Abstract
Adults with obesity may develop asthma that is ineffectively controlled by inhaled corticosteroids and long-acting beta-adrenoceptor agonists. Mechanistic and translational studies suggest that metabolic dysregulation that occurs with obesity, particularly hyperglycemia and insulin resistance, contributes to altered immune cell function and low-grade systemic inflammation. Importantly, in these cases, the same proinflammatory cytokines believed to contribute to insulin resistance may also be responsible for airway remodeling and hyperresponsiveness. In the past decade, new research has emerged assessing whether hypoglycemic therapies impact comorbid asthma as reflected by the incidence of asthma, asthma-related emergency department visits, asthma-related hospitalizations, and asthma-related exacerbations. The purpose of this review article is to discuss the mechanism of action, preclinical data, and existing clinical studies regarding the efficacy and safety of hypoglycemic therapies for adults with obesity and comorbid asthma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Obesity contributes to increased prevalence of comorbid asthma in adults. |
Adults with obesity and comorbid asthma exhibit increased asthma symptoms and exacerbation of risk and poorer response to inhaled corticosteroids. |
Observational studies of medical therapies approved for type 2 diabetes and/or obesity support the targeting of metabolic pathways for possible beneficial asthma outcomes. |
Head-to-head studies and inclusion of asthma endpoints in clinical trials of hypoglycemic therapies are needed for clarification of clinical benefit for adults with obesity and comorbid asthma. |
Introduction
Asthma is classically defined as variable airflow limitation and airway hyperresponsiveness occurring most often in the context of airway inflammation. Clinical evidence indicates that obesity negatively impacts asthma incidence, severity, symptoms, and therapeutic response. Mechanistic and translational studies suggest that the underlying metabolic dysregulation in patients with obesity, particularly hyperglycemia and insulin resistance, contributes to altered immune cell function and low-grade systemic inflammation. Over the past two decades, multiple pharmacologic interventions, targeting a variety of metabolic pathways, have been approved for the treatment of prediabetes, type 2 diabetes mellitus (T2DM), and obesity. Understanding the relevance of these therapeutic advances to asthma clinical outcomes and respiratory function is an essential step towards personalized medicine for high-risk patients with multimorbidity. Unexpectedly, recent studies also suggest that some of these medication classes may directly benefit airway inflammation, airflow limitation, and airway hyperresponsiveness through mechanisms not currently addressed by conventional asthma therapeutics. In this review, we describe the major pharmacologic interventions available for the treatment of metabolic dysregulation and the clinical implications in the context of adult asthma.
Asthma in Adults with Obesity: Prevalence and Challenges
Obesity and asthma often coexist through interrelated mechanical, inflammatory, nutritional, and behavioral pathways. In the USA, the National Center for Health Statistics determined that adults with obesity demonstrated higher rates of asthma (11.1%) compared to those classified as overweight (7.8%) or normal weight (7.1%) [1]. From 1999 to 2016, the rate of obesity in adults with asthma trended upwards to 1.75-fold that of the general population without asthma [2]. Patients with obesity and comorbid asthma additionally suffer from increased asthma severity and chronic morbidity, thereby incurring disproportionately large medical costs [3,4,5,6]. Long-term treatment options for asthma, the most prominent of which are inhaled corticosteroids (ICS) taken with or without long-acting beta-adrenoceptor agonists (LABA), illicit reduced responses in patients with obesity and comorbid asthma due to rapid medication clearance and poor absorption [7,8,9]. However, weight loss in individuals with obesity can independently reduce asthma symptoms and protect against a decline in measures of lung function, specifically forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) [10,11,12]. As a result, recommendations for patients with obesity and comorbid asthma typically involve changes in diet, exercise, or even bariatric surgery to achieve weight loss with secondary remission in asthma symptoms [13, 14].
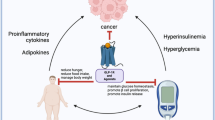
Obesity and the associated metabolic dysregulation alter the comorbid asthma clinical trajectory. The pathophysiological mechanism of asthma is largely understood as type (T)2-high inflammation, characterized by interleukin (IL)-4, IL-5, and IL-13 and airway eosinophilia, driving airway hyperresponsiveness (AHR) and airway remodeling and leading to airflow obstruction [15]. As asthma severity increases, T2-low airway inflammation, characterized by airway neutrophilia and inflammasome activation controlled by IL-10, alarmins (e.g., IL-25, IL-33), and tumor growth factor beta (TGF-β) becomes more prevalent [16]. Both classic T2-high and T2-low inflammatory patterns can be observed in obesity-associated asthma. Furthermore, in the context of obesity, accumulated visceral adipose tissue releases TGF-β, which induces M1 macrophages to secrete IL-6 and tumor necrosis factor alpha (TNF-α) (Fig. 1) [17]. Ultimately, the resulting low-grade inflammation causes remodeling and hyperresponsiveness in the airways, increasing the risk of asthma exacerbations, namely, flares in asthma symptoms that lead to an emergency department (ED) visit, hospitalization, or therapy intensification [18,19,20,21,22,23,24]. Obesity increases the risk of impaired fasting glucose and T2DM due to alterations in adipose tissue and β-cell function that altogether causes an inadequate production and response to insulin [25,26,27]. Increased levels of hemoglobin A1c (HbA1c), a marker for glucose control over a 3-month period used in the diagnosis and monitoring of T2DM, is weakly independently associated with asthma-related hospitalization [28]. Compared to those with normal HbA1c, individuals with HbA1c in the prediabetic/diabetic range have a higher asthma exacerbation rate [29]. Insulin resistance, determined by the homeostatic model assessment of insulin resistance (HOMA-IR) or euglycemic–hyperinsulinemic clamp, is negatively associated with lung function [30, 31]. Furthermore, the downstream effects of insulin resistance may intensify asthma symptoms through hypercontractility, vagally-induced bronchoconstriction, and fibrosis [32,33,34]. Invasive interventions, such as bariatric surgery, leads to decreased levels of visceral adipose tissue that in turn improves lung function, reduces inflammation, lowers asthma-related exacerbation risk, and decreases the intensity of medication needed to control asthma symptoms [35,36,37,38,39]. However, Forno et al. found that the presence of metabolic syndrome significantly attenuates the effect of bariatric surgery on asthma control [40]. The relative impact of excess fat mass, as in obesity, and metabolic dysregulation, as in insulin resistance and overt T2DM, on airway inflammation and clinical disease in asthma remain an area of active research.
