Abstract
Introduction
Reusable nebulizer–compressor combinations deliver inhaled medications for patients with chronic lung diseases. On hospital discharge, the patient may take home the disposable nebulizer that was packaged and combine it with their home compressor. Though this practice may reduce waste, it can increase variability in medication delivery. Our study compared several reusable and disposable nebulizers packaged with compressor kits used in the US. We included a common disposable hospital nebulizer that may not be supplied with popular home kits but may be brought home after a hospitalization or emergency department visit. We focused on fine droplet mass < 4.7 μm aerodynamic diameter (FDM<4.7 μm), associated with medication delivery to the airways of the lungs.
Methods
We evaluated the following nebulizer–compressor combinations (n = 5 replicates):
-
1.
OMBRA® Table Top Compressor with MC 300® reusable and Airlife™ MistyMax™ 10® disposable nebulizer,
-
2.
Sami-the-Seal® compressor with SideStream® reusable and disposable nebulizers and Airlife™ MistyMax 10™ disposable nebulizer,
-
3.
VIOS® compressor with LC Sprint® reusable, and VixOne® and Airlife™ MistyMax™ disposable nebulizers,
-
4.
Innospire® Elegance® compressor with SideStream® reusable and disposable nebulizers and Airlife™ MistyMax 10™ disposable nebulizer,
-
5.
Willis-the-Whale® compressor with SideStream® reusable and disposable nebulizers and Airlife™ MistyMax 10™ disposable nebulizer,
-
6.
Pari PRONEB® Max compressor with LC Sprint® reusable and Airlife™ MistyMax 10™ disposable nebulizer.
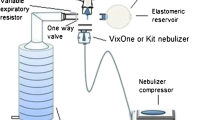
We placed a 3-ml albuterol solution (0.833 mg/ml) in each nebulizer. A bacterial/viral filter was attached to the nebulizer mouthpiece to capture emitted medication, with the filter exit coupled to a simulator of a tidal breathing adult (rate = 10 cycles/min; Vt = 600 ml; I/E ratio = 1:2). The filter was replaced at 1-min intervals until onset of sputter. Droplet size distributions (n = 5 replicates/system) were determined in parallel by laser diffractometry.
Results
Cumulative FDM<4.7 μm varied from 381 ± 33 μg for the best performing combination (Proneb/LC-Sprint) to 150 ± 21 μg for the system with the lowest output (VIOS®/MistyMax 10™).
Conclusions
Substituting one nebulizer for another can result in large differences in medication delivery to the lungs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
The study was undertaken to highlight the ongoing issue of large medication delivery variability with pneumatic (jet) nebulizer–compressor groupings with a selection of combinations prescribed in the US healthcare environment. |
What was learned from the study? |
The measurements confirmed that large variability between some reusable and disposable nebulizer–compressor combinations persists. |
Durable medical equipment (DME) suppliers in the US currently treat nebulizer–compressor kits as substitutable or interchangeable devices. The wide variability in medication delivery found between compressors and nebulizers when used in different combinations may impact disease management. |
Introduction
Although seldom used in a hospital environment, nebulizer–compressor combinations are widely prescribed in the US for home use to deliver inhaled medications to patients with chronic lung conditions such as cystic fibrosis (CF) [1], chronic obstructive pulmonary disease (COPD) [2], bronchiectasis or asthma [3] post hospitalization for preventative therapy following an exacerbation. Initially, these components are often supplied together, but there are numerous points in the treatment period where “mixing and matching” may occur. However, in cases where replacements are supplied separately, it is recognized that practitioners and patients may substitute either the compressor or the nebulizer while at home with a similar component from a different brand [4]. Even though a compressor may be intended by the manufacturer for use with a more robust reusable nebulizer, in the hospital environment a lower-cost disposable device may be substituted and given to the patient to take home after therapy to avoid discarding the nebulizer on departure and therefore reducing hospital waste [5]. Finally, inadvertent nebulizer substitution may arise in cases where a disposable version of the same nebulizer brand is co-packaged with DME suppliers. A recently published survey sponsored by the United States Cystic Fibrosis Foundation has identified that such replacements can result in inferior clinical performance and may alter the characteristics of the released aerosol, which can result in a reduction of medication deposition into the lungs for the treatment of pulmonary symptoms, potentially impairing airway clearance [5].
Beyond considerations for medication delivery to patients with cystic fibrosis, other studies have highlighted large variability in medication delivery from different nebulizer–compressor combinations [6, 7]. However, many of these investigations were undertaken more than 15 years ago. There is therefore a need to provide clinicians involved with the treatment of exacerbations associated with chronic lung diseases up-to-date information reflective of the newer designs of nebulizers and compressors currently approved for use in the US. The purpose of our study was therefore to quantify, in association with other key operating variables, the variability of fine droplet mass fraction < 4.7 μm in aerodynamic diameter (FDM<4.7 μm) as our primary in vitro performance measure. Airborne droplets or particles finer than approximately 5 μm aerodynamic diameter are associated with effectiveness of medication delivery for targeting receptors in the airways [8] for drug substances treating several lung conditions, in particular COPD, where exacerbations of symptoms are common [2].
