Abstract
The plant Silybum marianum (L.) Gaertn. has been used in a preliminary study to investigate its phytoremediation potential in moderately and heavily Cd-polluted Greek soils. For this purpose, a pot experiment was carried out using four soil treatments, two from agricultural and two from urban areas, contaminated with 3 and 30 mg of Cd per kg of soil. The modified Bureau of Community Reference (BCR) fractionation method was used to determine Cd levels in the soil fractions. The water-soluble, available-DTPA extractable, total and pseudo-total concentrations of Cd were also evaluated and attempts to find relationships between Cd soil fractions and Cd levels in the parts of the cultivated plants were made. Significant correlations among Cd soil fractions, Cd plant parts, and soil physicochemical parameters were obtained and discussed. High Cd translocation and accumulation rates were observed mainly in the root and the lower part of the stems, while in flowers and seeds no Cd was detected, leading to the conclusion that the high-value product contained in the seeds maintains its quality and is free of contaminants. The results are satisfactory indicating Silybum marianum (L.) Gaertn. as a promising plant for remediation practices in Cd-contaminated Mediterranean soils, promoting the principles of circular economy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, soil pollution and degradation have become major environmental problems of concern to societies worldwide (Cao et al. 2020). Heavy metals (HMs) or potential toxic elements (PTEs) when accumulated in the soil can easily and almost certainly disperse to different parts of the local ecosystem, causing serious environmental deterioration (Awasthi et al. 2022). Heavy metals that result from numerous human activities, such as burning coal and tyres, applying fertilizer, using hard water for irrigation, spilling petrochemicals, atmospheric deposition and using animal manure, are mainly absorbed by soil. The increasing use of heavy metals has put a significant amount of stress on soil ecosystems in agricultural and urban areas, and the consequences can also significantly increase dietary exposure via plant-food chain transfer (Oladoye et al. 2022). Thus, they can migrate to plants and accumulate in their different parts, both underground and above ground, i.e. in roots, leaves, stems, and fruits (Mawari et al. 2022). Toxic metals, after being absorbed by crops, enter the human body through the food chain (Zorpas et al. 2021; Zhang et al. 2023). After entering the human body, toxic metals accumulate and can cause both acute and chronic damages. Long-term and multi-year human exposure to metals have been linked to serious human health consequences such as cancer and various neurological disorders (Dasharathy et al. 2022; Wang et al. 2023). Studies on surface soils proved that of the eight common toxic elements (As, Cd, Cr, Cu, Hg, Ni, Pb, and Zn) show increasing trends, raising the risk to human health (Liang et al. 2023; Papadimou et al. 2023). Since Cd does not undergo metabolic degradation into less harmful forms and is not excreted, humans are unable to react to exposures to a number of metals, including in particular, Cd (Peana et al. 2022). The long biological half-life of Cd makes it essentially a cumulative toxin and chronic exposure cause harmful effects as it is easily stored in the organs of the human body (Zeng et al. 2019).
The ever-increasing number of degraded soils makes their remediation vital (Golia et al. 2021). Phytoremediation is a cost-effective, environmentally friendly, and aesthetically pleasing method that can be applied to highly degraded areas (Stylianou et al. 2020; Zhi et al. 2020; Golia et al. 2023). However, the use of edible crops, especially for areas with HM contamination, is not ecologically suitable because HM can enter the food chain (Ait Elallem et al. 2021; Zorpas et al. 2021). In contrast, plants that are non-edible, have a short life cycle, are fast-growing, deep-rooted, and metal-stabilizing with high biomass yield, and, in addition, can produce high-value products may be the ideal solution for soil remediation using plants (Gupta et al. 2013; Yang et al. 2019). Given the land under cultivation for food production is alarmingly concentrated, using moderately contaminated soils to produce edible and/or high-value products is a good option to reduce food shortage problems (Yang et al. 2017; Dobrikova et al. 2021). Therefore, phytoremediation of such neglected and abandoned soils for agricultural production is receiving global attention (Zehra et al. 2020), under the context of circular economy principles (Almendro-Candel et al. 2019; Golia 2023), considering at the same time sustainability principles (Papamichael et al. 2022, D'adamo and Costaldi 2023).
It is widely known that, in addition to food crops, several plants species—aromatic and medicinal—are cultivated, especially for their secondary metabolites (Perlein et al. 2021). As these crops are not directly associated with a food chain, they possess an additional advantage over food crops for phytoremediation purposes (Pandey et al. 2019), such as milk thistle, S. marianum, and industrial hemp (Cannabis sativa L.) (Jeswani et al. 2020; Golia et al. 2021, 2023). The significant yield potential of its tissues enables the accumulation and dilution of HMs, which leads to increased tolerance to HMs and to increased survivability at high pollutant concentrations (De Vos et al. 2022).
Asteraceae family includes the native annual or biennial plant S. marianum. It is a common weed that has been introduced as a medicinal plant for silymarin production (Karkanis et al. 2011; Liava et al. 2021). Therefore, milk thistle is considered a remarkable medicinal plant for the treatment of liver and gallbladder disorders (Hammami et al. 2018). Furthermore, several components of milk thistle have been shown to have antioxidant, anti-inflammatory, and anti-cancer properties (Bokelmann 2022). In addition, scientific studies conducted both in the field and in greenhouses show that there is potential use of certain species of milk thistle as bioenergy plants in soils contaminated by both HMs and petroleum products (Domínguez et al. 2017; Hammami et al. 2018). According to Hammami et al (2018) both Cd and Cd in the presence with diesel oil, S. marianum has the ability to retain more than 60% of the cadmium in the soil. However, research on the usage of this advantageous plant regarding its continued existence and ability to accumulate metals in its tissues, growing in Mediterranean soils is limited. In addition, Mediterranean soils have the highest overall rates of erosion, lowest levels of soil organic matter, and serious salinization problems. Additionally, they have high concentration of shallow soils, severe human pressures and a high susceptibility to climate change (Ferreira et al. 2022).