Proposed mechanisms of action of metabolic pathways in the lung of patients with asthma. This figure depicts the multiple immunological and inflammatory pathways related to asthma that result from metabolic dysfunction in patients with obesity. 1 Accumulated visceral adipose tissue, exacerbated or ameliorated by certain classes of hypoglycemic therapies, can contribute to circulating levels of proinflammatory cytokines, such as TGF-β. 2 These proinflammatory cytokines then activate M1 macrophages characteristic of the type 2-low asthma endotype, which contribute further to the low-grade inflammation. 3 Environmental exposures, including exposure to the common dust mite and mold allergens, result in proinflammatory cytokines upregulating genes in the airway responsible for fibrosis and hyperresponsiveness. Some classes of hypoglycemic therapies directly or indirectly inhibit these pathways. This figure was created with BioRender.com (https://biorender.com/). HFD high-fat diet, SGLT-2i sodium glucose co-transporter 2 inhibitors, TZD thiazolidinediones, TGF-β tumor growth factor beta, TGF-βR tumor growth factor beta receptor, TNF-α tumor necrosis factor alpha, IL-6 interleukin-6, TLR4 toll-like receptor 4, AMP/ATP adenosine monophosphate/adenosine triphosphate, AMPK AMP-activated protein kinase, SMAD3 mothers against decapentaplegic homolog 3, NF-κB nuclear factor-κB, PKA protein kinase A, DPP-4i dipeptidyl peptidase-4 inhibitors, GLP-1Ra glucagon-like peptide-1 receptor agonists
The complex interactions of obesity, metabolic dysregulation, and asthma preclude the establishment of a definitive therapeutic paradigm at this time. Therapies which can address insulin resistance and promote weight loss, while having limited risk for hypoglycemia, may hold the most promise (Fig. 2). There is a growing awareness of the clinical challenges of multimorbidity and polypharmacy facing patients with obesity and comorbid asthma, justifying a renewed approach to personalized medicine in this asthma subpopulation. In the following sections, we review the main therapeutic classes used for the treatment of prediabetes and T2DM in the context of asthma management (Table 1).
Impact of therapeutic classes for the treatment of type 2 diabetes mellitus on metabolic parameters relevant in asthma. This figure demonstrates the different effects of multiple classes of hypoglycemic therapies on hypoglycemia, insulin resistance, and weight. Gray shading denotes how insulin is the only hypoglycemic therapy that fails to improve insulin sensitivity. See Fig. 1 caption for abbreviations
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Biguanides
Most patients initiating hypoglycemic monotherapy are prescribed metformin [41]. Metformin is also approved as an adjunct to diet and exercise for the management of T2DM and routinely used off-label to address insulin resistance in women with polycystic ovarian syndrome and in prediabetes. Metformin reduces gluconeogenesis by inhibiting the mitochondria respiratory chain in hepatocytes, raising the ratio of adenosine monophosphate (AMP) to adenosine triphosphate (ATP) ratio and activating AMP-activated protein kinase (AMPK) [42]. In patients with metabolic dysregulation, reduced AMPK activity led to increased inflammatory gene expression [43]. Notably, AMPK also plays a crucial role in preserving respiratory function, as studies in animal models suggest AMPK suppresses TGF-β-induced proliferation of airway smooth muscle cells and AHR caused by hypoxia-inducible factor/vascular endothelial growth factor (HIF/VEGF) interaction [44, 45]. By indirectly upregulating AMPK expression, metformin demonstrates highly efficacious and tolerable anti-inflammatory properties systemically and in lung tissue in murine models [46,47,48,49].
Metformin use in patients with obesity and comorbid asthma is linked to the improvement of both chronic conditions. In a study involving temporarily induced insulin resistance through treatment of non-esterified fatty acid (NEFA), metformin improved extrahepatic glucose utilization in patients with T2DM potentially by promoting microvascular perfusion [50, 51]. However, two randomized clinical trials showed that other hypoglycemic therapies alone or in combination with metformin led to superior glycemic control compared to metformin by itself [52, 53]. While multiple studies show metformin yields insignificantly or modest weight loss, the U.S. Food and Drug Administration (FDA) does not recognize metformin as a weight loss agent [54]. Importantly, many large retrospective observational cohort studies examining metformin usage in adults with asthma provided evidence of superior asthma-related outcomes. Both Li et al. and Wu et al. used a claims-based cohort and arrived at the similar conclusion that adults with asthma and comorbid diabetes who took metformin demonstrated decreased rates of asthma exacerbations, asthma-related ED visits, and asthma-related hospitalizations [55,56,57]. Wu et al. later identified adults with asthma-chronic obstructive pulmonary disease (COPD) overlap within the COPDGene database and found metformin initiation improved quality of life as assessed by the St. George’s Respiratory Questionnaire [58]. Prospective controlled studies in asthma, with and without comorbid obesity, are needed to establish the utility of metformin.
Insulin
By improving glucose control, insulin substantially reduces the risk of diabetes-related endpoints, diabetes-related death, and all-cause mortality, compared to conventional dietary interventions in patients with T2DM [59]. Administering insulin can lower blood glucose level in a dose-dependent manner albeit with notable adverse effects [60]. Two basal insulin analogs, glargine and detemir, cause modest weight gain of 2 kg per 1% reduction in HbA1c, with weight gain worsened by twice-daily dosing as compared to once-daily dosing [61]. While hypoglycemia is more commonly reported by patients with type 1 diabetes mellitus, 46.5% of insulin users with T2DM reported hypoglycemic events at an average 2.5 severe hypoglycemic events/patient-year [62]. In a prospective cohort study of patients with T2DM without comorbid asthma or COPD, insulin initiation exacerbated the methacholine-induced decline in FEV1 in the first 60 days and serum total immunoglobulin E at 1 year [63]. Multiple retrospective observational cohort studies have identified associations between insulin usage and asthma onset in patients with diabetes. In one study, patients with T2DM who had ≥ 3 prescriptions of insulin per year were more than twice as likely to be subsequently diagnosed with asthma [64]. Similarly, Reynar et al. found that among adults diagnosed with T2DM, insulin usage, but not duration of therapy, glycemic control, or T2DM complications, increased the risk of incident asthma [65]. The addition of insulin to the treatment regime for T2DM in the context of asthma is unlikely to change from current T2DM guidelines where it is added as a last-line chronic agent.
Thiazolidinediones
Widely prescribed in the early 1990s, thiazolidinediones (TZD) have largely declined in popularity over the past two decades over concerns of side effects, including weight gain, skeletal fractures, and edema [66,67,68]. Rosiglitazone and pioglitazone may also increase the risk for heart failure and bladder cancer, respectively [69, 70]. TZD help control levels of excessive serum-free fatty acids by ligating to the gamma isoform of the peroxisome proliferator-activated receptor (PPARγ) and promoting the ability of adipose tissue to store fat [71, 72]. By addressing the underlying adipose tissue dysfunction that occurs during obesity, TZD showed potential for having anti-inflammatory properties with low hypoglycemic risk. In cultured human airway smooth muscle cells, troglitazone dose-dependently reduced the release of IL-6 via a pathway distinct from AMPK and PPARγ [73]. However, a systematic review found that usage of TZD across many clinical trials did not significantly affect IL-6 levels [74].
