Abstract
Purpose
A new Circulaire II (holding chamber) was designed to be used with VixOne nebulizer to increase the inhalable doses reaching the patient. The aim of this study was to evaluate the efficacy of this holding chamber with VixOne nebulizer and determine its usability with other nebulizers.
Methods
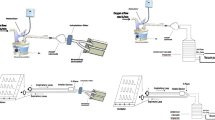
The aerodynamic characteristics of emitted dose of 2 ml salbutamol solution, 5000 μg/ml, were evaluated using cooled Andersen cascade impactor at inhalation flow 15 L/min. The respirable solutions were nebulized using 3JN (VixOne, NebuTech, and En full Kit) with both holding chamber and T-piece. To evaluate the pulmonary and systemic bioavailability, 12 non-smoking health subjects (6 females), > 18 years, with an average(SD) FEV1 > 90% of predicted, inhaled nebulized aerosol of 1 ml respirable solution (5000 μg salbutamol), diluted to 2 ml total volume using normal saline through the same nebulizer setting described above using normal tidal breathing. Subjects provided urine samples 30 min post-dosing (USAL0.5) as an index of pulmonary bioavailability and cumulatively collected their urine for 24 h (USAL24) as an index of systemic bioavailability. To determine the amount that would reach the patients (aerosol-emitted), subjects again inhaled 1 ml salbutamol-diluted respirable solution through the same nebulizer setting described above but with filters to capture salbutamol-emitted.
Results
Amount of salbutamol deposited in the holding chamber was significantly higher than in T-piece among all nebulizers (p < 0.001). The fine particle fraction (FPF), < 3 μm, of VixOne with holding chamber, was significantly higher (p < 0.001) and the mass median aerodynamic diameter (MMAD) was significantly lower (p = 0.022) than that with T-piece. The NebuTech and Kit had lower FPF and higher MMAD with holding chamber compared to T-piece (p < 0.05). There was no significant difference in the USAL24 and the emitted aerosol between the 3JNs with holding chamber or T-piece.
Conclusions
Holding chamber did not significantly increase the emitted aerosol or USAL24 responsible for the side effect but improved the FPF < 3 μm and MMAD, responsible for lung deposition, with VixOne-JN only. Holding chamber, or similar add-on device, can be used with other nebulizers other than the VixOne, not to increase the inhaled dose but as a fugitive aerosol saver.


Similar content being viewed by others
References
Dean RH. Nebulizers: principles and performance. Respir Care. 2000;45(6):609.
Pisut F. Comparison of medication delivery by T-nebulizer with inspiratory and expiratory reservoir. Respir Care. 1989;34(11):985–8.
Nwakaego CO. Cost-benefit analysis of a dosimetric nebulizer using Circulaire and aTraditional Vixone nebulizer. The College of Health and Human Science: Georgia State University; 2011.
Thomas S, Langford J, George R, Geddes D. Improving the efficiency of drug administration with jet nebulisers. Lancet. 1988;331(8577):126.
Marshall L, Francis P, Khafagi F. Aerosol deposition in cystic fibrosis using an aerosol conservation device and a conventional jet nebulizer. J Paediatr Child Health. 1994;30(1):65–7.
Dennis JH. A review of issues relating to nebulizer standards. J Aerosol Med. 1998;11(s1):S-73–9.
Rau JL, Ari A, Restrepo RD. Performance comparison of nebulizer designs: constant-output, breath-enhanced, and dosimetric. Respir Care. 2004;49(2):174–9.
Gardenhire DS, editor. Improved aerosol delivery with a conserver-type nebulizer system powered by 6 common home air compressors. Submitted to the American Association for Respiratory Care for the AARC Open Forum to be held November; 2013.
Gardenhire DS, editor. An in vitro comparison of two dosimetric nebulizers. American journal of respiratory and critical care medicine; 2011. Am Thoracic Soc.
Silkstone V, Dennis J, Pieron C, Chrystyn H. An investigation of in vitro/in vivo correlations for salbutamol nebulized by eight systems. J Aerosol Med. 2002;15(3):251–9.