The objectives of the present study are (a) to investigate the capacity of S. marianum to resist toxic exposure and grow in Cd-contaminated agricultural and urban soils; (b) to study the potential of S. marianum of Cd uptake and accumulation in its different parts, such as roots, shoots, leaves, flowers, and seeds; and (c) to find the possible relationships or interaction effects among Cd distributed in plant parts and Cd soil fractions, using the proper statistical tools.
Materials and methods
Descriptive characteristics of the sampling area
Four different soils constituted the basis of the study. The soils were slightly acidic and alkaline and were derived from rural and urban areas of central Greece. Urban soils for experiment (S3 and S4) were taken from the City of Volos (Papadimou et al. 2023). The rural soils for experiment (S1 and S2) were taken from intensively cultivated soils of the Thessalian plain (Golia et al. 2007).
For each examined sample, six subsamples were collected within an area of approximately 150 cm radius, using a wooden spade (ISO/ISO 10390:2005). The use of composite surface soils has been designed by reducing the number of analyses or replicates that need to be carried out and is intended to provide a representative sample of the area under investigation. The samples were delivered to the soil laboratory, where they were air-dried, passed through a 2-mm diameter sieve, and prepared for further physical and chemical analyses (pH, EC, CaCO3, particle size distribution, organic carbon content, water-soluble, available, pseudototal, and total metal concentrations) (Golia et al. 2007; Dimirkou et al. 2009).
Laboratory contamination of soils
Soils were subjected to contamination by the addition of appropriate calculated volumes of Cd solutions. Both urban and agricultural soil samples were selected to have an undetectable Cd concentration so that laboratory contamination could be performed. For this purpose, aqueous solutions of Cd2+ were prepared and added to the soils so that the final Cd concentration in the soils appeared at two levels (A and B), respectively polluted with 3 and 30 mg Cd kg−1.
The Cd solutions were added by spraying to the soils, followed by manual mixing for 10 min. The samples were transferred to a plastic bag where they remained for 18 days. Mixing was carried out at regular intervals to achieve absolute homogenization. To avoid anaerobic conditions, the soils were watered and aired throughout this time. In Fig. 1 the experimental procedure of soil contamination using the appropriate volumes of Cd solutions (A + B level) along with the cultivation of S. marianum in contaminated soils at all growth stages are presented. Specifically, (a) in rural soils the experiment began with soil preparation, i.e. tillage. Then soil sampling of both agricultural and urban samples was carried out. This procedure lasted 2 days. (b) Subsequently, soil samples were contaminated with Cd solutions (A + B level) and left to incubate for 18 days. (c) After incubation, the contaminated soil samples were placed in pots and seeded in the beginning of November. One week after seeding, seedlings of S. mariamum appeared. (d) The plants were grown by forming rosettes over the first 4 months of November until March, and the plant’s growth traits were noted. (e) The plants matured, flowered, and grew in height from March to April. (f) In May, the flowers matured and were harvested. (g) After the flowers were harvested, the seeds were extracted and kept under suitable environmental conditions until the next sowing.
Experimental procedure of soil contamination using the appropriate volume of Cd solutions (A + B level) and cultivation of S. marianum in contaminated soils at all its growth stages. a Field preparation and soil sampling, b contamination of soils using Cd solution, c sowing of thistle, d study of plant growth characteristics, e flowering, f flower maturity, g seed production
Pot experiment
The seeds of the plant S. marianum were collected from a crop grown on the farm of the University of Thessaly. Seeds were collected from nearly 100 individual plants in May 2020 and stored in plastic bags in the refrigerator (at 4 °C) and on the day of sowing were kept at room temperature for 5 h. The contaminated soils after the incubation period were transferred to plastic pots with a height of 15 cm and a base area of 40 cm2. A total of four soils were used, contaminated at two levels of Cd (3 and 30 mg Cd kg−1) and an additional one with 0 mg Cd kg−1, which we refer to as the control sample. In each case, there were nine replicates. Therefore, a completely randomized experiment was conducted with 3 (Cd concentrations) × 4 (soils) × 9 (replicates) = 108 pots. The moisture content of each pot was maintained at 70% by weighing the pots two or three times a week to prevent possible loss due to evaporation. The plants were harvested 7 months after their emergence in the pots. The pots were kept in the greenhouse from November to March, where the plants were only automatically watered. The pots were transferred outdoors in March, and automatic watering was continued. The experiment was conducted without fertilization.
Soil and plant physicochemical analyses
The plant after completing its cycle was divided into the following parts: underground and above ground. The underground part, namely the roots, was divided into two parts, the lower and the upper part of the root. The above-ground part was divided into the lower, middle, and upper stems and the lower, middle, and upper leaves. The plant’s length above ground was roughly one meter, and the root stretched the entire length of the pot. The root was split into two sections (0–8 and 8–15 cm), and the shoots and leaves were divided into lower, middle, and upper sections according to heights of 0–30, 30–60, and 60–90 cm.