The authors of a few studies have argued in favor of TZD for protection against asthma in patients with obesity and comorbid T2DM. Two large retrospective observational cohort studies utilizing data from the U.S. Veteran Affairs Medical Centers found that TZD lowered the risk of incident asthma, asthma exacerbation, and oral steroid prescriptions [75, 76]. These effects were strengthened by adherence to TZD and concurrent usage of angiotensin-converting enzyme inhibitors. However, a pilot randomized controlled trial (RCT) of pioglitazone in patients with poorly controlled asthma with comorbid obesity was prematurely discontinued due to new safety concerns around the risk for bladder cancer [77]. Additionally, the significant weight gain observed in the treatment group had the potential for harm without improving asthma control or lung function. In a second RCT of pioglitazone in severe asthma, patients in the pioglitazone arm showed both no improvement in the primary clinical endpoint of the asthma quality of life questionnaire score and a high rate of adverse effects [78]. Systemic TDZ are unlikely to advance as a therapeutic intervention for asthma due to these safety concerns and the lack of clinical benefit. The impact of local delivery of TZD to the airway and in adults with asthma without comorbid obesity remain unexplored.
Sulfonylureas
After metformin initiation, sulfonylureas are commonly used as second-line therapy to lower levels of HbA1c and blood glucose during the fasting and post-prandial periods [79]. Sulfonylureas raise plasma insulin concentration by binding to receptors on the cell membranes of β-pancreatic cells. This interaction blocks the inflow of potassium into the cytosol and subsequent depolarization, triggering the diffusion of calcium that contracts actomyosin responsible for the exocytosis of insulin [80]. However, adding on and switching to sulfonylureas may increase the risk of myocardial infarction, mortality, and severe hypoglycemia compared to other hypoglycemic agents [81, 82]. One retrospective observational cohort study found sulfonylureas provided modest protection in adults with T2DM against incident asthma, statistically equivalent to that of metformin initiation [65]. Cardiovascular and hypoglycemic risk limit the use of sulfonylureas in T2DM, independent of asthma comorbidity, and a strong rationale for clinical benefit would need to be observed to warrant their further study in the asthma context.
Dipeptidyl Peptidase-4 Inhibitors
Dipeptidyl peptidase-4 inhibitors (DPP-4i) may be considered over sulfonylureas as second-line therapy after metformin because of their reduced hypoglycemic risk and neutral effect on weight [83, 84]. Glucagon-like peptide-1 (GLP-1) stimulates insulin secretion from pancreatic β-cells in hyperglycemic conditions but is cleaved by dipeptidyl peptidase-4 (DPP-4), an adipokine released in excessive amounts by adipose tissue in patients with obesity [85, 86]. By preventing inactivation of GLP-1, DPP-4i has been found to significantly improve β-cell function and glycemic control, albeit its effects are attenuated in patients with high levels of insulin resistance [87, 88]. Studies employing in vitro models of human bronchial epithelial cells have indicated that DPP-4i blocks pathways contributing to oxidative stress and fibrosis [89, 90]. Saxagliptin has been shown to mitigate oxidative stress in ovalbumin (OVA)-sensitized mice by modulation of toll-like receptor 4 (TLR4) and nuclear factor-κB (NF-κB) signaling [91]. However, a retrospective observational matched cohort study showed that adults with asthma who utilized DPP-4i showed no improvement in asthma control, treatment stability, and asthma exacerbation compared to their counterparts on other hypoglycemic medications [92]. A recent network meta-analysis further established that DPP-4i did not reduce the risk of incident asthma relative to placebo [93]. Clinical data are currently lacking to support their preferential use in the context of comorbid asthma.
Sodium Glucose Co-Transporter 2 Channel Inhibitors
In the past decade, the U.S. FDA approved a new class of drugs for improving glucose control in adults with T2DM: sodium-glucose co-transporter 2 channel (SGLT-2) inhibitors (SGLT-2i) [94]. In the kidneys, SGLT-2 are responsible for reabsorption of 90–97% of filtered glucose [95]. By blocking SGLT-2, SGLT-2i induce glucosuria, thereby reducing signs of chronic hyperglycemia, as indicated by HbA1c levels and insulin sensitivity. The authors of an in vitro transcriptomics experiment assessing the progression of diabetic kidney disease in human proximal tubular cells concluded that the one of the SGLT-2i currently available, canagliflozin, reverses inflammation by decreasing the levels of TNF receptor 1, IL-6, matrix metalloproteinase 7, and fibronectin 1 compared to the sulfonylurea glimepiride [96]. The mechanism underlying the anti-inflammatory properties of SGLT-2i could be a downstream effect of its ability to lower uric acid and insulin production [97]. A network analysis pooling nine clinical trials of SGLT-2i evaluating cardiorenal outcomes in patients with T2DM, heart failure, and/or chronic kidney disease reported that SGLT-2i reduced the occurrence of asthma serious adverse events compared to placebo [98]. Wang et al. used a similar approach to identify 19 clinical trials and found that SGLT-2i decreased the risk of asthma, based on asthma-related adverse events reported in the studies, compared to GLP-1 receptor agonists (GLP-1Ra) and DPP-4i [93]. However, the validity of both meta-analyses is limited by the very low frequency of asthma outcomes in both the treatment and placebo groups. Adequately powered studies of SGLT-2i use in asthma populations with sufficient (e.g., at higher risk of) asthma-related events and with prespecified asthma outcomes are needed.
Glucagon Like Peptide-1 Receptor Agonists
Since 2005, GLP-1Ra have emerged as a highly effective drug class for glycemic control that also reduce the risk of cardiovascular events and promote weight loss [99]. GLP-1Ra improve pancreatic β-cell function by promoting cell proliferation, stimulating insulin secretion, and inhibiting glucagon release [100]. Additionally, GLP-1Ra limit weight gain by suppressing food intake through the induction of satiety and delayed gastric emptying. The authors of a randomized clinical trial that enrolled adults with obesity concluded that 68 weeks of once-weekly semaglutide 2.4 mg resulted in a mean change in body of − 14.9% compared to − 2.4% in adults on placebo [101]. GLP-1Ra potentially mediates multiple inflammatory pathways involved in the pathophysiology of comorbid asthma in patients with obesity [102]. Preclinical experiments have shown that GLP-1Ra decreased expression of proinflammatory cytokines, such as TNF-α, through protein kinase A-dependent inactivation of NF-κB (Fig. 1) [103,104,105]. Through inhibiting the release of IL-33, GLP-1Ra administered in murine models attenuated airway eosinophilia and neutrophilia, the release of T2 cytokines from type 2 innate lymphoid cells (ILC2), mucus production, and AHR following exposure to fungal allergens and viral antigens [106,107,108]. Interestingly, GLP-1Ra given to OVA-sensitized mice also depressed the activity of oligomerization domain-like receptor protein (NLRP)3, which induces airway inflammation and AHR in obesity by upregulating levels of IL-17 secretion by ILC3 via secretion of IL-1β [109,110,111].
