Abstract
Obstructive sleep apnea (OSA) is estimated to occur in 26% of adults and 2% to 7% of children. OSA is characterized by a partial or complete cessation of airflow in the upper airway. Classically, the main risk factors include obesity, age, and gender, although those outside the “overweight, middle-aged man” phenotype can certainly be at risk for the development of OSA, particularly when predisposing anatomic abnormalities are encountered. Common symptoms include excessive daytime sleepiness, snoring, witnessed apneas, choking or gasping, and unrefreshing sleep. OSA has also been linked to many systemic pathology including pulmonary and cardiovascular disease, neurocognitive and neuropsychiatric impairments, metabolic dysregulation, and ophthalmologic disorders. Due to this potential for influencing multi-system disease, accurate diagnosis and treatment is essential. Treatment methods are continuously developing and improving, but the traditional, gold standard treatment is positive airway pressure (PAP) treatment. There are several modalities within this category including continuous PAP and bilevel PAP. Other treatment alternatives which can improve OSA include upper airway surgery, orthodontic therapy, mandibular advancement devices, and weight loss. Novel treatments that target upper airway muscle tone include hypoglossal nerve stimulation and myofunctional exercises. The goal of this article was to summarize key aspects of patient presentation, potential comorbidities, and therapeutic options for multidisciplinary clinicians who play an integral role in the management of this syndrome from childhood to old-age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) syndrome is characterized by repetitive episodes of either partial (hypopnea) or complete (apnea) collapse of the upper airway with associated symptoms [1]. Increased resistance (due to anatomic factors such as nasal obstruction, lymphoid tissue hypertrophy) and increased compliance (increased collapsibility during inspiration) of the upper airway are among the factors leading to obstruction during sleep [2]. These events may lead to oxyhemoglobin desaturations, carbon dioxide retention, and arousal from sleep [2, 3]. Patients with OSA most commonly complain of daytime sleepiness and fatigue. Complaints may also include snoring, choking or gasping, witnessed apneas, morning headaches, and non-restorative sleep [2, 4].
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Risk Factors
Anatomy, obesity, gender, and age are all important risk factors when screening patients for OSA. Anatomically, many OSA patients often have a smaller upper airway compared to normal subjects. Deficits in maxillary and mandibular development can restrict the size of the upper airway [2, 4, 5]. An increase in the size of the soft tissues such as the tongue and lateral pharyngeal walls causes a decrease in airway circumference, thus increasing resistance [2, 4, 5]. Presence of lymphoid tissue (adenoids, tonsils) is an important anatomic finding that may increase the risk of OSA, particularly in the pediatric population [4]. A careful physical exam with attention to these factors is important when considering a diagnosis of OSA.
OSA does indeed have an intimate association with obesity. Studies have linked obesity with increased fat deposits in the lateral parapharyngeal fat pads and the tongue, which reduces the pharyngeal airway size and increases the size of the soft tissues, respectively [2, 4]. This becomes problematic because the tongue plays an important role in pharyngeal dilation, but fat deposits may inhibit muscle function as well as decrease airway size and increase collapsibility [6].
Gender differences have also been implicated as factors in OSA. Women have smaller upper airway, neck, tongue, and soft tissue sizes than men [4]. During puberty, adolescent boys have a longer pharyngeal airway, resulting in increased airway collapsibility [7, 8].
Aging also impacts OSA development for several reasons. Studies have reported that aging increases the risk of developing OSA due to shortening of the anterior–posterior length of the structures around the pharynx. This, in turn, may affect reflexes controlling airway patency, allowing the pharyngeal airway to become more collapsible. Furthermore, increased fat deposits around the upper airway during aging can lead to restricted air flow as seen in obesity. Moreover, whereas males show increased pharyngeal airway length early in life, females develop long pharyngeal airways and larger tongues around the time of menopause [8].
Diagnosis
While OSA remains persistently underdiagnosed, recent estimates suggest that 27% of men and 11% of women between the ages of 30 and 70 have OSA [9]. OSA can occur at any age (2% to 7% of children) but is more common in middle and older age [2, 5]. Underdiagnosis of OSA is common, with symptoms associated with the disorder often mistakenly attributed to other neurological or psychiatric disorders [10]. It is thus important for healthcare professionals to be aware of the symptoms and treatments for OSA, as this disorder can severely diminish quality of life. The importance of accurate diagnosis is perhaps not better highlighted than with the risk of drowsy driving and motor vehicle collisions in untreated patients with OSA [11].