Sarhan RM, Elberry AA, Abdelwahab NS, Rabea H, Salem MN, Abdelrahim MEA. Effect of a nebulizer holding chamber on aerosol delivery. Respir Care. 2018:(In Press;63:1125–31.
Madney YM, Fathy M, Elberry AA, Rabea H, Abdelrahim MEA. Nebulizers and spacers for aerosol delivery through adult nasal cannula at low oxygen flow rate: an in-vitro study. J Drug Deliv Sci Technol. 2017;39:260–5.
Harb HS, Elberry AA, Rabea H, Fathy M, Abdelrahim MEA. Is Combihaler usable for aerosol delivery in single limb non-invasive mechanical ventilation? J Drug Deliv Sci Technol. 2017 2017/08/01/;40:28–34.
ElHansy MHE, Boules ME, Farid H, Chrystyn H, El-Maraghi SK, Al-Kholy MB, et al. In vitro aerodynamic characteristics of aerosol delivered from different inhalation methods in mechanical ventilation. Pharm Dev Technol. 2017;22(6):844–9.
Abdelrahim ME. Aerodynamic characteristics of nebulized terbutaline sulphate using the Andersen cascade impactor compared to the next generation impactor. Pharm Dev Technol. 2011;16(2):137–45.
Hassan A, Rabea H, Hussein RR, Eldin RS, Abdelrahman MM, Said AS, et al. In-vitro characterization of the aerosolized dose during non-invasive automatic continuous positive airway pressure ventilation. Pulm Ther. 2016;2(1):115–26.
Abdelrahim ME, Plant P, Chrystyn H. In-vitro characterisation of the nebulised dose during non-invasive ventilation. J Pharm Pharmacol. 2010;62(8):966–72.
Abdelrahim ME, Chrystyn H. Aerodynamic characteristics of nebulized terbutaline sulphate using the next generation impactor (NGI) and CEN method. J Aerosol Med Pulm Drug Deliv. 2009;22(1):19–28.
Berg E, Svensson JO, Asking L. Determination of nebulizer droplet size distribution: a method based on impactor refrigeration. J Aerosol Med. 2007;20(2):97–104.
Rabea H, Ali AM, Eldin RS, Abdelrahman MM, Said AS, Abdelrahim ME. Modelling of in-vitro and in-vivo performance of aerosol emitted from different vibrating mesh nebulisers in non-invasive ventilation circuit. Eur J Pharm Sci. 2017;97:182–91.
Hindle M, Chrystyn H. Determination of the relative bioavailability of salbutamol to the lung following inhalation [see comments]. Br J Clin Pharmacol. 1992;34(4):311–5.
Mazhar SH, Ismail NE, Newton DA, Chrystyn H. Relative lung deposition of salbutamol following inhalation from a spacer and a Sidestream jet nebulizer following an acute exacerbation. Br J Clin Pharmacol. 2008;65(3):334–7.
Saeed H, Mohsen M, Fink JB, Dailey P, Salah Eldin A, Abdelrahman MM, et al. Fill volume, humidification and heat effects on aerosol delivery and fugitive emissions during noninvasive ventilation. J Drug Deliv Sci Technol. 2017;39:372–8.
Moustafa IOF, ElHansy MHE, Al Hallag M, Fink JB, Dailey P, Rabea H, et al. Clinical outcome associated with the use of different inhalation method with and without humidification in asthmatic mechanically ventilated patients. Pulm Pharmacol Ther. 2017;45:40–6.
Mohsen M, Elberry AE, Salah Eldin A, Hussein RR, Abdelrahim EM. Effects of heat and humidification on aerosol delivery during auto-CPAP noninvasive ventilation. Arch Pulmonol Respir Care. 2017;3(1):11–5.
Saeed H, Mohsen M, Salah Eldin A, Elberry AA, Abdelwahab NS, Hussein RRS, et al. Effects of fill volume and humidification on aerosol delivery during single limb non-invasive ventilation. Respir Care. 2018:(In Press;63:1370–8.
