Abstract
The ability of synthetic zeoliteNaX to remove Cr(VI) and Pb(II) from aqueous solutions was studied in fixed bed column. The effect of bed height (4, 6, 8 in.) and flow rate (0.825, 1.6, 2.14, 4.0, 5.0 L/h) was studied. The uptake and percentage removal of Cr(VI) Pb(II) and on zeoliteNaX for 4-in. column were found to be 5.66 and 8.896 mg/g and 84.99 and 89%, respectively. The bed depth service time (BDST) and Thomas model were used for the analysis of the column. The column experiments showed that the breakthrough time and the exhaustion time are found to increase with increase in bed height. Favorable results were obtained with the BDST model, which describe the experimental data well.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rapid development of industrial activities has intensified environmental pollution and the deterioration of ecosystems, with the accumulation of pollutants, such as heavy metals, synthetic compounds, nuclear wastes, etc.[2, 3]. Due to their high toxic nature even at very low concentrations, the ions of Pb, Cr, Cd, Zn, Hg, Cu, etc. gain importance [1, 4, 5]. In recent years, increasing concern about the effect of toxic metals in the environment has resulted in more strict environmental regulations for industrial applications that discharge metal bearing effluents [6]. As growing attention is being given to the potential health hazard arising from the existence of heavy metals in the environment, the need for cost effective and efficient methods for the removal of metals has resulted in the development of new separation technologies. Precipitation, adsorption–ion exchange, flocculation, absorption, electrochemical processes, and/or membrane processes, such as electrodialysis, nanofiltration, and reverse osmosis, are commonly applied for the treatment of industrial effluents [8, 9, 13]. Studies on the treatment of effluents containing heavy metals have revealed adsorption to be a highly effective technique for the removal of heavy metals from dilute aqueous metal solutions [15]. Additionally, adsorption has been found to be superior to other techniques for water reuse in terms of the initial cost, simplicity of design, and easiness of operation [11, 16, 17, 19]. In this study, Cr(VI) and Pb(II) ion removal from aqueous solutions by column adsorption method was investigated by using an adsorbent zeoliteNaX. ZeoliteNaX is found to be an effective adsorbent in the batch study for removal of heavy metals. The column study has been carried out to ascertain the effect of parameters such as flow rate, adsorbent bed height, and initial concentration of the two metallic ion solutions on the removal efficiency.
Materials and Methods
All chemicals used were of analytical grade (Central Drug House, India). Adsorbent zeoliteNaX of bulk density 600 g/L having cylindrical shape particle of diameter 1.5 mm used as adsorbent in this work was purchased from Central Drug House (CDH) India. The adsorbent was contacted with 1 M NaCl solution to bring it to a near homoionic state of Na since extensive exposure to high sodium concentration was required to displace ions (Cr(VI) and Pb(II)) from the zeolitic matrix. The exposure of natural zeolite to 1 M NaC1 solution led to the production of the sodium-rich sample [7] followed by washing with ultrapure water, followed by drying in the oven at 373 K. The dried adsorbent was stored in desiccators for subsequent use. Concentrations of Cr and Pb ions were determined as per standard methods for analysis of water and wastewater using atomic absorption spectrophotometer having air-acetylene flame (model AAS 4141, ECIL India).
Experimental Methods
The column study was carried out in three separate fixed beds of 10, 15, and 20 cm of zeoliteNaX adsorbent in PVC pipe columns of inner diameter 2.54 cm. The adsorbent was carefully placed in the column, and the bed was flushed several times with ultrapure water to ensure a close packing of the zeoliteNaX particles to minimize cracks/channels/voids. The bed was allowed to drain completely before loading it with the sorbate. An upward flow of the solution was maintained in the column using a peristaltic pump. Upward flow was preferred because it ensured complete coverage of the adsorbent and reduced formation of channels compared to the case of downward flow [10]. As the feed passes through the bed, the ion exchange zone moves downstream and in due course reaches the exit. When the concentration of the effluent reaches 5–10% of the influent, the flow is stopped. This point is commonly referred as breakthrough point or breakpoint and is fixed accordingly to the needs of the operation. Since only the last portion of the fluid that passes through the bed has this concentration level, the average fraction of the solute removed from the beginning until the breakpoint is generally very high [12]. In the present study, the break point is set at 10%, and the total volume of the treated solution until this point is used as a measure of the removal efficiency of the operation.