In addition to salient features of the history and physical, diagnostic polysomnography is an essential tool in the diagnosis of OSA (Table 1). Breathing events can be quantified during polysomnography, with summary reports including measurements such as the Apnea Hypopnea Index (AHI: number of apneas in addition to hypopneas per hour) and Oxygen Desaturation Index (ODI: number of oxyhemoglobin desaturations per hour observed during monitoring). Obstructive apneas are noted when there is at least a 90% drop in airflow associated with ongoing respiratory muscle effort [3]. Hypopneas are scored when there is at least a 30% drop in airflow, resulting in either an associated oxyhemoglobin desaturation or electroencephalographic arousal [3]. Other abnormalities in airflow measurements (such as flow limitation) leading to disruption of sleep not meeting the above criteria may also be reported as part of a Respiratory Disturbance Index (RDI) [3]. Through these measurements, an interpretive report attempts to summarize the presence and severity of sleep apnea from the overnight recording. This report, in addition to the history and physical examination, helps the clinician to make a diagnosis of OSA. [3]. By confirming the diagnosis and initiating treatment, the clinician can address the patients’ chief complaints, as well as mitigate the potential impacts of OSA on systemic disease.
OSA and Associated Morbidity: More than Just Snoring
The growing associations between OSA and systemic diseases have broadened the rationale for therapy beyond simply targeting snoring and daytime sleepiness. As a result, referrals to sleep physicians now come from a variety of sources, including both primary care providers as well as sub-specialists such as pulmonologists, neurologists, cardiologists, psychiatrists, endocrinologists, and ophthalmologists.
Cardiovascular Disease
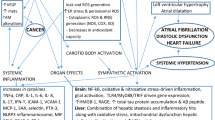
An increase in cardiovascular disease is predicted by the presence of OSA. This has been suggested to be mediated by a variety of mechanisms including increased sympathetic tone [12], endothelial dysfunction [13, 14], and platelet aggregation [15]. As a result, referrals to sleep physicians are increasingly common from primary care providers and cardiovascular specialists hoping to better address their patients’ risk factors for hypertension, stroke, and arrhythmia [16].
A dose–response association between the number of breathing events per hour on a diagnostic polysomnogram and the incidence of hypertension at four-year follow-up was demonstrated among participants in the Wisconsin Sleep Cohort Study [17]. While the odds ratios (ORs) for incident hypertension were strongest among those with an AHI of 15 events per hour or more [OR 2.89, 95% confidence interval (CI) 1.45–5.64], there was a significant association even among those with an AHI of 0.1 to 4.9/h (OR 1.42, 95% CI 1.13–1.78) relative to subjects with an AHI of 0/hr. Cardiac arrhythmias, including atrial fibrillation, are known to be associated with the presence of OSA [18] with some evidence suggesting that the treatment of OSA may reduce the frequency of arrhythmias [19]. Additionally, patients with OSA are at an increased risk (relative risk 2.57) of sudden cardiac death during typical sleeping hours (12:00AM–5:59AM) contrary to those without OSA who were more likely to have a fatal event in the morning hours (6:00AM–11:59AM) [20]. Treatment of OSA with continuous positive airway pressure (CPAP) has been associated with a decreased risk of fatal and non-fatal cardiovascular events [21].
Neurocognitive and Neuropsychiatric Dysfunction
While excessive daytime sleepiness is a cardinal daytime symptom among patients with OSA, other neurocognitive and neuropsychiatric complaints are frequently reasons for presentation to the sleep clinic. An analysis of 190 subjects with OSA demonstrated that patients often reported “lack of energy” or “tiredness” as opposed to “sleepiness” as their principal daytime complaint [22]. Treatment with CPAP demonstrated significant improvement in objective measurements of fatigue and energy compared to placebo-CPAP after only 3 weeks of therapy [23].