Soils for pot experiments were air-dried and sieved from 2 mm and underwent various physicochemical analyses, according to Golia et al. (2007); Dimirkou et al. (2009); Papadimou et al. (2023). They were analyzed to obtain soil pH potentiometrically in deionized water in the ratio of 1:1 (w/v) using pH-meter BASIC 20+ (Crison Instruments SA, Barcelona, Spain). Electrical conductivity (EC) was determined with EC-meter BASIC 30+ (Crison Instruments SA, Barcelona, Spain) values using a 1:1 (w/v) soil solution ratio (ISO/ISO 10390:2005). The determination of CaCO3% was carried out using a Bernard–Scheibler calcimeter, Gabbrielli, Italy. The particle size distribution was assessed by the Bouyoucos’ method, and the organic carbon content was measured after wet digestion, according to the modified Walkley–Black method (Page 1982). Water-soluble metal concentrations were extracted using 0.01 M CaCl2. Available to plants, Cd concentration was determinate after extraction using DTPA extraction solution in a ratio 1:2 (soil to DTPA). Total Cd concentrations were evaluated using 1 g of the fine fraction (< 2 mm) of each soil sample along with a combination of hydrochloric acid (HCl), hydrofluoric acid (HF), nitric acid (HNO3), and hydrogen peroxide (H2O2) after 30 min digestion into the microwave oven (JAOAC, 1984). Pseudototal Cd concentrations were determined using the aqua regia method (ISO/DIS 11466).
In addition, a fractionation method (using the three-step extraction procedure), developed under the European Commission's Standards, Measurements and Testing Programme (previously known as the Bureau of Community Reference, BCR) was used. The modified BCR method, as described by Golia et al. (2007), involves four steps, whereas an additional step, aqua regia was used for Cd extraction from the residue remaining after the three extraction steps. The aqua regia extraction method results in concentrations commonly referred to as "pseudototal”, as the silicates are not fully digested (Golia et al. 2007). Therefore, the following fractions of Cd were determined: (1) exchangeable and water-soluble, using 0.11 M acetic acid solution (2) bound to Fe and Mn oxides or reducible, using solution 0.5 M hydroxylammonium chloride (pH 1.5), (3) bound to soil organic matter using 8.8 M hydrogen peroxide (pH 2) and 1 M ammonium acetate (pH 2), and (4) residual Cd.
After completion of the plant cycle, the roots and the above-ground part of S. marianum were collected and washed using tap water and then rinsed with deionized water for two to three times. They were placed in paper bags and dried at 70 °C for 24 h until they reached a constant weight. The samples were subsequently weighed and ground for chemical analyses. The aqua regia solution extraction method (HCl–HNO3, 3:1) (ISO/DIS 11466) was followed for Cd determination in the plant roots, stems, leaves, flowers, and seeds.
Εvaluation of recovery and method accuracy
All extractants and blank analyses were carried out in triplicate. Cd was determined by atomic absorption spectrophotometry (AAS), AA-6300 Shimadzu (Tokyo, Japan) using flame (F-AAS) or the graphite furnace (GF) technique, Shimadzu Atomizer GFA-EX7i (Tokyo, Japan) (JAOAC, 1984) or using ICP–AES Spectruma GDA 150 (Spectro Analytical Instruments, Kleve, Germany), according to its detection limits. Analytical grade chemical reagents were used for the digestion and laboratory analyses. Glassware along with plastic containers used in the study were cleaned using 20% HNO3 and rinsed with distilled water before use. Cd standard solutions (1000 mg dm−3) were prepared from Titrisol® concentrate (Merck, Darmstadt, Germany), after a series of properly diluted solutions.
For the evaluation of the analytical methods a certified reference material BCR141R—calcareous loam soil (trace elements) (JRC—Joint Research Centre, Geel, Belgium)—was analyzed with the soil samples. Additionally, a standard reference material (SRM 1573a—Tomato Leaves) (National Institute of Standards and Technology, Gaithersburg, MD) was analyzed along with the plant tissues. The recovery ratio of standards ranged from 89.3% to 104.4%, and 88.1% to 103.7% for Cd in soil and plant, respectively.
Statistical processing of data
The Microsoft Office Excel package (v.11, 2022) (Microsoft, Redmond, WA) was used to study and evaluate statistically the results. The mean, maximum, and minimum values, as well as the standard deviation among treatments, were obtained for each data set. Tukey’s test with IBM SPSS Statistics software (IBM, Chicago, IL) was used to find the statistically significant difference between treatments, both at 95 and 99% levels of significance. After evaluating the sets of all paired comparisons simultaneously, the means of each treatment were analyzed using Tukey’s test to identify any difference between two means that is higher than the predicted standard error (Gurvich and Naumova 2021).
Furthermore, the experimental results were statistically processed using a one-way analysis of variance (ANOVA) and least significant difference (LSD) test at the significance level < 0.05 by SPSS software (version 26.1) with IBM SPSS Statistics software (IBM, Chicago, IL).
A two-way analysis of variance was performed to verify the differences existing in the plants grown in soils of different cadmium content. The same statistical procedure was used to find the changes in metal levels in roots, stems, and leaves in relation to water-soluble, available, and total cadmium concentrations in the soil. To properly complete the statistical treatment of the data, the average of three replicates was calculated for each data point.
Results and discussion
Soil physicochemical properties
Table 1 presents the values of physicochemical properties of the four soils used in this study. The first two soils (S1 and S2) are derived from rural areas, while soils S3 and S4 originate from urban soils of Volos City (central Greece), as mentioned extensively in the materials and methods chapter. Soils S1 and S4 are alkaline, while S2 and S3 are slightly acidic (slightly below neutral pH). The researchers Papadimou et al. (2023) found that the S2 soil sample has a lower pH value, while S3 and is an acidic soil, usually uncommon in urban environments. The research Mónok et al. (2021) found that urban soils had significantly higher pH, carbonate, and salt content but smaller organic carbon content than agricultural soils. While the urban soil S4 also has higher levels of organic matter than is typically seen in cities, the agricultural soil S2 had the greatest organic matter level of all the other soils. The high levels of organic matter found in samples taken in central and busy squares or in flower beds near main roads are probably due to greenery improvement works by the municipality’s services, as they supply them with manure or compost that increases soil fertility and plant flowering (Guilland et al. 2018). The CaCO3 percentages are fully associated with the pH value of the soils. A slight increase in the alkaline reaction of the soil is in most cases followed by an increase in the calcium carbonate level and vice versa (Nyamaizi et al. 2022).