Methods
Ethical Approval
Ethical approval was not necessary as the work presented was undertaken without the use of human or animal subjects.
Methodology
Several jet nebulizer–compressor systems were evaluated as packaged, and therefore in the configuration that they would be received at purchase. Additionally, a common hospital style standard jet nebulizer (MistyMax 10) was tested with each of the configurations available for purchase as a baseline to demonstrate the variability of medication delivery when used with the pre-packaged configurations.
Measurements of medication delivery were made (n = 5 devices; one determination per device) in the ambient temperature and relative humidity ranges of 19–23 °C and 33–41% RH, respectively. Each nebulizer-on-test delivered a 3-ml fill of aqueous albuterol sulfate inhalation solution (0.833 mg/ml; Ventolin®, GSK Inc., Mississauga, Canada) as a representative solution medication from the nebulizer mouthpiece as a droplet aerosol to a 47-mm diameter bacterial-viral filter. The filter was coupled to a breathing simulator (ASL 5000, Ingmar Medical, Pittsburgh, PA, USA), mimicking an adult breathing pattern (tidal volume (Vt) = 600 ml; inspiratory/expiratory (I:E) ratio 1:2; respiratory rate = 10 cycles/min). The nebulizer–compressor combinations investigated are listed in Table 1 and the common measurement setup used is illustrated schematically in Fig. 1.
Each performance test was conducted in accordance with a common protocol in which nebulization was temporarily interrupted to permit replacement of a collection filter at 1-min intervals during the duration of each test to ensure filter saturation was avoided, with bias arising from the consequent potential loss of medication [7]. Each interruption period was kept as short as possible and typically close to 1 s in duration to minimize potential bias caused by warming of the solution contained in the reservoir of the nebulizer-on-test.
The compressed gas supply conditions for each nebulizer–compressor system-on-test were quantified in terms of compressor pressure (P) and air flow rate (Q), both measured proximally to the nebulizer. Compressor pressure was established using a calibrated digital pressure gauge (0 to 100 psig; Cole-Parmer, Canada) and flow rate was determined by a thermal mass flowmeter (model 4040, TSI Inc, St. Paul MN, USA). The total mass of albuterol (TM) recovered was determined by an HPLC–UV spectrophotometric method from each filter assigned to each 1-min interval from the start of nebulization to the audible onset of sputter (tsputter). The small amount of albuterol that may have been released during the beginning of each exhalation portion of each breathing cycle was not captured. To do so by inserting a filter or alternative collection device in the exhalation pathway would have increased the resistance of the exhalation flow, potentially risking bias in medication output, especially for the air entrainment nebulizers that were evaluated. The treatment duration was determined as the sum of the nebulizer operating time from the start until first sputter was detected (tsputter). Droplet size distributions (n = 5 replicates/system) were determined by laser diffractometry (Malvern Spraytec®, Malvern Panalytical, Malvern, UK). The open bench method was used, choosing a 30-s duration for measurement once stability had been acquired, typically within a few seconds after initiation of nebulization. Preliminary testing (data not shown) was undertaken with each nebulizer–compressor combination to ensure that the reported LD measurements were representative of the stable nebulization period. The final LD measurements yielded time-averaged values of the volume (mass) median diameter (Dv,50) and the size distribution spread (Span), calculated as the difference in droplet sizes corresponding to the 90th and 10th volume (mass) percentiles of the distribution, normalized by Dv,50. The mass of albuterol, delivered as fine droplet mass (FDM<4.7 μm), was determined as the product of TM and fine droplet fraction (FDF<4.7 μm) derived from the LD measurements, assuming the droplets to be spherical (dynamic shape factor of unity) and their density to be 1.0 × 103 kg/m3 (density of water), irrespective of their size. The fine droplet delivery rate (FDM<4.7 μm rate) was calculated as (FDM<4.7 μm/tsputter). The residual mass (Rm) was determined by HPLC–UV spectrophotometric assay of the albuterol content of the solution in the nebulizer reservoir at completion of nebulization (the albuterol content at the start was taken as the label claim value).
Results
The performance measures for the nebulizer–compressor combinations are summarized in Table 2. The key comparisons that we identified, together with their significance value from two-sided t tests with p ≤ 0.5 as defining significantly different outcomes, are summarized in Table 3.
