Abstract
One of the main environmental concerns of today is the occurrence of arsenic in wastewater. Targeting a solution for this problem, several efforts have been made towards research and application of cost-effective and effortlessly adaptable processes. The ability of a biofilm of Bacillus arsenicus MTCC 4380 supported on sawdust/MnFe2O4 composite to biosorb/bioaccumulate As(III) and As(V) was investigated in batch experiments. Optimum biosorption/bioaccumulation conditions were determined as a function of contact time and temperature. The equilibrium was achieved after about 220 min at 30 °C temperature. Nonlinear regression analysis was done to determine the best-fit kinetic model based on three correlation coefficients and three error functions and also to predict the parameters involved in kinetic models. The results showed that both Brouers–Weron–Sotolongo and Avrami models for both As(III) and As(V) were capable of providing realistic explanation of biosorption/bioaccumulation kinetic. Applicability of mechanistic models showed that the rate controlling step in the biosorption/bioaccumulation of both As(III) and As(V) was film diffusion rather than intraparticle diffusion. The estimated thermodynamic parameters ΔG 0, ΔH 0, and ΔS 0 revealed that biosorption/bioaccumulation of both As(III) and As(V) was feasible, spontaneous, and exothermic under examined conditions. The activation energy (E a) estimated from Arrhenius equation specified the nature of biosorption/bioaccumulation being ion exchange type. The effect of co-existing ions such as Cu2+, Zn2+, Bi3+, Cd2+, Fe3+, Pb2+, Co2+, Ni2+, Cr6+, and SO4 2− at different concentrations was inspected.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Arsenic and its compounds are considered extremely noxious environmental pollutants to which millions of people are exposed over the world through ingestion of polluted water [1]. Arsenic is released to the environment typically through natural processes, because of the occurrence of arsenical minerals, leaching and soil erosion, inputs from geothermal sources, and volcanic emissions in addition to a result of anthropogenic activities, such as combustion of fossil fuels, smelting, mining activities, use of arsenical pesticides, production of glassware, dyes, and drugs, etc. [1, 2]. Copper smelting causes a huge volume of wastewater having large amounts of inorganic compounds for instance heavy metals like lead, copper, zinc, iron, cadmium, bismuth, etc., and highly carcinogenic metalloid-like arsenic species, which poses a serious threat to man, flora, and fauna of our ecosystem by contaminating the natural water tables (ground water and surface water) in the vicinity [3].
Chronic uptake of arsenic by humans through polluted food and water may result in severe health hazard through the damage of major body organs, such as cancers of the lung, skin, kidney, liver, and urinary bladder and other problems such as loss of appetite, neurological disorders, and skin thickening (hyperkeratosis) [1, 4]. Arsenic exists in the natural water mainly in inorganic species, arsenite (As(III)) and arsenate (As(V)). As(III) is the predominant arsenic form in reducing environment; whereas, As(V) is present mainly in oxidizing conditions. As(III) is considered 25–60-fold more poisonous than As(V) and inclines to be more mobile in the environment [2]. Because of these health hazards, in drinking water, the maximum contaminant level (MCL) of arsenic has been revised to 10 from 50 μg/L by the World Health Organization (WHO) in 1993 [5] and the European Commission in 2003 [6].
Several techniques have been employed for scavenging of arsenic from water and wastewater, for example, chemical oxidation [7], membrane processes [8], adsorption [4, 9], ion exchange [10], and electrocoagulation [4], but none of them is extensively recognized due to cost or maintenance of the process.
The technical exploration for special methods has been focused on the utilization of biosorption or bioaccumulation process. Many microorganisms are renowned for being capable of removing metal ions from dilute aqueous solution and for accumulating them within their cell structure.
Bioaccumulation can be stated as the metabolism-dependent active uptake of toxins by living cells [11]. Biosorption is a metabolism-independent passive uptake of toxins by certain classes of dead/inactive biological materials or by materials derived from biological sources which may bind and accumulate toxins from aqueous solution [12]. Biofilms can be well established as microorganism communities attached to a surface [13] There are four possible reasons for the formation of biofilm: defense (shield from risky situations), colonization (formation of biofilm as a mechanism for remaining in an encouraging position), community (use of cooperative profits), and default mode of growth. Bacteria exhibit the most of their usual activity by growing as a biofilm. It is probable that the existence of an appropriate substrate for attachment is all that is vital for encouraging formation of biofilm [14].
The mechanisms between biofilms and metal ions are well defined by Le Cloirec et al. and can be suggested as follows: bulk diffusion (diffusion of the metal ions remaining in solution to the external surface of the biofilm), external mass transfer (occurrence of mass transfer via the layer of high concentration round the biofilm), fast metal ion interactions with surface of solid and specifically with the bacterial wall (these interactions can be bioaccumulation, production of enzyme, biosorption on the bacterial surface, extracellular precipitation by metabolites formed by bacteria, oxidation and/or reduction, and extracellular complexation), gentle surface diffusion, diffusion into the biofilm prior to the interaction with bacteria, and last of all, interactions with bacteria existent inside the biofilm [13].
The objectives of the current study are (1) to characterize the prepared fresh biosorbent (SD/MnFe2O4 composite) and immobilized bacterial cells before and after metal loading with scanning electron micrograph (SEM), (2) to inspect the impact of contact time as well as temperature for removing As(III) and As(V) from synthetically prepared copper smelting wastewater, (3) to evaluate the kinetics and mechanism of current biosorption/bioaccumulation process, (4) to scrutinize the thermodynamics of biosorption/bioaccumulation in order to identify the mechanism of biosorption/bioaccumulation process, (5) to investigate the effect of initial arsenic concentration on kinetics of biosorption/bioaccumulation, and (6) to study the influence of temperature on kinetics of biosorption/bioaccumulation.
Materials and Methods
Materials
Sawdust of Syzygium cumini (Jamun tree) was collected from a timber working shop situated near the campus of Indian Institute of Technology, Roorkee, India. All the chemicals and reagents were of analytical reagent grade and used without additional purification. Standards, matrix modifier, and wash solutions were prepared by deionized double distilled water. The stock solutions of As(III) and As(V) were purchased from Himedia Laboratories Pvt. Ltd. Mumbai India. All other necessary chemicals used in the experiments were purchased from Himedia Laboratories Pvt. Ltd. Mumbai India.
Microorganism and Growth Medium
The microorganism utilized was the arsenic-resistant bacterium Bacillus arsenicus (MTCC 4380; Microbial Type Culture Collection and Gene Bank (MTCC), Chandigarh, India). Culture media was prepared as per the guidelines of microbial-type cell culture (MTCC). Composition of growth medium and cultivation conditions are exhibited in Table S1 of the Electronic Supplementary Material.
Acclimatization
B. arsenicus MTCC 4380 was cultivated in a 250-mL flask containing 100 mL of the growth media with As(III) and As(V). The cultures were acclimatized to As(III) and As(V) individually exposing the culture in a series of shake flasks [14].
The bacterial inoculum was prepared by transferring a loop full of bacterial culture from the nutrient agar tubes to the flask containing sterilized growth media, incubated at 30 °C for 24 h with moderate agitation in an incubator cum orbital shaker. Then, the acclimatization of B. arsenicus MTCC 4380 in arsenic environment was carried out as follows:
After 24 h, the synthetic medium in the flask had turned milky specifying significant bacterial growth in the flask. Appropriate amount of arsenic (either As(III) or As(V)) was added into the flask having 100 mL sterilized growth media to acquire a concentration of 50 mg/L of arsenic. Firstly, growth of B. arsenicus MTCC 4380 was inhibited and the growth started after 2 h. After 24 h of incubation at 30 °C, 5 mL of the arsenic-resistant bacterial inoculum was periodically added in a series of 250-mL flasks containing 100 mL of arsenic containing sterilized growth media (As(III) or As(V) concentration, 100, 200, 500, 800, 1000, 1200, 1500, and 1800 mg/L) under sterile conditions in a laminar hood chamber. After 24 h, another fresh growth media (As(III) or As(V) concentration, 2000 mg/L) was also inoculated with 5 mL of the last culture (As(III) or As(V) concentration, 1800 mg/L) to ensure that the bacteria was already adapted to both As(III) or As(V). For inoculum, a further subculturing was performed and all the inoculum transfers were done in exponential phase (OD value ∼1 at 600 nm).
Methods
Biosorbent Preparation
Sawdust (SD) was washed to clean the adhering dirt, rinsed thoroughly with double distilled water, and finally heated in an air oven at 105 °C for 4 h; 25 g of dried SD were added into a 500-mL conical flask containing 250 mL of 0.5 M HCl solution; thereafter, it was shaken for 4 h at 120 rpm at 25 °C. The mixture was left overnight, and then it was filtered to separate SD which was repeatedly washed with double-distilled water to obtain neutral pH. Then, the biosorbents were dried at 110 °C for 3 h for removing moisture, cooled to room temperature, and kept in plastic bags for further usage.
SD/MnFe2O4 composites were prepared by chemical coprecipitation method with few modifications [15]. In this procedure, 11.52 g of acid-treated SD was mixed into 200 mL solution containing dissolved ferric(III) chloride (FeCl3; 0.05 mol) and manganese(II) chloride (MnCl2; 0.025 mol) at room temperature. The quantity of acid-treated SD was fixed for acquiring SD/MnFe2O4 mass ratios of 2:1. The solution temperature was increased to 60 °C under energetic magnetic stirring, and after that, 5 mol/L of NaOH solution was added drop by drop to the above mixture till the pH of the solution attained 11. Thereafter, agitation was carried out for 1 h. Then, the suspension was heated in a water bath at 100 °C for 4 h. After cooling, the prepared composite was constantly washed with double-distilled water to eliminate the contaminations (e.g., Na+, Cl−) accompanied with the processes. Afterward, as-prepared composite was collected from the washed solution by filtering the mixture and then was oven dried at 110 °C. The reaction ionic equation [16] is as follows:
Immobilization of Microbial Cells on the Biosorbent
To immobilize B. arsenicus MTCC 4380 on prepared SD/MnFe2O4 composite, initially, 95 mL culture media was inoculated with 5 mL of bacterial suspension of B. arsenicus MTCC 4380 from both As(III)- and As(V)-acclimatized 24-h-old culture. The flasks were incubated at 30 °C for the next 24 h with moderate shaking at 120 rpm. Then, immobilization of bacterial cell was performed by adding weighed amount of prepared SD/MnFe2O4 composite to the above suspension containing 24-h-old culture. Then, the flasks were again incubated at 30 °C for the next 24 h with moderate shaking at 120 rpm. Bacterial cell immobilization was established by observing a small amount of bacterial-treated SD/MnFe2O4 composite through scanning electron microscopy.