OSA has also been associated with impairments in cognitive function, including the domains of executive function, memory, and attention [24–27]. In the pediatric population, symptoms of OSA and attention deficit hyperactivity disorder overlap, and screening for sleep-disordered breathing should be considered in this population [28]. The association between OSA and the development of dementia has also been a focus of recent attention, with one study suggesting that older women with an ODI of >15/h were at an increased risk for developing dementia (OR 2.04, 95% CI 1.10–3.78) [29]. A small study including participants with mild to moderate Alzheimer’s disease who continued with CPAP use through a three-year follow-up demonstrated less cognitive decline compared to subjects who discontinued therapy [30].
An association between OSA and depression has also been suggested in the literature. Participants in the Wisconsin Sleep Cohort Study demonstrated a relationship between sleep apnea severity at baseline and future risk of developing depressive symptoms [31]. A review of the National Health and Nutrition Examination Survey 2005–2008 survey data also suggests an association between survey-based indicators of OSA and depression in a sample of 11,329 adults [32]. However, the data are mixed with regard to the effects of treatment of OSA on outcomes associated with depression [33, 34]. Kawahara et al. found that CPAP treatment lessened participants’ scores on the Epworth Sleepiness Scale (ESS) and the Sung self-depression scale [33]. Gagnadoux et al. reported that after 1 year of CPAP treatment, persistent depressive symptoms were not resolved by treatment but were associated with ESS score [34]. This suggests that CPAP therapy may help with depressive symptoms if it is effective in lowering the ESS score.
Pulmonary Disease
While the relationship between OSA and obstructive lung diseases such as asthma and chronic obstructive pulmonary disease (COPD) is not fully understood, studies have demonstrated the importance of OSA diagnosis and treatment in patients with obstructive lung diseases. The Sleep Heart Health study looking at the overlap between OSA and COPD reported that patients who had both airflow obstruction as well as a RDI >10/h scored higher on the ESS, had less sleep time and efficiency, and had more oxygen desaturations compared to patients with either airflow obstruction or an RDI >10 [35].
Metabolic Dysregulation
Insulin resistance and impaired glucose metabolism have been shown to be associated with OSA, even when controlling for body mass index [36, 37]. Researchers have proposed that OSA increases stress on the body leading to the development of these conditions. Treatment of OSA has been associated with improvements in insulin resistance [38]. Patterns of fat distribution also may be driven by the presence of OSA, with one study suggesting that increased visceral fat deposition is more strongly associated with obese patients with OSA than obese controls [39].
Ophthalmologic Disorders
Ophthalmologic disorders and the potential link to OSA has been a target of increasing focus. The bulk of the focus has targeted the potential association between OSA and the development of glaucoma, with mixed data suggesting both the presence [40, 41] and absence [42] of a significant link. One study based in France found no difference in incidence of glaucoma between participants with and without OSA [42]. However, another study based in Taiwan found that within a five-year follow-up period participants with OSA had a 1.67 hazard ratio for glaucoma compared to participants without OSA [40]. An association between OSA and other ophthalmologic disorders, such as floppy eyelid syndrome [43, 44], optic neuropathy [45, 46], and papilledema [47] has also been suggested in the literature.
Treatment Options
CPAP: Tried-and-True
CPAP is currently the most effective treatment for OSA and is regarded as the gold standard [48]. Sullivan, in 1981, first demonstrated that CPAP prevents obstructive events by maintaining upper airway patency during sleep with delivered air pressure [49]. Goel et al. report specifically that using CPAP for a minimum of 4 h significantly improved symptoms such as daytime sleepiness and exercise capacity [50]. On the other hand, Weaver et al. showed that the cut-off point of 4 h is artificial, and all night usage is the goal [51]. Following 1 year of CPAP use, studies have demonstrated significant improvement in quality of life, decrease in ESS score, and improvement in blood pressure [52, 53].