DV,50 (μm) derived from the LD measurements varied from 1.79 ± 0.04 μm for the SideStream reusable/Willis-the-Whale compressor combination to a maximum of 5.6 ± 0.4 μm for the MistyMax 10/Innospire Elegance system. In general, DV,50 (μm) from combinations including the MistyMax 10 nebulizer were in the range between 4.9 and 5.6 μm with values in the range 2.0 ± 0.3 μm for the other systems evaluated. Mean values of Span clustered between 1.8 and 2.2 with no obvious trend apparent between the different combinations evaluated. For this reason, the remainder of the assessment focused on the other metrics that were more discriminating of in vitro medication delivery performance.
-
1.
Compressor operating pressures were assessed for each system, as this measure is related to the force available for droplet formation from a pneumatic nebulizer [9]. Pressures ranged from 20.9 ± 0.3 psig for the LC Sprint (reusable)/Proneb combination to 11.0 ± 0.31 psig for the VixOne/VIOS system (Fig. 2). The LC Sprint/VIOS combination operated at the second highest pressure (18.1 ± 0.4 psig). The operating pressure for the manufacturer-recommended MC 300 reusable/OMBRA combination was also in the highest group at 16.6 ± 0.3 psig. The pressure was reduced slightly to 15.2 ± 0.4 psig [two-sided unpaired t test, p = 0.003] if the MistyMax 10 disposable nebulizer was substituted. The operating pressures obtained with SideStream reusable (12.6 ± 0.4 psig), and disposable (12.7 ± 0.9 psig) nebulizers were comparable with the Sami-the-Seal compressor [p = 0.28]. Similar behavior to these nebulizers was observed with the Willis-the-Whale compressor (12.8 ± 0.9 psig reusable; 12.9 ± 0.7 psig disposable [p = 0.63]), and the Innospire Elegance compressor (12.2 ± 0.2 psig reusable; 11.9 ± 0.4 psig [p = 0.87]). In each case, replacing with the MistyMax 10 disposable nebulizer increased the operating pressure only marginally to 15.5 ± 0.5 psig (Sami-the-Seal compressor), 14.4 ± 1.7 psig (Willis-the-Whale compressor) and 14.2 ± 0.2 psig (Innospire Elegance compressor) [one-way ANOVA, p ≤ 0.001).
-
2.
Rank-ordered values of total mass of albuterol delivered from start to onset-of-sputter (TM) are summarized in Fig. 3 as an indication of overall output from each combination irrespective of droplet size. TM was significantly greater for the two compressor combinations tested with the LC Sprint nebulizer (VIOS: 660 ± 54 μg; Proneb: 654 ± 57 μg) compared with the other combinations (one-way ANOVA, p < 0.001). TM for the MC 300/OMBRA pairing was 376 ± 37 μg and comparable at 349 ± 27 μg (paired t test, p = 0.35) when the MistyMax 10 nebulizer was substituted. Values of TM for the two SideStream options were also comparable for each of the compressors evaluated (Willis-the-Whale: 319 ± 14 μg (reusable); 315 ± 20 μg (disposable) [p = 0.73]; Sami-the-Seal (299 ± 8 μg (reusable); 291 ± 9 μg (disposable) [p = 0.18]; Innospire Elegance (319 ± 12 μg; 305 ± 22 μg (disposable) [p = 0.27]). Corresponding values for the two disposable nebulizers (VixOne and MistyMax 10) tested with the VIOS compressor were also similar (MistyMax 10: 353 ± 60 μg; VixOne: 320 ± 19 μg [p = 0.29]).
-
3.
Rank-ordered values of residual mass of albuterol in the nebulizer–compressor combinations recovered after the onset of sputtering (Rm), representing wasted medication, are summarized in Fig. 4. Rm ranged from 913 ± 109 μg for the MC 300/OMBRA combination to as much as 1311 ± 158 μg for the MistyMax 10/Willis-the-Whale combination, with most values clustered in the range of 1050–1150 μg. Compressor combinations involving the MistyMax 10 nebulizer had mean Rm values varying between 1143 and 1311 μg. Rm values for the reusable and disposable versions of the SideStream nebulizer were comparable and mid-range for the three different compressors with which these devices were evaluated (Innospire Elegance: 1076.4 ± 41.7 μg – reusable; 1054.8 ± 80.9 μg – disposable [p = 0.63]; Willis-the-Whale: 1090.5 ± 40.6 μg – reusable; 1092.5 ± 45.4 μg – disposable [p = 0.94]; Sami-the Seal: 1095.5 ± 51.9 μg – reusable; 1136.8 ± 33.2 μg – disposable [p = 0.17]).
-
4.