The soils have electrical conductivity values ranging from 1947 to 3350 μS cm−1. The higher values of electrical conductivity in agricultural soils may be due to the use of fertilizers, i.e., salts, so the presence of ions is obvious. Furthermore, the investigators Romaneckas et al (2023) showed during a field experiment that soil electrical conductivity was highly correlated with sand. In urban soils, the high values of electrical conductivity are probably due to two causes: (a) the vicinity of the samples to the sea, as the soil is located at a distance of 200 m from the sea, and (b) the intense anthropogenic activities developed in the area, which is a favorite tourist destination (Mónok et al. 2021; Papadimou et al. 2023).
The clay and sand content of the soils are presented in Table 1. The clay content of the soils is similar, as the samples that have the most common particle size distribution in both agricultural and urban soils were selected. In urban soils, the sand content is relatively higher than the clay content, while in agricultural soils, farming practices may reduce clay levels (Sowiński et al. 2023). Medriano et al. (2022) studied the influence of different types of land use on soil physicochemical properties, along with the abundance of nitrogen levels and forms. Urban soils suffer from a gradual depletion of nutrients and augmentation or accumulation of toxic substances.
Total, pseudototal, available, and water-soluble soil Cd content
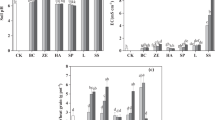
Figure 2 presents the amounts of total and pseudototal Cd concentrations. Furthermore, the potentially available fractions of Cd for uptake by plants, i.e., the available concentrations as extracted using DTPA solution and the water-soluble content of Cd, are also displayed.
The ratio of pseudototal Cd to total Cd concentration shows values from 84.5 to 99.3%, which leads to the hypothesis that despite the fact that silicates are not fully assimilated, the method of pseudototal Cd quantification satisfactorily yields the total Cd content in both urban and agricultural soils, as well as being cost effective, as HF and H2O2 are not used. The researchers Bossert et al. (2019) proposed an effective and less hazardous hydrofluoric acid-free method to dissolve silica particles in solid matrices. On the other hand, in the agricultural soil with the highest percentage of organic matter, there is an underestimation of the total concentration as the ratio of the Cd concentration extracted with aqua regia to the total concentration extracted with the strongest mixture of acids along with H2O2 shows the lowest value (84.5%). It is evident, therefore, that extraction in the presence of H2O2 leads to the destruction of organic matter and the release of Cd resulting in the quantification of its total concentration. Shahbazi and Beheshti (2019) conducted a critical comparison of three methods for measuring HMs in calcareous soils of Iran, leading to matching conclusions.
The percentage of the water-soluble concentration of Cd compared with the available concentration, i.e., the concentration extracted with the DTPA solution, shows percentages ranging from 62.5 to 86.9%. The slightly alkaline soil (S1) shows the highest percentage, indicating that the water-soluble amount of Cd extracted using DTPA solution is comparable to that extracted using a diluted CaCl2 solution, a faster and lower cost method (Degryse et al. 2020).
Cd fractions using the modified BCR method
Figure 3 shows the Cd concentrations determined using the modified BCR fractionation method (Golia et al. 2007). The percentage of the water-soluble concentration of Cd over the Cd concentration found in the first fraction of the BCR method indicates percentages ranging from 38.5 to 61.5%. The first fraction of the BCR method includes both the water-soluble and the exchangeable concentration of the Cd, i.e., represents a fraction that is the most available to plants. In comparison with the Cd-DTPA, an overestimation of Cd available concentration was observed both in alkaline and slightly acid soils, indicating that the water-soluble Cd can be a fairly reliable indicator of Cd availability, especially in slightly alkaline soils (Navarro-Pedreño et al. 2018; Liu et al. 2023).
The second fraction of the BCR method corresponds to the amount of metal bound to the Fe and Mn oxides. Often this fraction represents the effect of anthropogenic activity on soil (Golia et al. 2007; Skorbiłowicz et al. 2021; Scharenbroch et al. 2022) indicated in their study, conducted in roadside soils, that significant metal-binding factors are Fe and Mn oxides. The most commonly occurring Fe oxides in urban soils are ferrihydrite, hematite, magnetite, and goethite (Sun et al. 2018), adsorbing and regulating metal mobility and availability. In urban soils (S3 and S4), the proportion of Cd bound to Fe and Mn oxides was found to be the dominant, indicating that Cd from anthropogenic activity accounts for 46.9% of the total Cd concentration.
As presented in Table 1 and Fig. 2, the percentage of Cd related to soil organic matter is much higher in the second soil (S2), reaching 25.9% of its total concentration. This is the soil with the highest organic matter content, as presented in Table 1. Lu et al. (2022) concluded that among the various methods used to speciate metals in soils, the BCR, or modified BCR, method generally classifies soil metals into several pools. Nevertheless, levels of metals and basic properties of soils (e.g., cation exchange capacity, organic matter, and soil reaction) are not equivalent among soil types, making a necessity to use additional analysis as DTPA or water-soluble extractions.
The sum of Cd concentrations in the different fractions obtained by applying the BCR method ranges from 98.9 to 102.1% of the total Cd concentration and from 99.0 to 101.3% of the pseudototal Cd concentration in the study soils.