Characterization
The measurements of SEM were done for observing the surface morphologies of the B. arsenicus MTCC 4380 immobilized on the surface of SD/MnFe2O4 composite (i.e., immobilized bacterial cells) before and after biosorption/bioaccumulation process (SEM; LEO electron Microscopy, England). Infrared spectra of the unloaded and metal-loaded immobilized bacterial cells were acquired utilizing a Fourier transform infrared spectrometer (NICHOLET 6700, coupled with OMNIC software version 6.2).
Batch Experimental Studies and Analytical Methods
A medium with 1.0 g/L of beef extract and 2.0 g/L of yeast extract, 5.0 g/L of peptone, and 5.0 g/L of NaCl was utilized for the growth of the microorganism. The media was sterilized at 121 °C for 15 min, cooled to room temperature, inoculated with bacteria, and kept at 30 °C for 24 h with moderate agitation (120 rpm) in an incubator cum orbital shaker. All biosorption/bioaccumulation experiments were done by shaking optimum biosorbent dose of 0.9 g/L of the SD/MnFe2O4 composite (optimized in previous study) with 100 mL of B. arsenicus MTCC 4380 bacterial suspension as a test solution and required amount of arsenic (As(III) or As(V)) was supplemented to give a final requisite concentration of As(III) or As(V) at an optimum initial pH value around 7.0 (optimized in previous study) and at a preferred temperature in an incubator cum orbital shaker (REMI Laboratory instruments) at 120 rpm. 1.0 N NaOH and 1.0 N HCl solutions were used to adjust the initial pH of the solution using a digital pH meter (HACH® India).
To study the biosorption/bioaccumulation kinetics, batch experiments were conducted by contacting an optimum biosorbent dose (0.9 g/L) of the SD/MnFe2O4 composite with As(III) or As(V) solution of a fixed concentration (50 mg/L) at a constant temperature of 30 °C at a range of contact time (5–500 min).
To inspect the impact of temperature on biosorption/bioaccumulation, batch experiments were done by contacting an optimum biosorbent dose (0.9 g/L) of the SD/MnFe2O4 composite with As(III) or As(V) solution of a fixed concentration (50 mg/L) at an optimum contact time of 220 min at a range of temperatures (20, 25, 30, 40, 45, and 50 °C).
To observe the influence of initial concentration on the kinetics of biosorption/bioaccumulation of both As(III) and As(V) by B. arsenicus MTCC 4380 immobilized on SD/MnFe2O4 composite, all batch experiments were performed by agitating 0.9 g/L of SD/MnFe2O4 composite with 100 mL of B. arsenicus MTCC 4380 bacterial suspension as a test solution supplemented with required amount of arsenic (As(III) or As(V)) to give final concentration As(III) or As(V) of 50, 100, 500, 1000, 1500, and 2000 mg/L at a constant temperature (30 °C).
To investigate the influence of temperature on the kinetics of biosorption/bioaccumulation of both As(III) and As(V) by immobilized bacterial cells, 0.9 g/L SD/MnFe2O4 composite was added to each round bottom flasks containing 50 mg/L B. arsenicus MTCC 4380 culture media as a test solution of As(III) or As(V). Experiments were conducted at three temperatures (30, 40, and 50 °C) by shaking the flasks.
The samples were withdrawn from the flasks through filtration using Whatman Filter paper (cat no. 1001 125; Remi Instruments Ltd., Mumbai, India) at predetermined time intervals for kinetic studies and after fixed contact time for thermodynamic studies. Then, the samples were centrifuged at 10000 rpm for 10 min for kinetic studies, a portion of filtrate was diluted with HNO3 solution (10 %, v/v).
Interference Studies
The As(III) and As(V) binding capacity experiments were again done with synthetically prepared wastewater containing the co-existing ions commonly existing in copper smelting wastewater with arsenic solution. The effect of ions such as Cu2+, Zn2+, and SO4 2− (100–2000 mg/L); Fe3+, Cd2+, and Bi3+ (10–500 mg/L); and Pb2+, Co2+, Ni2+, and Cr6+ (1–70 mg/L) on elimination of As(III) and As(V) were inspected.
Arsenic Quantification
The total arsenic was measured in a ThermoFisher Scientific iCE 3000 Series AA graphite furnace atomic absorption (GFAA) spectrometer, with Zeeman background correction (GF-AAS). A 0.5-nm slit width and a wavelength of 193.7 nm were chosen. An arsenic high-intensity hollow-cathode lamp was utilized for determining As <20 μg/L. Pyrolytic graphite-coated tubes with forked pyrolytic platforms were utilized. Argon (Ar) was utilized as a protective gas all throughout. To determine the dissolved arsenic, 8.0 mL of a centrifuged sample was added to 1 mL of 1 % HNO3 and 1.0 mL of chemical matrix modifier (50 g/L of nickel nitrate solution) in a flask. The prepared sample injected was 20 μL. All measurements were conducted at least in duplicate and on the basis of integrated absorbance.
Theoretical Background
With the goal of assessing biosorption capacity by mass balance, the amount of adsorbate molecules adsorbed per unit mass of biosorbent at time t (mg/g) was calculated:
The amount of adsorbate molecules adsorbed in terms of percentage was calculated as follows:
where C 0 and C t are arsenic concentrations at time 0 and t (mg/L), respectively, V is the volume of the solution (mL), and M is the mass of biosorbent used (g).
Determining Adsorption Kinetic Parameters by Nonlinear Regression
The kinetic parameter sets are calculated by nonlinear regression due to the inherent bias affecting after linearization. This delivers a mathematically laborious method to estimate kinetic parameters utilizing the original kinetic equation [17]. Usually, Gauss–Newton methods or Levenberg–Marquardt-based algorithms [18] are used. The biosorption kinetic data of arsenic on the surface of immobilized bacterial cells were analyzed by nonlinear curve fitting analysis using professional graphics software package OriginPro (8.5.1 version) for fitting the kinetic models.
The optimization method needs the selection of a goodness-of-fit measure (GoFM) with the aim of esteeming the fitting of the kinetics to the experimental data. In the current study, six GoFM (residual sum of squares (SSE), reduced Chi-square test (reduced χ 2), coefficient of determination (R 2), adjusted R-square (\( \overline{R^2} \)), R value (R), and root-MSE value) were employed for assessing kinetic parameters using the OriginPro software by considering 95 % confidence interval (The details of GoFM are provided with the Electronic Supplementary Material).
The adjusted coefficient of determination (\( \overline{R^2} \)), which generally takes into account the number of variables and sample size in the model, is considered superior to the coefficient of determination (R 2), since it revises the overestimation by R 2 [19]. While dealing with small samples, specifically it is more exact than R 2.
Adsorption Kinetic Modeling
There are many models for kinetics of biosorption at the interface of solid/solution. Fractional power model (FP), pseudo first order (PFO), and pseudo second order (PSO) are common empirical models. However, their major drawback is that they may simply define the kinetics of adsorption at some restrictive situations [20]. Elovich is a two-parametric semi-empirical model and FP, Ritchie second-order, and exponential (EXP) kinetic models are two-parametric empirical models to analyze kinetic data at near to equilibrium. As well as other empirical models for instance Avrami, modified pseudo second-order (MPSO) and mixed 1,2-order model (MOE) equations have been recommended for modeling the adsorption kinetics at the interface of solid/solution. These models are mainly three parametric equations. These new models are expected to describe the performance of a given biosorbent more accurately and to help gain insight in biosorption mechanism [21].
The prior kinetic models of adsorption at the interface of solid/solution can be generalized by taking into consideration the fractal-like methodology, i.e., time dependence of adsorption rate coefficient. A physical significance of the concept of fractal-like has been considered for adsorption kinetics on solid surfaces that are energetically heterogeneous. The fractal-like study demonstrates that the kinetics of adsorption at interface of the solid/solution in a real system with various kinds of surface sites and with various affinities for adsorption can be demarcated by a fractal-like methodology. Based on this study, the history of process can impact the process. Besides, the achieved rate coefficient of adsorption is a function of time. Table 1 presents representative kinetic models for a biosorptive reaction (The details of adsorption kinetic modeling are provided with the Electronic Supplementary Material).
Adsorption Mechanistic Modeling
The removal of adsorbate species from the liquid by the solid phase is occurring in three successive steps as follows [22, 23]. The three stages tangled in the mechanism of biosorption process are as follows:
-
(i)
Firstly, the adsorbate mass transfer from the aqueous phase on the biosorbent surface, i.e., film diffusion or surface diffusion takes place;
-
(ii)
Secondly, internal diffusion of adsorbate via either a pore diffusion model or homogeneous solid-phase diffusion model, i.e., particle diffusion occurs; and
-
(iii)
The third stage is the biosorption of adsorbate on the surface sites. Due to its very fast nature, it cannot be considered for the rate determining step.
So, as to know the rate controlling step, the following different models (Table 2) were applied using the experimental data of kinetic study (The details of mechanistic model equations are provided with the Electronic Supplementary Material).
Adsorption Thermodynamic Modeling
The entropy and Gibbs free energy parameters should be measured with the intention of deciding if the processes will happen spontaneously. Thermodynamic parameters, for example, ΔG 0, ΔH 0, and ΔS 0 can be calculated utilizing equilibrium constant while the temperature varies (Table 3), and their evaluation gives an insight into the possible nature of adsorption (The details of thermodynamic equations are provided with the Electronic Supplementary Material).
Adsorption Activation Energy
The adsorption activation energy (E a) was calculated by considering the equilibrium constants under the different experimental conditions (Table 3) (The detailed equation is provided with the Electronic Supplementary Material).
