol 69:1787–1816. https://doi.org/10.1007/s10722-021-01340-z
FAO (2014) Growing greener cities in Latin America and the Caribbean. Food and Agricultural Organization of the United Nations, Rome. http://www.fao.org/3/a-i3696e.pdf
Flowers TJ (1985) Physiology of halophytes. Plant Soil 89:41–56
Flowers TJ, Colmer TD (2008) Salinity tolerance in halophytes. New Phytol 179(4):945–963
Fuller S, Beck E, Salman H, Tapsell L (2016) New horizons for the study of dietary fiber and health: a review. Plant Foods Hum Nutr 71:1–12. https://doi.org/10.1007/s11130-016-0529-6
Gasparini B, Rasch PJ, Hartmann DL, Wall CJ, Dütsch M (2021) A Lagrangian perspective on tropical anvil cloud lifecycle in present and future climate. J Geophys Res Atmos 126:e2020JD033487. https://doi.org/10.1029/2020JD033487
Gonçalo Filho F, da Silva DN, Suddarth SRP, Ferreira JFS, Anderson RG, dos Santos FC, de Lira RB, Neto MF, Cosme CR (2019) Reclaiming tropical saline-sodic soils with gypsum and cow manure. Water 12:57
Guil-Guerrero JL, Giménez-Giménez A, Rodríguez-García I, Torija-Isasa ME (1998) Nutritional composition of Sonchus species (S. asper L., S. oleraceus L. and S. tenerrimus L.). J Sci Food Agric 76:628–632
Gupta UC, Gupta SC (2014) Sources and deficiency diseases of mineral nutrients in human health and nutrition: a review. Pedosphere 24(1):13–38. https://doi.org/10.1016/S1002-0160(13)60077-6
Hameed A, Khan M (2011) Halophytes: biology and economic potentials. Karachi Univ J Sci 39(1):40–44
Hamouda I, Badri M, Mejri M, Cruz C, Siddique KHM, Hessini K (2016) Salt tolerance of Beta macrocarpa is associated with efficient osmotic adjustment and increased apoplastic water content. Plant Biol 18:369–375
Harisha RP, Setty S, Ravikanth G (2021) Dependency and economic benefits of the use of wild food plants among tribal communities in Malai Madeshawara Hills wildlife sanctuary, Southern India. Future Food J Food Agric Soc 9(2):1–19. https://doi.org/10.17170/kobra-202011192211
Hessini K, Jeddi K, El Shaer HM, Smaoui A, Ben Salem H, Siddique KHM (2020) Potential of herbaceous vegetation as animal feed in semi-arid Mediterranean saline environments: the case for Tunisia. Agron J 112:2445–2455. https://doi.org/10.1002/agj2.20196
Heywood V (1999) Use and potential of wild plants in farm households. Farm Systems Management, Series 15. FAO Corporate Document Repository. FAO, Rome
Hoffland E, Kuyper TW, Comans RNJ, Creamer RE (2020) Eco-functionality of organic matter in soils. Plant Soil 455:1–22
Hohmann S, Kadeireit JW, Kadeireit G (2006) Understanding Mediterranean-Californian disjunctions: molecular evidence from Chenopodiaceae-Betoideae. Taxon 55:67–78
Hopkins B, Ellsworth J (2005) Phosphorus availability with alkaline/calcareous soil. In: Proc Western Nutrient Manag Conf, Salt Lake City, UT, USA, 3–4 Mar 2005, 6(3–4):88–93
Hounsome N, Hounsome B (2011) Biochemistry of vegetables: major classes of primary (carbohydrates, amino acids, fatty acids, vitamins, and organic acids) and secondary metabolites (terpenoids, phenolics, alkaloids, and sulfur‐containing compounds) in vegetables. In: Sinha NK (ed) Handbook of vegetables and vegetable processing. Blackwell, Ames. https://doi.org/10.1002/9780470958346
Keller GB, Mndiga H, Maass BL (2005) Diversity and genetic erosion of traditional vegetables in Tanzania from the farmer’s point of view. Plant Genet Resour 3:400–413. https://doi.org/10.1079/PGR200594