Cd distribution in S. marianum cultivated in the four soils
Figure 4 depicts the Cd concentrations in the various parts of the S. marianum plant cultivated in the four soils of the study, which were treated at two concentration levels. According to the European Union’s (EU) maximum allowable level of Cd in soils, EU regulation, and incorporation into Greek legislation (Papadimou et al. 2023), the first contamination level corresponds to that value, whereas the second level corresponds to a Cd concentration that is 10 times higher, or 30 mg Cd kg−1. As a result, the first level describes moderately Cd-contaminated soils, while the second level describes substantially Cd-contaminated soils, both in rural and urban environments. The flowers and seeds were below the detection limits of the instrument Cd concentrations (0.02 μg L−1) and are therefore not shown in the Fig. 4.
S. marianum in this study of all four soils showed that most of the soil cadmium was taken up and transported to the upper root and lower part of the stem. Cd translocation is significantly reduced in the upper parts of the stem and the leaves. No indication of Cd was identified in the plant’s flowers or seeds in any soil regardless of pollution level. Cadmium intake is proportional to the concentration of soil cadmium, so it is higher in heavily contaminated soils (i.e., at the second level of contamination).
The accumulation of metals, mainly in the lower part of the shoot, and the inverse variation in concentration with plant height is something that has been observed in several studies and in many plants. Golia et al. (2021, 2023) observed that industrial hemp accumulates trace elements and toxic metals, mainly in the root, less in the lower part of the stem, while the upper stem and upper leaves are almost free of metals. Cao et al. (2019) investigated the distribution, availability and translocation of HMs in Brassica napus L. Cao et al. (2020) continued their research on accumulation and distribution of both Cd and Pb in 28 oilseed rape cultivars grown in a contaminated field. Since the studied plants can and largely accumulate the soil metals in its underground part, it indicates that the most likely mechanism of phytoremediation is phytostabilization (Simiele et al. 2021). Metal-tolerant plant species are used in phytostabilization to immobilize HMs underground and lower their bioavailability. This prevents the metals from migrating into the ecosystem and lowers the risk of metals getting into the food chain (Sharma et al. 2023).
Correlations among soil physicochemical parameters, Cd concentrations in soil fractions, and parts of cultivated plants in the study soils
Tables 2 and 3 demonstrate the correlation coefficients between the physical and chemical properties of the soils, the Cd concentrations in the different soil fractions, and the Cd levels in the various plant parts of S. marianum. Some symbols and labels were used to ensure that variables in the tables were represented uniformly. According to the BCR method, the soil Cd fractions 1, 2, 3, and 4 are, respectively, exchangeable and water soluble, bounded to Mn and Fe oxides, bounded to soil organic matter, and residual. Furthermore, a color gradient is used in these tables (as a footnote) to show the positive or negative correlation between the associated values.
Clarification and interpretation of colors used for the correlation coefficients between soil physicochemical properties, Cd concentrations in soil fractions, and Cd concentration in parts of S. marianum.

Table 2 depicts the relationships that arise between soil characteristics, soil Cd fractions, and Cd levels in plant parts when the plant is cultivated in heavily polluted agricultural soil. On the other hand, Table 3, presents the correlation coefficients among Cd in S. marianum and Cd in urban soil 3 (S3), which is also heavily contaminated (B level).
Soil pH appears to be the key parameter for controlling Cd in soil-to-plant system, in both agricultural and urban soils. The concentration of Cd in the plant parts, where it appears to accumulate the greatest amount, notably the top section of the root and the lower part of the stems, apparently negatively correlated with soil pH (Table 1). The available soil concentration and the concentration in the plant portions of greatest accumulation have a positive correlation. Zhang et al. (2023) also found that Cd accumulation in stems and roots, along with subcellular distribution in Persicaria hydropiper (L.) Delarbre, in paddy soils was highly affected by soil pH. Furthermore, Lu et al. (2022) in their research indicated that the variation in soil pH was responsible for the transformation of Cd speciation in paddy soils during submerging/draining alternation. Organic matter is positively and statistically significant connected with plant Cd that absorb the greatest amount of Cd of soil 2 (S2), a typical agricultural soil with the highest percentage of organic matter (Golia et al. 2007). On the other hand, as far as urban soil (S3) is concerned (Table 3b), the high association between Cd bound to Mn and Fe oxides suggests the plant root gains cadmium from this pool. The Cd content linked to Fe and Mn oxides, or in the second soil fraction of the BCR technique, which reflects the anthropogenic burden on the soil (Scharenbroch et al. 2022).
Plant metal levels were significantly (0.01 level of significance) associated with soil levels in both soil groups of the present study. It is well known that a lot of the work on predicting soil metal levels is based on mathematical models that take into account soil pH along with available metal concentrations (Lv 2019; Lv and Liu 2019; Golia and Diakoloukas 2022). The bioavailability of the metal in the parts of plants where it predominantly accumulates is greatly influenced by the accessible concentration of Cd (extracted using DTPA), both in agricultural and urban soils, particularly in soils that are highly polluted by hazardous Cd.
Conclusions
A pot experiment was conducted investigating the potential of S. marianum for the remediation of Cd-contaminated soils. The experiment included four soils, slightly acidic and alkaline, originating from rural and urban areas. Laboratory contamination was performed in two levels to eventually produce two soil groups representing moderately and heavily contaminated soils. The water-soluble, available, pseudototal and total Cd concentrations, and Cd-soil fractions were determined using the modified BCR method. The highest percentages of Cd concentration were bound in the organic matter fraction in agricultural soils, and in the Fe and Mn oxide fraction in urban soils, indicating the extent of contamination due to anthropogenic features.