Rank-ordered values of fine droplet mass fraction of albuterol < 4.7 μm aerodynamic diameter (FDF<4.7 μm), representing medication potentially capable of reaching the airways of the lungs, and obtained by laser diffractometry, are summarized in Fig. 5 as a measure of likely deposition efficiency distal to the oropharyngeal region [9]. Values of FDF<4.7 μm ranged from as low as 41.6 ± 2.9% for the MistyMax 10/Innospire Elegance combination to 68.9 ± 1.6% for the MC 300/OMBRA pairing. Importantly, all the combinations in which the MistyMax 10 nebulizer was paired had values of FDF<4.7 μm < 47.6 ± 3.1%, achieved with the Proneb compressor that operated at the highest pressure. The performance of the VixOne/VIOS compressor was slightly better with the MistyMax 10/Proneb combination, with FPF<4.7 μm at 49.7 ± 2.3% compared with 42.5 ± 4.0% for the VixOne nebulizer [p = 0.007]. As before, the SideStream reusable and disposable options performed similarly in terms of this measure with each of the different compressors with which they were evaluated (Willis-the-Whale: reusable 67.5 ± 4.2%, disposable 67.1 ± 1.2% [p = 0.87]; Sami-the-Seal: reusable 66.5 ± 2.7%, disposable 65.5 ± 1.4% [p = 0.49]; Innospire Elegance: 67.3 ± 2.5%, disposable 66.2 ± 2.2% [p = 0.45]).
-
5.
Rank-ordered values of fine droplet mass of albuterol < 4.7 μm aerodynamic diameter also delivered from start to onset-of-sputter (FDM<4.7 μm) are presented in Fig. 6 as the primary measure of medication delivery to the airways of the lungs, sometimes referred to as ‘respirable mass’ for oral inhaled aerosol delivery [10]. The five combinations delivering the greatest values of FDM<4.7 μm, each pairing a reusable nebulizer with a compressor, were as follows: LC-Sprint/Proneb: 381 ± 33 μg; LC-Sprint/VIOS: 353 ± 29 μg; MC 300/OMBRA: 259 ± 25 μg; SideStream reusable/Willis-the-Whale: 215 ± 9 μg; SideStream reusable/Innospire Elegance: 215 ± 8 μg. In contrast, the highest value of FDM<4.7 μm with any MistyMax 10 disposable nebulizer–compressor combination occurred when it was paired with the Proneb compressor (178 ± 17 μg). However, this value was significantly smaller than 381 ± 33 μg obtained with the manufacturer-intended LC-Sprint pairing [p = 0.003]. Likewise, FDM<4.7 μm for the reusable MC300 nebulizer (259 ± 25 μg) was significantly greater than the corresponding value when the MistyMax 10 disposable nebulizer was substituted (158 ± 12 μg) [p < 0.001]. However, the same measure with the disposable VixOne nebulizer/VIOS compressor combination (160 ± 9 μg) was comparable with values of this measure obtained with the various MistyMax 10 nebulizer–compressor combinations [p > 0.05].
-
6.
Rank-ordered values of time to sputter (tsputter), as an indicator of treatment time per session, are presented in Fig. 7. The shortest times were associated with the two nebulizers evaluated with the Proneb compressor (LC Sprint: 314 ± 33 s; MistyMax 10: 336 ± 48 s). Recall that this compressor operated at the highest pressure of the group evaluated (Fig. 2). The two SideStream variants had similar values of tsputter for two of the three different compressors with which they were paired (Sami-the-Seal: 340 ± 35 s reusable [p = 0.40]; 359 ± 32 s; disposable: Willis-the-Whale: 358 ± 55 s reusable [p = 0.99]; 358 ± 54 s disposable). However, tsputter for the reusable version was 359 ± 34 s, but very slightly longer at 398 ± 16 s for the disposable version when paired with the Innospire Elegance compressor [p = 0.04]. Apart from the relatively short tsputter times with the Proneb compressor just described, combinations paired with the MistyMax 10 nebulizer in general were insignificantly different (i.e., SideStream reusable/Sami-the-Seal: 340 ± 35 s; MistyMax 10/Sami-the-Seal: 392 ± 38 [p = 0.54]) or had slightly longer times. However, the longest value of tsputter was associated with the VIOS/VixOne combination at 527 ± 85 s.
Figures 8 and 9 depict correlations between the nebulizer–compressor combination on test and TM and FDM<4.7 μm, respectively. Although there was a trend towards larger values of either measure with increasing compressor pressure, the case for claiming linearity between these variables was weak, with correlation coefficients (r2) being 0.731 and 0.486 for TM and FDM<4.7 μm, respectively. Removing the two largest values of TM and FDM<4.7 μm reduced the slope of the correlation (data not shown) but barely increased r2 in either case.