Analysis of Column Data
Successful design of a column sorption process required prediction of the concentration-time profile or breakthrough curve for the effluent. Various mathematical models have been developed for analysis and prediction of the shape of the breakthrough curves. Among these, the bed depth service time (BDST) model is simple to use in the design of a fixed bed adsorption column developed by Bohart and Adams. Bohart and Adams developed an eq. (1) to describe the relationship between service time (t) and bed height (X) for a fixed bed absorber. Service time is the time needed by the adsorption column to reach the breakthrough point with specific saturation percentage at adsorption operating conditions. The eq. (1) is as follows:
Since \( {e}^{K{N}_{o\left(\frac{X}{V}\right)}} \) is usually much greater than unity, the above equation can be simplified to
Solving the eq. (2) for t
where C o is the initial concentration of solute (mg/L), C B the desired concentration of solute at breakthrough (mg/L), K the adsorption rate constant (L/mg h), N o the adsorption capacity (mg/L), X the bed depth of column (cm), V the linear flow velocity of feed to bed (cm/h), and t is the service time of column under above conditions (h).
Setting t = 0 and solving eq. (3) for X yields
where X o is the minimum column height necessary to produce an effluent concentration CB, also known as critical bed depth.
Thomas Model
Theoretical model proposed by Thomas is widely used to describe the column performance. The linear form of Thomas model can be expressed as eq. (5), where k Th (mlit/min mg) is the Thomas model constant, q 0 (mg/g) is the adsorption capacity. The values of k Th and q 0 can be determined from the linear plot of ln[(C0/Ct) − 1] against t.
Weber and Morris Intra-Particle Diffusion Model
Intra-particle diffusion model was based on the theory proposed by Weber and Morris. The model is as follows:
qt = kintt1/2 + C (6) where k int is the intra-particle diffusion rate constant and C is the intercept related to the thickness of the boundary layer. The value of the intra-particle diffusion rate constant is 0.55 (mg/g/min1/2).
Results and Discussion
Effect of Bed Height
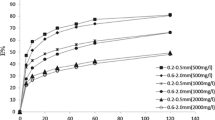
The adsorption of metal ions in packed bed column is influenced by the amount of adsorbent present. Three beds of 4, 6, and 8 in. height having 30, 45, and 60 g of the adsorbent, respectively were used for the column study. Solution of 10 mg/L initial concentration of each of the two metal ions was used at a flow rate of 0.825 L/h to get column study data. The breakthrough curves for adsorption of the two metal ions on various bed heights of zeoliteNaX are shown in Figs. 1 and 2. The breakthrough time and the exhaustion time are found to increase with increase in bed height. It can be attributed to the increase in sorption sited and the contact time due to increased amount of adsorbent available in the column [14].
Effect of Flow Rate
Heavy metal ion removal from industrial effluent is mostly carried out in continuous mode. The study of the effect of flow rate assumes importance as it is one of the useful parameters in the design of an adsorber. The sorption capacity data of zeoliteNaX for solution of the two metal ions at initial concentration of 10 mg/L in a bed height of 4 in. is presented here for flow rates of 0.825, 1.6, 2.14, 4.0, and 5.0 L/h.
It is clear from Figs. 3 and 4 that rapid uptake of ions in the initial stages is followed by decreasing adsorption rate to eventually reach saturation point. As the flow rate is increased, the breakthrough curves become steeper and reach the breakthrough point quickly. At higher flow rate, the contact time between the adsorbate and adsorbent is minimized, leading to an early breakthrough [18]. The uptake of Cr(VI) and Pb(II) on zeoliteNaX for 4-in. column was found to be 5.66 and 8.896 mg/g, respectively.
Analysis of Column Data
Mathematical analysis was carried out for packed bed of adsorbent. The S shaped curves obtained from the experimental data were evaluated. As per the BDST model, the slope of the breakthrough curve is equal to the reciprocal of velocity in the adsorption zone. The design parameters like K, No, and Xo were calculated and shown in Table 1. Thomas model constant was found to be 4.12 x10-2, 3.21x10-2, and 2.21x10-2 mlit/min mg and 5.34 x10-2, 4.23x10-2, and 3.33x10-2 mlit/min mg for Pb(II) and Cr(VI).
Regeneration Study
Regeneration and reuse of an adsorbent are crucial for cost reduction so that its industrial applications may be developed. Use of such adsorbents for recovery of heavy metal ions from industrial waste is desirable. Fixed bed/fluidized bed adsorbers with zeolite with regeneration capacity to adsorb and desorbs several times the heavy metal ions from industrial wastewater are desirable to meet the stringent pollution control requirements. The regeneration study was carried out by 0.1 N NaCl solution through the bed height of 4 in. at a flow rate of 0.1 L/min for the two metal ions. The concentration of metal ions was monitored after different time intervals. It was observed that desorption collection time took 20 min after which further desorption was negligible. The total volume was 2 L. The average for each 1-L collection was calculated and is given in Table (2). In the first collection, 169.93 mg of Cr(VI) was adsorbed from which 157.69 mg could be desorbed, indicating an excellent desorption efficiency of 92.80%. The remaining was desorbed in the second collection. Similar data have been reported for Pb(II) metal ion also. Total desorption of metal ion was more than 98%. The total volume taken for adsorption purpose was 35 L while it was only 2 L for desorption purpose. There is a huge gap of regeneration efficiency as shown in Table 2. Initially, the zeoliteNaX-regenerated adsorbent has more sites for ion exchange of metals Pb(II) and Cr(VI) and as the process takes place, the sites have been filled up and efficiency of the adsorbent decreased.