Kotchen TA, Cowley AW Jr, Frohlich ED (2013) Salt in health and disease—a delicate balance. N Engl J Med 368:1229–1237. https://doi.org/10.1056/NEJMra1212606
Lange W, de Bock SM (1989) The diploidised meiosis of tetraploid beta macrocarpa and its possible application in breeding sugar beet. Plant Breeding 103:196–206. https://doi.org/10.1111/j.1439-0523.1989.tb00371.x
Leogrande R, Vitti C, Castellini M, Mastrangelo M, Pedrero F, Vivaldi GA, Stellacci AM (2021) Comparison of two methods for total inorganic carbon estimation in three soil types in Mediterranean area. Land 10:409. https://doi.org/10.3390/land10040409
Letschert JPW (1993) Beta section Beta: biogeographical patterns of variation and taxonomy. Wageningen Agricultural University Papers 93-1. Veenman Drukkers BV, Wageningen
Long SP, Ort DR (2010) More than taking the heat: crops and global change. Curr Opin Plant Biol 13(3):240–247. https://doi.org/10.1016/j.pbi.2010.04.008
Maatallah-Zaier M, Ciudad-Mulero M, Cámara M, Pereira C, Ferreira ICFR, Achour L, Kacem A, Patricia Morales P (2020) Revalorization of Tunisian wild Amaranthaceae halophytes: nutritional composition variation at two different phenotypes stages. J Food Compost Anal 89:103463. https://doi.org/10.1016/j.jfca.2020.103463
Marschner H (1995) Mineral nutrition of higher plants. Academic, London
Martins-Noguerol R, Matías L, Pérez-Ramos IM, Xoaquín Moreira X, Francisco M, Pedroche J, De Andrés-Gil C, Gutiérrez E, Salas JJ, Moreno-Pérez AJ, Davy AJ, Muñoz-Vallés S, Figueroa ME, Cambrollé J (2023) Soil physicochemical properties associated with the yield and phytochemical composition of the edible halophyte Crithmum maritimum. Sci Total Environ 869:61806. https://doi.org/10.1016/j.scitotenv.2023.161806
Matar A, Torrent J, Ryan J (1992) Soil and fertilizer phosphorus and crop responses in the dryland Mediterranean zone. Adv Soil Sci 18:81–146
Maxted N, Kell S (2009) Establishment of a global network for the in situ conservation of crop wild relatives: status and needs. Background study paper 39. FAO, Rome
Maxted N, Vincent H (2021) Review of congruence between global crop wild relative hotspots and centres of crop origin/diversity. Genet Resour Crop Evol 68:1283–1297. https://doi.org/10.1007/s10722-021-01114-7
Maxted N, Ford-Lloyd BV, Jury S, Kell S, Scholten M (2006) Towards a definition of a crop wild relative. Biodivers Conserv 15:2673–2685
McMichael A, Woodruff RE, Hales S (2006) Climate change and human health: present and future risks. Lancet 367:859–869
Mehalaine S, Chenchouni H (2020) Plants of the same place do not have the same metabolic pace: soil properties affect differently essential oil yields of plants growing wild. Arab J Geosci 13:1263
Meyers LD, Hellwig JP, Otten JJ (eds) (2006) Dietary reference intakes: the essential guide to nutrient requirements. National Academies Press, Washington, DC
Mohanty BP, Sankar TV, Ganguly S, Mahanty A, Anandan R, Chakraborty K, Paul BN, Sarma D, Dayal JS, Mathew S, Asha KK, Mitra T, Karunakaran D, Chanda S, Shahi N, Das P, Das P, Akhtar MS, Vijayagopal P, Sridhar N (2016) Micronutrient composition of 35 food fishes from India and their significance in human nutrition. Biol Trace Elem Res 174(2):448–458. https://doi.org/10.1007/s12011-016-0714-3
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
Mzoughi Z, Chahdoura H, Chakroun Y, Cámara M, Fernández-Ruiz V, Morales P, Mosbah H, Flamini G, Mejdi Snoussi M, Hatem Majdoub H (2019) Wild edible Swiss chard leaves (Beta vulgaris L. var. cicla): Nutritional, phytochemical composition and biological activities. Food Res Int 119:612–621. https://doi.org/10.1016/j.foodres.2018.10.039