Results and Discussion
Effect of Chemical Treatment on Biosorption
The acid-treated sawdust itself has the lower capacity to adsorb As(III) and As(V). Its main function was to provide a template with high specific area for MnFe2O4 loading. Untreated sawdust (SD), utilized in water treatment facilities, occupies mainly negatively charged surface due to hydroxyl group at neutral pH and therefore was not a good biosorbent for negatively charged/neutral arsenic. The hydroxyl groups can offer chemical reaction sites and adsorb iron and manganese ions to grow MnFe2O4 particles. The reason may be that the template can prevent MnFe2O4 particles from aggregating in biosorption process and enhance the effective biosorption area, resulting in the highly enhancement of biosorption capacity [4]. Loading of MnFe2O4 increased the positive charge density on the biosorbent surface by neutralizing negative surface charge and creating positive charge in its place [24]. Mondal et al. reported that impregnation of Ca on the surface of GAC also increased the positive charge on the surface of GAC and finally improved the adsorption capacity of GAC [25, 26]. By comparing the arsenic adsorption capacity of MnFe2O4 with and without the template in Table 4, it was observed that the adsorption capacities of MnFe2O4 loading on the acid-treated sawdust are much higher than the bare ones. Hereby, the introduction of acid-treated sawdust template can efficiently improve the adsorption capacity. The reason may be that the template can prevent MnFe2O4 particles from aggregating in adsorption process and enhance the effective adsorption area, resulting in the highly enhancement of adsorption capacity. A similar finding has been found for the loading of Fe3O4 on pure wheat straw [27].
Effect of Contact Time
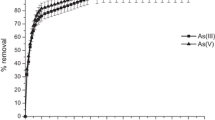
Figure 1 shows the effect of contact time on the percent removal of As(III) and As(V) utilizing immobilized bacterium. The time needed to attain equilibrium for removal of both As(III) and As(V) was 220 min. With further increase in time, no remarkable improvement was observed in removing arsenic. So, further biosorption/bioaccumulation studies were done for a contact time of 220 min.
From the outcomes, it is further clear that in all the systems, the saturation time are not influenced by the adsorbate concentration in the solution. The change in the rate of removal might be due to the fact that initially all sites of surfaces of immobilized bacterial cells are easily accessible and also the concentration gradient of adsorbate is very high. At optimum pH, the fast kinetics of interaction of adsorbate-immobilized bacterial cells might be accepted to increase availability of the active sites of the surface of immobilized bacterial cells. So, the scavenging of adsorbate was rapid in the early stages and gradually decreases with the interval of time until equilibrium in each case. The reduction in removal of metal ion at the later stage of the process was due to the decreasing of concentration of metal ions [28]. Furthermore, the characteristic of the equilibrium time curve exhibited that the SBB process approaches the equilibrium in a short span of time [29]. So, the curves found were single, smooth, and continuous, leading to equilibrium and suggested the probability of monolayer coverage of the adsorbate on the surface of immobilized bacterial cells [30].
Biosorption Kinetic Studies
Information on the kinetics of adsorbate uptake is important to find optimum operating conditions for full-scale batch process. As a nonlinearizable kinetic model and with the determination of comparing its fitting capability with the previous considered models, a nonlinear regression analysis was carried out to 14 adsorption kinetic model. Table S2 of the Electronic Supplementary Material shows the values of kinetic constants of all the models for the biosorption/bioaccumulation of both As(III) and As(V) by immobilized bacterial cells. The results exhibited that there was no notable relationship between the kinetic data for both As(III) and As(V) (Fig. S1) with low correlation coefficients and high error values signifying that these models (fractional power, pseudo first order, Elovich, and exponential) are not suitable in the present case.
On the basis of the maximum correlation coefficients (R and R 2) and the lowest error values (reduced χ 2), the superior and perfect fitting of the experimental results was observed using Brouers–Weron–Sotolongo for both As(III) and As(V) (Fig. S1) among all the tested kinetic models; whereas on the basis of maximum correlation coefficients (\( \overline{R^2} \)) and the lowest error values (SSE and root-MSE), the better and perfect demonstration of the experimental results was attained using Avrami kinetic model (Fig. S1) among all the well-known kinetic models for both As(III) and As(V).
The equilibrium uptake (q e ; 51.72 and 54.975 mg/g for As(III) and As(V), respectively), acquired from Brouers–Weron–Sotolongo model was closely in line with the experimental value (51.449 and 54.402 mg/g for As(III) and As(V), respectively). Returning to the theory beyond the best fitting model (i.e., BWS), the biosorption/bioaccumulation phenomenon of both As(III) and As(V) on the surface of immobilized bacterial cells would be controlled by chemisorption interactions type, enlightening that the rate governing step might be chemical adsorption, including valency forces by means of exchange or sharing of electrons between As(III) or As(V) anions and immobilized bacterial cells. Also, BWS model expresses another remarkable data which is the time important to adsorb half the maximum amount (τ nBWS, α ). As presented in Table S2 of the Electronic Supplementary Material, with respect to initial arsenic concentration, 23.226 and 12.45 min (the lowest value) were sufficient to immobilized bacterial cells to attain half of the As(III) and As(V) uptake capacities, respectively, which is significant in addition to valuable parameter for calculating the reaction speed. As for BWS model itself, it has a good fitting behavior and more implicitly, it expresses such quality data (i.e., adsorption capacity nearest to experimental value (q e(BWS)) and the time of half reaction (τ nBWS, α )), which are valued aimed at industrial treatment design purposes [31].
Avrami exponent (n AV; 0.599 for As(III) and 0.49 for As(V)) is a fractionary number connected with the possible variations of the biosorption/bioaccumulation mechanism that happens all over the biosorption/bioaccumulation process [32]. In place of following only an integer-kinetic order, the mechanism of biosorption/bioaccumulation could follow multiple kinetic orders that are altered throughout the contact of the adsorbate with immobilized bacterial cells [32]. n AV is a resultant of the multiple kinetic order of the biosorption/bioaccumulation procedure.
On the basis of good correlation coefficient (R, R 2, and \( \overline{R^2} \)) and low error values (SSE, reduced χ 2, and root-MSE), it can be confirmed that other kinetic models, for example, pseudo second order, modified second order, Ritchie second order, fractal-like pseudo second order, and fractal-like mixed 1,2 order, fractal-like exponential models also exhibited good fitting of biosorption/bioaccumulation kinetic data for both As(III) and As(V) on the surface of immobilized bacterial cells.
The value of good correlation coefficients (R, R 2, and \( \overline{R^2} \)) and low error values (SSE, reduced χ 2, and root-MSE) for pseudo second-order model forecasts that mechanism of biosorption/bioaccumulation is a chemisorption type.
It is understood that the mixed 1,2-order rate equation is linear in the Lagergren coordinates (i.e., it behaves like the first-order equation) near to the equilibrium and somewhat in the initial portion of the experiment. Precisely, we may conclude that it contains two linear segments connected with a curved one. Actually, the second-order equation shows linear behavior in the initial portion of the experiment also, though the linear section is quite short. On the other hand, MOE may be may be treated as a Langmuir equation for energetically homogeneous surfaces or as purely empirical equation for energetically heterogeneous surfaces.
The vital assumption of modified second-order model was that a number of surface sites (n R ) are used by each adsorbate. It was supposed that the pre-adsorbed stage occurred on immobilized bacterial cells biosorption/bioaccumulation.
The vital supposition of the Ritchie second-order model was that one adsorbate was adsorbed on two surface sites. It was supposed that the pre-adsorbed stage has not occurred on immobilized bacterial cell biosorption/bioaccumulation [33]. The biosorbent surface coverage has been typically presumed to be zero.
In case of fractal-like models (fractal-like pseudo first order, fractal-like pseudo second order, fractal-like exponential, and fractal-like mixed 1,2 order), the biosorption/bioaccumulation rate coefficient is considered a function of time by using the fractal-like idea. One of the probable physical significances of fractal-like kinetics was that the biosorption/bioaccumulation of As(III) and As(V) occurred at solid/solution interface. In this approach, it was showed that by passing time, various paths for biosorption/bioaccumulation of As(III) and As(V) on surface appears [34].
Final Remarks on Biosorption/Bioaccumulation Kinetic Studies
The value of correlation coefficient (R and R 2) and error value (reduced χ 2) for Brouers–Weron–Sotolongo model was better than achieved using the other kinetic model for both As(III) and As(V), indicating a complex mechanism of biosorption/bioaccumulation. But based on the obtained correlation coefficient (\( \overline{R^2} \)) and error values (SSE and root-MSE), the Avrami model showed the best fit to the biosorption/bioaccumulation kinetic data of both As(III) and As(V) among all the models, indicating that the mechanism of biosorption/bioaccumulation models followed multiple kinetic order. So, the kinetics of As(III) and As(V) biosorption/bioaccumulation using immobilized bacterial cells as a biosorbent can be well elucidated by Brouers–Weron–Sotolongo as well as Avrami kinetic models.
Among the models, pseudo second-order model also possessed a good fitness with experimental kinetic data. This tendency comes as a suggestion that the rate-limiting step in biosorption/bioaccumulation of arsenic (either As(III) or As(V)) are chemisorption involving valence forces through the sharing or exchange of electrons between immobilized bacterial cells and arsenic (either As(III) or As(V)) ions [35], complexation, coordination, and/or chelation [36].
It is important to find the rate at which As(III) or As(V) is biosorbed/bioaccumulated on the surface of immobilized bacterial cells, which is vital for designing the fixed bed biosorption column. Using the biosorption/bioaccumulation rate, kinetic constants are evaluated to define the equilibrium capacity of immobilized bacterial cells and mass transfer coefficient at various aqueous phase concentrations. Amount of As(III) or As(V) biosorbed/bioaccumulated on solid surface is calculated using the kinetic equation, which is vital for esteeming the concentration of the aqueous phase in fixed bed column operation. The key design factors of fixed bed adsorption column, the breakthrough time in addition to the shape of breakthrough curve are dependent on biosorption/bioaccumulation rate. For the faster biosorption/bioaccumulation rate, breakpoint time is attained earlier and the breakthrough curve shape is also steeper.
The descriptive models from the best to worst for As(III) and As(V) were sorted according to GoFM values and shown in Tables S3 and S4, respectively.
So, at this point, the fitting “ambiguity” influenced by R, R 2, \( \overline{R^2} \), SSE, reduced χ 2, and root-MSE were for both As(III) and As(V), because R, R 2, and reduced χ 2 established the Brouers–Weron–Sotolongo as best fitting model whereas \( \overline{R^2} \), SSE, and root-MSE showed that the Avrami kinetic model is the best fitting model. So, for overcoming this doubt, it would be modest and practical to compare the theoretically evaluated q e values with the experimental ones.
