Abstract
Introduction
Long-term oxygen therapy (LTOT) is reported to improve survival in patients with chronic respiratory failure. We aimed to describe effectiveness, burden, and cost of illness of patients treated with portable oxygen concentrators (POC) compared to other LTOT options.
Methods
This retrospective comparative analysis included adult patients with chronic respiratory insufficiency and failure (CRF) upon a first delivery of LTOT between 2014 and 2019 and followed until December 2020, based on the French national healthcare database SNDS. Patients using POC, alone or in combination, were compared with patients using stationary concentrators alone (aSC), or compressed tanks (CTC) or liquid oxygen (LO2), matched on the basis of age, gender, comorbidities, and stationary concentrator use.
Results
Among 244,719 LTOT patients (mean age 75 ± 12, 48% women) included, 38% used aSC, 46% mobile oxygen in the form of LO2 (29%) and POC (18%), whereas 9% used CTC. The risk of death over the 72-month follow-up was estimated to be 13%, 15%, and 12% lower for patients in the POC group compared to aSC, CTC, and LO2, respectively. In the POC group yearly mean total costs per patient were 5% higher and 4% lower compared to aSC and CTC groups, respectively, and comparable in the LO2 group. The incremental cost-effectiveness ratio (ICER) of POC was €8895, €6288, and €13,152 per year of life gained compared to aSC, CTC, and LO2, respectively.
Conclusion
Within the POC group, we detected an association between higher mobility (POCs autonomy higher than 5 h), improved survival, lower costs, and ICER − €6 238, compared to lower mobility POCs users.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Long-term oxygen therapy (LTOT) is reported to improve survival and to reduce hospitalizations of patients with chronic respiratory insufficiency and failure (CRF) due to chronic obstructive pulmonary disease (COPD) or other causes. In LTOT patients, the high numbers of hospital admissions and frequent specialist visits significantly contribute to disease burden and aggravate the healthcare costs of the underlining respiratory diseases. Studies assessing potential clinical benefits and cost-effectiveness of portable oxygen concentrators (POCs) compared to other oxygen delivery solutions are scarce. |
What was learned from the study? |
In France CRF adult patients with LTOT delivered between 2014 to 2019 were elderly (mean age 75 ± 12), with equal gender distribution (48% women) and with at least one comorbidity. |
POC use, either alone or in combination, was associated with improved survival, and similar healthcare resource use (e.g., hospitalizations, specialist visits) compared to the other oxygen delivery devices. |
On the basis of the incremental cost-effectiveness ratio (ICER), POCs were cost-effective compared to stationary concentrators, compressed tanks, and liquid oxygen. |
Within the POC group patients using higher-mobility devices (POCs with an autonomy higher than 5 h) was cost-saving, being associated with better survival, lower healthcare resource use and related costs. |
Introduction
Long-term oxygen therapy (LTOT) is the recommended standard of care for patients with chronic respiratory insufficiency and failure (CRF) due to severe chronic obstructive pulmonary disease (COPD) or other causes [1, 2]. Besides obesity or pulmonary hypertension, other comorbid conditions, such as heart failure and its various etiologies, or lung cancer, can aggravate CRF [3, 4]. Although supplemental oxygen is an established therapy for patients with CRF, studies reporting the effect of LTOT devices on clinical outcomes, such as hospitalizations and mortality, and on the costs related to the healthcare resource use, are scarce and with a limited sample size [5,6,7].
Oxygen delivery devices allow mobility as needed, depending on the model. On the basis of reimbursement, logistics, and performance, patients have often a combination of devices for home oxygen delivery, a non-portable, fixed, device with continuous flow oxygen dispensation for in-home use, a portable device for ambulation, and a backup system. In real-life settings it is practically impossible to estimate which oxygen delivery device is the one bringing the most relevant clinical benefits to a patient with CRF, as in LTOT standard of care fixed and portable devices are often used in combination. The majority of the LTOT clinical trials focusing on the portable oxygen concentrator (POC) device, which operates by concentrating oxygen from the ambient air, have compared it with other portable solutions, such as liquid oxygen devices, or continuous flow oxygen cylinder [8,9,10,11,12]. These trials have demonstrated no difference between the various modalities of portable oxygen devices (activities of daily living, oxygen saturation, Borg score or 6-min walking test [6MWT]), underlying the importance of patients’ mobility backed by current guidelines [1, 13, 14]. Appropriate LTOT use reduces hospitalizations and overall mortality in severely hypoxemic patients with COPD, improving cognitive function, emotional status, and potentially slowing the progressive impairments in independent living, affecting also young subjects [15,16,17,18].
Different types of POCs are available on the market. When selecting a POC, certain measurements and specifications of the device should be considered in relation to the disease requirements and patient’s lifestyle. These measurements are weight, oxygen flow rate, battery life, oxygen purity (typically around 90% or higher), noise level, durability, and additional features such as pulse dose delivery, continuous flow mode, travel-friendly accessories, and user-friendly interfaces. When the oxygen flow is intermittent, the pulse dose delivery can be adjusted manually or automatically (oxygen sensing technology), depending on the degree of physical activity and needs [19]. The weight can go from the smaller models (2–5 lb, approximately 0.9–2.3 kg) to the larger, more feature-rich models (10–20 lb, approximately 4.5–9 kg). Patients’ preference for portable options is recognized, since they support autonomy, independent living, and well-being of patients [20,21,22]. Clinicians are becoming more attentive to the patient’s perspectives and are aware that guidelines recommend keeping LTOT patients active and able to work [14, 23].
The economic and social burden of chronic respiratory diseases is substantial across countries. Lost productivity and low mobility due to COPD are particularly worrisome in patients with comorbidities and expected to increase in the coming years [24, 25]. The American Thoracic Society raised awareness on the increase of respiratory diseases in association with multimorbidity, especially in the context of worldwide aging trends [26, 27]. Studies assessing the clinical and economic burden of LTOT on patients in France, and throughout Europe, are limited. As such, bringing new evidence on the current use of domiciliary LTOT in France could help improve care management for severe respiratory insufficiencies conditions. Also considering the country-specific reimbursement constraints, cost-effectiveness studies could elevate the quality of home oxygen services available to patients [14, 28].
The primary objective of this study was to evaluate the health impact of LTOT according to the different oxygen delivery strategies in patients treated for CRF due to COPD or other causes, using the French national healthcare system database, SNDS. We examined clinical outcomes, such as survival, hospitalizations, and specialist visits, to estimate the cost-effectiveness of POC, used alone or in combination, compared with other oxygen delivery devices, such as stationary concentrators, used alone (aSC), compressed tanks, alone or in combination (CTC), and liquid oxygen, alone or in combination (LO2). The secondary objective was to estimate and compare cost-effectiveness of POCs based on the different level of autonomy.