In multiple studies, GLP-1Ra use was associated with improvements in asthma outcomes in adults with T2DM and comorbid asthma. The first preliminary uncontrolled study of nine patients treated with a GLP-1Ra found that 1 year of a GLP-1Ra improved asthma symptoms and reduced asthma exacerbation rate in those with weight loss in excess of the population median [112]. In a new user, active comparator study design using data available in the electronic health record, adults with T2DM and comorbid asthma who initiated a GLP-1Ra for treatment intensification for T2DM experienced the lowest risk of asthma exacerbation in the following 6 months compared to those initiating sulfonylureas, SGLT-2i, or DPP-4 inhibitors [113]. A claims-based analysis comparing users of GLP-1Ra and DPP-4i in patients with T2DM and comorbid chronic lower respiratory disease (CLRD)—a term encompassing asthma and COPD—similarly found that GLP-1Ra led to reduced incidence of CLRD-related hospitalizations and exacerbations [114]. Although tangential to asthma, a randomized clinical trial which recruited patients with T2DM revealed that liraglutide reduced serum levels of surfactant protein D which independently predicted improvements in FVC [115]. Additionally in a prospective cohort of 32 adults with T2DM but without co-existing obstructive lung disease, the addition of a GLP-1Ra to metformin as treatment improved lung function (FEV1 and FVC) over metformin alone or metformin + insulin [116]. GLP-1Ra are an active area of research in clinical and translational asthma studies, and a prospective clinical trial of the GLP-1Ra semaglutide in adults with asthma and comorbid obesity is forthcoming [117].
Future Directions
The majority of studies examining asthma-related outcomes in adults with obesity and/or comorbid diabetes taking hypoglycemic agents are large retrospective observational cohort studies. To strengthen the observed associations and move findings into practice requires additional investigation. First, prospective studies in adults with asthma and comorbid obesity stratified by the extent of metabolic dysfunction or weight loss can help determine whether hypoglycemic agents exhibit independent, beneficial effects in the airway, as suggested by preclinical studies. Second, head-to-head studies comparing hypoglycemic agents in adults with obesity and comorbid asthma should consider endpoints beyond asthma-related exacerbations, such as levels of circulating and airway inflammation markers that could help inform response to therapy or identify asthma endotypes to target.
The area of study reviewed in this paper continues to evolve as new hypoglycemic agents as well as non-pharmacologic interventions advance. Two recent phase 3 multicenter randomized trials in adults with obesity showed that tirzepatide, a novel dual gastric inhibitory peptide\GLP-1R agonist, caused substantial weight loss in patients who failed to lose weight via dietary changes and noninferior and superior glycemic control compared to semaglutide in those with comorbid T2DM [118, 119]. Limited data are currently available on the impact of tirzepatide on comorbidities seen in patients with obesity, such as asthma. Hypoglycemic therapies should also be compared to non-pharmacologic interventions, such as bariatric surgery, to develop rational approaches to the use of these interventions in patients with obesity and comorbid asthma and to identify who may benefit most from each approach. In doing so, clinicians and researchers may more fully expand the growing armamentarium of personalized medicine for patients with obesity and comorbid asthma.
Conclusion
The current therapeutic paradigm for adults with obesity and comorbid asthma requires a balancing act between improving clinically meaningful asthma-related outcomes, avoiding weight gain, minimizing hypoglycemia, and preventing unintended severe adverse events. An expanding volume of observational evidence suggests that certain classes of hypoglycemic agents could be used to address both chronic conditions by targeting the underlying metabolic dysregulation. However, until randomized clinical trials of these pharmacologic tools for obesity and metabolic dysfunction evaluate asthma outcomes as a primary endpoint, the costly management of comorbid asthma in adults with obesity will still heavily rely on the paradigm of inhaled ICS with or without LABA.
References
Akinbami LJ, Fryar CD. Current asthma prevalence by weight status among adults: United States, 2001–2014. NCHS Data Brief. 2016;239:1–8.
Lurbet MF, Rojano B, Whittaker Brown SA, et al. Obesity trends among asthma patients in the United States: a population-based study. Ann Glob Health. 2019;85(1):10. https://doi.org/10.5334/aogh.2420.
Luthe SK, Hirayama A, Goto T, Faridi MK, Camargo CA Jr, Hasegawa K. Association between obesity and acute severity among patients hospitalized for asthma exacerbation. J Allergy Clin Immunol Pract. 2018;6(6):1936-1941.e4. https://doi.org/10.1016/j.jaip.2018.02.001.
Schatz M, Zeiger RS, Zhang F, Chen W, Yang SJ, Camargo CA Jr. Overweight/obesity and risk of seasonal asthma exacerbations. J Allergy Clin Immunol Pract. 2013;1(6):618–22. https://doi.org/10.1016/j.jaip.2013.07.009.
Chang C, Lee SM, Choi BW, et al. Costs Attributable to overweight and obesity in working asthma patients in the United States. Yonsei Med J. 2017;58(1):187–94. https://doi.org/10.3349/ymj.2017.58.1.187.
Ilmarinen P, Pardo A, Tuomisto LE, et al. Long-term prognosis of new adult-onset asthma in obese patients. Eur Respir J. 2021;57(4):2001209. https://doi.org/10.1183/13993003.01209-2020.
Pradeepan S, Garrison G, Dixon AE. Obesity in asthma: approaches to treatment. Curr Allergy Asthma Rep. 2013;13(5):434–42. https://doi.org/10.1007/s11882-013-0354-z.
Goleva E, Covar R, Martin RJ, Leung DY. Corticosteroid pharmacokinetic abnormalities in overweight and obese corticosteroid resistant asthmatics. J Allergy Clin Immunol Pract. 2016;4(2):357-60.e2. https://doi.org/10.1016/j.jaip.2015.11.013.
Forno E, Lescher R, Strunk R, et al. Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol. 2011;127(3):741–9. https://doi.org/10.1016/j.jaci.2010.12.010.
Peralta GP, Marcon A, Carsin AE, et al. Body mass index and weight change are associated with adult lung function trajectories: the prospective ECRHS study. Thorax. 2020;75(4):313–20. https://doi.org/10.1136/thoraxjnl-2019-213880.
Stenius-Aarniala B, Poussa T, Kvarnström J, Grönlund EL, Ylikahri M, Mustajoki P. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ. 2000;320(7238):827–32. https://doi.org/10.1136/bmj.320.7238.827. [Published correction appears in BMJ 2000 Apr 8;320(7240):984].
Scott HA, Gibson PG, Garg ML, et al. Dietary restriction and exercise improve airway inflammation and clinical outcomes in overweight and obese asthma: a randomized trial. Clin Exp Allergy. 2013;43(1):36–49. https://doi.org/10.1111/cea.12004.
Juel CT, Ali Z, Nilas L, Ulrik CS. Asthma and obesity: does weight loss improve asthma control? a systematic review. J Asthma Allergy. 2012;5:21–6. https://doi.org/10.2147/JAA.S32232.