According to correlation coefficient \( \overline{R^2} \) values the fitness of the models for all kinetic models are almost equivalent to each other. So, based on equivalent adsorption capacity, the orders followed by the models in decreasing manner are shown in Table S5. From Table S5, it can be concluded that on the basis of equivalent biosorption/bioaccumulation capacity, fractal-like pseudo second-order kinetic model is the best fitting model for both As(III) and As(V) owing to the highest value.
Biosorption Mechanistic Studies
Intraparticle Diffusion Model
Outcomes of intraparticle model for both As(III) and As(V) are shown in Table 5. Figure S2 indicated the multi-linear nature of intraparticle model. As the plot did not pass through the origin, so intraparticle diffusion was not the only rate governing step. So, there were three processes leading the rate of biosorption/bioaccumulation; however, only one was rate controlling in any certain time range. The intraparticle diffusion rate constant k int2 for both As(III) and As(V) was valued from the slope of the second linear portion (Fig. S2; Table 5). The multi-linear curve of the intraparticle model with intercept C int2 indicated the fact that both intraparticle diffusion of adsorbate through the mesoporus openings filled with liquid and film/external mass transfer across the thickness of boundary layer were the rate governing steps in the biosorption/bioaccumulation of both As(III) and As(V) kinetics on the surface of immobilized bacterial cells. The value of intercept C int acquired through the model conveyed the value of thickness of boundary layer of liquid surrounding the immobilized bacterial cells [37].
The explanation of the random data points found in Fig. S2 and Table 5 specified that intraparticle mechanistic model had been appropriate to cost-effectively and competently describe the biosorption/bioaccumulation of both As(III) and As(V)) on the surface of immobilized bacterial cells in terms of linear regression coefficient adjusted R-square (\( \overline{R^2} \)), ranging between 0.927 and 0.993 in both case. Larger value of intercepts achieved for second linear portion, i.e., C int2 suggested that the film diffusion had played a vital role as the rate governing step [38].
Determination of Rate-Limiting Step
The values of the film diffusion coefficient D 1 and the pore diffusion coefficient D 2 for both As(III) and As(V) are revealed in Table 5. The high negative exponential of almost nearest values specified that both pore diffusion and film diffusion had controlled the mechanism of biosorption/bioaccumulation for both As(III) and As(V).
Dumwald–Wagner Model
The plot (Fig. S3) of log(1 − F 2) versus t has delivered almost linear curves (≥0.947) for the scavenging of both As(III) and As(V) by immobilized bacterial cells, respectively, but did not pass through the origin, suggesting that the diffusion of adsorbate into pores of the immobilized bacterial cells was not the only rate-limiting step in both cases. Table 5 displays the evaluated results of Dumwald–Wagner model. In the current study, the linearity of the plots intersected the origin of coordinates; so, film diffusion process was the rate controlling step.
Richenberg Model or Boyd Plot
The consequences of the corresponding model for both As(III) and As(V) are exhibited in Table 5 and Fig. S4, respectively. From the plots of B b t versus t, it was detected that the plots had dynamic linear form with a correlation coefficient adjusted R-square (\( \overline{R^2} \)) of 0.964 for As(III) and 0.948 for As(V) at studied temperature but did not pass through origin which had selected the ongoing biosorption/bioaccumulation processes to be governed by film diffusion mechanism.
Film Diffusion Mass Transfer Rate Equation or McKay Plot
The values achieved for ln(1 − F) as a function of time t, were plotted in Fig. S5 for As(III) and As(V), respectively, and the results of the respective model for both As(III) and As(V) are revealed in Table 5. The rate constant of the initial fast process (k M1) was evaluated from the slope of the first straight line (Fig. S5). As can be understood from Fig. S5, the rate constant of the slow process (k M2) was attained from the slope of the second linear portion. It is clear that the initial fast process was directed by film diffusion. Also the values of k M2 are smaller than the values of k M1 for both As(III) and As(V). So, the acquired values of k M2 were evidence of a pore diffusion mechanism in the second stage of the biosorption/bioaccumulation [39]. The existence of two straight lines for both As(III) and As(V) indicated that two processes, i.e., film diffusion and pore diffusion had involved in these processes.
The plots were about linear for both As(III) and As(V), but it did not yield perfect linearity. So, pore diffusion was not the rate controlling step. In the current study film diffusion was the rate governing step.
Bangham’s Model
The results of the respective model for both As(III) and As(V) are exposed in Table 5 and Fig. S6. The goodness of fit of curve for Bangham’s model was connected in terms of correlation coefficient adjusted R-square (\( \overline{R^2} \)). Values of Bangham parameters k b and α are 20.828 L/g and 0.494 for biosorption/bioaccumulation of As(III) and 32.849 L/g and 0.46 for biosorption/bioaccumulation of As(V) with a correlation coefficients of 0.984 and 0.987, respectively. The double logarithmic plot (Fig. S6) for As(III) and As(V), respectively, as specified by the above equation did not return perfect linear curves, and some data were dispersed (\( \overline{R^2} \) ≤ 0.987) for the removal of As(III) and As(V) by immobilized bacterial cells illuminating that the diffusion of adsorbate within pores of the immobilized bacterial cells was not only the rate governing step [40], film diffusion also had impact on the rate-limiting step.
Determination of Diffusion Coefficient
The D p and D f values for As(III) were 1.11 × 10−7 and 1.96 × 10−7 cm2/s, respectively. Likewise, these values for As(V) were 1.71 × 10−7 and 1.07 × 10−6 cm2/s, respectively (Table 5).
Thus, in the current system, pore diffusion was not the only rate governing step for both As(III) and As(V). For As(III), the value of D f (1.96 × 10−7 cm2/s) falls within the range of 10−6 and 10−8 cm2/s, and for As(V), the D f value (1.07 × 10−6 cm2/s) is also in the range. So, it was noticed that the film diffusion had been the rate controlling step for both As(III) and As(V).
Higher value of D f of As(V) than that of As(III) was owing to the presence of As(V) as totally positively charged species and As(III) as usually neutral species at the experimental pH. Due to negative charge of As(V), it was freely transported from the bulk solution to generally the positively charged immobilized bacterial cells and acquired biosorbed/bioaccumulated on the active sites of surface of immobilized bacterial cells in place of the interior pores.
Determination of Diffusivity
From the slope π2 D e /r 2 of the plot of ln(1/(1 − F 2)) versus t (Fig. S7; Table 5), the value of diffusion coefficient (D e ) as assessed, was detected to be 5.95 × 10−14 and 5.87 × 10−14 m2/s for As(III) and As(V) biosorption/bioaccumulation on the surface of immobilized bacterial cells, respectively. For the current study, the value of D e lies within the range of 10−9 to 10−17 m2 /s, so the system was chemisorption system. The values of the diffusion coefficient, D e fell well within the magnitudes reported in literature [41].
Final Remarks on Biosorption/Bioaccumulation Mechanistic Studies
The above-considered models for biosorption/bioaccumulation of both As(III) and As(V) specified that two processes, i.e., film diffusion (diffusion of adsorbate through the solution to the external surface of biosorbent or boundary layer diffusion of adsorbate) and pore diffusion (diffusion of the adsorbate from the surface film into the pores) were involved in the current biosorption/bioaccumulation processes for both As(III) and As(V) and pore diffusion was not the only rate controlling step. Usually, film diffusion controlled the rate governing step in both cases. The uptake of arsenic (As(III) or As(V)) species from the liquid to the solid phase was carried out in three consecutive steps [23]. Firstly, film diffusion occurred. Secondly, pore diffusion took place. The third step is the biosorption, which is being very rapid in nature and cannot be taken into account for the rate determining [22].
Effect of Temperature
The temperature has two main influences on the biosorption/bioaccumulation process. Temperature essential for the biosorption/bioaccumulation system commands the biosorption/bioaccumulation as endothermic or exothermic. Increasing the temperature is familiar to raise the rate of diffusion of the adsorbate, due to the reduction in the viscosity of the solution. Besides, changing the temperature will alter the equilibrium biosorption capacity of the immobilized bacterial cells for a specific biosorbent [42].
The effect of temperature on the removal efficiency of As(III) and As(V) was inspected in the range of 20–50 °C during the equilibrium time. The consequences of influence of temperature have been exposed in Fig. 2. It was observed from Fig. 2 that with a little increase in temperature from 20 to 30 °C, the biosorption/bioaccumulation of arsenic (either As(III) or As(V)) ions on immobilized bacterial cell surface increased. After 30 °C temperature, the removal of arsenic (As(III) or As(V)) ions by immobilized bacterial cell decreased with the increase in temperature. For As(III) and As(V), percent removal decreased from 92.609 to 82.174 % and from 97.923 to 91.769 %, respectively, with increase in temperature from 30 to 50 °C.
The favorable temperature for the growth of B. arsenicus MTCC 4380 is 30 °C [43]. The result specified that the highest removal was at a temperature of 30 °C in biosorption/bioaccumulation system. Further increase in temperature resulted in lower scavenging efficiency for arsenic removal in biosorption/bioaccumulation system.
This can also be clarified by the exothermicity of the biosorption/bioaccumulation process. Temperature influences the interaction between the biomass and the metal ions, generally by impacting the stability of the metal-sorbent complex and the ionization of the cell wall moieties [44]. The temperature of the biosorption solution could be vital for energy-dependent mechanisms in metal binding process [45]. Energy independent mechanisms are less expected to be influenced by temperature because the processes responsible for biosorption are mainly physico-chemical in nature [46, 47]. The results acquired in the current study indicated that the metal ion sorption in SBB system was exothermic. Kacar et al. [46] and Ozdemir et al. [47] reported in their work about the exothermic behavior of metal ion sorption on the bacterial surface. The authors correlated their research outcomes as an energy-independent mediated sorption of metal ions.
This can also be clarified by the spontaneity of the biosorption/bioaccumulation process. Initial rise in removal efficiency up to 30 °C is mainly because of the rise in collision frequency between biosorbent and adsorbate. Further rise in temperature (>30 °C) caused in lower removal efficiency for arsenic removal by immobilized bacterial cells. This reduction in scavenging efficiency might be owing to many factors: the relative increase in the dodging tendency of the arsenic ions from the solid biosorbent phase to the bulk liquid phase; deactivating immobilized bacterial cells or destructing some active sites on the surface of immobilized bacterial cells due to bond ruptures [48] or owing to the weakening of biosorptive forces between the adsorbate species and the active sites of the immobilized bacterial cells and also between the adjacent molecules of adsorbed phase for high temperatures or movement of biosorbents with more speed, so, lower interaction time with the biosorbent active sites was available for them [49].