Methods
Data Source
The study consisted of secondary data analysis based on claims data from the Système National des Données de Santé (SNDS) [national healthcare system database], which includes health information (hospital care and primary care) for 66 million people, more than 99% of the French population, with over 10 years of follow-up, owned by the Caisse Nationale d’Assurance Maladie (CNAM) [National Health Insurance] [29, 30]. The SNDS provides pseudonymous, comprehensive, and individualized data through a unique personal identification number. Details on the data available have been previously described [31].
In accordance with French regulations, the study protocol was approved by the ethics and scientific committee for health research, studies, and evaluations (CESREES) and by the French data privacy committee (CNIL; Decision DR-2021-228). Data access was delivered by CNAM after agreement. In the study personal data processing is intended for a research project not involving human subjects [32].
Study Design
This is a retrospective comparative observational study based on the SNDS medico-administrative dataset. The study report was completed following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [33].
Study Period
The study was conducted from 1 January 2013 to 31 December 2020. Long-term oxygen treatment (LTOT) was defined by at least 3 months of continuous oxygen supplementation. The LTOT index date was defined as the date of first prescription of a LTOT device happening during the inclusion period, from now on referred to as the index date. The inclusion period was defined from 1 January 2014 to 31 December 2019. The historical period corresponded to 12 months prior to the LTOT index date and was specified to enable the description of patient baseline characteristics. Patients had an individual follow-up period up to the date of death or up to 31 December 2020 (for maximum of 72 months). To maximize patient inclusion, a minimum time of follow-up was not specified.
Study Population
The study population encompassed all French adults (aged ≥ 18 years) with a recorded diagnosis of COPD or CRF, due to COPD or other causes, during the study period, and a reimbursement for a first-time prescription of a LTOT device occurring from 1 January 2014 to 31 December 2019 (incident and prevalent cases). The list of ICD-10 codes used to identify the diagnosis of COPD or CRF is provided in the Supplementary Material (Table S1). The list of the codes used to identify medical devices associated with LTOT [stationary concentrator (SC); compressed tanks (CTC); liquid oxygen (LO2); portable oxygen concentrator (POC)] is provided according to the French nomenclature list of codes, Liste des produits et prestations (LPP) [34] (Table S2).
Patients treated with LTOT are often equipped with multiple oxygen delivery devices, according to the French guidelines for LTOT standard of care [35]. For the analysis we identified different groups determined by the higher frequency of the device used, alone or in combination, during the follow-up period (Table 1). The groups were mutually exclusive, as patients were considered only once in a specific group and remained in the assigned group until the end of the follow-up.
Only in the SC group were patients SC exclusive users, identified as SC used alone (aSC). In the other groups analyzed, the main device, used alone or in combination with other devices following standard of care, was used to identify the patients: CTC, LO2, and POC.
In addition, the POC group was further examined. A secondary analysis was performed in two subpopulations of patients with potentially higher mobility (HM) (POCs autonomy higher than 5 h, Inogen only devices) and lower mobility (LM) (POCs autonomy lower than 5 h, all non-Inogen devices). The oxygen delivery devices included in the analysis reflect their availability on the French market and their level of autonomy between 2013 and 2020. Of note, in the higher mobility group only Inogen devices were detected in the SNDS dataset, as they were the ones approved for reimbursement within France during the prescription period considered in the study.
Study Endpoints
Patient characteristics, including gender and age, were described for all patients at index date. Comorbidities were selected as known to be significantly associated with CRF severity, and identified during the historical period according to algorithms used in the mapping published by the CNAM [36].
Overall survival, healthcare resource use (HCRU), and the associated costs were assessed. HCRU included all-cause private and public hospitalizations, respiratory-related hospitalizations, and rehospitalization (30-day gap between two respiratory hospitalizations), emergency room (ER) visits, outpatient general practitioner (GP) and specialist visits, and dispensing of observable LTOT medical devices.
Patients’ level of mobility, level of frailty during hospital stays, and access to healthcare were estimated. The patient’s level of mobility was estimated using the French nomenclature list of LPP codes related to mobility assistance equipment (Table S3). The hospital frailty risk score (HFRS) was calculated using diagnoses recorded during hospital stay in the 2-year historical period (except for the patients included in 2014, who only had 1 year of historical before the index date, based on the data extraction provided by CNAM) [37]. Potential territorial healthcare access disparities were estimated considering rural/urban areas distribution and the social deprivation index [38, 39].
Statistical Analysis
Descriptive Analysis
Descriptive analyses were conducted depending on the nature of the variable. Qualitative variables were described by the number and frequency for each modality and quantitative variables by the mean, median, min/max values, quartiles, and standard deviation (SD).
The healthcare use results were reported as the rate per patient per year.
Comparative Analysis
Propensity Score Matching
A propensity score was generated for matching subjects of the “treated” and “control” groups as described. The studied outcomes were analyzed between the two comparable groups; a 1:1 non-discounted matching between patients was performed using the propensity score method. Matching variables, including the comorbidities associated with the diagnosis of CRF, due to COPD or other causes, are listed in Table 2. The probability of using a POC (alone or in combination) device was modeled using a multivariate logistic regression including the following covariates: (a) the sociodemographic characteristics at the time of the LTOT index (age, gender); (b) comorbidity type and number per patient; (c) the use of a stationary concentrator (at least one recorded delivery during the follow-up period); (d) index date LTOT delivery before or after 2018, considering how the SNDS listing changed in terms of reimbursement for this prescription [40]. Of note, matching was not performed on respiratory disease severity and LTOT adherence/utilization, as related data are not available in the SNDS database. Three different matchings were implemented. Conditional on the propensity score, the distribution of observed baseline covariates will be similar between the “treated” group (POC-equipped patients) and “control” group (aSC-, CTC-, LO2-equipped patients). Standardized mean differences were used to analyze the balance in measured baseline variables before and after matching. For this analysis, SD was expressed as a percentage, and the result was reported as “excellent balance” (SD < 5%) [41]. The treated and control groups were compared two by two using the Student’s t test (quantitative variables) and chi-square (qualitative variables). All tests performed were two-sided and considered significant at p = 0.05.
Survival Analysis
Overall survival (OS) was estimated using the Kaplan–Meier method considering all-cause death as an event. Patients were censured at the end of follow-up period, loss to follow-up, or death. In this case the number of days/months between the index date and the date of last observed care (up to 31 December 2020) was considered during follow-up. Whereas for the time to death the number of days/months between the index date and the date of death was considered.
The comparison between the two groups of interest was performed with log-rank test and 95% confidence interval. The hazard ratio (HR) was estimated with the Cox method.
