Abstract
Partial replacement of Portland cement by supplementary cementitious materials results in decreased concentration of calcium hydroxide, leading to a reduction in the pH of the pore solution and influencing the formation of passive films on steel. Decreases in pH are often equated to lower-quality passive films, but, as has been recently shown, that is not always the case. This study evaluates the effect of partial replacement of cement Portland by silica fume, both on the pH of pore solution and corrosion resistance of reinforced concrete. Three concrete mixtures with variations of cement replacement by silica fume (0, 5 and 10% by volume) were produced. Pore solution chemical composition, corrosion current density and polarization resistance were measured up to 91 days. Results show that, especially in early ages—when concrete resistivity remains low and unchanged among the different mixtures tested—partial replacement of cement by silica fume led to an improved passivation process and increased corrosion resistance, even with a related decrease in calcium hydroxide concentration and pH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Currently, a greater durability of reinforced concrete structures is required due to problems originated by corrosion of the steel reinforcement, new competitive requirements and sustainability demands from the civil construction field. The cement industry has become aware of the necessity of a sustainable thinking partly because, for each kilogram of clinker produced, nearly 0.9 kg of CO2 are released into the atmosphere [1]. If a coproduct or industrial waste is incorporated in the cementitious matrix, the average of carbon dioxide emissions is inferior [2] due to the reduction in the clinker consumption. The utilization of pozzolanic materials such as fly ash and silica fume in the composition of cementitious materials generally exhibits economic, technical and environmental feasibility [3]. Regarding the durability of reinforced concrete structures, the corrosion process is one of the main determining agents of the structure’s service life. Such deterioration is developed in the reinforcement steel through spontaneous electrochemical reactions. In case there are no mechanisms that prevent the development of corrosion, additional resources will be necessary for replacing the deteriorated materials [4–6].
When a polozzanic admixture, rich in amorphous silicon, in used the concrete, the calcium hydroxide formed during cement hydration reacts with such admixture and alters the physical and chemical characteristics of the cement paste [7]. The chemical alteration occurs with the consumption of calcium hydroxide by the pozzolan, originating hydrated calcium silicate (C–S–H), enhancing resistance of the cementitious paste but reducing the alkalinity of the pore solution [8, 9]. The physical effects are caused by the change in the microstructure and in the interfacial transition zone of concrete, which fills the pores left by the cement particulates, improves the distribution of the size of the pores, increases the resistance and the electrical resistivity and reduces the permeability of the system [7, 10].
The liquid phase of concrete typically shows a pH between 12.5 and 13.3, favoring the formation of the compact passive film on the surface of the reinforcing steel [11]. When there is a disruption in the system by some external event, the pH of concrete and steel can be reduced, which can possibly lead to a deterioration of the passive film and culminate in corrosion processes [7, 12], although the reduction in pH caused by pozzolanic admixtures is not capable of reaching critical values of steel despassivation [9]. However, since the seminal work by Shalon and Raphael [21]—which evaluated the amount of rust formed on steel as a function of OH− concentration—it is generally accepted in the field that the higher the pH, the higher is the corrosion resistance of steel embedded in concrete. Nonetheless, more recent research has challenged this long-held belief, showing that, depending on the ionic strength of the electrolyte, small reductions on pH of the pore solution can in fact lead to better-quality passive films and improved corrosion resistance [22–26].
One of the most widely used techniques to evaluate the condition of corrosion is based on the principle of polarization resistance (Rp). This principle represents the inertia that a system has when developing the corrosion process, based on parameters that determine the corrosion rate [13, 14]. For this reason, the greater the polarization resistance, the less intense the corrosion rate will be [13, 14].
Another important method considered to evaluate corrosion resistance in reinforced concrete structures is electrical resistivity. Such parameter is related to the dissolved ions in the liquid phase of concrete that have the capacity of conducting electric current. Such conductivity depends on the humidity ratio, the permeability and the degree of ionization of concrete [7, 13, 15, 16]. When there is a small amount of electrolyte in advanced degree of hydration or when there are mineral admixtures the resistivity of the system is greater and the corrosion rate is lower [17]. The greater the resistivity value, the lower the risk of corrosion in reinforced concrete structures.
This paper aims to quantify the pH of pore solutions correlating the pH with the corrosion behavior of reinforced concrete under different concrete mixing ratios with partial substitution of cement by silica fume.
2 Materials and methods
2.1 Materials
A high early strength Portland cement (CPV-ARI) was used in this research. This type of cement was chosen due to the absence of blended pozzolanic admixtures and high content of C3S in the clinker used, thus leading to a greater availability of calcium hydroxide for the pozzolanic reactions. The specific gravity of the cement is 3.12 g/cm3 and the loss on ignition is 3.35%. The chemical composition, obtained by quantitative X-ray fluorescence (XRF), is shown in Table 1.
The pozzolan employed in this study was silica fume, from silicon metal production, non-densified type and light gray color. The specific gravity is 2.09 g/cm3 and the loss on ignition 2.40%. The chemical composition, determined by a qualitative X-ray fluorescence (XRF) test, is shown in Table 2.