Throughout the past decades, PAP therapy has made vast improvements not only in pressure delivery, patient comfort, and accessibility. Older devices were large, heavy, loud, and did not provide feedback on compliance or effectiveness (Fig. 1). Devices are now much smaller, portable, and quiet. Current machines can report usage, mask fit, and AHI to the patient and prescriber. In addition, newer devices allow physicians to remotely monitor use and efficacy, as well as adjust settings. Many devices now have algorithms to auto-adjust pressures in relation to detected obstructive events. These devices can increase pressures during periods of sleep where obstruction may be more prominent (e.g., rapid eye movement sleep or supine position sleep), and decrease when obstruction is less problematic. This may potentially increase the tolerability of PAP therapy [54].
One of the first continuous positive airway pressure (CPAP) machines used in the United States, weighing 12 kilograms. Equipment was created at the Stanford Sleep Clinic in December 1981 based on advice from Colin Sullivan, MD (University of Sydney, Australia). Newer PAP devices are small, portable, quiet, and provide objective feedback on use and efficacy
While PAP therapy has evolved, adherence remains as a primary obstacle to therapy. Factors impacting adherence include nasal resistance, optimal pressures, mask fit, and adverse side effects [55]. To mitigate these issues, allergies, nasal congestion, and nasal septum deviation should be well treated. Physicians should work with patients to find the most comfortable and effective pressures, proper mask fittings should be employed, and side effects should be minimalized. Sleeping positions such as elevation of head and trunk may help reduce nasal resistance [56].
Alternatives to PAP Therapy: It Takes a Village
While PAP therapy is considered the treatment of choice for OSA, alternative therapies may be a consideration based on treatment preference and disease context [48]. Pursuing these treatment options requires a multi-disciplinary approach, involving otolaryngologists, general surgeons, orthodontists, oral and maxillofacial surgeons, dentists, and nutritionists.
Upper Airway Surgery
Surgical interventions, particularly in the realm of pediatrics, are a mainstay of therapy in the management of OSA. The American Academy of Pediatrics recommends adenotonsillectomy for those with evidence of hypertrophy without contraindication to surgery as the first line of treatment for pediatric OSA [57]. For adults (and much less commonly in select pediatric cases), uvulopalatopharyngoplasty (UPPP) and tongue base reduction surgeries may also be a consideration. However, surgical improvement (defined as an AHI of <20/h and a decrease of 50% from baseline) was noted in just over 50% of patients with UPPP in one case series [58]. Even among those with surgical improvement, some residual disease may remain. Much of this is due to the fact that soft tissue surgeries may not address the underlying skeletal abnormalities that predispose these patients to the development of upper airway obstruction.
As a result, further attention has been directed to skeletal based surgeries. Maxillomandibular advancement (MMA), achieved by osteotomy of the maxilla and mandible, increases airway dimensions and decreases airway resistance. A meta-analysis demonstrated favorable surgical success rates (86.0%) for patients undergoing the procedure [59]. Moreover, 43% of patients attained surgical cure (AHI <5/h), with the mean AHI decreasing from 63.9 (±26.7/h) pre-MMA to 9.5 (±10.7/h) post-MMA surgery.
Orthodontic Therapy
Rapid maxillary expansion in pediatric orthodontics has played an important role in the management of malocclusion and dental crowding. However, recent evidence suggests that palatal expansion plays an important role in the management of pediatric OSA, taking advantage of the not yet fused maxillary suture. Maxillary expansion has been associated with cephalometric increases in nasal base and nasal cavity, thereby decreasing total airway resistance [60]. A study involving 31 children between the ages of 5 and 8 who underwent rapid maxillary expansion resulted in AHI improvement that continued through adulthood [61] (Fig. 2). Surgically assisted rapid maxillary expansion may be a consideration in adults once the palatal suture has fused, with data suggesting a 56% reduction in AHI and significant improvement in validated measures of sleepiness after the procedure [62].
Mandibular Advancement Devices
Oral appliances, targeting anterior advancement of the mandible, have been used as an alternative to PAP therapy for decades [63]. These custom devices, fashioned by a dentist, work by enlarging the oropharyngeal airway. These can be considered as an initial treatment for patients with mild to moderate OSA who elect to defer treatment with PAP therapy [48]. The use of Mandibular Advancement Devices (MAD) was associated with a 62.5% improvement in AHI among patients with OSA in a randomized controlled trial, with 37.5% demonstrating complete response (a treatment AHI <5/h) [64]. Another randomized controlled trial demonstrated improvement in measures of objective and subjective sleepiness with MAD use [65]. While this may be an attractive option for patients, potential limitations such as aggravation of the temporomandibular joint, occlusal changes, tooth discomfort, and sub-optimal therapy may make this a less desirable treatment option for some patients [66].