Global Initiative for Asthma (GINA). 2022 GINA main report. 2022 GINA report, global strategy for asthma management and prevention. 2022. https://ginasthma.org/gina-reports/. Accessed 28 Oct 2022.
Kudo M, Ishigatsubo Y, Aoki I. Pathology of asthma. Front Microbiol. 2013;4:263. https://doi.org/10.3389/fmicb.2013.00263.
Bantulà M, Roca-Ferrer J, Arismendi E, Picado C. Asthma and obesity: two diseases on the rise and bridged by inflammation. J Clin Med. 2021;10(2):169. https://doi.org/10.3390/jcm10020169.
Periyalil HA, Wood LG, Wright TA, et al. Obese asthmatics are characterized by altered adipose tissue macrophage activation. Clin Exp Allergy. 2018;48(6):641–9. https://doi.org/10.1111/cea.13109.
Saraiva SA, Silva AL, Xisto DG, et al. Impact of obesity on airway and lung parenchyma remodeling in experimental chronic allergic asthma. Respir Physiol Neurobiol. 2011;177(2):141–8. https://doi.org/10.1016/j.resp.2011.03.019.
Tan CK, Chong HC, Tan EH, Tan NS. Getting ‘Smad’ about obesity and diabetes. Nutr Diabetes. 2012;2(3): e29. https://doi.org/10.1038/nutd.2012.1.
Hong SH, Kang M, Lee KS, Yu K. High fat diet-induced TGF-β/Gbb signaling provokes insulin resistance through the tribbles expression. Sci Rep. 2016;6:30265. https://doi.org/10.1038/srep30265.
Yan J, Zhang H, Yin Y, et al. Obesity- and aging-induced excess of central transforming growth factor-β potentiates diabetic development via an RNA stress response. Nat Med. 2014;20(9):1001–8. https://doi.org/10.1038/nm.3616.
Peters MC, Mauger D, Ross KR, et al. Evidence for exacerbation-prone asthma and predictive biomarkers of exacerbation frequency. Am J Respir Crit Care Med. 2020;202(7):973–82. https://doi.org/10.1164/rccm.201909-1813OC.
Peters MC, McGrath KW, Hawkins GA, et al. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med. 2016;4(7):574–84. https://doi.org/10.1016/S2213-2600(16)30048-0. [Published correction appears in Lancet Respir Med. 2018 Mar; 6(3):e10].
Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010;2010:289645. https://doi.org/10.1155/2010/289645.
Shin JA, Lee JH, Lim SY, et al. Metabolic syndrome as a predictor of type 2 diabetes, and its clinical interpretations and usefulness. J Diabetes Investig. 2013;4(4):334–43. https://doi.org/10.1111/jdi.12075.
Klein S, Gastaldelli A, Yki-Järvinen H, Scherer PE. Why does obesity cause diabetes? Cell Metab. 2022;34(1):11–20. https://doi.org/10.1016/j.cmet.2021.12.012.
Karakelides H, Irving BA, Short KR, O’Brien P, Nair KS. Age, obesity, and sex effects on insulin sensitivity and skeletal muscle mitochondrial function. Diabetes. 2010;59(1):89–97. https://doi.org/10.2337/db09-0591.
Yang G, Han YY, Forno E, et al. Glycated hemoglobin A1c, lung function, and hospitalizations among adults with asthma. J Allergy Clin Immunol Pract. 2020;8(10):3409-15.e1. https://doi.org/10.1016/j.jaip.2020.06.017.
Wu TD, Brigham EP, Keet CA, Brown TT, Hansel NN, McCormack MC. Association between prediabetes/diabetes and asthma exacerbations in a claims-based obese asthma cohort. J Allergy Clin Immunol Pract. 2019;7(6):1868-73.e5. https://doi.org/10.1016/j.jaip.2019.02.029.
Cardet JC, Ash S, Kusa T, Camargo CA Jr, Israel E. Insulin resistance modifies the association between obesity and current asthma in adults. Eur Respir J. 2016;48(2):403–10. https://doi.org/10.1183/13993003.00246-2016.
Forno E, Han YY, Muzumdar RH, Celedón JC. Insulin resistance, metabolic syndrome, and lung function in US adolescents with and without asthma. J Allergy Clin Immunol. 2015;136(2):304-311.e8. https://doi.org/10.1016/j.jaci.2015.01.010.
Dekkers BG, Schaafsma D, Tran T, Zaagsma J, Meurs H. Insulin-induced laminin expression promotes a hypercontractile airway smooth muscle phenotype. Am J Respir Cell Mol Biol. 2009;41(4):494–504. https://doi.org/10.1165/rcmb.2008-0251OC.
Nie Z, Jacoby DB, Fryer AD. Hyperinsulinemia potentiates airway responsiveness to parasympathetic nerve stimulation in obese rats. Am J Respir Cell Mol Biol. 2014;51(2):251–61. https://doi.org/10.1165/rcmb.2013-0452OC.
Park YH, Oh EY, Han H, et al. Insulin resistance mediates high-fat diet-induced pulmonary fibrosis and airway hyperresponsiveness through the TGF-β1 pathway. Exp Mol Med. 2019;51(5):1–12. https://doi.org/10.1038/s12276-019-0258-7.
Galanakis CG, Daskalakis M, Manios A, Xyda A, Karantanas AH, Melissas J. Computed tomography-based assessment of abdominal adiposity changes and their impact on metabolic alterations following bariatric surgery. World J Surg. 2015;39(2):417–23. https://doi.org/10.1007/s00268-014-2826-2.
van Huisstede A, Rudolphus A, Castro Cabezas M, et al. Effect of bariatric surgery on asthma control, lung function and bronchial and systemic inflammation in morbidly obese subjects with asthma. Thorax. 2015;70(7):659–67. https://doi.org/10.1136/thoraxjnl-2014-206712.
Reddy RC, Baptist AP, Fan Z, Carlin AM, Birkmeyer NJ. The effects of bariatric surgery on asthma severity. Obes Surg. 2011;21(2):200–6. https://doi.org/10.1007/s11695-010-0155-6.
Hasegawa K, Tsugawa Y, Chang Y, Camargo CA Jr. Risk of an asthma exacerbation after bariatric surgery in adults. J Allergy Clin Immunol. 2015;136(2):288-94.e8. https://doi.org/10.1016/j.jaci.2014.12.1931.
Boulet LP, Turcotte H, Martin J, Poirier P. Effect of bariatric surgery on airway response and lung function in obese subjects with asthma. Respir Med. 2012;106(5):651–60. https://doi.org/10.1016/j.rmed.2011.12.012.
Forno E, Zhang P, Nouraie M, et al. The impact of bariatric surgery on asthma control differs among obese individuals with reported prior or current asthma, with or without metabolic syndrome. PLoS ONE. 2019;14(4): e0214730.