Nelson DW, Sommers LE (1996) Chapter 34: Total carbon, organic carbon, and organic matter. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner ME (eds) Methods of soil analysis. Part 3: Chemical methods, 5.3. Wiley, New York
Nguyen KA, Liou YA, Tran HP, Hoang PP, Nguyen TH (2020) Soil salinity assessment by using near-infrared channel and vegetation soil salinity index derived from Landsat 8 OLI data: a case study in the Tra Vinh Province, Mekong Delta, Vietnam. Prog Earth Planet Sci 7:1. https://doi.org/10.1186/s40645-019-0311-0
Nirmala C, Shahar B, Dolma N, Santosh O (2022) Promising underutilized wild plants of cold desert Ladakh, India for nutritional security and health benefits. App Food Res 2:100145. https://doi.org/10.1016/j.afres.2022.100145
Okello J, Okullo JBL, Eilu G, Nyeko Ph, Obua J (2018) Proximate composition of wild and on-farm Tamarindus indica LINN fruits in the agro-ecological zones of Uganda. J Nutr Health Food Eng 8(4):310–317
Olsen S, Sommers LE (1982) Phosphorus. In: Page AL (ed) Methods of soil analysis, part 2: chemical and microbiological properties. American Society of Agronomy, Soil Science Society of America, Madison, pp 403–430
Papafilippaki A, Paranychianakis N, Nikolaidis NP (2015) Effects of soil type and municipal solid waste compost as soil amendment on Cichorium spinosum (spiny chicory) growth. Sci Hortic 195:195–205. https://doi.org/10.1016/j.scienta.2015.09.030
Parry ML, Rosenzweig C, Iglesias A, Livermore M, Fischer G (2004) Effects of climate change on global food production under SRES emissions and socio-economic scenarios. Glob Environ Chang 14(1):53–67
Patz JA, Kovats RS (2002) Hot spots in climate change and human health: present and future risks. Lancet 368:859–869
Perrino EV, Calabrese G (2014) Vascular flora of the ancient olive groves of Apulia (Southern Italy). Nat Croat 23(1):189–218
Perrino EV, Perrino P (2020) Crop wild relatives: know how past and present to improve future research, conservation and utilization strategies, especially in Italy: a review. Genet Resour Crop Evol 67(5):1067–1105. https://doi.org/10.1007/s10722-020-00930-7
Perrino EV, Wagensommer RP (2021) Crop wild relatives (CWR) priority in Italy: distribution, ecology, in situ and ex situ conservation and expected actions. Sustainability 13(4):1682. https://doi.org/10.3390/su13041682
Perrino EV, Wagensommer RP, Medagli P (2014) The genus Aegilops L. (Poaceae) in Italy: taxonomy, geographical distribution, ecology, vulnerability and conservation. Syst Biodivers 12(3):331–349
Perrino EV, Valerio F, Jallali S, Trani A, Mezzapesa GN (2021) Ecological and biological properties of Satureja cuneifolia Ten. and Thymus spinulosus Ten.: two wild officinal species of conservation concern in Apulia (Italy). A preliminary survey. Plants 10:1952. https://doi.org/10.3390/plants10091952
Pinela J, Carvalho AM, Ferreira IC (2017) Wild edible plants: Nutritional and toxicological characteristics, retrieval strategies and importance for today’s society. Food Chem Toxicol 110:165–188. https://doi.org/10.1016/j.fct.2017.10.020
Piper CS (1947) Soil and plant analysis. Interscience, New York
Piscitelli L, Shaaban A, Mondelli D, Mezzapesa GN, Miano TM, Dumontet S (2015) Use of olive mill pomace biochar as a support for soil microbial communities in an Italian sandy soil. Soil Horizons 56(6):1–7. https://doi.org/10.2136/sh15-02-0006