Biosorption Thermodynamic Studies
The estimated values of the thermodynamic parameters from the plot of ln k d versus 1/T (Fig. 3) are shown in Table 6. The equilibrium constant k d was evaluated while the temperature was altered between 20 and 50 °C for both As(III) and As(V). The maximum scavenging of adsorbate was achieved at 30 °C in both cases. The removal efficiency primarily rose with rising the temperature from 20 to 30 °C. Then, it reduced with rising the temperature from 30 to 50 °C. The ΔH 0 values attained for the biosorption/bioaccumulation of both As(III) and As(V) were negative due to the exothermic nature of the biosorption/bioaccumulation process. The value achieved for As(V) biosorption/bioaccumulation was more than the value achieved for As(III). A negative value of ΔG 0 specified the spontaneous nature of the biosorption/bioaccumulation process; however, the negative value of ΔS 0 presented a decrease in the randomness at the solid/solution interface during the biosorption/bioaccumulation process [50]. Higher negative value of ΔG 0 at a temperature of 30 °C, as was achieved in the study, concluded more driving force for biosorption/bioaccumulation at 30 °C [51]. The values of ΔG 0 achieved in the current research were between −22.68 and −24.04 and −25.08 and −27.37 kJ/mol for As(III) and As(V), respectively. Thus, it indicated that the mechanism of current biosorption/bioaccumulation process had happened via ion exchange and/or surface complexation mechanism.
Effect of Initial Arsenic Concentration on Biosorption/Bioaccumulation Kinetics
To inspect the influence of initial concentration on kinetic studies, a series of contact time experiments for both As(III) and As(V) was performed at various initial concentration (50–2000 mg/L) at temperature of 30 °C. Figure S8 shows the contact time essential for both As(III) and As(V) with an initial concentration of 50–1000 mg/L for attaining equilibrium at 220 min. However, for both As(III) and As(V) with higher initial concentration (>1000–2000 mg/L), higher equilibrium time of 300 min was needed. As can be decided from Fig. S8, the amount of the biosorbed/bioaccumulated As(III) and As(V) on the surface of immobilized bacterial cells changed with time and at some point in time achieved a constant value beyond which no more As(III) or As(V) was scavenged from solution. At the moment, the amount of both As(III) and As(V) desorbing from the biosorbent was in a state of dynamic equilibrium with the amount of the both As(III) and As(V) being biosorbed/bioaccumulated on the surface of immobilized bacterial cells. The time vital for achievement of this state of equilibrium is called the equilibrium time. Thus, the rate of biosorption/bioaccumulation reduced with time till it tended to a plateau owing to the constant reduction in the concentration driving force.
Determination of Initial Sorption Rate
From Fig. 4 and Table 7, it is noted that the pseudo second-order rate constants (k PSO) were detected to decrease and the initial sorption rates (h) were agreed to rise with higher initial concentration of both As(III) and As(V). The initial sorption rate was larger for higher initial As(III) and As(V) concentrations as the resistance to uptake both As(III) and As(V) decreased as the mass transfer driving was force enhanced.
The estimated q e(PSO) values confirmed just appropriate with the experimental data, and high correlation coefficient (R, R 2, and \( \overline{R^2} \)) and low error values (SSE, reduced χ 2, and root-MSE) revealed that the model can be utilized for the whole biosorption/bioaccumulation process and allowed the chemisorption of both As(III) and As(V) on the surface of immobilized bacterial cells
Effect of Temperature on Biosorption/Bioaccumulation Kinetic
Temperature is a notable factor controlling the biosorption/bioaccumulation process. The influence of temperature on the of biosorption/bioaccumulation of both As(III) and As(V) by immobilized bacterial cells was carried out from 30 to 50 °C at C 0 is 50 mg/L and SD/MnFe2O4 composite loading is 0.9 g/L. A declining biosorption rate of both As(III) and As(V) with increasing temperature from 30 to 50 °C recognized the process to be exothermic (Fig. S9).
Biosorption Activation Energy
Biosorption/bioaccumulation rate constants k PSO of both As(III) and As(V) were estimated from experimental data at different temperatures assuming nonlinear form of pseudo second-order kinetics. Parameters of Arrhenius equation were fitted employing these rate constants for valuing temperature-independent rate parameters and biosorption/bioaccumulation type. A plot of ln k PSO versus 1/T exhibited a straight line with slope − E a/R (Fig. 5; Table 8). The magnitude of activation energy for As(III) and As(V) biosorption/bioaccumulation on the surface of immobilized bacterial cells were 12.35 and 10.61 kJ/mol, signifying that both the biosorption/bioaccumulation of As(III) and As(V) on the surface of immobilized bacterial cells was activated by chemisorption.
Studies of Influencing Co-existing Ions
The effects of concentrations of initial co-existing ions on the biosorption/bioaccumulation process of As(III) and As(V) on Corynebacterium glutamicum MTCC 2745 immobilized on surface of SD/MnFe2O4 composite are revealed in Figs. 6a–c and 7a–c, respectively. Consequences clearly showed that the percent removal of As(III) and As(V) were observed to decrease with the presence of a very low concentration of various ions, for example, copper (Cu2+), zinc (Zn2+), bismuth (Bi3+), lead (Pb2+), cobalt (Co2+), nickel (Ni2+), chromium (Cr6+), and sulfate (SO4 2−). On the other hand, in the presence of iron (Fe3+) and cadmium (Cd2+), the removal efficiencies were observed to increase. The reduction of percent removal in the existence of Cu2+, Zn2, Bi3+, Pb2+, Co2+, Ni2+ Cr6+, and SO4 2− ions may be clarified on the basis of the ionic radii. All these ions are bigger than As(III) and As(V), and therefore, in their attendance, the percent removal reduced [52, 53]. This might be owing to the competition of these ions with arsenic for the biosorption sites and/or owing to masking of biosorption sites by the bigger ions [54]. Conversely, the ionic radii of Cd2+ and Fe3+ are almost the same, so, there was no reducing effect [54]. The percent removal of As(III) and As(V) increased in presence of both Cd2+ and Fe3+ ions. Mostly Cd2+ and Fe3+ ions could be biosorbed/bioaccumulated by immobilized bacterial cells and efficiently compensated the surface negative charge generated by specific biosorption/bioaccumulation of As(III) and As(V) and acted as a bridge between immobilized bacterial cells and As(III) and As(V) ions, which has preferred the biosorption/bioaccumulation of As(III) and As(V) anions [52, 55]. While the ionic radius of Cd2+ is nearly the same, so there was no reducing effect [53]. As well as this, Fe3+ ions being an outstanding adsorbent for arsenic, the current effect in the occurrence of Fe3+ may also be owing to adsorption of arsenic on ferric hydroxides (Fe(OH)3), which would form while Fe3+ ions are present in wastewater under oxic conditions [56]. Sulfate is a divalent oxyanion and has competition with anions of both As(III) and As(V) through enhanced electrostatic interaction [57]. Sulfate had an negligible influence on As(III) and As(V) biosorption/bioaccumulation. This is due to the fact that the sulfate binding affinity for the immobilized bacterial cells is much weaker than arsenic [55]. These consequences are also consistent with other study [58, 59].