Economic Evaluation
All-cause HCRU was assessed for the following categories: private and public hospitalizations, medical visits defined by the ACE (actes et consultations externes), such as outpatient physician and paramedic visits, technical medical, imaging, or biological procedures, laboratory tests, dispensing of observable drugs and medical devices, financial sickness benefits, and invalidity pensions.
For each oxygen delivery device, total direct costs associated with the HCRU parameters during the follow-up period were annualized to 2020 prices using Consumer Price Index and reported as the mean annualized cost per patient per year and quoted in EUR. The economic evaluation was conducted from the payer perspective, considering the actual rate expenditures reimbursed by the French national health insurance reported in the SNDS. The cost-effectiveness of POC compared to other oxygen delivery devices (aSC, CTC, LO2) was assessed by the incremental cost-effectiveness ratio (ICER), which was expressed as cost per life-year gained [42]. ICER was calculated as the ratio between the difference of total care costs between the POC (treated) and the control groups and the difference of overall survival (expressed by the number of life-years gained, LYG) over the study period. The total LYG in the POC group compared to the control groups was therefore estimated by the difference in number of life-years lost between the two groups during the study.
All analyses were performed in R Studio Version 4.3.1 (R Studio Inc., Boston, MA).
Results
Study Population
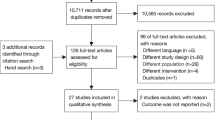
The study population included 244,719 adult patients receiving LTOT between January 2014 and December 2019 (Fig. 1). Among them, five groups were identified according to the main oxygen delivery device used (either alone or in combination) during the inclusion period. The following four groups were described further: compressed tank, alone or in combination (CTC); liquid oxygen, alone or in combination (LO2); portable oxygen compressor, alone or in combination (POC); stationary concentrator, alone (aSC).
Study flowchart of the population before matching. CTC compressed tanks, alone or in combination; LO2 liquid oxygen, alone or in combination; LTOT long-term oxygen therapy; POC portable oxygen concentrator, alone or in combination; aSC stationary concentrator, alone. All percentages are calculated using N = 244719 as denominator
Overall, the study population comprised elderly patients, with a mean age of 75 (SD 12) years, and similar gender proportion (48% women). Only 20% were younger than 65 years old. Sociodemographic and clinical characteristics of the study population are reported in Table S4.
According to group assignment, before matching most of the patients were in the stationary concentrator group (aSC 38%), and in the mobile oxygen therapy group (46%), of which 29% were equipped with liquid oxygen (LO2) and 18% equipped with portable oxygen compressor (POC). Of note, among POC users, the majority used Inogen concentrators (49%) and/or other devices [Philips (31%), Caire Eclipse (14%), Invacare (12%), GCE Zen-O (2%), and Resmed (1%)]. Unmatched patients included in the mobile oxygen therapy were younger (73–74 years old), with lower proportion of female patients (approximately 45%), whereas patients equipped with aSC were older (78 years old), as a stationary concentrator is often prescribed at the end of life, with 53% female patients (Tables 3, 5). Six percent of the patients were included in the CTC group. The remaining 4%, representing patients with other combinations of LTOT, were not analyzed in the study as a result of the high heterogeneity of LTOT combination.
At baseline, 88% of the LTOT patients presented at least one comorbidity, with the majority having more than one concomitantly (Table S4, Fig. 2). The most prevalent comorbidities were chronic respiratory diseases, excluding cystic fibrosis (86%), and cardiovascular disease (70%). For other main chronic conditions, such as chronic heart failure, cancer, and vascular risk treatments (without pathologies), the prevalence was 38%, 32%, and 30%, respectively. Obesity was recorded in only 5% of patients; however, this is likely an underestimation since diagnoses are only captured by recorded ICD-10 codes used to describe the underlying reason for the hospital stays, whereas weight and BMI are not available in the SNDS. A total of 13% of patients had concomitant chronic respiratory disease and cardiovascular disease. More specifically patients presented chronic respiratory disease overlapping with cardiovascular diseases, and chronic heart failure (13%) or vascular risk treatments (5%). Chronic respiratory diseases overlapped also with cancer and chronic heart failure and cardiovascular disease (6%). Remarkably, only 5% of the patients had chronic respiratory disease as a single disorder, whereas dyspnea, which is one of the symptoms of patients with CRF, was reported for 4% of patients.
Venn diagram showing overlap between the main comorbid conditions known to be significantly associated with chronic respiratory insufficiencies and failure severity. This analysis was implemented to include patients with at least one comorbidity. Color coding is in accordance with the number of patients in each category of comorbid pathology, and within the Venn diagram the percentage of patients is reported
Besides sociodemographic and clinical characteristics, baseline differences between POC, alone or in combination, compared to aSC, CTC, and LO2 groups, were evaluated assessing the proportion of patients with a stationary concentrator equipment, in addition to the estimation of patients’ mobility level, hospital frailty risk score, and potential healthcare access disparities, considering rural/urban areas distribution and the French social deprivation index.
In the study population most patients were equipped with a stationary concentrator device, independently of group assignment (Table S5). In the total LTOT population, 24% received assistive mobility devices, with a higher proportion of patients in the aSC group compared to the POC group (28% vs. 19%, respectively). The four groups had comparable mobility needs, with the walking cane being the most frequently prescribed device compared to medical walkers and wheelchairs. According to the hospital frailty risk score, 64% of the total LTOT patients were not vulnerable, with the aSC group presenting a higher proportion of patients with low and intermediate frailty scores (36% and 2%, respectively; Table S6). Regarding potential healthcare access disparities, no major differences were observed in terms of rural/urban residence (Table S7) and social deprivation index (Table S8) between groups. Most patients lived in urban areas and a high proportion belonged to the more disadvantaged categories (approximately 70–75% in the three lowest levels).
In summary, patients’ distribution among groups was similar in terms of residence and deprivation index; only minor differences were observed regarding the use of assistive mobility devices and the estimation of hospital frailty risk. Once considered in the matching, these variables did not impact the results. For this reason, only the use of SC equipment was considered in the propensity score matching.
Following the descriptive analyses, patients in the POC group, using POC alone or in combination, were matched to aSC, CTC, and LO2 treated patients, respectively, on the basis of age, gender, comorbidities, use of stationary concentrator equipment, and LTOT index date before or after 2018. The excellent balance between treated (POC) and control groups (aSC, CTC, LO2) achieved by the propensity score matching (Figs. S1–S3) allowed us to assess the cost-effectiveness of this LTOT device. The overall survival, HCRU, and the associated costs were compared between the matched groups, starting from the oxygen delivery solution more distant to POC in terms of mobility and flexibility—the stationary concentrators.