Discussion
Clinicians continue to choose small-volume jet nebulizers for both hospital and domiciliary use [2, 11, 12] for a variety of reasons, despite treatment times being lengthy compared with alternative therapeutic modes. The rationales include the avoidance of patient adherence to achieve an optimal inhalation profile needed to use pressurized metered-dose inhalers without a valved holding chamber and with dry powder inhalers [13]. They also include delivery of drug products only available for inhalation via the nebulizer route, and convenience. A further driver in favor of nebulization for COPD therapy is patient limitation associated with poor inhaler technique associated with the more challenging operation of the other dosage forms with patients who are generally elderly [14]. Another important aspect, particularly pertinent for CF patients, is the non-availability of important medications (e.g., antibiotics) for delivery by the other inhaled medication delivery platforms [15]. Nevertheless, the variability in medication delivery performance from the variety of marketed nebulizer–compressor systems in North America has not improved, despite the issue being highlighted several years ago [6, 16]. Much of this variability arises from the variable pressure characteristics of home-based compressors for use with this class of inhaler [7]. Wide performance variability has also been reported by Alvine et al. as coming from poor quality control, especially with disposable nebulizers [17], although that study was undertaken more than 30 years ago and may therefore not reflect current manufacturing practices.
Given this situation, the selection of nebulizer–compressor systems to evaluate was carefully made to include examples comparing, where possible, reusable with disposable devices of the same nebulizer brand. Our intention was to find out if significant variability still existed between the two types of nebulizers, as well as to draw attention to the potential for adverse impacts on medication delivery performance arising from the difference between the device combinations that prescribing clinicians intend their patients to receive and what they might be given by their DME supplier. We also intentionally focused our attention on the unique healthcare management system in US hospitals associated with outpatient aftercare in the domiciliary environment.
We chose only a single reference breathing pattern having Vt of 600 ml to represent an average 100 kg individual, based on normal physiologic Vt for adults is approximately 6 ml/kg body weight [18]. Our study clearly showed that in several, but not all instances, large variability existed in several key metrics between the 16 nebulizer–compressor combinations evaluated (Table 2). In addition, we found that the albuterol delivery rate, expressed either in terms of TM or FDM<4.7 μm, was loosely correlated with compressor pressure in a linear relationship (Figs. 8 and 9, respectively). In general, therefore, this more general outcome supports the observation of Nerbrink and Dahlbäck [19] that the air pressure applied to a jet nebulizer is a good indicator of the energy available for droplet formation. However, clinicians need to be aware that merely measuring this pressure by itself as a diagnostic when switching from a manufacturer-recommended nebulizer for in-hospital use to an alternative, for example when discharging a patient home, may not reveal a poor performing nebulizer with a particular compressor.
From the standpoint of medication delivery efficiency, our findings for the values of Rm are of particular interest, as this measure potentially represents wasted medication. However, before discussing the outcomes in relation to this measure, we highlight the limitation that these differences were partly obscured by the large variability with some measurements that was associated with the capability of the operator to define audibly the onset of sputter from one nebulizer to another. Nevertheless, we found that mean values of Rm varied by as much as 43% across all the combinations evaluated from the smallest mass retained (913 μg) that was recovered from the MC 300/OMBRA system to 1311 μg for the MistyMax/Willis-the-Whale combination. In contrast, Rm for the SideStream reusable and disposable options were comparable with whichever of the three compressors that were paired with these devices (Innospire Elegance, Willis-the-Whale, Sami-the Seal). Yet, values of Rm for the same compressors in which the MistyMax 10 disposable nebulizer was substituted for either SideStream option, were increased by between 10 and 20%. We also observed a 20% increase in Rm with the Proneb Max compressor, changing from the recommended reusable LC Sprint to the MistyMax 10 device. Likewise, switching the recommended reusable MC 300 to the MistyMax 10 nebulizer was associated with a 25% increase in Rm in association with the OMBRA compressor. However, countering the trend previously described, we found that Rm for the VIOS compressor with the disposable VixOne nebulizer was 6% lower than with the LC-Sprint reusable device. This outcome implies that, for this compressor at least, a firm ruling that just because a nebulizer is disposable may not be predictive by itself of the magnitude of retained medication.
Though less important with a low-cost medication such as albuterol, Rm would likely be a factor to consider when choosing the most appropriate nebulizer–compressor combination for more expensive medications such as, for example, those prescribed for airway clearance in CF both in the US [4] and in Europe [20]. Expanding on this consideration, clinical practice guidelines developed by the US Cystic Fibrosis Foundation recommend the use of several inhaled medications, in particular mucolytics and airway hydrators for mucus thinning as well as antibiotics for eradication and long-term suppression of bacteria in the airway to manage the disease effectively [21, 22]. These inhaled medications are typically delivered in the home by nebulizer–compressor combinations, and when coupled with other airway clearance techniques [23], are the mainstay in slowing the progression of lung function deterioration [4]. Given their very high unit therapy cost, the importance of Rm could become more acute should inhaled biologic medications become available for treating chronic lung conditions by the jet nebulizer–compressor route.
We found that mean values of tsputter were all within the 300 to 420 s (5-to-7-min) range except for the VixOne/VIOS combination at 527 s. This outcome is consistent with an observation that treatment times per therapy for almost all the nebulizer–compressor systems would likely be comparable, regardless of the system chosen. However, the uncertainty in these measurements was relatively high, largely because of the difficulty in precisely defining the onset of sputter from one nebulizer to another, a limitation already mentioned in connection with the measurements of Rm.