Weight Loss and Bariatric Surgery
Obesity has been consistently shown to be an independent (although not the solitary) predictor of OSA. While achieving weight loss through behavioral or dietary interventions may be challenging, small reductions in weight can make a significant impact on the severity of associated OSA. In a prospective cohort of 690 subjects followed over a 4-year period, a 10% reduction in body weight was associated with a 26% decrease in AHI (a 20% reduction was associated with a 48% decrease in AHI) [67]. As a result, attention to weight reduction should be a part of the treatment discussion for overweight and obese patients with OSA. The role of bariatric surgery should be part of this discussion. A clinical trial comparing lifestyle modification to bariatric surgery among 133 morbidly obese patients with OSA demonstrated favorable improvements in weight status (30% vs. 8% weight reduction) and AHI among those undergoing surgical intervention [68]. Among those undergoing bariatric surgery, 66% had remission of OSA.
Emerging Therapies for OSA: Strength in Muscles
While many of the previously discussed therapies target mechanical solutions to upper airway obstruction, attention is being directed towards emerging strategies that address underlying neuromuscular weakness that is a major component of OSA.
Myofunctional Therapy
Myofunctional therapy, utilizing a series of coordinated exercises to strengthen orofacial muscles, has been discussed in the literature as a means of targeting orthodontic issues such as malocclusion for a century [69]. The effects of adjacent muscles on occlusal status and jaw growth suggest that there may be a role for targeting tone and position of these muscles to improve airway patency during sleep in patients with OSA [70]. A number of studies have now suggested demonstrable improvements in objective measures among patients with OSA undergoing a regimented myofunctional exercise program [70–72]. A recent meta-analysis of myofunctional therapy in the treatment of OSA demonstrated a reduction in AHI by 50% in adults and 62% in children [73]. This suggests an important treatment and prevention option, particularly among children where the trajectory of jaw growth is still dynamic. Furthermore, a common source of airway obstruction during sleep is when the tongue relaxes and shifts into a more posterior position, thus blocking the airway. Myofunctional therapy trains and strengthens the tongue muscle to prevent this obstruction.
Hypoglossal Nerve Stimulation
Collapsibility of the upper airway muscles (particularly the genioglossus) is thought to be a major contributing factor to OSA, and contraction has been demonstrated to improve airway patency [74, 75]. In recent years, implantable Hypoglossal Nerve Stimulation (HNS) has been approved for use in patients who fail first line therapies for OSA. A meta-analysis including 200 patients who underwent implantation of a HNS demonstrated an approximate 50% reduction in AHI and ODI following placement [76]. The generalizability of these data remains to be seen as most studies had very stringent inclusion criteria [76]. However, this may be a reasonable option for patients who have failed alternative modalities of therapy.
Conclusion
The impacts of untreated OSA on systemic health cannot be understated. As the pathophysiology of OSA is further elucidated, the impacts on health will likely only become more profound. As a result, identification of patients by primary care providers and sub-specialists will be essential in managing co-morbid and associated conditions.
Given the anatomic and skeletal influences on the development of OSA, recognizing the process early in childhood may allow for an opportunity to reverse this trajectory. Pediatricians and orthodontists may have a unique role in managing this preventative strategy by identifying patients who may benefit from treatment options such as myofunctional therapy and palatal expansion that address underlying issues with jaw growth.
Personalized medicine will likely play a more pivotal role in the management of patients with OSA. As treatment options expand, a “one size fits all” approach will no longer suffice. As such, a multi-disciplinary team will be essential to maximizing treatment efficacy for patients with OSA.
References
Douglas N, Polo O. Pathogenesis of obstructive sleep apnoea/hypopnea syndrome. Lancet. 1994;344:653–5.
Deegan P, McNicholas W. Pathophysiology of obstructive sleep apnoea. Eur Respir J. 1995;8:1161–78.