Baseline Characteristics and Survival of Matched POC Versus aSC Groups
Baseline Characteristics
Before matching, patients in the POC group had younger age and male prevalence compared to patients in the aSC group (Table 3). Patients in the POC group had higher chronic respiratory diseases (POC 93% vs. aSC 83%), cancers (POC 37% vs. aSC 29%), and vascular risk treatments (POC 36% vs. aSC 29%). Whereas aSC-equipped patients presented more often neurological or degenerative diseases (aSC 22% vs. POC 13%) and slightly more cardiovascular diseases (aSC 73% vs. POC 71%), in line with their older age.
Propensity score matching between POC (treated) and aSC (control) groups yielded 18,295 matched pairs, which represented 20% of the aSC and 43% of the POC populations before matching. The two groups were comparable after matching (Fig. S1).
Overall Survival
The Kaplan–Meier curves demonstrated that patients with POC, alone or in combination, had a significantly better median overall survival (mOS) than those matched with aSC (48.8 months vs. 41.4 months, respectively) over the 72-month follow-up period (Fig. 3). The mortality risk was significantly reduced by 13% (HR 0.87 [95% CI 0.84–0.89], p < 0.0001) in patients in the POC group. The survival rate was estimated to be favorable for patients in the POC group at 12 months, with 86% of patients alive versus 80% in the aSC group.
Baseline Characteristics and Survival of Matched POC Versus CTC Groups
Baseline Characteristics
Before matching, patients in the POC and CTC groups had similar age and gender distribution. Differences were observed only in terms of comorbidities (Table 4). Patients in the POC group had high prevalence of chronic respiratory diseases (POC 93% vs. CTC 86%), vascular risk treatments (POC 36% vs. CTC 31%), and slightly more psychiatric illness (POC 24% vs. CTC 20%).
Propensity score matching between POC (treated) and CTC (control) groups yielded 21,552 matched pairs, which represented 98% of the CTC and 50% of the POC populations before matching. The two groups were comparable after matching (Fig. S2).
Overall Survival
Patients in the POC group had significantly better median overall survival (mOS), which was 41.2 vs. 33.3 months than in the CTC group (Fig. 4). The estimated mortality risk was significantly reduced by 15% (HR 0.85 [95% CI 0.82–0.87], p < 0.0001) in POC compared to patients in the CTC group. The survival rate was estimated to be favorable for POC, alone or in combination, patients at 12 months, with 78% of patients alive versus 73% in the CTC group.
Baseline Characteristics and Survival of Matched POC Versus LO2 Groups
Baseline Characteristics
The subpopulations included in the mobile oxygen therapy options, liquid oxygen, and portable oxygen compressor were compared (Table 5). Before matching, patients in the POC and LO2 groups had similar age and gender distribution. Regarding comorbidities, patients in the POC group had higher chronic respiratory diseases (POC 93% vs. LO2 82%), cancers (POC 37% vs. LO2 32%), vascular risk treatments (POC 36% vs. LO2 27%), and psychiatric illness (POC 24% vs. LO2 18%). LO2-equipped patients presented more often pneumonia (LO2 23% vs. POC 14%).
Propensity score matching between POC (treated) and LO2 (control) groups yielded 35 294 matched pairs, which represented 50% of the LO2 and 82% of the POC populations before matching. The two groups were comparable after matching (Fig. S3).
Overall Survival
In patients using POC, alone or in combination, the estimated overall survival (mOS) was 46.7 (45.9–47.6) months compared to 39.6 (38.9–40.4) months for patients in the LO2 group (Fig. 5). Although comparing two mobile oxygen therapeutical devices, the reduced estimated mortality risk, previously observed for POC compared to aSC and CTC groups, remained evident. Patients in the POC group had 12% (HR 0.88 [95% CI 0.86–0.9], p < 0.0001) lower risk of death compared to LO2 users. The survival rate was estimated to be more favorable for patients in the POC group at 24 months of follow-up, with 68% of patients alive versus 66% in the LO2 group.
In summary, the patients equipped with portable oxygen concentrators, alone or in combination, showed consistently improved survival compared to the three other oxygen delivery solutions analyzed: stationary concentrators, compressed tanks, and liquid oxygen.
Cost-effectiveness Analysis
To further evaluate the effectivness of POC, alone or in combination, compared to the different therapeutical options, the HCRU and the associated costs were analyzed over the 72 months of follow-up. The HCRU and costs are presented in Table S9 (POC compared to aSC), Table S10 (POC compared to CTC), and Table S11 (POC compared to LO2). The respective costs analyzed from the index date up to the end of 72-month follow-up are expressed per patient per year.
HCRU and Costs of Matched POC Versus aSC Groups
Patients equipped with POC, alone or in combination, had higher all-cause hospitalization rate and relative risk compared to patients in the aSC group, represented by respiratory-related hospitalizations, but reduced readmissions and ER visits (Table S9). Medical visits (primary/community care or hospital visits) were comparable between groups, except for a higher relative risk of consulting a pulmonologist, a geriatrician, or a hematologist in the POC group.
Total healthcare costs were evaluated per category in POC, alone or in combination, and aSC groups (Fig. 6). Yearly mean total healthcare costs per patient were 5% (€579) higher in the POC group (€10,861) compared to aSC (€10,282). All-cause hospitalizations were the main cost driver, followed by LTOT-related expenses, higher in POC patients.
The survival benefit over the 72 months of follow-up was estimated through the cost-effectiveness analysis using the ICER.
The cost-effectiveness analysis showed that POC, alone or in combination, was cost-effective. Incremental cost and efficacy were in favor of POC (Table 6). The ICER was equal to €8895 per life-year gained for patients in the POC group compared to aSC group.
HCRU and Costs of Matched POC Versus CTC Groups
Patients equipped with POC and CTC, alone or in combination, had comparable all-cause hospitalization rate, but patients in the POC group had lower ER visits (Table S10). Patients in the POC group had lower consultation rate with GP, but higher with pneumonologists, compared to the CTC group.
Yearly mean healthcare costs per patient were 4% (− €502) lower in the POC group (€12,166) compared to CTC (€12,668) (Fig. 7). All-cause hospitalizations were the main cost driver, followed by LTOT-related expenses, in both groups.
According to cost-effectiveness analysis, incremental cost was higher for POC with a positive incremental efficacy (Table 7). POC presented an ICER of €6288 per life-year gained compared to the CTC group.
HCRU and Costs of Matched POC Versus LO2 Groups
Patients in the POC, alone or in combination, group had slightly higher respiratory-related hospitalization and readmission rates; however, the respective relative risks were comparable with the LO2, alone or in combination, group (Table S11). Patients in the POC group had lower rate of medical visits, consulting less often the general practitioner and geriatrics.
Yearly mean total healthcare costs per patient were comparable, with €59 difference between POC (€12,094) and LO2 (€12,035) groups (Fig. 8). All-cause hospitalizations were the main cost driver, with no differences between groups. Whereas patients in the LO2 group had higher LTOT-related expenses.