Looking at the measures of medication delivery, we observed that higher output from the premium LC Sprint (reusable) nebulizer defined by measures of both TM (Fig. 3) and FDM<4.7 μm (Fig. 6) with both the associated manufacturer-recommended Proneb Max compressor, which had the highest operating pressure (Fig. 2) and with the VIOS compressor, which operated at the second highest pressure. This outcome is in broad agreement with the results from a laboratory-based comparison of 30 jet nebulizer–compressor combinations undertaken several years ago by Berg and Picard [24] for the delivery of budesonide in aqueous suspension. However, the compressors that this group evaluated with the LC Sprint were previous models (Pari Boy, Pari Boy S, Pari Master and Pari Turbo Boy S) to the currently available Proneb Max compressor, and Berg and Picard based their comparisons on pediatric tidal breathing patterns rather than that for an adult, as was chosen as the reference breathing pattern for the present investigation.
The most important in vitro measure of likely nebulizer performance in clinical use is the respirable mass (dose) of medication (FDM<4.7 μm) delivered per treatment [25]. This value is indicative of the amount of medication that has the potential to deposit in the airways of the lungs and therefore provide therapeutic benefit, neglecting medication that may be lost through exhalation before deposition [26]. Our purpose was to examine variations in readily available laboratory measures of medication delivery between nebulizer–compressor combinations encountered currently in the US hospital and domiciliary environments using performance attributes defined in an international standard for nebulizing systems [27] and chapter < 1601 > of the US pharmacopeia [28]. We therefore treated changes to deposition location caused by condensation-driven growth during passage through the respiratory tract where the relative humidity is close to saturation at body temperature [29] as being beyond scope. Importantly, as found by other investigators [16, 25], we observed that measures of performance based on TM in the present study were not predictive of FDM<4.7 μm. The underlying driver was that FPF<4.7 μm, obtained from the laser diffraction measurements, and used to calculate values of FDM<4.7 μm, varied from about 42% for the MistyMax 10 nebulizer with Innospire Elegance and VIOS compressors to as much as 69% for the MC 300 nebulizer/OMBRA compressor.
Our findings concerning the large variation in FDM<4.7 μm from one nebulizer–compressor combination to another largely mirrored those of previously published studies [6, 7], suggesting that the variability associated with these systems has not improved materially. Our objective was to address concerns raised by Lester et al. [5], in their recent comprehensive survey of accessibility of nebulizer–compressor combinations for CF patients. They highlighted the fact that prescriptions for specific nebulizer–compressor combinations are frequently treated as generic by DME companies. It follows that substitution of either the compressor or nebulizer that may take place at the point of purchase could potentially affect the ability for the patient to fully manage their disease process or pulmonary symptoms. Furthermore, some compressors are packaged with two different styles of nebulizer (typically a more expensive reusable device that may have a life span up to 6 months and a disposable nebulizer with a significantly shorter life span). In this context, notably, we found that measures of FDM<4.7 μm for the SideStream reusable and disposable nebulizer options were comparable with the three different home-based compressors that we evaluated with these devices. However, we found that such concordance in values of FDM<4.7 μm between reusable and disposable nebulizers was not the norm, as exemplified by the trend towards lower values of FDM<4.7 μm that we found for each combination in which the disposable MistyMax 10 or VixOne nebulizer was substituted for the manufacturer-recommended reusable nebulizer for a given compressor. It follows that without careful control of whether the nebulizer supplied for home use following hospital discharge is reusable, there could be a risk that patients may be receiving substantially inferior medication delivery as therapeutically beneficial fine droplets. Further, as non-clinicians, DME suppliers will unlikely be aware of the performance variation and life cycle of different nebulizers packaged with these compressors. In this context, we also note also that Lester et al. [5] suggested that a possible driving cause for the substitution of either nebulizer or compressor unit may be low reimbursement for DME companies by third-party payors and/or limited availability of the prescribed nebulizer and/or compressor to these companies from distributors of these devices. However, regardless of the underlying reason(s), without firm clinical evidence for doing so, we maintain that substitution of nebulizers with a given compressor, or vice versa has the potential to increase the risk to the patient of long-term consequences for disease management and symptom control. Specifically in the CF population, non-adherence has been reported by Eakin et al., as being enhanced by the lack of efficient devices and/or the use of inefficient/deteriorating devices [30]. A similar situation may also exist for patients on perceived ineffective nebulizer-portable compressor therapy for symptom control in chronic obstructive pulmonary disease [31]. However, to the best of our knowledge, a similar study to that undertaken by Eakin et al. [30] for this much larger group of patients has not yet been undertaken.