Characterization
FT-IR Analysis
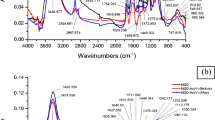
The biosorption capacity of heavy metal on many biosorbents is governed by the presence of several active functional groups on the surface of biosorbent. The Fourier transform infrared spectra (FT-IR) study of fresh SD/MnFe2O4 composite as well as As(III)-acclimatized B. arsenicus MTCC 4380 immobilized on surface of SD/MnFe2O4 composite at unloaded and metal-loaded stage (Fig. 8a) and fresh SD/MnFe2O4 composite plus As(V)-acclimatized B. arsenicus MTCC 4380 immobilized on surface of SD/MnFe2O4 composite at unloaded and metal-loaded stage (Fig. 8b) at optimized batch experimental condition was observed for identifying the function groups responsible mainly for the process of biosorption/bioaccumulation. Tables S6 and S7 of the Electronic Supplementary Material specify the wavenumber for the many functional groups present in the fresh SD/MnFe2O4 composite and As(III)-acclimatized B. arsenicus MTCC 4380 immobilized on surface of SD/MnFe2O4 composite at As(III)-unloaded and As(III)-loaded stages and fresh SD/MnFe2O4 composite and As(V)-acclimatized B. arsenicus MTCC 4380 immobilized on surface of SD/MnFe2O4 composite at As(V)-unloaded and As(V)-loaded stages, respectively. Surface –OH and –NH groups were main active functional groups answerable for biosorption/bioaccumulation as the wavenumber shifted from 3432.725 to 3426.977 cm−1 (As(III)) and from 3432.725 to 3422.016 cm−1 (As(V)) which may be probably owing to the complexation of –OH groups with As(III) or As(V) ions [41]. Some researchers also specified that after the adsorption of arsenic on the Fe–Ce and Fe–Mn adsorbents, the peak of hydroxyl groups reduced or vanished [60, 61]. Aliphatic C–H stretching may be responsible for As(III) and As(V) biosorption/bioaccumulation on As(III) and As(V) acclimatized B. arsenicus MTCC 4380 immobilized on surface of SD/MnFe2O4 composite as wavenumber shifted from 2929.612 to 2919.69 cm−1 and from 2923.602 to 2919.69 cm−1, respectively, possibly owing to the complexation of C–H stretching vibration of alkyl chains [62]. Aldehyde C–H stretching may be responsible for As(III) and As(V) biosorption/bioaccumulation on As(III)- and As(V)-acclimatized B. arsenicus MTCC 4380 immobilized on surface of SD/MnFe2O4 composite as wavenumber shifted from 2854.176 to 2857.674 cm−1 and from 2854.176 to 2852.713 cm−1, respectively [63]. Tables S6 and S7 of the Electronic Supplementary Material also display the responsibility of aliphatic acid C=O stretching for As(III) and As(V) biosorption/bioaccumulation by shifting the wavenumber from 1735.647 to 1745.116 cm−1 and from 1729.862 to 1735.647 cm−1, respectively [41]. The next biosorption/bioaccumulation peaks at 1640.93 cm−1 shifted to 1629.58 cm−1 for As(III) and 1640.93 cm−1 shifted to 1630.388 cm−1 for As(V), perhaps due to the complexation of amide group (N–H stretching and C=O stretching vibration) with As(III) and As(V) ions [64]. Wave number shifted from 1625.426 to 1615.504 cm−1 (As(III)) and from 1657.054 to 1641.55 cm−1 (As(V)) which gave the reactivity of unsaturated group like alkene for the biosorption/bioaccumulation process. Tables S6 and S7 of the Electronic Supplementary Material also exhibit the intense bands at 1470.388 cm−1 which shifted to 1459.845 cm−1 (As(III)) and at 1527.442 cm−1, shifted to 1517.727 cm−1 (As(V)) that indicated the responsibility of aromatic –NO2 group for the biosorption/bioaccumulation process, respectively. Another shift was detected from 1449.922 to 1442.516 cm−1 (As(III)) and from 1442.516 to 1430.945 cm−1 (As(V)), corresponding to the complexation of nitrogen with As(III) and As(V) ions of the N–H group [65]. Wavenumber shifted from 1366.822 to 1375.018 cm−1 (As(III)) and from 1375.018 to 1382.326 cm−1 (As(V)) assigned the reactivity of carboxylate anion C=O stretching for the biosorption/bioaccumulation process [53]. Wavenumber 1231.628 cm−1 shifted to 1237.209 cm−1 and 1240.024 cm−1 shifted to 1237.829 cm−1 assigned for –SO3 stretching for the biosorption/bioaccumulation of As(III) and As(V), respectively. The peaks at 1082.1705 cm−1 (As(III)) and 1087.132 cm−1 As(V) may be attributed to the C–N stretching vibrations of amino groups which shifted to higher frequency and appeared at 1113.178 and 1114.671 cm−1, respectively, due to the interaction of nitrogen from the amino group with As(III) and As(V) ions [66]. Wavenumber 1043.317 cm−1 shifted to 1049.102 cm−1 (As(III)) and shifting from 1056.124 to 1052.959 cm−1 (As(V)) indicated the Si–O stretching, active for the biosorption/bioaccumulation process. The other weak biosorption/bioaccumulation peak shifted from 885.581 to 901.085 cm−1 (As(III)) and from 885.581 to 900.608 cm−1, corresponding to the O–C–O scissoring vibration of polysaccharide [67]. The band at 590.12 cm−1 (Fig. 8a) and 603.62 cm−1 (Fig. 8b) could be attributed to the existence of Fe–O bond [68], but then it shifted to 603.62 cm−1 after biosorption/bioaccumulation of As(III) (Fig. 8a) and to 600.93 cm−1 after biosorption/bioaccumulation of As(V) (Fig. 8b), respectively. A typical peak at 566.978 cm−1 (Fig. 8a) and 559.38 cm−1 (Fig. 8b) could be assigned to Mn–O bond [69] and it had a different variability to 563.121 cm−1 (Fig. 1a) and 561.193 cm−1 (Fig. 8b) for biosorption/bioaccumulation of As(III) and As(V), respectively. The change in wavenumber of Me–O bonds after biosorption/bioaccumulation of As(III) and As(V) indicated that both Fe–O and Mn–O bonds were responsible for both MnFe2O4–As(III) and MnFe2O4–As(V) [68, 70]. Presence of As(III) and As(V) on the As(III) and As(V) acclimatized B. arsenicus MTCC 4380 immobilized on surface of SD/MnFe2O4 composite can be assured from the bands appeared at 797.519 cm−1 (Fig. 8a) and 828.527 cm−1 (Fig. 8b), respectively [71]. It has to be cited here, that a clear band was very hard to be got in the case of As(III), compared with the distinctive band of As(III) found at 797.519 cm−1 and As(V) found at 828.527 cm−1. This may be because of different mechanisms involved in As(III) and As(V) biosorption/bioaccumulation. It should be distinguished that the As–O band after biosorption/bioaccumulation of arsenic was not clearly observed because of the broad overlapping peaks in this region [70].
FT-IR spectra of a fresh SD/MnFe2O4 composite (MSD), As(III)-acclimatized B. arsenicus MTCC 4380 immobilized on SD/MnFe2O4 composite before and after As(III) biosorption/bioaccumulation and b fresh SD/MnFe2O4 composite (MSD), As(V)-acclimatized B. arsenicus MTCC 4380 immobilized on SD/MnFe2O4 composite before and after As(V) biosorption/bioaccumulation
SEM-EDX Analysis
Figure 9a, b exhibits the morphology of As(III) and As(V) acclimatized living bacterial cells of B. arsenicus MTCC 4380, respectively. From the figure, it is obvious that they were basically rod-like shape. The SEM images of the prepared fresh SD/MnFe2O4 composite (Fig. 9c) and As(III) acclimatized the B. arsenicus MTCC 4380 immobilized on surface of SD/MnFe2O4 composite at stage of unloaded and loaded with As(III) (Fig. 9d, e) and As(V) acclimatized the B. arsenicus MTCC 4380 immobilized on surface of SD/MnFe2O4 composite at stage of unloaded and loaded with As(V) (Fig. 9f, g) were also shown. It can be understood from Fig. 9c, manganese ferrite (MnFe2O4) particles with numerous diameters were arbitrarily distributed on the acid treated SD surface. It is clear from Fig. 9d, f that most of the active sites of SD/MnFe2O4 composite were shielded owing to the formation of biofilm on it. The bacterial mass partially occupies the void spaces of the biosorbent surface, so formation of biolayer on the surface of biosorbent decreases its surface porosity [72]. It can be observed from Fig. 9e, g that the immobilized bacterial cells have bulky structure with no porosity. A change in surface morphology from being smooth to rough and occupation of pores specified the As(III) and As(V) biosorption/bioaccumulation on the surface and pores of SD/MnFe2O4 composite providing it a rough texture (Fig. 9e, g) [73].
Scanning electron micrographs (SEM) of a As(III)-acclimatized living cells of B. arsenicus MTCC 4380, b As(V)-acclimatized living cells of B. arsenicus MTCC 4380 (original magnification, ×10000), c fresh SD/MnFe2O4 composite, d As(III)-acclimatized B. arsenicus MTCC 4380 immobilized on SD/MnFe2O4 composite at unloaded stage, e As(III)-acclimatized B. arsenicus MTCC 4380 immobilized on SD/MnFe2O4 composite at loaded stage, f As(V)-acclimatized B. arsenicus MTCC 4380 immobilized on SD/MnFe2O4 composite at unloaded stage, and g As(V)-acclimatized B. arsenicus MTCC 4380 immobilized on SD/MnFe2O4 composite at loaded stage (original magnification, ×500)
Conclusions
-
The current study showed that the B. arsenicus MTCC 4380 immobilized on surface of SD/MnFe2O4 composite was applied fruitfully for the biosorption/bioaccumulation of both As(III) and As(V) from synthetically prepared wastewater.
-
The optimum contact time and temperature for biosorption/bioaccumulation of both As(III) and As(V) were 220 min and 30 °C, respectively.
-
Contact time desirable for achievement of equilibrium improved with increasing concentration, though remained virtually unaffected by increasing temperature.
-
The rate of biosorption/bioaccumulation of both As(III) and As(V) by immobilized bacterial cells decreased with increasing concentration.
-
By applying 14 different kinetic models and using method of the nonlinear regression for curve fitting analysis (maximizing the correlation coefficient (R, R 2, and \( \overline{R^2} \)) and minimizing the error values (SSE, reduced χ 2, and root-MSE)) to evaluate optimum parameter sets, Brouers–Weron–Sotolongo as well as Avrami models were found suitable to predict the kinetic of biosorption/bioaccumulation of both As(III) and As(V) on the surface of immobilized bacterial cells according to GoFM values.
-
The current experimental data also followed pseudo second-order model very well, hence it can be concluded that chemisorption was the possible route of biosorption/bioaccumulation on immobilized bacterial cells.
-
The results achieved from different mechanistic models indicated the controller of film diffusion over pore diffusion.
-
The effective diffusivity estimated by using Vermeulen’s approximation was indicated that the interaction between arsenic ions (either As(III) or As(V)) and immobilized bacterial cells were chemical in nature.
-
The related thermodynamic parameters revealed that the biosorption/bioaccumulation of both As(III) and As(V) had been a feasible and exothermic process and spontaneous.
-
From Arrhenius equation, it was confirmed that the mechanism of biosorption/bioaccumulation of As(III) and As(V) by immobilized bacterial cells might be an ion exchange type.
-
EDX analysis documented the attendance of iron and manganese in the SD/MnFe2O4 composite, and it also established the fact that both As(III) and As(V) was biosorbed/bioaccumulated on the immobilized bacterial cells.
-
Spectroscopic studies (Fe-SEM and FT-IR) confirmed that ion exchange process was responsible for the uptake of arsenic (As(III) or As(V)) on immobilized bacterial cells.
-
B. arsenicus MTCC 4380 immobilized on surface of SD/MnFe2O4 composite can be used as a capable biosorbent for removal of both As(III) and As(V) from contaminated water sources.