According to the cost-effectiveness analysis, incremental cost was higher for POC with a positive incremental efficacy (Table 8). POC presented an ICER of €13,152 per life-year gained compared to patients in the LO2 group.
Differences in Mobility Between Two Subpopulations Within the POC Group
In a secondary analysis, the cost-effectiveness of the device was assessed within the POC group in relation to the autonomy levels, and consequently patients’ mobility, of the portable oxygen concentrators recorded between 2013 and 2020 in the SNDS database. Two subpopulations distinguished by higher mobility (HM) (POCs with an autonomy higher than 5 h, Inogen only devices) and lower mobility (LM) (POCs with an autonomy lower than 5 h, all non-Inogen devices) were described and compared for baseline characteristics, overall survival, HCRU and costs. Among the 40,617 patients with COPD and CRF equipped with POCs, 18,630 and 21,987 were identified in the HM and LM groups, respectively. Before matching, the HM (treated) group had higher a proportion of men compared to the LM (control) group (57% vs. 52%), and an average age of 72 (SD 11) years for HM and 75 (SD 12) for LM. In addition to POCs, some patients used SC (40% HM vs. 45% LM).
After matching, 17,099 were included in each group and the subpopulations were comparable: mean age 72 ± 11, 56% male patients, 42% of SC equipment. The Kaplan–Meier curves showed that POC HM patients had higher mOS compared to the LM group (52.7 vs. 43.4 months in LM and HM, respectively) (Fig. 9). The estimated mortality risk was 19% lower (HR 0.81 [95% CI 0.78–0.83], p < 0.0001) in the HM group. The survival rate was estimated to be more favorable for HM patients at 12 months of follow-up, with 84% of patients alive versus 78% in the LM group.
POC HM and POC LM groups had similar risk of all-cause and respiratory hospitalizations, and ER visits. However, despite the matching for age and comorbidities, POC LM patients had higher geriatric care visits (Table S12).
Yearly mean total healthcare costs per patient were 12% higher (€1640) in the LM group, €13,503 LM vs. €11,863 HM (Fig. 10). All-cause hospitalizations were the main cost driver, being 14% higher for POC LM patients.
The calculation of the ICER showed that POCs with HM were cost-saving (− €6238 per life-year gained) compared to LM (Table 9). Incremental cost and incremental efficacy were in favor of POC HM subpopulation.
Discussion
In the current study evaluating portable oxygen concentrators compared to other LTOT therapeutical strategies, we addressed a current lack of evidence evaluating the use of domiciliary LTOT, with a focus on portable oxygen concentrators, using a French nationwide database that included all adult patients with COPD and CRF, due to COPD or other causes, with a prescription of a LTOT between 2014 to 2019. The study population comprised elderly patients, 90% of whom had at least one comorbidity affecting the disease severity. COPD has been shown to be the strongest predictor for a higher number of comorbidities, followed by the cumulative number of other factors, such smoking, male gender, and older age, in association with mortality [44, 45]. However, the prevalence of COPD is predicted to increase to 2.8 million by 2025 in France, especially among women and subjects aged ≥ 75 years [46]. In our analysis the average age of matched patients was 75, presenting often coexisting comorbidities, such as chronic respiratory diseases concomitant with cardiovascular diseases, and cancer. In patients with COPD, comorbidities can significantly change the disease severity, affecting life expectancy and contributing to incremental healthcare costs in France [47]. It is becoming evident that the current “individual disease-centered” management of patients with chronic respiratory diseases is costly and inappropriate, especially in older multimorbid patients [26]. In the analysis patients were matched for comorbidities; however, the current study did not examine the impact of specific comorbidities or CRF-associated disease types on the cost-effectiveness analysis of LTOT devices.
The use of different modalities of oxygen therapy depends on the level of daily activity, exercise capacity, patient’s specific needs, including comorbidities. Among the four groups identified by the main medical device used, either alone or in combination, the POC group was associated with more favorable survival rates compared to other oxygen delivery solutions: stationary concentrators, compressed tanks, and liquid oxygen (aSC, CTC, LO2). Although this therapeutic intervention seems to be more effective for the selected clinical outcome, this should be interpreted with caution, considering the limitations intrinsic to the real-world setting. Being a retrospective study based on a medico-administrative database, details on the level of mobility/autonomy of the patient at the time of LTOT prescription are unavailable. We could hypothesize that patients included in the POC group had a more favorable phenotype at the time of LTOT prescription, with higher baseline mobility compared to the other groups, considering that POC is prescribed to allow a more active life. The survival benefit observed in patients using POC, alone or in combination, may be the result of several factors not available in the SNDS, including socio-psychological implications related to the level of mobility and freedom offered by POC itself.
Stakeholders need to be informed on the healthcare costs associated with LTOT patients depending on the different therapeutic strategies, and the potential benefits arising from higher autonomy of the device, to facilitate proper resource allocation and inform future policies. To the best of our knowledge this is the first study evaluating the cost-effectiveness of portable oxygen concentrators, used either alone or in combination, compared to other oxygen delivery solutions.
The clinical performance of POCs compared to traditional portable systems such as compressed oxygen cylinders has been demonstrated to be equivalent during 6-min walk tests in patients with COPD and interstitial lung disease (ILD) [48]. More recently, POCs have been shown to improve muscle oxygenation during walking in patients with ILD [49]. Guidelines, previous trials, and ongoing center-based and home-based programs, including digital enabled interventions, reflect the importance of mobility in pulmonary rehabilitation in terms of improvements in exercise capacity, health status, and quality of life for the LTOT patients, including healthcare cost reduction as a result [50, 51]. Compared with other devices, portable systems are recognized to provide numerous advantages favoring compliance with LTOT, as recommended [1, 2]. The weight of portable oxygen cylinders can limit their use. When patients need to collect their cylinders from the hospital themselves or to follow ambulatory oxygen therapy, usage and treatment compliance are likely to be affected by the device weight [13]. Today technology has made portable oxygen concentrators smaller, lightweight, and better performing. Considerable attention is paid to active patients, but also to those with a lower degree of physical ability. This analysis suggests that relevant clinical benefits might be associated with POC use, alone or in combination, compared to more burdensome solutions such as SC and CTC. Patients in the POC group had superior overall survival compared with all the groups analyzed. In addition, within the POC group, the higher mobility (POCs autonomy higher than 5 h) was associated with an even more favorable estimation, with 10 months higher survival (52.7 months vs. 43.4 months in low mobility subpopulation). This secondary analysis highlighted the key role of mobility in LTOT patients’ life expectancy as reduced mobility might increase the risk of chronic respiratory disease progression and severity.