The initial outcomes of the experiment are encouraging, as milk thistle seems to thrive and survive even in heavily Cd-contaminated soils, promoting circular economy issues. It includes characteristics and attributes that permit its classification among accumulator plants, and as it collects the metal pollutant in its subsurface part, it appears to undergo phytostabilization mechanisms. When grown in heavily Cd-contaminated soils, Cd appears to accumulate in the upper part of the root and the lower part of the shoot, leaving the rest of the plant parts essentially unaffected by Cd.
Studying the correlation coefficients among Cd in plant parts along with Cd soil levels, it appears that soil pH is a key parameter controlling the availability and uptake of Cd by the plant tissues.
However, future experiments should be carried out in naturally contaminated soils, investigating the synergistic or antagonistic effects among metals and the ability of S. marianum to accumulate HMs when cultivated in agricultural and urban polluted Mediterranean soils.
Data availability
Data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ait Elallem K, Sobeh M, Boularbah A, Yasri A (2021) Chemically degraded soil rehabilitation process using medicinal and aromatic plants: review. Environ Sci Pollut Res 28:73–93. https://doi.org/10.1007/s11356-020-10742-y
Almendro-Candel MB, Navarro-Pedreño J, Lucas IG, Zorpas AA, Voukkali I, Loizia P (2019) The use of composted municipal solid waste under the concept of circular economy and as a source of plant nutrients and pollutants. Municipal Solid Waste Manag. https://doi.org/10.5772/intechopen.83386
Awasthi G, Nagar V, Mandzhieva S et al (2022) Sustainable amelioration of heavy metals in soil ecosystem: existing developments to emerging trends. Minerals 12:85. https://doi.org/10.3390/min12010085
Bokelmann JM (2022) Milk thistle (Silybum marianum): seeds, flower heads. AJHP 56:495–509. https://doi.org/10.1016/B978-0-323-84676-9.00061-1
Bossert D, Urban DA, Maceroni M et al (2019) A hydrofluoric acid-free method to dissolve and quantify silica nanoparticles in aqueous and solid matrices. Sci Rep 9:7938. https://doi.org/10.1038/s41598-019-44128-z
Cao X, Wang X, Tong W et al (2019) Distribution, availability and translocation of heavy metals in soil-oilseed rape (Brassica napus L.) system related to soil properties. Environ Pollut 252:733–741. https://doi.org/10.1016/j.envpol.2019.05.147
Cao X, Wang X, Tong W et al (2020) Accumulation and distribution of cadmium and lead in 28 oilseed rape cultivars grown in a contaminated field. Environ Sci Pollut Res 27:2400–2411. https://doi.org/10.1007/s11356-019-06826-z
D’Adamo I, Gastaldi M (2023) Perspectives and challenges on sustainability: drivers, opportunities and policy implications in universities. Sustainability 15(4):3564
Dasharathy S, Arjunan S, Maliyur Basavaraju A et al (2022) Mutagenic, carcinogenic, and teratogenic effect of heavy metals. Evid Based Complementary Altern Med 2022:8011953. https://doi.org/10.1155/2022/8011953
De Vos B, Souza MF, Michels E, Meers E (2022) Industrial hemp (Cannabis sativa L) in a phytoattenuation strategy: Remediation potential of a Cd, Pb and Zn contaminated soil and valorization potential of the fibers for textile production. Ind Crops Prod 178:114592. https://doi.org/10.1016/j.indcrop.2022.114592
Degryse F, da Silva RC, Baird R, Cakmak I, Yazici MA, McLaughlin MJ (2020) Comparison and modelling of extraction methods to assess agronomic effectiveness of fertilizer zinc. J Plant Nutr Soil Sci. https://doi.org/10.1002/jpln.201900340
Dimirkou A, Ioannou Z, Golia EE, Danalatos N, Mitsios IK (2009) Sorption of cadmium and arsenic by goethite and clinoptilolite. Commun Soil Sci Plant Anal. https://doi.org/10.1080/00103620802623539
Dobrikova A, Apostolova E, Hanć A, Yotsova E, Borisova P, Sperdouli I, Adamakis I-DS (2021) Tolerance mechanisms of the aromatic and medicinal plant salvia sclarea l. To Excess Zinc Plants 10:194. https://doi.org/10.3390/plants10020194
Domínguez MT, Montiel-Rozas MM, Madejón P et al (2017) The potential of native species as bioenergy crops on trace-element contaminated Mediterranean lands. Sci Total Environ 590–591:29–39. https://doi.org/10.1016/j.scitotenv.2017.03.018
Ferreira CS, Seifollahi-Aghmiuni S, Destouni G, Ghajarnia N, Kalantari Z (2022) Soil degradation in the European Mediterranean region: processes, status and consequences. Sci Total Environ 805:150106
Golia EE (2023) The impact of heavy metal contamination on soil quality and plant nutrition. Sustainable management of moderate contaminated agricultural and urban soils, using low cost materials and promoting circular economy. Sustain Chem Pharm 33:101046. https://doi.org/10.1016/j.scp.2023.101046
Golia EE, Diakoloukas V (2022) Soil parameters affecting the levels of potentially harmful metals in Thessaly area, Greece: a robust quadratic regression approach of soil pollution prediction. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-14673-0
Golia EE, Tsiropoulos NG, Dimirkou A, Mitsios I (2007) Distribution of heavy metals of agricultural soils of central Greece using the modified BCR sequential extraction method. Int J Environ Anal Chem 87:1053–1063. https://doi.org/10.1080/03067310701451012