Saeed H, Ali AMA, Elberry AA, Eldin AS, Rabea H, Abdelrahim MEA. Modeling and optimization of nebulizers’ performance in non-invasive ventilation using different fill volumes: comparative study between vibrating mesh and jet nebulizers. Pulm Pharmacol Ther. 2018 2018/06/01/;50:62–71.
Harb HS, Elberry AA, Rabea H, Fathy M, Abdelrahim MEA. Performance of large spacer versus nebulizer T-piece in single limb non-invasive ventilation. Respir Care. 2018:(In Press;63:1360–9.
Hassan A, Salah Eldin R, Abdelrahman MM, Abdelrahim ME. In-vitro/in-vivo comparison of inhaled salbutamol dose delivered by jet nebulizer, vibrating mesh nebulizer and metered dose inhaler with spacer during non-invasive ventilation. Exp Lung Res. 2017;43(1):19–28.
ElHansy MH, Boules ME, El Essawy AFM, Al-Kholy MB, Abdelrahman MM, Said AS, et al. Inhaled salbutamol dose delivered by jet nebulizer, vibrating mesh nebulizer and metered dose inhaler with spacer during invasive mechanical ventilation. Pulm Pharmacol Ther. 2017;45:159–63.
Moustafa IO, Ali MR-A, Al Hallag M, Rabea H, Fink JB, Dailey P, et al. Lung deposition and systemic bioavailability of different aerosol devices with and without humidification in mechanically ventilated patients. Heart Lung: J Acute Crit Care. 2017;46(6):464–7.
Hussein RR, MA Ali A, Salem HF, Abdelrahman MM, Said AS, Abdelrahim ME. In vitro/in vivo correlation and modeling of emitted dose and lung deposition of inhaled salbutamol from metered dose inhalers with different types of spacers in noninvasively ventilated patients. Pharm Dev Technol. 2017;22(7):871–80.
Madney YM, Fathy M, Elberry AA, Rabea H, Abdelrahim MEA. Aerosol delivery through adult nasal-cannula via HFNC circuit at a low-oxygen flow. Respir Care. 2018. (In press).
Evans ME, Walker S, Brittain R, Paterson J. The metabolism of salbutamol in man. Xenobiotica. 1973;3(2):113–20.
Abdelrahim M, Plant P, Chrystyn H. The relative lung and systemic bioavailability of terbutaline following nebulisation in non-invasively ventilated patients. Int J Pharm. 2011;420(2):313–8.
Dimich-Ward H, Wymer ML, Chan-Yeung M. Respiratory health survey of respiratory therapists. CHEST J. 2004;126(4):1048–53.
Christiani DC, Kern DG. Asthma risk and occupation as a respiratory therapist. Am Rev Respir Dis. 1993;148:671–4.
Carnathan B, Martin B, Colice G. Second hand (S)-albuterol: RT exposure risk following racemic albuterol. Respir Care. 2001;46(10):1084.
Kern DG, Frumkin H. Asthma in respiratory therapists. Ann Intern Med. 1989;110(10):767–73.
Demers B. Drugs prescribed for patients shouldn’t be taken by caregivers. CHEST J. 2004;126(4):1012–3.
Delclos GL, Gimeno D, Arif AA, Burau KD, Carson A, Lusk C, et al. Occupational risk factors and asthma among health care professionals. Am J Respir Crit Care Med. 2007;175(7):667–75.
Witt-Enderby PA, Yamamura HI, Halonen M, Palmer JD, Bloom JW. Chronic exposure to a β2-adrenoceptor agonist increases the airway response to methacholine. Eur J Pharmacol. 1993;241(1):121–3.
Piper SD. In vitro comparison of the circulaire and AeroTee to a traditional nebulizer T-piece with corrugated tubing. Respir Care. 2000;45(3):313–9.