Berry R, Budhiraja R, Gottlieb D, et al. Rules for scoring respiratory events in sleep: update on the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2010;8:597–619.
Schwab R, Kuna S, Remmers J. Anatomy and physiology of upper airway obstruction. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia: Elsevier Saunders; 2005. p. 983–93.
Guilleminault C, Akhtar F. Pediatric sleep-disordered breathing: new evidence on its development. Sleep Med Rev. 2014;24:46–56.
Kim AM, Keenan BT, Jackson N, et al. Tongue fat and its relationship to obstructive sleep apnea. Sleep. 2014;37:1639–48.
Eckert K, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:144–53.
Malhotra A, Huang Y, Fogel R, Lazic S, Pillar G, Jakab M. Aging influences on pharyngeal anatomy and physiology: the predisposition to pharyngeal collapse. Am J Med. 2006;119:72.e9–72.14.
Peppard P, Young T, Barnet J, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14.
Stores G. Clinical diagnosis and misdiagnosis of sleep disorders. J Neurol Neurosurg Psychiatry. 2007;78:1293–7.
George C. Sleep apnea, alertness, and motor vehicle crashes. Am J Respir Crit Care Med. 2007;10:954–6.
Aydin M, Altin R, Oeren A, Kart L, Bilge M, Unalacak M. Cardiac autonomic activity in obstructive sleep apnea: time-dependent and spectral analysis of heart rate variability using 24-hour Holter electrocardiograms. Tex Heart Inst J. 2004;31:132–6.
Foster G, Poulin M, Hanley P. Intermittent hypoxia and vascular function: implications for obstructive sleep apnoea. Exp Physiol. 2007;92:51–65.
Lurie A. Endothelial dysfunction in adults with obstructive sleep apnea. Adv Cardiol. 2011;46:139–70.
Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation scientific statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. Circulation. 2008;118:1080–111.
Oga T, Chin K, Tabuchi A, et al. Effects of obstructive sleep apnea with intermittent hypoxia on platelet aggregability. J Atheroscler Thromb. 2009;16:862–9.
Peppard P, Young T, Palta M, Skaturd J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84.
Guilleminault C, Connoly S, Winkle R. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol. 1983;52:490–4.
Bazan V, Grau N, Valles E, et al. Obstructive sleep apnea in patients with typical atrial flutter: prevalence and impact on arrhythmia control outcome. Chest. 2013;143:1277–83.
Cami A, Howard D, Olson E, Somers V. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–14.
Marin J, Carrizo S, Vicente E, Agusti A. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53.
Chervin R. Sleepiness, fatigue, tiredness, and lack of energy in obstructive sleep apnea. Chest. 2000;118:372–9.
Tomfohr L, Ancoli-Israel S, Loredo J, Dimsdale J. Effects of continuous positive airway pressure on fatigue and sleepiness in patients with obstructive sleep apnea: data from a randomized controlled trial. Sleep. 2001;34:121–6.
Findley L, Barth J, Powers D, Wilhoit S, Bod D, Suratt P. Cognitive impairment in patients with obstructive sleep apnea and associated hypoxemia. Chest. 1986;90:686–90.
Saunamaki T, Jehkonen M. A review of executive functions in obstructive sleep apnea syndrome. Acta Neurol Scand. 2007;115:1–11.
Beebe D, Gozal D. Obstructive sleep apnea and the pre-frontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioural deficits. J Sleep Res. 2002;11:1–16.
Naegele B, Thouvard V, Pepin J, et al. Deficits of cognitive executive functions in patients with sleep apnea syndrome. Sleep. 1995;18:43–52.
Sedky K, Bennet D, Carvalho K. Attention deficit hyperactivity disorder and sleep disordered breathing in pediatric populations: a meta-analysis. Sleep Med Rev. 2014;18:349–56.
Yaffe K, Laffan A, Harrison S, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2001;306:613–9.
Cooke J, Ayalon L, Palmer B, et al. Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer’s disease and obstructive sleep apnea: a preliminary study. J Clin Sleep Med. 2009;5:305–9.
Peppard P, Szklo-Coxe M, Hla K, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med. 2006;166:1709–19.