Nomenclature
a E Elovich coefficient representing the initial adsorption rate (mg/g min)
b E Elovich coefficient representing desorption constant (g/mg)
A the frequency factor
B b t a mathematical function of F (F = q t /q e )
C 0 initial concentration of arsenic in the solution (mg/L)
C e equilibrium concentration of arsenic in the solution (mg/L)
C s the concentration of arsenic on the biosorbent and in the solution (mg/L)
C int the intercept of the intraparticle diffusion plot (mg/g)
d the thickness of the water film adhered to the biosorbent (cm)
D 1 film diffusion constant (cm2/s)
D 2 pore diffusion constant (cm2/s)
D e diffusivity (m2/s)
D f film diffusion coefficient (cm2/s)
D p pore diffusion coefficient (cm2/s)
E a the activation energy of adsorption (kJ/mol)
F the adsorption progress (F = q t /q e )
f 2 the involvement of pseudo second-order model
h the initial sorption rate (mg/g min)
k AV the Avrami kinetic rate coefficient (1/min)
k b the Bangham model constant (L/g)
k d the equilibrium constant (L/g),
k DW the Dumwald–Wagner rate constant (1/min)
k EXP the exponential rate coefficient (mg/g min)
k int the intraparticle diffusion rate coefficient (mg/g min0.5)
k M the film diffusion rate constant (1/min)
k MOE the mixed 1,2-order rate coefficient (1/min)
k nBWS, α the Brouers–Weron–Sotolongo reaction constant (1/min)
k PF the fractional power model rate coefficient (mg/g min)
k PFO the pseudo first-order rate coefficient (1/min)
k PSO the pseudo second-order rate coefficient (g/mg min)
k′1,0 the fractal-like mixed 1,2-order rate coefficient (1/min)α
k′2R the modified second-order rate coefficient (1/min)
k″2R the Ritchie second-order rate coefficient (1/min)
k′EXP division of k EXP by q e (1/min)
k″EXP the fractal-like exponential rate coefficient (1/min)α
k′PFO the fractal-like pseudo first-order kinetic rate coefficient (1/min)α
k′FPSO is the fractal-like pseudo second-order kinetic rate coefficient (g/mg min)α
k′PSO multiplication of q e and k PSO (1/min)
k″FPSO multiplication of q e and k′FPSO (1/min)
m the weight of the biosorbent per liter of solution (g/L)
m b an integer
n the number of observations in the experimental study
n AV constant corresponding to the mechanism of adsorption
n BWS a fractional reaction order
n R number of surface sites
p the number of parameters to be estimated
q e the amount of adsorbate adsorbed on the biosorbent surface at equilibrium (mg/g)
q t the amount of adsorbate adsorbed on the biosorbent surface at time t (mg/g)
r mean radius of biosorbent particle (cm)
R the universal gas constant (8.314 J/mol K)
R e percent removal of adsorbate
t contact time (min)
t α a fractal time
T the solution temperature (K)
v adjustment parameter
V the volume of the solution (mL)
Greek symbols
α the fractal time exponent
α b Bangham model constant
θ the biosorbent surface coverage at pre-adsorbed stage, (θ = q t /q e ) dimensionless
θ 0 the biosorbent surface coverage at time t, (θ 0 = q 0/q e ) dimensionless
θ e the equilibrium surface coverage
τ 1/2 half-reaction time (min)
τ nBWS, α the time required for adsorbing half the maximum amount
ΔG 0 Gibbs free energy change (kJ/mol)
ΔH 0 enthalpy change (kJ/mol)
ΔS 0 entropy change (J/mol K)
References
Mondal P, Majumder CB, Mohanty B (2006) Laboratory based approaches for arsenic remediation from contaminated water: recent developments. J Hazard Mater 137(1):464–479. doi:10.1016/j.jhazmat.2006.02.023
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17(5):517–568. doi:10.1016/S0883-2927(02)00018-5
Basha CA, Bhadrinarayana NS, Anantharaman N, Begum KM (2008) Heavy metal removal from copper smelting effluent using electrochemical cylindrical flow reactor. J Hazard Mater 152(1):71–78. doi:10.1016/j.jhazmat.2007.06.069
Mohan D, Pittman CU (2007) Arsenic removal from water/wastewater using adsorbents—a critical review. J Hazard Mater 142(1):1–53. doi:10.1016/j.jhazmat.2007.01.006
WHO (1993) Guidelines for drinking water quality. World Health Organization, Geneva, p 41
European Commission Directive (1998) 98/83/EC, related with drinking water quality intended for human consumption, Brussels, Belgium
Emett MT, Khoe GH (2001) Photochemical oxidation of arsenic by oxygen and iron in acidic solutions. Water Res 35(3):649–656. doi:10.1016/S0043-1354(00)00294-3
Nguyen VT, Vigneswaran S, Ngo HH, Shon HK, Kandasamy J (2009) Arsenic removal by a membrane hybrid filtration system. Desalination 236(1):363–369. doi:10.1016/j.desal.2007.10.088
Chutia P, Kato S, Kojima T, Satokawa S (2009) Arsenic adsorption from aqueous solution on synthetic zeolites. J Hazard Mater 162(1):440–447. doi:10.1016/j.jhazmat.2008.05.061
Shao W, Li X, Cao Q, Luo F, Li J, Du Y (2008) Adsorption of arsenate and arsenite anions from aqueous medium by using metal (III)-loaded amberlite resins. Hydrometallurgy 91(1):138–143. doi:10.1016/j.hydromet.2008.01.005
Malik A (2004) Metal bioremediation through growing cells. Environ Int 30(2):261–278. doi:10.1016/j.envint.2003.08.001
Naja G, Volesky B (2006) Behavior of the mass transfer zone in a biosorption column. Environ Sci Technol 40(12):3996–4003. doi:10.1021/es051542p
Le Cloirec P, Andrè Y, Faur-Brasquet C, Gerente C (2003) Engineered biofilms for metal ion removal. Rev Environ Sci Biotechnol 2(2-4):177–192
Mondal P, Majumder CB, Mohanty B (2008) Treatment of arsenic contaminated water in a batch reactor by using Ralstonia eutropha MTCC 2487 and granular activated carbon. J Hazard Mater 153(1):588–599. doi:10.1016/j.jhazmat.2007.09.028
Shao L, Ren Z, Zhang G, Chen L (2012) Facile synthesis, characterization of a MnFe2 O4/activated carbon magnetic composite and its effectiveness in tetracycline removal. Mater Chem Phys 135(1):16–24. doi:10.1016/j.matchemphys.2012.03.035
Bianfang Z, Guide T, Zonglin Y, Zhenbiao W, Qingfen Y, Jianpo C (2007) Synthesis of magnetic manganese ferrite. J Wuhan Univ Technol Mater Sci Ed 22:514–517
Hadi M, McKay G, Samarghandi MR, Maleki A, Solaimany Aminabad M (2012) Prediction of optimum adsorption isotherm: comparison of Chi-square and Log-likelihood statistics. Desalin Water Treat 49(1-3):81–94. doi:10.1080/19443994.2012.708202
Hanna OT, Sandall OC (1995) Computational methods in chemical engineering. Prentice–Hall International, New Jersey
Warrens MJ (2008) On similarity coefficients for 2 × 2 tables and correction for chance. Psychometrika 73(3):487–502. doi:10.1007/s11336-008-9059-y
Haerifar M, Azizian S (2013) An exponential kinetic model for adsorption at solid/solution interface. Chem Eng J 215–216:65–71. doi:10.1016/j.cej.2012.11.017
Qiu H, Lv L, Pan BC, Zhang QJ, Zhang WM, Zhang QX (2009) Critical review in adsorption kinetic models. J Zhejiang Univ Sci A 10(5):716–724
Singh TS, Pant KK (2006) Kinetics and mass transfer studies on the adsorption of arsenic onto activated alumina and iron oxide impregnated activated alumina. Water Qual Res J Can 41(2):147–156
Al-Degs YS, El-Barghouthi MI, El-Sheikh AH, Walker GM (2008) Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigments 77(1):16–23. doi:10.1016/j.dyepig.2007.03.001
Mondal P, Mohanty B, Majumder CB (2009) Treatment of simulated arsenic contaminated groundwater using GAC‐Cu in batch reactor: optimization of process parameters. Can J Chem Eng 87(5):766–778. doi:10.1002/cjce.20214
Mondal P, Balo Majumder C, Mohanty B (2007) Removal of trivalent arsenic (As (III)) from contaminated water by calcium chloride (CaCl2)-impregnated rice husk carbon. Ind Eng Chem Res 46(8):2550–2557. doi:10.1021/ie060702i
Mondal P, Mohanty B, Balomajumder C (2010) Treatment of arsenic contaminated groundwater using calcium impregnated granular activated carbon in a batch reactor: optimization of process parameters. CLEAN-Soil, Air, Water 38(2):129–139. doi:10.1002/clen.200900081
Tian Y, Wu M, Lin X, Huang P, Huang Y (2011) Synthesis of magnetic wheat straw for arsenic adsorption. J Hazard Mater 193:10–16. doi:10.1016/j.jhazmat.2011.04.093
Mishra V, Balomajumder C, Agarwal VK (2010) Zn (II) ion biosorption onto surface of eucalyptus leaf biomass: isotherm, kinetic, and mechanistic modeling. CLEAN-Soil, Air, Water 38(11):1062–1073. doi:10.1002/clen.201000030
Mishra V, Balomajumder C, Agarwal VK (2013) Design and optimization of simultaneous biosorption and bioaccumulation (SBB) system: a potential method for removal of Zn (II) ion from liquid phase. Desalin Water Treat 51(16-18):3179–3188. doi:10.1080/19443994.2012.749027
Ranjan D, Talat M, Hasan SH (2009) Biosorption of arsenic from aqueous solution using agricultural residue ‘rice polish’. J Hazard Mater 166(2):1050–1059. doi:10.1016/j.jhazmat.2008.12.013
Ncibi MC, Ranguin R, Pintor MJ, Jeanne-Rose V, Sillanpää M, Gaspard S (2014) Preparation and characterization of chemically activated carbons derived from Mediterranean Posidonia oceanica (L.) fibres. J Anal Appl Pyrolysis 109:205–214. doi:10.1016/j.jaap.2014.06.010
Vaghetti JC, Lima EC, Royer B, da Cunha BM, Cardoso NF, Brasil JL, Dias SL (2009) Pecan nutshell as biosorbent to remove Cu (II), Mn (II) and Pb (II) from aqueous solutions. J Hazard Mater 162(1):270–280. doi:10.1016/j.jhazmat.2008.05.039
Cheung CW, Porter JF, McKay G (2001) Sorption kinetic analysis for the removal of cadmium ions from effluents using bone char. Water Res 35(3):605–612. doi:10.1016/S0043-1354(00)00306-7