Hess D, Fisher D, Williams P, Pooler S, Kacmarek RM. Medication nebulizer performance: effects of diluent volume, nebulizer flow, and nebulizer brand. Chest. 1996;110(2):498–505.
Mason JW, Miller WC. Comparison of aerosol delivery via circulaire nebulizer system versus a disposable nebulizer in COPD patients. Respir Care. 1996;41(11):1006–8.
Mason JW, Miller WC, Small S. Comparison of aerosol delivery via Circulaire system vs conventional small volume nebulizer. Respir Care. 1994;39(12):1157–61.
Hoffman L, Smithline H. Comparison of Circulaire to conventional small volume nebulizer for the treatment of bronchospasm in the emergency department. Respir Care. 1997;42(12):1170–4.
Raabe O, Howard R, Cross C. Aerosol considerations in asthma. Bronchial asthma, principles of diagnosis and treatment. 2nd ed. Orlando: Grune & Stratton; 1986. p. 495–514.
Saeed H, Elberry AA, Eldin AS, Rabea H, Abdelrahim ME. Effect of nebulizer designs on aerosol delivery during non-invasive mechanical ventilation: a modeling study of in vitro data. Pulm Ther. 2017;3(1):233–41.
Ari A, Fink JB, Pilbeam S. Secondhand aerosol exposure during mechanical ventilation with and without expiratory filters: an in-vitro study. Indian J Respir Care. 2016;5(1):677.
Mitchell J. Inhalation and nasal formulations. In: Developing drug products in an aging society. Springer; 2016. p. 331–82.
Loffert DT, Ikle D, Nelson HS. A comparison of commercial jet nebulizers. Chest. 1994;106(6):1788–92.
Alvine GF, Rodgers P, Fitzsimmons KM, Ahrens RC. Disposable jet nebulizers: how reliable are they? Chest. 1992;101(2):316–9.
Langford S, Allen M. Salbutamol output from two jet nebulizers. Respir Med. 1993;87(2):99–103.
Ho SL, Coates AL. Effect of dead volume on the efficiency and the cost to deliver medications in cystic fibrosis with four disposable nebulizers. Can Respir J. 1999;6(3):253–60.
Coates AL, MacNeish CF, Lands LC, Meisner D, Kelemen S, Vadas EB. A comparison of the availability of tobramycin for inhalation from vented vs unvented nebulizers. Chest. 1998;113(4):951–6.
Waldrep JC, Keyhani K, Black M, Knight V. Operating characteristics of 18 different continuous-flow jet nebulizers with beclomethasone dipropionate liposome aerosol. Chest. 1994;105(1):106–10.
Vecellio L, Abdelrahim ME, Montharu J, Galle J, Diot P, Dubus J-C. Disposable versus reusable jet nebulizers for cystic fibrosis treatment with tobramycin. J Cyst Fibros. 2011;10(2):86–92.
Bosco AP, Rhem RG, Dolovich MB. In vitro estimations of in vivo jet nebulizer efficiency using actual and simulated tidal breathing patterns. J Aerosol Med. 2005;18(4):427–38.
Dhand R. Aerosol delivery during mechanical ventilation: from basic techniques to new devices. J Aerosol Med Pulm Drug Deliv. 2008;21(1):45–60.
Author information
Authors and Affiliations
Contributions
Mona Ahmed: Experiment, data entry, statistics, and writing.
Raghda R.S. Hussein: Experiment and study design.
Ahmed A. Elberry: Concept and study design.
Mohamed E. Abdelrahim: Concept, planning of study design, statistics, and writing.
Corresponding author
Ethics declarations
This study was conducted in accordance with the amended Declaration of Helsinki. Local institutional review boards and independent ethics committees approved the protocol and written informed consent was obtained from all volunteers.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdelrahman, M.A., Elberry, A.A., Hussein, R.R.S. et al. Effect of Holding Chamber as an Add-on Device on Aerosol Delivery and Fugitive Aerosol from Different Jet Nebulizers. J Pharm Innov 15, 73–79 (2020). https://doi.org/10.1007/s12247-018-9369-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-018-9369-2