Our laboratory-based investigation was limited in scope to nebulizer–compressor combinations based on an internal and unpublished survey of current US hospital use and did not include a clinical component. We recognize that further work is needed both to quantify the extent to which nebulizer and/or compressor substitutions are made and to determine the clinical impact of such substitutions, focusing on inhaled therapeutics in widespread use for the home maintenance treatment of chronic pulmonary conditions, such as CF, COPD, or bronchiectasis. A further limitation was the use of the open rather than closed bench approach for the LD measurements. However, most nebulizers we evaluated had an air-entrainment capability that protected the droplet cloud from evaporation into the lower relative humidity of the ambient environment [32]. We therefore propose that future studies are merited to investigate the importance of using a closed cell for droplet transport through the laser diffractometer.
Conclusions
Our laboratory-based investigation has provided further evidence of the large variability in key performance measures for a selection of 16 common nebulizer–compressor combinations currently available in the US. We focused on pairing the manufacturer-recommended reusable and at least one disposable nebulizer with each of the home-based compressors to evaluate the likelihood of a significant drop in fine droplet medication delivery by substituting the disposable for the reusable version. We found that such a change had little impact on performance for the two SideStream variants evaluated with three different compressors with which they are likely to be paired. However, we observed that substitution with either the VixOne or MistyMax disposable devices with any of the six compressors resulted in significant degradation of fine droplet delivery.
Although our investigation was of necessity limited in scope, given the large number of potential nebulizer–compressor combinations available worldwide, it lends support to the observation of Lester et al. [5] that the quality of aerosol therapy could be compromised if the prescribed nebulizer–compressor combination is substituted for generic alternatives to either the nebulizer or compressor. Further work perhaps involving in vitro or in silico models of the respiratory tract is needed to evaluate how the significant differences in the measures, FDM<4.7 μm and secondary measure, Rm, might affect the efficiency of medication delivery to the airways. Finally, we suggest that the outcome from the present work may also be applicable for other chronic airflow-restrictive lung diseases beyond CF, in particular COPD, which are often treated at home with one or more medications delivered by nebulizer–compressor.
Data Availability
The data are not stored in a repository as this work was not a clinical trial. The datasets generated during and/or analyzed during the study are available from Trudell Medical International (TMI), 725 Baransway, London, Ontario, N5V 5G4, Canada on reasonable request.
References
Collins N. Nebulizer therapy in cystic fibrosis: an overview. J R Soc Med. 2009;102:S11–7.
Dhand R, Dolovich M, Chipps B, Myers TR, Restrepo R, Farrar JR. The role of nebulized therapy in the management of COPD: evidence and recommendations. COPD: J Chron Obstr Dis. 2011;9(1):58–72.
Sockrider M. Nebulizer breathing treatments at home. American Thoracic Society Patient Information Series. 2020. Available at: https://www.thoracic.org/patients/patient-resources/resources/nebulizer-breathing-treatments-home.pdf, visited March 21, 2023.
Awad SM, Berlinski A. Crossover evaluation of compressors and nebulizers typically used by cystic fibrosis patients. Respir Care. 2018;63(3):294–300.
Lester M, Eidson D, Blair S, et al. Cystic Fibrosis Foundation nebulizer and compressor survey. Respir Care. 2021;66(12):1840–7.
Smith EC, Denyer J, Kendrick AH. Comparison of twenty-three nebulizer/compressor combinations for domiciliary use. Eur Respir J. 1995;8:1214–21.
Reisner C, Katial RK, Bartelson BB, Buchmeir A, Rosenwasser LJ, Nelson HS. Characterization of aerosol output from various nebulizer/compressor combinations. Ann Allergy Asthma Immunol. 2002;86(5):566–74.
Heyder J, Bebhard J, Rudolf G, Schiller CF, Stahlhofen W. Deposition of particles in the human respiratory tract in the size range 0.005–15 um. J Aerosol Sci. 1986;17:811–25.
Finlay WH, Stapleton WH, Zuberbuhler P. Fine particle fraction as a measure of mass depositing in the lung during inhalation of nearly isotonic nebulized aerosols. J Aerosol Sci. 1997;28(7):1301–9.
Everard ML, Devadason SG, Summers QA, Le Souef PN. Factors affecting total and “respirable” dose delivered by a salbutamol metered dose inhaler. Thorax. 1995;50(7):746–9.
Tashkin DP. A review of nebulized drug delivery in COPD. Int J COPD. 2016;11:2585–96.
Ari A, Restrepo RD. Aerosol delivery device selection for spontaneously breathing patients: 2012. Respir Care. 2012;57(4):613–26.
Laube BL, Janssens HM, de Jongh FHC, et al. Chrystyn H. ERS/ISAM Task Force Report: What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. 2011;37:1308–31.
Usmani OS, Lavorini F, Marshall J, et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res. 2018;19: Article 10.