Pokluda R, Kuben J (2002) Comparison of selected Swiss chard (Beta vulgaris ssp. cicla L.) varieties. Hortic Sci 29(3):114–118
Pottier Alapetite G (1979) Flore de la Tunisie. Angiospermes-dicotylédones, Gamopétales. Programme flore et végétation tunisiennes. Ministère de l'Enseignement Supérieur et de la Recherche Scientifique et le Ministère de l'Agriculture, Tunis, p 44
POWO (2023) The International Plant Names Index and World Checklist of Vascular Plants. http://www.ipni.org and https://powo.science.kew.org/. Accessed 19 July 2023
Qadir M, Ghafoor A, Murtaza G (2000) Amelioration strategies for saline soils: a review. Land Degrad Dev 11:501–521
Quintaes KD, Diez-Garcia RW (2015) The importance of minerals in the human diet. In: de la Guardia M, Guarriges S (eds) Handbook of mineral elements in food. Wiley-Blackwell, Oxford. https://doi.org/10.1108/RR-03-2016-0075
Ratnayake SS, Kariyawasam CS, Kumar L, Hunter D, Liyanage ASU (2021) Potential distribution of crop wild relatives under climate change in Sri Lanka: implications for conservation of agricultural biodiversity. Curr Res Environ Sustain 3:100092
Ray DK, Gerber JS, MacDonald GK, West PC (2015) Climate variation explains a third of global crop yield variability. Nat Commun 6(1):1–9
Rebman JP, Gibson J, Rich K (2016) Annotated checklist of the vascular plants of Baja California, Mexico. Proc San Diego Soc Nat Hist 45:1–352
Redden R (2015) Wild relatives for the crop improvement challenges of climate change: the adaptation range of crops. In: Redden R, Yadav SS, Maxted N, Dulloo ME, Guarino L, Smith P (eds) Crop wild relatives and climate change. Wiley-Blackwell, Hoboken, pp 61–79
Rodrigues MJ, Castañeda-Loaiza V, Monteiro I, Pinela J, Barros L, Abreu RMV, Oliveira MC, Reis C, Soares F, Pousão-Ferreira P, Pereira CG, Custódio L (2020) Growth performance, in vitro antioxidant properties and chemical composition of the halophyte Limonium algarvense Erben are strongly influenced by the irrigation salinity. Ind Crops Prod 143:111930. https://doi.org/10.1016/j.indcrop.2019.111930
Romojaro A, Ángeles Botella M, Obón C, Teresa Pretel M (2013) Nutritional and antioxidant properties of wild edible plants and their use as potential ingredients in the modern diet. Int J Food Sci Nutr 64(8):944–952. https://doi.org/10.3109/09637486.2013.821695
Satter MMA, Khan MMRL, Jabin SA, Abedin N, Islam MF, Shaha B (2016) Nutritional quality and safety aspects of wild vegetables consume in Bangladesh. Asian Pac J Trop Biomed 6(2):125–131
Schlüter U, Colmsee C, Scholz U, Bräutigam A, Weber AP, Zellerhoff N, Bucher M, Fahnenstich H, Sonnewald U (2013) Adaptation of maize source leaf metabolism to stress related disturbances in carbon, nitrogen and phosphorus balance. BMC Genom 14(1):1–25. https://doi.org/10.1186/1471-2164-14-442
Schröder JJ (2014) The position of mineral nitrogen fertilizer in efficient use of nitrogen land: a review. Nat Resour 5:936–948
Señoráns FJ, Luna P (2012) Sample preparation techniques for the determination of fats in food. Compr Sample Sample Prep 4:203–211
Shad A, Shah HU, Bakht J (2013) Ethnobotanical assessment and nutritive potential of wild food plants. J Anim Plant Sci 23(1):92–99
Slavin JL, Lloyd B (2012) Health benefits of fruits and vegetables. Am Soc Nutr Adv Nutr 3:506–516. https://doi.org/10.3945/an.112.002154
Suh MC, Hahne G, Liu JR, Neal StewartJr C (2015) Plant lipid biology and biotechnology. Plant Cell Rep 34:517–518