Conclusions
The adsorbent synthetic zeoliteNaX is an effective adsorbent for the removal of Cr(VI) and Pb(II) from wastewaters in continuous mode. The uptake of Cr(VI) and Pb(II) on zeoliteNaX for 4-in. column was found to be 5.660 and 8.896 mg/g, respectively at pH of 4.0 and 30 °C. The results obtained agree to the bed depth service time (BDST) model well. Thomas model constant was found to be 4.12 x10-2, 3.21x10-2, and 2.21x10-2 mlit/min mg and 5.34 x10-2, 4.23x10-2, and 3.33x10-2 mlit/min mg for Pb(II) and Cr(VI).
References
Aggarwal D, Goyal M, Bansal RC (1999) Adsorption of chromium by activated carbon from aqueous solution. Carbon 37:1989–1997
Aksu Z, Tezer S (2005) Biosorption of reactive dyes on the green alga Chlorella vulgaris. Process Biochem 40:1347–1361
Alvarez-Ayuso E, Garsia AS, Querol X (2003) Purification of metal electroplating waste waters using zeolites. Water Res 37:4855–4862
Bailey SE, Olin TJ, Bricka RM, Adrian DD (1999) A review of potentially low cost sorbent for heavy metals. Water Res 33:2469–2479
Barros MASD, JrIF A, Arroyo PA, Sousa-aguiar EF, CRG T (2003) Multicomponent ion exchange isotherms in NaX zeolite. Lat Am Appl Res 3:339–345
Candela PM, Martin MJ, Marcia TR (1995) Chromium (VI) removal with activated carbons. Water Res 29(9):2174–2180
Curkovic L, Cerjan-Stefanovic S, Filipan T (1997) Metal ion exchange by natural and modified zeolites. Water Res 31(6):1379–1382
Dantas TNDC, Dantas AAN, Moura MCPDA (2001) Removal of chromium from aqueous solutions by diatomite treated with microemulsion. Water Res 35(9):2219–2224
Garsia SA, Alastuey A, Querol X (1999) Heavy metal adsorption by different minerals: application to the remediation of polluted soils. Sci Total Environ 242:179
Harland CE (1994) Ion exchange: theory and practice, Second edn. The Royal Society of Chemistry, London
Hasan FM (2014) Removal of cadmium (II) and lead (II) ions from aqueous solution by zeolite A4 supported on natural carbon. Int J Sci Technol 3(7):391–399
Inglezakis VJ, Loizidou MD, Grigoropoulou HP (2003) Ion exchange of Pb2+, Cu2+, Fe3+ and Cr3+ on natural clinoptilolite: selectivity determination and influence of acidity on metal uptake. J Colloid Interf Sci 261:49–54
Kocasoy G (1999) Removal of heavy metal from industrial wastewater by using natural zeolites. Proceedings of International Conference on Urban Pollution Control Technology 209-214
Kumar U, Manas B (2005) Fixed bed column study for Cd(II) removal from wastewater using treated rice husk. J Hazard Mater 129:253–259
Meshko VD, Markovska LT, Mirko SM (2006) Modeling of the adsorption kinetics of zinc onto granular activated carbon and natural zeolite. J Serb Chem Soc 71(8–9):957–967
Mier MV, Raymundo LC, Gehr R, Blanca EJC, Pedro JJA (2001) Heavy metal removal with Mexican clinoptilolite: multi-component ionic exchange. Water Res 35(2):373–378
Padmesh TVN, Vijayaraghvan K, Sekaran G, Velan M (2006) Biosorption of acid blue 15 using fresh water macroalga Azolla filiculoides: batch and column studies. Dyes Pigments 71:77–82
Sivaprakash B, Rajamohan N, Sadhik AM (2010) Batch and column sorption of heavy metal from aqueous solution using a marine alga Sargassum tenerrimun. Int J Chem Tech Res 2(1):155–162
Wan CT, Mark DGL, Hanna LPBA, Cybelle MF, James IC, Meng WW (2016, 2016) Competitive fixed-bed adsorption of Pb(II), Cu(II), and Ni(II) from aqueous solution using chitosan-coated bentonite. Int J Polym Sci 1:–11
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pandey, P.K., Sharma, S.K. Removal of Cr(VI) and Pb(II) from Wastewater by ZeoliteNaX in Fixed Bed Column. Water Conserv Sci Eng 2, 61–65 (2017). https://doi.org/10.1007/s41101-017-0026-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41101-017-0026-2