Agent P, Parrott H. Inhaled therapy in cystic fibrosis: agents, devices and regimens. Breathe. 2015;11(2):111–8.
Loffert D, Ikle D, Nelson H. A comparison of commercial jet nebulizers. Chest. 1994;106(6):1788–92.
Alvine GF, Rodgers P, Fitzsimmons KM, Ahrens RC. Disposable jet nebulizers. How reliable are they? Chest. 1992;101(2):316–9.
Tenney S, Remmers J. Comparative quantitative morphology of the mammalian lung: diffusing area. Nature. 1963;197:54–6.
Nerbrink O, Dahlbäck M. Basic nebulizer function. J Aerosol Med. 1994;7(Suppl 1):S7-11.
Eidt-Koch D, Wagner TOF, Mittendorf F, von der Schulenberg J-M. Outpatient medication costs of patients with cystic fibrosis in Germany. Appl Health Econ Health Policy. 2010;8(2):111–8.
Flume PA, O’sullivan BP, Robinson KA, et al. Cystic fibrosis foundation, pulmonary therapies committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176(10):957–69.
Mogayzel PJ Jr, Naureckas ET, Robinson KA, et al. Pulmonary Clinical Practice Guidelines Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2013;187(7):680–9.
Pryor JA, Tannenbaum E, Scott SF. Beyond postural drainage and percussion: airway clearance in people with cystic fibrosis. J Cystic Fibrosis. 2010;9:187–92.
Berg EB, Picard RJ. In vitro delivery of budesonide from 30 jet nebulizer/compressor combinations using infant and child breathing patterns. Respir Care. 2009;54(12):1671–8.
Hess DR. Nebulizers: principles and performance. Respir Care. 2000;45(6):609–22.
Labiris NR, Dolovich MB. Pulmonary drug delivery. Part II: The role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):600–12.
International Standards Organization: ISO/CD 27427. Anaesthetic and respiratory equipment—Nebulizing systems and components. International Standards Organization (ISO), Geneva, Switzerland. Available at: https://www.iso.org/standard/78542.html. Accessed Oct 3, 2023.
United States Pharmacopeial Convention. Chapter <1601>: Products for nebulization – Characterization tests. United States Pharmacopeia, Rockville, MD, USA. Available at: https://www.uspnf.com/. Accessed Oct 3, 2023.
Grasmeijer N, Frijlink HW, Hinrichs WLU. An adaptable model for growth and/or shrinkage of droplets in the respiratory tract during inhalation of aqueous particles. J Aerosol Sci. 2016;93:21–34.
Eakin MN, Bilderback MS, Boyle MP, Mogayzel PJ, Riekert KA. Longitudinal association between medication adherence and lung health in people with cystic fibrosis. J Cyst Fibros. 2011;10(4):258–64.
Sims MW. Aerosol therapy for obstructive lung diseases: device selection and practice management issues. Chest. 2011;140(3):781–8.
Dennis JH, Pieron CA, Pagels J, Smurthwaite M, Nerbrink O. Development and application of a low flow cascade impactor to size nebulized aerosols. J Aerosol Med. 1999;1999(12):134.
Acknowledgements
The authors wish to acknowledge Cathy Doyle and Rubina Ali, both of Trudell Medical International for making the measurements.
Funding
Financial support was provided by the study sponsor, Monaghan Medical Corporation (MMC Corp), 153 Industrial Blvd., Plattsburgh, NY 12901, USA. The study sponsor is also funding the journal’s Rapid Service Fee.
Author information
Authors and Affiliations
Contributions
All authors (Jolyon Mitchell, Jason Suggett, Judy Schloss, Dominic Coppolo and Mark Nagel) contributed to the study conception and design. Data collection and analysis were performed by Mark Nagel and Jolyon Mitchell. The first draft of the manuscript was written by Jolyon Mitchell in his role as consultant to MMC Corp, with the assistance of Judy Schloss. All authors provided feedback on subsequent versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Dominic Coppolo and Judy Schloss are employees of Monaghan Medical Corporation (MMC) who market the MC 300 nebulizer/OMBRA compressor in the US; Jason Suggett and Mark Nagel are employees of Trudell Medical International, manufacturer of both devices and distributor worldwide, except for the US and Mexico; Jolyon Mitchell is a private consultant who has received funding from TMI and MMC.
Ethical Approval
Ethical committee approval was not sought because this is a laboratory-based study and does not contain any experimental work with animals or human participants performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Schloss, J., Coppolo, D.P., Suggett, J.A. et al. Interchanging Reusable and Disposable Nebulizers Used with Home-Based Compressors May Result in Inconsistent Dosing: A Laboratory Investigation with Device Combinations Supplied to the US Healthcare Environment. Pulm Ther (2024). https://doi.org/10.1007/s41030-024-00256-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41030-024-00256-0