Golia EE, Angelaki A, Giannoulis KD, Skoufogianni E, Bartzialis D, Cavalaris C, Vleioras S (2021) Evaluation of soil properties, irrigation and solid waste application levels on Cu and Zn uptake by industrial hemp. Agron Res. https://doi.org/10.15159/AR.21.016
Golia EE, Bethanis J, Ntinopoulos N, Kaffe GG, Komnou AA, Vasilou C (2023) Investigating the potential of heavy metal accumulation from hemp. The use of industrial hemp (Cannabis sativa L.) for phytoremediation of heavily and moderated polluted soils. Sustain Chem Pharm 31:100961. https://doi.org/10.1016/j.scp.2022.100961
Guilland C, Maron PA, Damas O, Ranjard L (2018) Biodiversity of urban soils for sustainable cities. Environ Chem Lett 16:2
Gupta AK, Verma SK, Khan K, Verma RK (2013) Phytoremediation using aromatic plants: A sustainable approach for remediation of heavy metals polluted sites. Environ Sci Technol 47(18):10115–10116. https://doi.org/10.1021/es403469c
Gurvich V, Naumova M (2021) Logical contradictions in the one-way ANOVA and Tukey-Kramer multiple comparisons tests with more than two groups of observations. Symmetry (basel) 13:1387. https://doi.org/10.3390/sym13081387
Hammami H, Alaie E, Dastgheib SMM (2018) The ability of Silybum marianum to phytoremediate cadmium and/or diesel oil from the soil. Int J Phytoremediation 20:756–763. https://doi.org/10.1080/15226514.2018.1425664
ISO/DIS 11466 (1994) Environment Soil Quality. By ISO Standards Compendium, Switzerland. Geneva, Switzerland
ISO/ISO 10390:2005 Soil Quality, Determination of pH. International Standards Organization; Geneve, Switzerland: 2005
JAOAC (1984) Chemists, A. of official, Ed.; 14th ed.; Inc. Virginia, USA, 1984; Vol. 6; ISBN 9788578110796
Jeswani HK, Chilvers A, Azapagic A (2020) Environmental sustainability of biofuels: a review: environmental sustainability of biofuels. Proc R Soc A Math Phys Eng Sci 476:2
Karkanis A, Bilalis D, Efthimiadou A (2011) Cultivation of milk thistle (Silybum marianum L. Gaertn.), a medicinal weed. Ind Crops Prod 34:2
Liang J, Liu Z, Tian Y et al (2023) Research on health risk assessment of heavy metals in soil based on multi-factor source apportionment: a case study in Guangdong Province. China Sci Total Environ 858:159991. https://doi.org/10.1016/J.SCITOTENV.2022.159991
Liava V, Karkanis A, Tsiropoulos N (2021) Yield and silymarin content in milk thistle (Silybum marianum (L.) Gaertn.) fruits affected by the nitrogen fertilizers. Ind Crops Prod. https://doi.org/10.1016/j.indcrop.2021.113955
Liu YR, van der Heijden MGA, Riedo J et al (2023) Soil contamination in nearby natural areas mirrors that in urban greenspaces worldwide. Nat Commun. https://doi.org/10.1038/s41467-023-37428-6
Lu H, Li K, Nkoh JN et al (2022) Effects of pH variations caused by redox reactions and pH buffering capacity on Cd(II) speciation in paddy soils during submerging/draining alternation. Ecotoxicol Environ Saf. https://doi.org/10.1016/j.ecoenv.2022.113409
Lv J (2019) Multivariate receptor models and robust geostatistics to estimate source apportionment of heavy metals in soils. Environ Pollut. https://doi.org/10.1016/j.envpol.2018.09.147
Lv J, Liu Y (2019) An integrated approach to identify quantitative sources and hazardous areas of heavy metals in soils. Sci Total Environ 646:19–28. https://doi.org/10.1016/j.scitotenv.2018.07.257
Mawari G, Kumar N, Sarkar S et al (2022) Heavy metal accumulation in fruits and vegetables and human health risk assessment: findings from Maharashtra, India. Environ Health Insights 16(1–10):117863022211191. https://doi.org/10.1177/11786302221119151
Medriano CA, Chan A, De SR, Bae S (2022) Different types of land use influence soil physiochemical properties, the abundance of nitrifying bacteria, and microbial interactions in tropical urban soil. SSRN Electron J. https://doi.org/10.2139/ssrn.4250780
Mónok D, Kardos L, Pabar SA et al (2021) Comparison of soil properties in urban and non-urban grasslands in Budapest area. Soil Use Manag. https://doi.org/10.1111/sum.12632
Navarro-Pedreño J, Almendro-Candel MB, Gómez Lucas I, Jordán Vidal MM, Bech Borras J, Zorpas AA (2018) Trace metal content and availability of essential metals in agricultural soils of Alicante (Spain). Sustainability 10(12):4534
Nyamaizi S, Messiga AJ, Cornelis JT, Smukler SM (2022) Effects of increasing soil pH to near-neutral using lime on phosphorus saturation index and water-extractable phosphorus. Can J Soil Sci. https://doi.org/10.1139/cjss-2021-0197
Oladoye PO, Olowe OM, Asemoloye MD (2022) Phytoremediation technology and food security impacts of heavy metal contaminated soils: a review of literature. Chemosphere 288:132555
Page AL (1982) Methods of soil analysis-Part 2: chemical and microbiological properties. (2nd edition). Am Soc Agron Inc PublMadison, USA 9:421–422 (Phosphurus)
Pandey J, Verma RK, Singh S (2019) Suitability of aromatic plants for phytoremediation of heavy metal contaminated areas: a review. Int J Phytoremed 21:25
Papadimou SG, Kantzou OD, Chartodiplomenou MA, Golia EE (2023) Urban Soil pollution by heavy metals: effect of the lockdown during the period of COVID-19 on pollutant levels over a five-year study. Soil Syst. https://doi.org/10.3390/soilsystems7010028
Papamichael I, Pappas G, Siegel JE, Zorpas AA (2022) Unified waste metrics: a gamified tool in next-generation strategic planning. Sci Total Environ 833:154835