Hayley A, Williams L, Venugopal K, Kennedy G, Berk M, Pasco J. The relationships between insomnia, sleep apnoea and depression: findings from the American National Health and Nutrition Examination Survey, 2005–2008. Aust N Z J Psychiatry. 2015;49:156–70.
Kawahara S, Akashiba T, Akahoshi T, Horie T. Nasal CPAP improves the quality of life and lessens the depressive symptoms in patients with obstructive sleep apnea syndrome. Intern Med. 2005;44:422–7.
Gagnadoux F, Le Vaillant M, Goupil F, et al. Depressive symptoms before and after long-term CPAP therapy in patients with sleep apnea. Chest. 2014;145:1025–31.
Mieczkowski B, Ezzie ME. Update on obstructive sleep apnea and its relation to COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:349–62.
Ip M, Lam B, Ng M, Lam W, Tsang K, Lam K. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–6.
Baburao A, Souza G. Insulin resistance in moderate to severe obstructive sleep apnea in nondiabetics and its response to continuous positive airway pressure treatment. N Am J Med Sci. 2014;6:500–4.
Chen L, Pei J, Chen H. Effects of continuous positive airway pressure treatment on glycaemic control and insulin sensitivity in patients with obstructive sleep apnoea and type 2 diabetes: a meta-analysis. Arch Med Sci. 2014;10:637–42.
Vgontzas A, Papanicolaou D, Bixler E, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–8.
Lin C, Hu C, Ho J, Chiu H, Lin H. Obstructive sleep apnea and increased risk of glaucoma: a population-based matched-cohort study. Ophthalmology. 2013;120:1559–64.
Hashim S, Al Mansouri F, Farouk M, Al Hashemi A, Singh R. Prevalence of glaucoma in patients with moderate to severe obstructive sleep apnea: Ocular morbidity and outcomes in a 3 year follow-up study. Eye (Lond). 2014;28:1304–9.
Aptel F, Chiquet C, Tamisier R, et al. Association between glaucoma and sleep apnea in a large French multicenter prospective cohort. Sleep Med. 2014;15:576–81.
McNab A. Floppy eyelid syndrome and obstructive sleep apnea. Ophthal Plast Reconstr Surg. 1997;13:98–114.
Robert PY, Adenis JP, Tapie P, et al. Eyelid hyperlaxity and obstructive sleep apnea (O.S.A.) syndrome. Eur J Ophthalmol. 1997;7:211–5.
Mojon DS, Hedges TR, Ehrenberg B, et al. Association between sleep apnea syndrome and nonarteritic anterior ischemic optic neuropathy. Arch Ophthamol. 2002;120:601–5.
Li J, McGwin G, Vaphiades MS, Owsley C. Non-arteritic anterior ischemic optic neuropathy and presumed sleep apnea syndrome screened by the sleep apnea scale of the sleep disorders questionnaire (SA-SDQ). Br J Ophthalmol. 2007;91:1524–7.
Purvin V, Kawasaki A, Yee R. Papilledema and obstructive sleep apnea syndrome. Arch Ophthalmol. 2000;118:1626–30.
Epstein L, Kristo D, Strollo P, et al. Clinical guideline for the evaluation, management, and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76.
Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–5.
Goel A, Talwar D, Jain S. Evaluation of short-term use of nocturnal nasal continuous positive airway pressure for a clinical profile and exercise capacity in adult patients with obstructive sleep apnea-hypopnea syndrome. Lung India. 2015;32:225–32.
Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–9.
Balk E, Moorthy D, Obadan NO, et al. Diagnosis and treatment of obstructive sleep apnea in adults. Comparative Effectiveness Reviews. 2011;32.
Rizzi CF, Ferraz MB, Poyares D, Tufik S. Quality-adjusted life-years gain and health status in patients with OSAS after one year of continuous positive airway pressure use. Sleep. 2014;37:1963–8.
Ip S, D’Ambrosio C, Patel K et al. Auto-titrating versus fixed continuous positive airway pressure for the treatment of obstructive sleep apnea: a systematic review with meta-analyses. Syst Rev. 2012;10:1–24.
Sawyer AM, Gooneratne N, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15:343–56.