Consistent with other recent studies, this analysis confirmed that COPD (and chronic respiratory insufficiencies) generate substantial costs for the health system in France [52]. Among the 66 publications reporting data on the healthcare resource use associated with moderate to very severe COPD, this study is among those with the longest follow-up period. In this study the economic burden of LTOT adult patients with COPD and CRF was assessed over 72 months. The yearly mean total costs per patient were estimated at between €10,000 and €13,000 in the four LTOT groups. Among the healthcare resource use considered, inpatient hospitalizations were the main cost driver, consistent with the steady increase in hospitalization rates and costs since 2000 in the area [53, 54]. In this analysis, hospitalization costs ranged between €9000 and €11,000, representing 78.8% of total HCRU costs in the LO2 group and 84.5% in aSC and CTC groups, followed by LTOT-related costs, ranging between 10% and 16% of the total HCRU costs. This distribution is consistent with an economic analysis comparing COPD in North America and Europe (France, Italy, the Netherlands, Spain, the UK), in which the majority (52–84%) of direct costs associated with COPD were due to inpatient hospitalizations [55]. In our study the POC group did not show a significant reduction in all-cause hospitalizations during the 72 months follow-up period compared to CTC and LO2. However, the length of hospital stay could be different, and it was not analyzed. Moreover, it is important to note that the number of visits for geriatric care was lower in the POC compared to LO2 group, similar to what observed when patients equipped with higher autonomy POCs were considered. Regarding the other specialties, the number of visits remained comparable between groups. In patients equipped with POCs the higher autonomy allows them to visit pneumologists and physical and rehabilitation specialists more regularly for follow-up visits, not necessarily being related to a direct effect of POC usage per se.
The current economic analysis demonstrated that POC-equipped patients, used alone or in combination, presented higher average annual costs compared to patients in the aSC group (€579, 5% higher), lower compared to the CTC group (− €502, 4% lower), and comparable costs to the LO2 group. According to the ICER, considering the standard “per life-year gained” used to evaluate high-value therapeutic interventions, at a willingness-to-pay higher than €8 895, €6288, and €13,152 per life-year gained POC would remain cost-effective compared to aSC, CTC, and LO2, respectively. Moreover, when the two subpopulations within the POC group based on the higher and lower mobility level were considered, POCs with an autonomy higher than 5 h were cost-saving (− €6238 per life-year gained) compared to LM.
In summary, POC, used alone or in combination, might contribute to promoting longer survival. On the basis of this observation and on the HCRU evaluation, the portable concentrators could be a cost-effective alternative to CTC and LO2 at comparable costs.
Strengths and Limitations
The use of the French Healthcare database SNDS, which is a large, unbiased, and potentially the most comprehensive healthcare database in Europe, is the key strength of the current analysis. The size of the population analyzed and the long follow-up are advantages derived by using this database. For the analysis of the HCRU, the costs represent actual costs accrued available in the database, representing an accurate estimate for routine clinical care for LTOT patients in both inpatient and outpatient settings in France. However, the SNDS also has intrinsic limitations due to the health data reported for health insurance reimbursement purposes. The database has limited clinical information and biological results of patients. Data collected to evaluate degree of respiratory symptoms, including dyspnea, exercise capacities (e.g., 6MWT) and severity of respiratory diseases (e.g., spirometry parameters), or parameters related to quality of life, are not available in the SNDS. As a result of this limitation the effect of LTOT on the severity of respiratory diseases could not be evaluated beyond number of hospitalizations and mortality. Moreover, there is the possibility that some patients may have been miscoded. Data on patients’ BMI are not available in the database; hence, the prevalence of obesity reported in the included population is likely an underestimation as only cases admitted to the hospital with obesity are reported. The patient’s perspective is not included in the SNDS, since data on patients’ adherence, such as how the patients use their oxygen device (e.g., duration of oxygen use hour/day), or reasons for their preference, are not available in the database.
Regarding the study design, the results should be interpreted with caution because of the main limitation of the study, the inability to dissociate treatment effect of a particular oxygen delivery device from the patient’s mobility level at the time of initial LTOT prescription, which one might be tempted to assume the clinical status of the patient from the choice of the device's autonomy. In addition, the analysis of the potential impact of the autonomy of oxygen delivery devices was limited to devices prescribed between 2013 and 2020, recognizing that devices currently on the market have evolved. in relation to cost estimation, the analysis was conducted from a third-party payer’s perspective, and therefore indirect costs such as costs associated with absence from work and reduced productivity from disease severity were not included. Also cost estimation was limited to a 6-year horizon. Additional data from clinical studies would be necessary to precisely estimate the cost-effectiveness of POC and LTOT in general beyond this time frame, and controlling for severity, number of prior hospitalizations, and other potential confounders.
Conclusion
Despite the limitations of the study, these results provide up-to-date evidence on the improved overall survival rates and cost-effectiveness associated with the use of POCs, either alone or in combination with other LTOT devices, in adult patients with CRF, informing stakeholders about healthcare costs for the different LTOT solutions. Future comparative and controlled interventional studies with adequate sample sizes are required to fully understand the value of POC use regarding clinical outcomes, mobility, and health-related quality of life.
Data Availability
The datasets generated for this study can be found in the SNDS database upon request to regulatory authorities.
Change history
17 July 2024
A Correction to this paper has been published: https://doi.org/10.1007/s41030-024-00266-y
References
Disease GIfCOL: GOLD 2023 - Global Strategy for Prevention, Diagnosis and Management of COPD: 2023 Report. 2023. https://goldcopd.org/2023-gold-report-2/
Branson RD. Oxygen therapy in COPD. Respir Care. 2018;63:734–48. https://doi.org/10.4187/respcare.06312.
Mirabile VS, Shebl E, Sankari A, Burns B. Respiratory failure. In: StatPearls. Treasure Island: StatPearls; 2023.
Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: role of comorbidities. Eur Respir J. 2006;28:1245–57. https://doi.org/10.1183/09031936.00133805.
Duck A. Cost-effectiveness and efficacy in long-term oxygen therapy. Nurs Times. 2006;102:46–50.
Oba Y. Cost-effectiveness of long-term oxygen therapy for chronic obstructive disease. Am J Manag Care. 2009;15:97–104.
Kyuchukov NPP, Glogovska P, et al. Hospital treatment costs of exacerbations in COPD patients with indications for LTOT. Eur Respir J. 2015;46(PA1053):2015.
NCT03174210. Effects of supplemental oxygen delivered by a portable oxygen concentrator compared to a liquid oxygen device in COPD. 2018.
NCT00875719: Oxygen portable concentrator during exercises in patients with COPD. 2011.
NCT04107493: Evaluation of a portable oxygen concentrator during ambulation for patients who require supplemental oxygen (POC-LEAN). 2021.