Desai NR, Shrank WH, Fischer MA, et al. Patterns of medication initiation in newly diagnosed diabetes mellitus: quality and cost implications. Am J Med. 2012;125(3):302.e1-302.e3027. https://doi.org/10.1016/j.amjmed.2011.07.033.
Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577–85. https://doi.org/10.1007/s00125-017-4342-z.
Gauthier MS, O’Brien EL, Bigornia S, et al. Decreased AMP-activated protein kinase activity is associated with increased inflammation in visceral adipose tissue and with whole-body insulin resistance in morbidly obese humans. Biochem Biophys Res Commun. 2011;404(1):382–7. https://doi.org/10.1016/j.bbrc.2010.11.127.
Pan Y, Liu L, Li S, et al. Activation of AMPK inhibits TGF-β1-induced airway smooth muscle cells proliferation and its potential mechanisms. Sci Rep. 2018;8(1):3624. https://doi.org/10.1038/s41598-018-21812-0.
Park SJ, Lee KS, Kim SR, et al. AMPK activation reduces vascular permeability and airway inflammation by regulating HIF/VEGFA pathway in a murine model of toluene diisocyanate-induced asthma. Inflamm Res. 2012;61(10):1069–83. https://doi.org/10.1007/s00011-012-0499-6.
Calixto MC, Lintomen L, André DM, et al. Metformin attenuates the exacerbation of the allergic eosinophilic inflammation in high fat-diet-induced obesity in mice. PLoS ONE. 2013;8(10):e76786. https://doi.org/10.1371/journal.pone.0076786.
Adeshirlarijaney A, Zou J, Tran HQ, Chassaing B, Gewirtz AT. Amelioration of metabolic syndrome by metformin associates with reduced indices of low-grade inflammation independently of the gut microbiota. Am J Physiol Endocrinol Metab. 2019;317(6):E1121–30. https://doi.org/10.1152/ajpendo.00245.2019.
Guo Y, Shi J, Wang Q, et al. Metformin alleviates allergic airway inflammation and increases Treg cells in obese asthma. J Cell Mol Med. 2021;25(4):2279–84. https://doi.org/10.1111/jcmm.16269.
Ma W, Jin Q, Guo H, et al. Metformin ameliorates inflammation and airway remodeling of experimental allergic asthma in mice by restoring AMPKα activity. Front Pharmacol. 2022;13:780148. https://doi.org/10.3389/fphar.2022.780148. [Published correction appears in Front Pharmacol. 2022 Apr 21;13:900127].
Basu R, Basu A, Chandramouli V, et al. Effects of pioglitazone and metformin on NEFA-induced insulin resistance in type 2 diabetes. Diabetologia. 2008;51(11):2031–40. https://doi.org/10.1007/s00125-008-1138-1.
Jahn LA, Hartline L, Liu Z, Barrett EJ. Metformin improves skeletal muscle microvascular insulin resistance in metabolic syndrome. Am J Physiol Endocrinol Metab. 2022;322(2):E173–80. https://doi.org/10.1152/ajpendo.00287.2021.
Kahn SE, Lachin JM, Zinman B, et al. Effects of rosiglitazone, glyburide, and metformin on β-cell function and insulin sensitivity in ADOPT. Diabetes. 2011;60(5):1552–60. https://doi.org/10.2337/db10-1392.
Bermúdez-Pirela VJ, Cano C, Medina MT, et al. Metformin plus low-dose glimeperide significantly improves homeostasis model assessment for insulin resistance (HOMA (IR)) and beta-cell function (HOMA (beta-cell)) without hyperinsulinemia in patients with type 2 diabetes mellitus. Am J Ther. 2007;14(2):194–202. https://doi.org/10.1097/01.pap.0000249909.54047.0e.
Yerevanian A, Soukas AA. Metformin: mechanisms in human obesity and weight loss. Curr Obes Rep. 2019;8(2):156–64. https://doi.org/10.1007/s13679-019-00335-3.
Li CY, Erickson SR, Wu CH. Metformin use and asthma outcomes among patients with concurrent asthma and diabetes. Respirology. 2016;21(7):1210–8. https://doi.org/10.1111/resp.12818.
Wu TD, Keet CA, Fawzy A, Segal JB, Brigham EP, McCormack MC. Association of metformin initiation and risk of asthma exacerbation. A claims-based cohort study. Ann Am Thorac Soc. 2019;16(12):1527–33. https://doi.org/10.1513/AnnalsATS.201812-897OC.
Wu TD, Fawzy A, Akenroye A, Keet C, Hansel NN, McCormack MC. Metformin use and risk of asthma exacerbation among asthma patients with glycemic dysfunction. J Allergy Clin Immunol Pract. 2021;9(11):4014-20.e4. https://doi.org/10.1016/j.jaip.2021.07.007.
Wu TD, Fawzy A, Kinney GL, et al. Metformin use and respiratory outcomes in asthma-COPD overlap. Respir Res. 2021;22(1):70. https://doi.org/10.1186/s12931-021-01658-3.
Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753-864. https://doi.org/10.2337/dci22-0034
[No authors listed]. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352(9131):837–53. [Published correction appears in Lancet 1999 Aug 14;354(9178):602].
Dailey G, Admane K, Mercier F, Owens D. Relationship of insulin dose, A1c lowering, and weight in type 2 diabetes: comparing insulin glargine and insulin detemir. Diabetes Technol Ther. 2010;12(12):1019–27. https://doi.org/10.1089/dia.2010.0063.
Khunti K, Alsifri S, Aronson R, et al. Rates and predictors of hypoglycaemia in 27 585 people from 24 countries with insulin-treated type 1 and type 2 diabetes: the global HAT study. Diabetes Obes Metab. 2016;18(9):907–15. https://doi.org/10.1111/dom.12689.
Terzano C, Morano S, Ceccarelli D, et al. Effect of insulin on airway responsiveness in patients with type 2 diabetes mellitus: a cohort study. J Asthma. 2009;46(7):703–7. https://doi.org/10.1080/02770900903056203.
Chen CZ, Hsu CH, Li CY, Hsiue TR. Insulin use increases risk of asthma but metformin use reduces the risk in patients with diabetes in a Taiwanese population cohort. J Asthma. 2017;54(10):1019–25. https://doi.org/10.1080/02770903.2017.1283698.
Rayner LH, Mcgovern A, Sherlock J, et al. The impact of therapy on the risk of asthma in type 2 diabetes. Clin Respir J. 2019;13(5):299–305. https://doi.org/10.1111/crj.13011.
Wang W, Zhou X, Kwong JSW, Li L, Li Y, Sun X. Efficacy and safety of thiazolidinediones in diabetes patients with renal impairment: a systematic review and meta-analysis. Sci Rep. 2017;7(1):1717. https://doi.org/10.1038/s41598-017-01965-0.
Fonseca V. Effect of thiazolidinediones on body weight in patients with diabetes mellitus. Am J Med. 2003;115(Suppl 8A):42S-48S. https://doi.org/10.1016/j.amjmed.2003.09.005.