Sun X, Chen J, Liu L, Rosanoff A, Xiong X, Zhang Y, Pei T (2018) Effects of magnesium fertilizer on the forage crude protein content depend upon available soil nitrogen. J Agric Food Chem 66(8):1743–1750. https://doi.org/10.1021/acs.jafc.7b04028
Tan A, Adanacioglu N, Karabak S, Aysar N, Tan AS, Aykas L (2017) Sea beets [Beta vulgaris subsp. maritima (L.) Arcang.] wild edible beets and home garden beets of Turkey. Anadolu J Aegean Agr Res Ins 27(2):54–61
Termote C, Van Damme P, Dhed’a Djailo B (2011) Eating from the wild: Turumbu, Mbole and Bali traditional knowledge on non-cultivated edible plants, District Tshopo, DR Congo. Genet Resour Crop Evol 58:585–618. https://doi.org/10.1007/s10722-010-9602-4
Tipirdamaz R, Gagneul D, Duhazé C, Aïnouche A, Monnier C, Özkum D, Larher F (2006) Clustering of halophytes from an inland salt marsh in Turkey according to their ability to accumulate sodium and nitrogenous osmolytes. Environ Exp Bot 57:139–153
Tutin TG et al (eds) (1993) Flora Europaea, vol 2, no 1. Cambridge University Press, Cambridge, pp 1–581
Ulian T, Diazgranados M, Pironon S, Padulosi S, Liu U, Davies L, Howes MJR, Borrell JS, Ondo I, Pérez-Escobar OA, Sharrock S, Ryan P, Hunter D, Lee MA, Barstow C, Łuczaj L, Pieroni A, Cámara-Leret R, Noorani A, Mba C, Womdim RN, Muminjanov H, Antonelli A, Pritchard HW, Mattana E (2020) Unlocking plant resources to support food security and promote sustainable agriculture. Plants People Planet 2(5):421–445. https://doi.org/10.1002/ppp3.10145
United Nations (2019) World population prospects 2019: Volume I: Comprehensive tables. Department of Economic and Social Affairs, Population Division, United Nations: New York
Vermerris W, Nicholson R (2008) Phenolic compounds and their effects on human health. In: Vermerris W, Nicholson R (eds) Phenolic compound biochemistry, vol 7. Springer, Dordrecht, pp 235–255. https://doi.org/10.1007/978-1-4020-5164-7_7
Vimlesh K, Giri AK (2011) Nitrogen mineralization in soil amended with crop residue: an incubation experiment under flooding conditions. Eur J Chem 8(1):25–28
Wahba MM, Labib F, Zaghloul A (2019) Management of calcareous soils in arid region. Int J Environ Pollut Environ Model 2:248–258
WHO (2005) Micronutrient deficiencies: iron deficiency anaemia. http://www.who.int/nutrition/topics/ida/en/. Accessed 13 Sept 2023
Yamanouchi M, Tanaka S, Fujiyama H (1997) The cultivarietal differences in salt-tolerance and the effect of NaCl on the absorption and translocation of K, Ca and Mg ions in Phaseolus vulgaris L. J Jpn Soc Hortic Sci 65:737–745. https://doi.org/10.2503/jjshs.65.737
Zait Y, Schwartz A (2018) Climate-related limitations on photosynthesis and drought-resistance strategies of Ziziphus spina-christi. Front Glob Change. https://doi.org/10.3389/ffgc.2018.00003
Acknowledgements
The authors are thankful to all those colleagues who kindly provided information and explanations concerning their papers, which we consulted on the subject.
Funding
Open access funding provided by Università di Foggia within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Philippe Michaud.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahmoud, K.B., Abdelkefi, F., Mezzapesa, G.N. et al. Nutritional value and functional properties of an underexploited Tunisian wild beet (Beta macrocarpa Guss.) in relation to soil characteristics. Euro-Mediterr J Environ Integr 9, 705–720 (2024). https://doi.org/10.1007/s41207-024-00468-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41207-024-00468-5