Toh ST, Lin CH, Guilleminault C. Usage of 4-phase high resolution rhinomanometry and measurement of nasal resistance in sleep-disordered breathing. Laryngoscope. 2012;122:2343–9.
Marcus C, Brooks L, Draper K, et al. Clinical practice guideline: diagnosis and management of obstructive sleep apnea syndrome. Pediatrics. 2012;130:576–84.
Xiong Y, Yi H, Yin S, et al. Predictors of surgical outcomes of uvulopalatopharyngoplasty for obstructive sleep apnea hypopnea syndrome. Otolaryngol Head Neck Surg. 2011;145:1049–54.
Holty J, Guilleminault C. Maxillomandibular advancement for the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2010;14:287–97.
Baratieri C, Alves M, Mattos C, Lau G, Nojima L, de Souza M. Transverse effects on the nasomaxillary complex one year after rapid maxillary expansion as the only intervention: a controlled study. Dent Press J Orthod. 2014;19:79–87.
Pirelli P, Saponara M, Guilleminault C. Rapid maxillary expansion (RME) for pediatric obstructive apnea: a 12-year follow-up. Sleep Med. 2015;16:933–5.
Vinha P, Eckeli A, Faria A, Xavier S, Mello-Filho F. Effects of surgically assisted rapid maxillary expansion on obstructive sleep apnea and daytime sleepiness. Sleep Breath. 2015;1–8. doi:10.1007/s11325-015-1214-y.
Schmidt-Nowara WW, Meade TE, Hays MB. Treatment of snoring and obstructive sleep apnea with a dental orthosis. Chest. 1991;99:1378–85.
Mehta A, Qian J, Petocz P, Darendeliler M, Cistuli P. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1457–61.
Gotsopoulos H, Chen C, Qian J, Cistulli PA. Oral appliance therapy improves symptoms in obstructive sleep apnea: a randomized, controlled trial. Am J Respir Crit Care Med. 2002;166:743–8.
Fritsch K, Iseli A, Russi E, Bloch K. Side effects of mandibular advancement devices for sleep apnea treatment. Am J Respir Crit Care Med. 2001;64:813–8.
Peppard P, Young T, Palta M, Dempsey J, Skaturd J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–21.
Fredheim JM, Rollheim J, Sandbu R, et al. Obstructive sleep apnea after weight loss: a clinical trial comparing gastric bypass and intensive lifestyle intervention. J Clin Sleep Med. 2013;9:427–32.
Rogers A. Exercises for the development of the muscles of the face, with a view to increasing their functional activity. Dent Cosm. 1918;60:857–76.
Guimaraes K, Drager L, Genta P, Marcondes B, Lorenzi-Filho G. Effects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2009;179:962–6.
Guilleminault C, Huang Y, Monteyrol P, Sato R, Quo S, Lin C. Critical role of myofascial reeducation in pediatric sleep-disordered breating. Sleep Med. 2013;14:518–25.
Suzuki H, Watanabe A, Akihiro Y, et al. Pilot study to assess the potential of oral myofunctional therapy for improving respiration during sleep. J Prosthodont Res. 2013;57:195–9.
Camacho M, Certal V, Abdullatif J, et al. Myofunctional therapy to treat obstructive sleep apnea: a systematic review and meta-analysis. Sleep. 2015;38:669–75.
Guilleminault C, Powell N, Bowman B, Stoohs R. The effect of electrical stimulation on obstructive sleep apnea syndrome. Chest. 1995;107:67–73.
Oliven A, Tov N, Geitini L, et al. Effect of genioglossus contraction on pharyngeal lumen and airflow in sleep apnoea patients. Eur Respir J. 2007;30:748–58.
Certal V, Zaghi S, Riaz M, et al. Hypoglossal nerve stimulation in the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope. 2015;125:1254–64.
Acknowledgments
No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Mustafa Bseikri, Lauren Lo and Christian Guilleminault have nothing to disclose.
Compliance with ethics guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bseikri, M., Lo, L. & Guilleminault, C. Obstructive Sleep Apnea: A Syndrome from Childhood to Old-Age. Pulm Ther 1, 31–42 (2015). https://doi.org/10.1007/s41030-015-0005-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41030-015-0005-8