Hernandez AV, Usmani A, Rajamanickam A, Moheet A. Thiazolidinediones and risk of heart failure in patients with or at high risk of type 2 diabetes mellitus: a meta-analysis and meta-regression analysis of placebo-controlled randomized clinical trials. Am J Cardiovasc Drugs. 2011;11(2):115–28. https://doi.org/10.2165/11587580-000000000-00000.
Tang H, Shi W, Fu S, et al. Pioglitazone and bladder cancer risk: a systematic review and meta-analysis. Cancer Med. 2018;7(4):1070–80. https://doi.org/10.1002/cam4.1354.
Loke YK, Kwok CS, Singh S. Comparative cardiovascular effects of thiazolidinediones: systematic review and meta-analysis of observational studies. BMJ. 2011;342:d1309. https://doi.org/10.1136/bmj.d1309.
Boden G, Cheung P, Mozzoli M, Fried SK. Effect of thiazolidinediones on glucose and fatty acid metabolism in patients with type 2 diabetes. Metabolism. 2003;52(6):753–9. https://doi.org/10.1016/s0026-0495(03)00055-6.
Soccio RE, Chen ER, Lazar MA. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014;20(4):573–91. https://doi.org/10.1016/j.cmet.2014.08.005.
Zhu M, Flynt L, Ghosh S, et al. Anti-inflammatory effects of thiazolidinediones in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2011;45(1):111–9. https://doi.org/10.1165/rcmb.2009-0445OC.
Chen R, Yan J, Liu P, Wang Z. Effects of thiazolidinedione therapy on inflammatory markers of type 2 diabetes: a meta-analysis of randomized controlled trials. PLoS ONE. 2015;10(4):e0123703. https://doi.org/10.1371/journal.pone.0123703.
Rinne ST, Feemster LC, Collins BF, et al. Thiazolidinediones and the risk of asthma exacerbation among patients with diabetes: a cohort study. Allergy Asthma Clin Immunol. 2014;10(1):34. https://doi.org/10.1186/1710-1492-10-34.
Sood A, Qualls C, Murata A, et al. Potential for repurposing oral hypertension/diabetes drugs to decrease asthma risk in obesity. J Asthma. 2022;1–9. https://doi.org/10.1080/02770903.2022.2097919.
Dixon AE, Subramanian M, DeSarno M, Black K, Lane L, Holguin F. A pilot randomized controlled trial of pioglitazone for the treatment of poorly controlled asthma in obesity. Respir Res. 2015;16:143. https://doi.org/10.1186/s12931-015-0303-6.
Kaler M, Barochia AV, Weir NA, et al. A randomized, placebo-controlled, double-blinded, crossover trial of pioglitazone for severe asthma. J Allergy Clin Immunol. 2017;140(6):1716–8. https://doi.org/10.1016/j.jaci.2017.05.033.
Kudaravalli J, Vijayalakshmi G, Kiran KK. Safety and efficacy of sulfonylurea drugs in type 2 diabetes mellitus. Apollo Med. 2013;10(2):165–8. https://doi.org/10.1016/j.apme.2013.05.002.
Sola D, Rossi L, Schianca GP, et al. Sulfonylureas and their use in clinical practice. Arch Med Sci. 2015;11(4):840–8. https://doi.org/10.5114/aoms.2015.53304.
Hougen I, Whitlock RH, Komenda P, Rigatto C, Clemens KK, Tangri N. Safety of add-on sulfonylurea therapy in patients with type 2 diabetes using metformin: a population-based real-world study. BMJ Open Diabetes Res Care. 2021;9(2):e002352. https://doi.org/10.1136/bmjdrc-2021-002352.
Douros A, Dell’Aniello S, Yu OHY, Filion KB, Azoulay L, Suissa S. Sulfonylureas as second line drugs in type 2 diabetes and the risk of cardiovascular and hypoglycaemic events: population based cohort study. BMJ. 2018;362:k2693. https://doi.org/10.1136/bmj.k2693.
Gallwitz B. Clinical use of DPP-4 inhibitors. Front Endocrinol (Lausanne). 2019;10:389. https://doi.org/10.3389/fendo.2019.00389.
GRADE Study Research Group, Nathan DM, Lachin JM, et al. Glycemia reduction in type 2 diabetes—glycemic outcomes. N Engl J Med. 2022;387(12):1063–74. https://doi.org/10.1056/NEJMoa2200433.
Lee YS, Jun HS. Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells. Metabolism. 2014;63(1):9–19. https://doi.org/10.1016/j.metabol.2013.09.010.
Sell H, Blüher M, Klöting N, et al. Adipose dipeptidyl peptidase-4 and obesity: correlation with insulin resistance and depot-specific release from adipose tissue in vivo and in vitro. Diabetes Care. 2013;36(12):4083–90. https://doi.org/10.2337/dc13-0496.
Dennis JM, Shields BM, Hill AV, et al. Precision medicine in type 2 diabetes: clinical markers of insulin resistance are associated with altered short- and long-term glycemic response to DPP-4 inhibitor therapy. Diabetes Care. 2018;41(4):705–12. https://doi.org/10.2337/dc17-1827.
Lyu X, Zhu X, Zhao B, et al. Effects of dipeptidyl peptidase-4 inhibitors on beta-cell function and insulin resistance in type 2 diabetes: meta-analysis of randomized controlled trials. Sci Rep. 2017;7:44865. https://doi.org/10.1038/srep44865.
Sun J, Chu S, Lu M, et al. The roles of dipeptidyl peptidase-4 and its inhibitors in the regulation of airway epithelial-mesenchymal transition. Exp Lung Res. 2020;46(6):163–73. https://doi.org/10.1080/01902148.2020.1753853.
Ma L, Chang E, Ruan X, Zhang B, Tang F, Zhang J. The protective effects of omarigliptin against lipopolysaccharide (LPS)-induced inflammatory response and expression of mucin 5AC (MUC5AC) in human bronchial epithelial cells. Mol Immunol. 2022;141:108–15. https://doi.org/10.1016/j.molimm.2021.11.013.
Helal MG, Megahed NA, Abd Elhameed AG. Saxagliptin mitigates airway inflammation in a mouse model of acute asthma via modulation of NF-kB and TLR4. Life Sci. 2019;239:117017. https://doi.org/10.1016/j.lfs.2019.117017.
Colice G, Price D, Gerhardsson de Verdier M, et al. The effect of DPP-4 inhibitors on asthma control: an administrative database study to evaluate a potential pathophysiological relationship. Pragmat Obs Res. 2017;8:231–40. https://doi.org/10.2147/POR.S144018.
Wang A, Tang H, Zhang N, Feng X. Association between novel glucose-lowering drugs and risk of asthma: a network meta-analysis of cardiorenal outcome trials. Diabetes Res Clin Pract. 2022;183: 109080. https://doi.org/10.1016/j.diabres.2021.109080.