NCT04299178. Usability evaluation of a portable oxygen concentrator. 2020.
NCT01673685. Trial of portable oxygen cylinders versus battery powered portable oxygen concentrators. 2017.
Hardinge MAJ, Bourne S, et al. British Thoracic Society, BTS Home Oxygen Guideline Group: BTS guidelines for home oxygen use in adults. Thorax. 2015;70:i1–43.
Jacobs SS, Krishnan JA, Lederer DJ, et al. Home oxygen therapy for adults with chronic lung disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202:e121–41. https://doi.org/10.1164/rccm.202009-203608ST.
Dal Negro RW, Bonadiman L, Bricolo FP, Tognella S, Turco P. Cognitive dysfunction in severe chronic obstructive pulmonary disease (COPD) with or without long-term oxygen therapy (LTOT). Multidiscip Respir Med. 2015;10:17. https://doi.org/10.1186/s40248-40015-40013-40244.
Sami R, Savari MA, Mansourian M, Ghazavi R, Meamar R. Effect of long-term oxygen therapy on reducing rehospitalization of patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Pulm Ther. 2023;9:255–70. https://doi.org/10.1007/s41030-41023-00221-41033.
Eisner MD, Iribarren C, Blanc PD, et al. Development of disability in chronic obstructive pulmonary disease: beyond lung function. Thorax. 2011;66:108–14. https://doi.org/10.1136/thx.2010.137661.
Djibo DA, Goldstein J, Ford JG. Prevalence of disability among adults with chronic obstructive pulmonary disease, Behavioral Risk Factor Surveillance System 2016–2017. PLoS ONE. 2020;15:e0229404. https://doi.org/10.1371/journal.pone.0229404.
Sanchez-Morillo D, Muñoz-Zara P, Lara-Doña A, Leon-Jimenez A. Automated home oxygen delivery for patients with COPD and respiratory failure: a new approach. Sensors (Basel). 2020;20:1178.
Dakkak J, Tang W, Smith JT, et al. Burden and unmet needs with portable oxygen in patients on long-term oxygen therapy. Ann Am Thorac Soc. 2021;18:1498–505. https://doi.org/10.1513/AnnalsATS.202005-202487OC.
Moretta P, Molino A, Martucci M, et al. Subject preferences and psychological implications of portable oxygen concentrator versus compressed oxygen cylinder in chronic lung disease. Respir Care. 2021;66:33–40. https://doi.org/10.4187/respcare.07829.
Möller S, Ivarsson B, Nordström L, Johansson A. LTOT patients’ experience of a portable oxygen unit and health-related quality of life-a cross-sectional study. Healthcare (Basel). 2020. https://doi.org/10.3390/healthcare8020182.
Melani AS, Sestini P, Rottoli P. Home oxygen therapy: re-thinking the role of devices. Expert Rev Clin Pharmacol. 2018;11:279–89. https://doi.org/10.1080/17512433.17512018.11421457.
Foo J, Landis SH, Maskell J, et al. Continuing to confront COPD international patient survey: economic impact of COPD in 12 countries. PLoS ONE. 2016;11:e0152618. https://doi.org/10.1371/journal.pone.0152618.
Srivastava K, Thakur D, Sharma S, Punekar YS. Systematic review of humanistic and economic burden of symptomatic chronic obstructive pulmonary disease. Pharmacoeconomics. 2015;33:467–88. https://doi.org/10.1007/s40273-40015-40252-40274.
Wilson KC, Gould MK, Krishnan JA, et al. An official American Thoracic Society Workshop Report. A framework for addressing multimorbidity in clinical practice guidelines for pulmonary disease, critical illness, and sleep disorders. Ann Am Thorac Soc. 2016;13:S12–21. https://doi.org/10.1513/AnnalsATS.201601-201007ST.
Olaya B, Moneta MV, Doménech-Abella J, et al. Mobility difficulties, physical activity, and all-cause mortality risk in a nationally representative sample of older adults. J Gerontol A Biol Sci Med Sci. 2018;73:1272–9. https://doi.org/10.1093/gerona/glx1121.
Jacobs SS, Lederer DJ, Garvey CM, et al. Optimizing home oxygen therapy. An official American Thoracic Society Workshop Report. Ann Am Thorac Soc. 2018;15:1369–81.
SNDS. Composantes du Système National des Données de Santé. https://www.snds.gouv.fr/SNDS/Accueil. Accessed 1 Jan 2023.
Tuppin P, Rudant J, Constantinou P, et al. Value of a national administrative database to guide public decisions: from the système national d’information interrégimes de l’Assurance Maladie (SNIIRAM) to the système national des données de santé (SNDS) in France. Rev Epidemiol Sante Publique. 2017;65(Suppl 4):S149-s167.
Lartigau M, Barateau M, Rosé M, Petricã N, Salles N. Pressure ulcer prevention devices in the management of older patients at risk after hospital discharge: an SNDS study. J Wound Care. 2023. https://doi.org/10.12968/jowc.12023.12932.Sup12969a.clxxi.
CNIL: Système National des Données de Santé. https://www.cnil.fr/fr/snds-systeme-national-des-donnees-de-sante. 2021. Accessed 1 Jan 2023.
Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4:e297. https://doi.org/10.1371/journal.pmed.0040297.
Dispositifs medicaux inscrits a la liste des produits et prestations - LPP https://www.ameli.fr/val-de-marne/medecin/exercice-liberal/facturation-remuneration/consultations-actes/nomenclatures-codage/liste-produits-prestations-lpp. Accessed 1 Jan 2023.
Therapy HAdS-Aoho: Oxygénothérapie à long terme: choisir la source la mieux adaptée. 2012. Haute Autorité de Santé - Assessment of home oxygen therapy
Cartographie des pathologies et des dépenses de l’Assurance Maladie. https://assurance-maladie.ameli.fr/etudes-et-donnees/par-theme/pathologies/cartographie-assurance-maladie. Accessed 1 Jan 2023.
Gilbert T, Cordier Q, Polazzi S, et al. External validation of the Hospital Frailty Risk Score in France. Age Ageing. 2022;51:afab126.
La France et ses territoires https://www.insee.fr/fr/statistiques/5039991?sommaire=5040030. Accessed 1 Jan 2023.
Répartition de l’indice de défavorisation sociale FDep15 à l’échelle communale https://geo.data.gouv.fr/fr/datasets/4db7e21edb3c4c0aef6e2149d9988b15f7d4fdb5. Accessed 1 Jan 2023.
OMéDIT-Obeservatoire des Médicaments Dispositifs médicaux Innovations Thérapeutiques. Forfaits hebdomadaires relatifs à l’oxygénothérapie - Guide to good practice and proper use of oxygenotherapy. 2018.