Peana M, Pelucelli A, Chasapis CT, Perlepes SP, Bekiari V, Medici S, Zoroddu MA (2022) Biological effects of human exposure to environmental cadmium. Biomolecules 13(1):36. https://doi.org/10.3390/biom13010036
Perlein A, Zdanevitch I, Gaucher R et al (2021) Phytomanagement of a metal(loid)-contaminated agricultural site using aromatic and medicinal plants to produce essential oils: analysis of the metal(loid) fate in the value chain. Environ Sci Pollut Res 28:62155–62173. https://doi.org/10.1007/s11356-021-15045-4
Romaneckas K, Buragienė S, Kazlauskas M et al (2023) Effects of soil electrical conductivity and physical properties on seeding depth maintenance and winter wheat germination, development and productivity. Agronomy 13:2. https://doi.org/10.3390/agronomy13010190
Scharenbroch BC, Trammell TL, Paltseva A et al (2022) Editorial: urban soil formation, properties, classification, management, and function. Front Ecol Evol 10:25
Shahbazi K, Beheshti M (2019) Comparison of three methods for measuring heavy metals in calcareous soils of Iran. SN Appl Sci. https://doi.org/10.1007/s42452-019-1578-x
Sharma JK, Kumar N, Singh NP, Santal AR (2023) Phytoremediation technologies and their mechanism for removal of heavy metal from contaminated soil: an approach for a sustainable environment. Front Plant Sci 14:2
Simiele M, Sferra G, Lebrun M et al (2021) In-depth study to decipher mechanisms underlying Arabidopsis thaliana tolerance to metal(loid) soil contamination in association with biochar and/or bacteria. Environ Exp Bot 182:104335. https://doi.org/10.1016/j.envexpbot.2020.104335
Skorbiłowicz M, Skorbiłowicz E, Rogowska W (2021) Heavy metal concentrations in roadside soils on the białystok-budzisko route in Northeastern Poland. Minerals. https://doi.org/10.3390/min11111290
Sowiński P, Smólczyński S, Orzechowski M et al (2023) Effect of soil agricultural use on particle-size distribution in young glacial landscape slopes. Agriculture. https://doi.org/10.3390/agriculture13030584
Stylianou M, Gavriel I, Vogiatzakis IN, Zorpas A, Agapiou A (2020) Native plants for the remediation of abandoned sulphide mines in cyprus: a preliminary assessment. J Environ Manage 274:110531
Sun J, Mailloux BJ, Chillrud SN et al (2018) Simultaneously quantifying ferrihydrite and goethite in natural sediments using the method of standard additions with X-ray absorption spectroscopy. Chem Geol. https://doi.org/10.1016/j.chemgeo.2017.11.021
Wang Y, Cao D, Qin J et al (2023) Deterministic and probabilistic health risk assessment of toxic metals in the daily diets of residents in industrial regions of Northern Ningxia, China. Biol Trace Elem Res 201:4334–4348. https://doi.org/10.1007/s12011-022-03538-3
Yang Y, Zhou X, Tie B, Peng L, Li H, Wang K, Zeng Q (2017) Comparison of three types of oil crop rotation systems for effective use and remediation of heavy metal contaminated agricultural soil. Chemosphere 188:148–156. https://doi.org/10.1016/j.chemosphere.2017.08.140
Yang Y, Ge Y, Tu P, Zeng H, Zhou X, Zou D, Wang K, Zeng Q (2019) Phytoextraction of Cd from a contaminated soil by tobacco and safe use of its metal-enriched biomass. J Hazard Mater 363:385–393. https://doi.org/10.1016/j.jhazmat.2018.09.093
Zehra A, Sahito ZA, Tong W, Tang L, Hamid Y, Khan MB, Ali Z, Naqvi B, Yang X (2020) Assessment of sunflower germplasm for phytoremediation of lead-polluted soil and production of seed oil and seed meal for human and animal consumption. J Environ Sci. https://doi.org/10.1016/j.jes.2019.05.031
Zeng H, Chen L, Zhou X, Zeng Q (2019) Cadmium accumulation in winter crops and the assessment of paddy soil phytoremediation in southern China. Environ Sci Pollut Res 26:17173–17182. https://doi.org/10.1007/s11356-019-05054-9
Zhang J, Liu K, He X et al (2023) Evaluation of heavy metal contamination of soil and the health risks in four potato-producing areas. Front Environ Sci 11:1071353. https://doi.org/10.3389/fenvs.2023.1071353
Zhi Y, Zhou Q, Leng X, Zhao C (2020) Mechanism of remediation of cadmium-contaminated soil with low-energy plant snapdragon. Front Chem 8:22. https://doi.org/10.3389/fchem.2020.00222
Zorpas AA, Pedreño JN, Candel MBA (2021) Heavy metal treatment and removal using natural zeolites from sewage sludge, compost, and agricultural soils: a review. Arab J Geosci 14:1098. https://doi.org/10.1007/s12517-021-07443-2
Funding
Open access funding provided by HEAL-Link Greece. This research received no external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Consent to participate
Not applicable.
Consent for publication
All the authors approved the final manuscript and agreed to its submission to the Journal.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Responsible Editor: Antonis Zorpas.
This paper belongs to the special issue: Sustainable Production and Consumption.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Papadimou, S.G., Barbayiannis, Ν. & Golia, E.E. Preliminary investigation of the use of Silybum marianum (L.) Gaertn. as a Cd accumulator in contaminated Mediterranean soils: the relationships among cadmium (Cd) soil fractions and plant Cd content. Euro-Mediterr J Environ Integr 9, 405–417 (2024). https://doi.org/10.1007/s41207-023-00430-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41207-023-00430-x