National Institute of Diabetes and Digestive and Kidney Diseases. Story of discovery: SGLT2 inhibitors: harnessing the kidneys to help treat diabetes. https://www.niddk.nih.gov/news/archive/2016/story-discovery-sglt2-inhibitors-harnessing-kidneys-help-treat-diabetes. Accessed September 14, 2022.
Fonseca-Correa JI, Correa-Rotter R. Sodium-glucose cotransporter 2 inhibitors mechanisms of action: a review. Front Med (Lausanne). 2021;8:777861. https://doi.org/10.3389/fmed.2021.777861.
Heerspink HJL, Perco P, Mulder S, et al. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia. 2019;62(7):1154–66. https://doi.org/10.1007/s00125-019-4859-4.
La Grotta R, de Candia P, Olivieri F, et al. Anti-inflammatory effect of SGLT-2 inhibitors via uric acid and insulin. Cell Mol Life Sci. 2022;79(5):273. https://doi.org/10.1007/s00018-022-04289-z.
Qiu M, Ding LL, Zhan ZL, Liu SY. Use of SGLT2 inhibitors and occurrence of noninfectious respiratory disorders: a meta-analysis of large randomized trials of SGLT2 inhibitors. Endocrine. 2021;73(1):31–6. https://doi.org/10.1007/s12020-021-02644-x.
Vaduganathan M, Patel RB, Singh A, et al. Prescription of glucagon-like peptide-1 receptor agonists by cardiologists. J Am Coll Cardiol. 2019;73(12):1596–8. https://doi.org/10.1016/j.jacc.2019.01.029.
Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(4):740–56. https://doi.org/10.1016/j.cmet.2018.03.001.
Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002. https://doi.org/10.1056/NEJMoa2032183.
Bendotti G, Montefusco L, Lunati ME, et al. The anti-inflammatory and immunological properties of GLP-1 receptor agonists. Pharmacol Res. 2022;182:106320. https://doi.org/10.1016/j.phrs.2022.106320.
Li Z, Li S, Wang N, Xue P, Li Y. Liraglutide, a glucagon-like peptide-1 receptor agonist, suppresses osteoclastogenesis through the inhibition of NF-κB and MAPK pathways via GLP-1R. Biomed Pharmacother. 2020;130: 110523. https://doi.org/10.1016/j.biopha.2020.110523.
Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011;13(1):11–22. https://doi.org/10.1016/j.cmet.2010.12.008.
Zhu T, Wu XL, Zhang W, Xiao M. Glucagon like peptide-1 (GLP-1) modulates OVA-induced airway inflammation and mucus secretion involving a protein kinase A (PKA)-dependent nuclear factor-κB (NF-κB) signaling pathway in mice. Int J Mol Sci. 2015;16(9):20195–211. https://doi.org/10.3390/ijms160920195.
Toki S, Goleniewska K, Reiss S, et al. Glucagon-like peptide 1 signaling inhibits allergen-induced lung IL-33 release and reduces group 2 innate lymphoid cell cytokine production in vivo. J Allergy Clin Immunol. 2018;142(5):1515-28.e8. https://doi.org/10.1016/j.jaci.2017.11.043.
Bloodworth MH, Rusznak M, Pfister CC, et al. Glucagon-like peptide 1 receptor signaling attenuates respiratory syncytial virus-induced type 2 responses and immunopathology. J Allergy Clin Immunol. 2018;142(2):683-7.e12. https://doi.org/10.1016/j.jaci.2018.01.053.
Toki S, Newcomb DC, Printz RL, et al. Glucagon-like peptide-1 receptor agonist inhibits aeroallergen-induced activation of ILC2 and neutrophilic airway inflammation in obese mice. Allergy. 2021;76(11):3433–45. https://doi.org/10.1111/all.14879.
Hur J, Kang JY, Kim YK, Lee SY, Lee HY. Glucagon-like peptide 1 receptor (GLP-1R) agonist relieved asthmatic airway inflammation via suppression of NLRP3 inflammasome activation in obese asthma mice model. Pulm Pharmacol Ther. 2021;67:102003. https://doi.org/10.1016/j.pupt.2021.102003.
Kim HY, Lee HJ, Chang YJ, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med. 2014;20(1):54–61. https://doi.org/10.1038/nm.3423.
Liang L, Hur J, Kang JY, Rhee CK, Kim YK, Lee SY. Effect of the anti-IL-17 antibody on allergic inflammation in an obesity-related asthma model. Korean J Intern Med. 2018;33(6):1210–23. https://doi.org/10.3904/kjim.2017.207.
Khan F, Mat A, Hogan AE, et al. Preliminary asthma-related outcomes following glucagon-like peptide 1 agonist therapy. QJM. 2017;110(12):853–4. https://doi.org/10.1093/qjmed/hcx125.
Foer D, Beeler PE, Cui J, Karlson EW, Bates DW, Cahill KN. Asthma exacerbations in patients with type 2 diabetes and asthma on glucagon-like peptide-1 receptor agonists. Am J Respir Crit Care Med. 2021;203(7):831–40. https://doi.org/10.1164/rccm.202004-0993OC.
Albogami Y, Cusi K, Daniels MJ, Wei YJ, Winterstein AG. Glucagon-like peptide 1 receptor agonists and chronic lower respiratory disease exacerbations among patients with type 2 diabetes. Diabetes Care. 2021;44(6):1344–52. https://doi.org/10.2337/dc20-1794.
López-Cano C, Ciudin A, Sánchez E, et al. Liraglutide improves forced vital capacity in individuals with type 2 diabetes: data from the randomized crossover LIRALUNG study. Diabetes. 2022;71(2):315–20. https://doi.org/10.2337/db21-0688.
Rogliani P, Matera MG, Calzetta L, et al. Long-term observational study on the impact of GLP-1R agonists on lung function in diabetic patients. Respir Med. 2019;154:86–92. https://doi.org/10.1016/j.rmed.2019.06.015.
Cahill KN. Glucagon-like peptide-1 receptor agonist in the treatment of adult, obesity-related, symptomatic asthma (GATA-3) (GATA-3). ClinicalTrials.gov identifier: NCT05254314. Updated 14 Sep 2022. https://clinicaltrials.gov/ct2/show/NCT05254314. Accessed 28 Oct 2022.
Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–16. https://doi.org/10.1056/NEJMoa2206038.
Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503–15. https://doi.org/10.1056/NEJMoa2107519.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
Katherine N Cahill contributed to the conception of this review. Material preparation and writing of the first draft of the manuscript and figures was done by Derek Ge. All authors critically reviewed and approved the final manuscript.
Disclosures
Derek Ge declares that he has no competing interests. Dinah Foer has received research grants from the NIH and CRICO. Katherine N. Cahill reports investigational product support from Novo Nordisk.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ge, D., Foer, D. & Cahill, K.N. Utility of Hypoglycemic Agents to Treat Asthma with Comorbid Obesity. Pulm Ther 9, 71–89 (2023). https://doi.org/10.1007/s41030-022-00211-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41030-022-00211-x