Li Y, Li L. Propensity score analysis methods with balancing constraints: a Monte Carlo study. Stat Methods Med Res. 2021;30:1119–42. https://doi.org/10.1177/0962280220983512.
NICE: The guidelines manual 2012. https://www.nice.org.uk/process/pmg6/chapter/assessing-cost-effectiveness.
Facteurs d’évolution des séjours hospitaliers MCO. https://assurance-maladie.ameli.fr/etudes-et-donnees/2014-facteurs-evolution-sejours-hospitaliers-mco. Accessed 1 Jan 2023.
Divo MJ, Marin JM, Casanova C, et al. Comorbidities and mortality risk in adults younger than 50 years of age with chronic obstructive pulmonary disease. Respir Res. 2022;23:267. https://doi.org/10.1186/s12931-12022-02191-12937.
Argano C, Scichilone N, Natoli G, et al. Pattern of comorbidities and 1-year mortality in elderly patients with COPD hospitalized in internal medicine wards: data from the RePoSI Registry. Intern Emerg Med. 2021;16:389–400. https://doi.org/10.1007/s11739-11020-02412-11731.
Burgel PR, Laurendeau C, Raherison C, Fuhrman C, Roche N. An attempt at modeling COPD epidemiological trends in France. Respir Res. 2018;19:130. https://doi.org/10.1186/s12931-12018-10827-12937.
Raherison-Semjen C, Ouaalaya H, Bernady A, et al. Profils de comorbidités et sévérité de la BPCO: données de la cohorte PALOMB. Rev Mal Respir. 2017;34:A4–5. https://doi.org/10.1016/j.rmr.2016.1010.1014.
Khor YH, McDonald CF, Hazard A, et al. Portable oxygen concentrators versus oxygen cylinder during walking in interstitial lung disease: a randomized crossover trial. Respirology. 2017;22:1598–603. https://doi.org/10.1111/resp.13083.
Hun Kim S, Beom Shin Y, Shin MJ, et al. Effects of walking with a portable oxygen concentrator on muscle oxygenation while performing normal or pursed-lip breathing in patients with interstitial lung disease: a randomized crossover trial. Ther Adv Respir Dis. 2023;17:17534666231186732. https://doi.org/10.1177/17534666231186732.
Wootton SL, Dale MT, Alison JA, et al. Mobile Health pulmonary rehabilitation compared to a center-based program for cost-effectiveness and effects on exercise capacity, health status, and quality of life in people with chronic obstructive pulmonary disease: a protocol for a randomized controlled trial. Phys Ther. 2023. https://doi.org/10.1093/ptj/pzad1044.
Pimenta S, Hansen H, Demeyer H, Slevin P, Cruz J. Role of digital health in pulmonary rehabilitation and beyond: shaping the future. ERJ Open Res. 2023. https://doi.org/10.1183/23120541.23100212-23122022.
Iheanacho I, Zhang S, King D, Rizzo M, Ismaila AS. Economic burden of chronic obstructive pulmonary disease (COPD): a systematic literature review. Int J Chron Obstruct Pulmon Dis. 2020;15:439–60. https://doi.org/10.2147/copd.S234942.
Duan KI, Birger M, Au DH, Spece LJ, Feemster LC, Dieleman JL. Health care spending on respiratory diseases in the United States, 1996–2016. Am J Respir Crit Care Med. 2023;207:183–92. https://doi.org/10.1164/rccm.202202-200294OC.
Laurendeau C, Chouaid C, Roche N, Terrioux P, Gourmelen J, Detournay B. Management and costs of chronic pulmonary obstructive disease in France in 2011. Rev Mal Respir. 2015;32:682–91. https://doi.org/10.1016/j.rmr.2014.1010.1731.
Wouters EF. Economic analysis of the confronting COPD survey: an overview of results. Respir Med. 2003;97(Suppl C):S3-14. https://doi.org/10.1016/s0954-6111(1003)80020-80023.
Acknowledgements
The authors would like to thank Inogen for subsidizing this study and for providing technical support for the identification and good understanding of the nomenclature of the medical devices concerned. The authors would also like to thank Mathieu Rosé, Director RWS in Alira Health, for the contribution in the study design, the protocol development and the SNDS database access procedures. The authors appreciate the support of the unit in charge of the data extraction (Demandes D’extraction, DEMEX) at the National Health Insurance Fund CNAM.
Funding
This work and the journal’s Rapid Service Fee were supported by Inogen Inc.
Author information
Authors and Affiliations
Contributions
Stanislav Glezer and Abhijith Pg conceived the study and oversaw overall direction and planning, including the study design and protocol development. Nicoleta Petrica requested SNDS database access to regulatory authorities. Nicoleta Petrica designed the model and the computational framework, performed the statistical analysis. Maria Pini drafted the manuscript with input from all authors. Gregoire Mercier, Jean-Marc Coursier, Nicoleta Petrica, Stanislav Glezer and Abhijith Pg critically reviewed the manuscript. All authors approved the last version to be submitted; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy of integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of Interest
The study was sponsored and funded by Inogen Inc., which has neither participated in the conduct of the study nor in the analysis of the data. At the time of study conduct and manuscript submission, Dr. Stanislav Glezer was an employee at Inogen Inc. and a shareholder of Inogen Inc. He worked as the Executive Vice President, R&D and Chief Medical Officer. Dr. Abhijith Pg is an employee at Inogen Inc. and a shareholder of Inogen Inc. He works as Director Medical Affairs. Dr. Gregoire Mercier served as French medical expert. He works as the Head of Data Science at the Montpellier University Hospital and the Desbrest Institute of Epidemiology and Public Health (IDESP), Montpellier, France. Dr. Jean-Marc Coursier served as French medical expert. He works as a pneumologist at the Antony private hospital, Antony, France. Nicoleta Petrica served as consultant data scientist, Alira Health, Paris, France. Maria Pini served as medical writer, Alira Health, Paris, France.
Ethical Approval
In accordance with French regulations, the study protocol was approved by the ethics and scientific committee for health research, studies, and evaluations (CESREES) and by the French data privacy committee (CNIL; Decision DR-2021-228). Data access was delivered by CNAM after agreement. In the study personal data processing is intended for a research project not involving human subjects.
Additional information
The original online version of this article was revised: A typo in Fig. 1 and also in the Abstract Results section have been corrected.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Glezer, S., Mercier, G., Coursier, JM. et al. Health and Economic Impact of Different Long-Term Oxygen Therapeutic Strategies in Patients with Chronic Respiratory Failure: A French Nationwide Health Claims Database (SNDS) Study. Pulm Ther 10, 237–262 (2024). https://doi.org/10.1007/s41030-024-00259-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41030-024-00259-x