Haerifar M, Azizian S (2012) Fractal-like adsorption kinetics at the solid/solution interface. J Phys Chem C 116(24):13111–13119. doi:10.1021/jp301261h
Miretzky P, Muñoz C, Carrillo-Chávez A (2008) Experimental binding of lead to a low cost on biosorbent: Nopal (Opuntia streptacantha). Bioresour Technol 99(5):1211–1217. doi:10.1016/j.biortech.2007.02.045
Lu S, Gibb SW (2008) Copper removal from wastewater using spent-grain as biosorbent. Bioresour Technol 99(6):1509–1517. doi:10.1016/j.biortech.2007.04.024
Kavitha D, Namasivayam C (2007) Experimental and kinetic studies on methylene blue adsorption by coir pith carbon. Bioresour Technol 98(1):14–21. doi:10.1016/j.biortech.2005.12.008
Rengaraj S, Yeon JW, Kim Y, Jung Y, Ha YK, Kim WH (2007) Adsorption characteristics of Cu (II) onto ion exchange resins 252H and 1500H: kinetics, isotherms and error analysis. J Hazard Mater 143(1):469–477. doi:10.1016/j.jhazmat.2006.09.064
Atun G, Hisarli G (2003) Adsorption of carminic acid, a dye onto glass powder. Chem Eng J 95(1):241–249. doi:10.1016/S1385-8947(03)00110-4
Weber WJ, Morris JC (1963) Intraparticle diffusion during the sorption of surfactants onto activated carbon. J Sanit Eng Div Am Soc Civ Eng 89(1):53–61
Singha B, Das SK (2011) Biosorption of Cr (VI) ions from aqueous solutions: kinetics, equilibrium, thermodynamics and desorption studies. Colloids Surf B 84(1):221–232. doi:10.1016/j.colsurfb.2011.01.004
Nouri L, Ghodbane I, Hamdaoui O, Chiha M (2007) Batch sorption dynamics and equilibrium for the removal of cadmium ions from aqueous phase using wheat bran. J Hazard Mater 149(1):115–125. doi:10.1016/j.jhazmat.2007.03.055
Shivaji S, Suresh K, Chaturvedi P, Dube S, Sengupta S (2005) Bacillus arsenicus sp. nov., an arsenic-resistant bacterium isolated from a siderite concretion in West Bengal, India. Int J Syst Evol Microbiol 55(3):1123–1127. doi:10.1099/ijs.0.63476-0
Sag Y, Kutsal T (1995) Biosorption of heavy metals by Zoogloea ramigera: use of adsorption isotherms and a comparison of biosorption characteristics. Chem Eng J 60(1):181–188. doi:10.1016/0923-0467(95)03014-X
Green-Ruiz C, Rodriguez-Tirado V, Gomez-Gil B (2008) Cadmium and zinc removal from aqueous solutions by Bacillus jeotgali: pH, salinity and temperature effects. Bioresour Technol 99(9):3864–3870. doi:10.1016/j.biortech.2007.06.047
Kacar Y, Arpa Ç, Tan S, Denizli A, Genç Ö, Arıca MY (2002) Biosorption of Hg (II) and Cd (II) from aqueous solutions: comparison of biosorptive capacity of alginate and immobilized live and heat inactivated Phanerochaete chrysosporium. Process Biochem 37(6):601–610. doi:10.1016/S0032-9592(01)00248-5
Özdemir S, Kilinc E, Poli A, Nicolaus B, Güven K (2009) Biosorption of Cd, Cu, Ni, Mn and Zn from aqueous solutions by thermophilic bacteria, Geobacillus toebii sub. sp. decanicus and Geobacillus thermoleovorans sub. sp. stromboliensis: equilibrium, kinetic and thermodynamic studies. Chem Eng J 152(1):195–206. doi:10.1016/j.cej.2009.04.041
Meena AK, Mishra GK, Rai PK, Rajagopal C, Nagar PN (2005) Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent. J Hazard Mater 122(1):161–170. doi:10.1016/j.jhazmat.2005.03.024
Roy P, Mondal NK, Das K (2014) Modeling of the adsorptive removal of arsenic: a statistical approach. J Environ Chem Eng 2(1):585–597. doi:10.1016/j.jece.2013.10.014
Ngah WW, Hanafiah MA (2008) Adsorption of copper on rubber (Hevea brasiliensis) leaf powder: kinetic, equilibrium and thermodynamic studies. Biochem Eng J 39(3):521–530. doi:10.1016/j.bej.2007.11.006
Crini G, Badot PM (2008) Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: a review of recent literature. Prog Polym Sci 33(4):399–447. doi:10.1016/j.progpolymsci.2007.11.001
Pandey PK, Choubey S, Verma Y, Pandey M, Chandrashekhar K (2009) Biosorptive removal of arsenic from drinking water. Bioresour Technol 100(2):634–637. doi:10.1016/j.biortech.2008.07.063
Baig JA, Kazi TG, Shah AQ, Kandhro GA, Afridi HI, Khan S, Kolachi NF (2010) Biosorption studies on powder of stem of Acacia nilotica: removal of arsenic from surface water. J Hazard Mater 178(1):941–948. doi:10.1016/j.jhazmat.2010.02.028
Maliyekkal SM, Philip L, Pradeep T (2009) As (III) removal from drinking water using manganese oxide-coated-alumina: performance evaluation and mechanistic details of surface binding. Chem Eng J 153(1):101–107. doi:10.1016/j.cej.2009.06.026
Zhang G, Liu H, Liu R, Qu J (2009) Adsorption behavior and mechanism of arsenate at Fe–Mn binary oxide/water interface. J Hazard Mater 168(2):820–825. doi:10.1016/j.jhazmat.2009.02.137
Kundu S, Gupta AK (2006) Adsorptive removal of As (III) from aqueous solution using iron oxide coated cement (IOCC): evaluation of kinetic, equilibrium and thermodynamic models. Sep Purif Technol 51(2):165–172. doi:10.1016/j.seppur.2006.01.007
Awual MR, Jyo A, Ihara T, Seko N, Tamada M, Lim KT (2011) Enhanced trace phosphate removal from water by zirconium (IV) loaded fibrous adsorbent. Water Res 45(15):4592–4600. doi:10.1016/j.watres.2011.06.009
Balaji T, Yokoyama T, Matsunaga H (2005) Adsorption and removal of As (V) and As (III) using Zr-loaded lysine diacetic acid chelating resin. Chemosphere 59(8):1169–1174. doi:10.1016/j.chemosphere.2004.12.007
Chiban M, Carja G, Lehutu G, Sinan F (2011) Equilibrium and thermodynamic studies for the removal of As (V) ions from aqueous solution using dried plants as adsorbents. Arab J Chem. doi:10.1016/j.arabjc.2011.10.002
Zhang Y, Yang M, Dou XM, He H, Wang DS (2005) Arsenate adsorption on an Fe-Ce bimetal oxide adsorbent: role of surface properties. Environ Sci Technol 39(18):7246–7253. doi:10.1021/es050775d
Zhang GS, Qu JH, Liu HJ, Liu RP, Li GT (2007) Removal mechanism of As (III) by a novel Fe-Mn binary oxide adsorbent: oxidation and sorption. Environ Sci Technol 41(13):4613–4619. doi:10.1021/es063010u
Sarı A, Tuzen M (2009) Biosorption of As (III) and As (V) from aqueous solution by macrofungus (Inonotus hispidus) biomass: equilibrium and kinetic studies. J Hazard Mater 164(2):1372–1378. doi:10.1016/j.jhazmat.2008.09.047
Tuzen M, Sarı A, Mendil D, Uluozlu OD, Soylak M, Dogan M (2009) Characterization of biosorption process of As (III) on green algae Ulothrix cylindricum. J Hazard Mater 165(1):566–572. doi:10.1016/j.jhazmat.2008.10.020
Seki H, Suzuki A, Maruyama H (2005) Biosorption of chromium (VI) and arsenic (V) onto methylated yeast biomass. J Colloid Interface Sci 281(2):261–266. doi:10.1016/j.jcis.2004.08.167
François F, Lombard C, Guigner JM, Soreau P, Brian-Jaisson F, Martino G, Vandervennet M, Garcia D, Molinier AL, Pignol D, Peduzzi J (2012) Isolation and characterization of environmental bacteria capable of extracellular biosorption of mercury. Appl Environ Microbiol 78(4):1097–1106. doi:10.1128/AEM.06522-11
Giri AK, Patel RK, Mahapatra SS (2011) Artificial neural network (ANN) approach for modelling of arsenic (III) biosorption from aqueous solution by living cells of Bacillus cereus biomass. Chem Eng J 178:15–25. doi:10.1016/j.cej.2011.09.111
Pokhrel D, Viraraghavan T (2007) Arsenic removal from an aqueous solution by modified A. niger biomass: batch kinetic and isotherm studies. J Hazard Mater 150(3):818–825. doi:10.1016/j.jhazmat.2007.05.041
Ren Y, Li N, Feng J, Luan T, Wen Q, Li Z, Zhang M (2012) Adsorption of Pb (II) and Cu (II) from aqueous solution on magnetic porous ferrospinel MnFe2O4. J Colloid Interface Sci 367(1):415–421. doi:10.1016/j.jcis.2011.10.022
Parida KM, Mallick S, Mohapatra BK, Misra VN (2004) Studies on manganese-nodule leached residues: 1. Physicochemical characterization and its adsorption behavior toward Ni2+ in aqueous system. J Colloid Interface Sci 277(1):48–54. doi:10.1016/j.jcis.2004.04.057
Li Z, Deng S, Yu G, Huang J, Lim VC (2010) As (V) and As (III) removal from water by a Ce–Ti oxide adsorbent: behavior and mechanism. Chem Eng J 161(1):106–113. doi:10.1016/j.cej.2010.04.039
Aryal M, Ziagova M, Liakopoulou-Kyriakides M (2010) Study on arsenic biosorption using Fe (III)-treated biomass of Staphylococcus xylosus. Chem Eng J 162(1):178–185. doi:10.1016/j.cej.2010.05.026
Mondal P, Majumder CB, Mohanty B (2008) Treatment of arsenic contaminated water in a batch reactor by using Ralstonia eutropha MTCC 2487 and granular activated carbon. J Hazard Mater 153(1):588–599. doi:10.1016/j.jhazmat.2007.09.028
Agarwal B, Balomajumder C, Thakur PK (2013) Simultaneous co-adsorptive removal of phenol and cyanide from binary solution using granular activated carbon. Chem Eng J 228:655–664. doi:10.1016/j.cej.2013.05.030
Acknowledgments
Our thanks to the Indian Institute of Technology, Roorkee for providing necessary facilities and to the Ministry of Human Resource Development, Government of India for financial support. The thoughtful comments by Dr. Apostolos Giannis, the Editor, and two anonymous reviewers are highly appreciated.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 10476 kb)
Rights and permissions
About this article
Cite this article
Podder, M.S., Majumder, C.B. Biosorptive Performance of Bacillus arsenicus MTCC 4380 Biofilm Supported on Sawdust/MnFe2O4 Composite for the Removal of As(III) and As(V). Water Conserv Sci Eng 1, 103–125 (2016). https://doi.org/10.1007/s41101-016-0007-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41101-016-0007-x