The grain size distribution of the silica fume was performed by laser diffraction in a sodium hexametaphosphate solution (5), after being exposed to ultrasonic cleaning (60 s of ultrasound at a power of 40 W) for deffloculation. The results of the equivalent diameters are displayed in Table 3.
The coarse aggregate, originated from basalt, has a fineness modulus of 5.75, specific gravity of 2.67 g/cm3, maximum diameter of 12.5 mm and fits into the particle size zone 4.75/12.5. The fine aggregate utilized was a natural quartz sand with fineness modulus of 2.32, specific gravity of 2.63 g/cm3 and maximum diameter of 2.4 mm.
The steel reinforcement utilized was a low-carbon steel (CA50) with nominal diameter of 12.5 mm.
2.2 Specimens production
The unit proportions of the constituent materials are shown in Table 4 for each concrete mixture. In all cases, the total volume of binder was kept constant. In the mixtures with silica fume, 5 and 10% of the binder volume was replaced by the mineral admixture.
The water/cement ratio was fixed in 0.65 because, according to Kulakowski [18], in order to obtain enough pore solution to analyze the pH, the w/c ratio must be near 0.7.
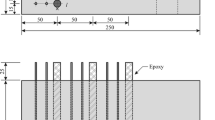
The specimens subjected to the determination of corrosion resistance were built with reinforced concrete in the dimensions of 100 mm of height, 260 mm of width and 260 mm of length containing a bar of low-carbon steel inserted according to Fig. 1. Two specimens were built for each concrete mixing ratio.
To verify the evolution of resistivity, two specimens were molded in a cylindrical shape of 100 mm of diameter and 200 mm of height, without reinforcement, for each concrete mixing ratio.
Aiming to extract the pore solution and determine the pH, the specimens were built with cement paste in the same proportions as of the partial substitution of cement shown in Table 4. The specimens had a cylindrical geometry of 40 mm of diameter and 80 mm of height. These specimens, after being removed from the mold, within 24 h, were enveloped with a plastic film and put into plastic bags to avoid any interaction with the environment, and then taken to the curing chamber (at 100% relative humidity) until the appropriate testing age.
2.3 Methods
2.3.1 Corrosion evaluation tests
The polarization resistance was determined from the corrosion current density parameter. Experiments were conducted with the assistance of a GECOR 8 equipment, manufactured by NDT James Instruments and provided by the Structural Models and Tests Laboratory (LEME) of the Federal University of Rio Grande do Sul (UFRGS). For this test only the sensor A was employed, which utilizes a copper/copper sulfate reference electrode. In order to obtain the polarization resistance (Rp) value, Eq. 1 was used, along with the values of current density (icorr) and the Stern-Geary (B) constant of 52 mV.
These determinations were performed at the ages of 3, 7, 28, 42, 56, 70, 84 and 91 days, and three replicate tests were conducted on each specimen.
To verify the resistivity of concrete, the equipment RESIPOD, manufactured by Proceq, was utilized. Such equipment adopts a Wenner array, which is also known as the method of four electrodes. The four measuring probes of the equipment have a spacing of 50 mm. The standard reading adopted was two measurements in each sample at the ages of 3, 7, 28, 42, 46 and 70 days, at specific points of the sample, being one opposed to the other.
2.3.2 Extracting the pore solution
To extract the pore solution, it was utilized an equipment that is similar to the one developed by Barneyback and Diamond [19], reproduced by Kulakowski [18]. Figure 2a explains schematically how the equipment functions and Fig. 2b shows the equipment utilized and developed by Writing and Mohamad [17] and Kulakowski [18].
The pore solutions of the cylinder specimens were extracted at ages 3, 7, 28 and 91 days.
To obtain the solution, it is necessary to apply a load on the specimen with the intention to promote confinement tension and expel the pore solution. The maximum tension applied by the hydraulic press was 300 MPa.
Some solution was also extracted after 4 h of the beginning of cement hydration due to the fact that the paste did not show a completely hardened consistency. In this case extraction was performed manually.
2.3.3 Obtaining the pH of the solution
Solutions extracted were kept in sealed containers without contact to the external environment. The analysis of the concentration of OH− ions from the samples collected was performed using the method of acid–base volumetric titration.
In order neutralize the alkaline pore solution, hydrogen chloride (HCl) at the concentration of 0.0102 N was employed. Moreover, an alcoholic solution of 1% (mass) phenolphthalein was utilized as a chemical indicator. Such indicator, when in basic pH, becomes carmine red; however, when in pH below 9, the indicator remains colorless. It was added 3 ml of phenolphthalein solution into a 0.5 ml solution of the sample extracted. To complete the dilution, 50 ml of deionized water was added as well.
When the solution turns from carmine red into colorless, the volume of acid consumed in the titration necessary to neutralize the volume of 0.5 ml of the solution diluted is reached. This happens because the deionized water and the phenolphthalein are neutral. The control of neutralization occurs visually.
By having the volume of acid consumed, it is possible to determine the concentration in moles per liter of OH− in the neutralized solution using Eq. 2.
where N = concentration of acid in Normal; Vac = volume of acid consumed to neutralize the solution; Vi = volume of the titrated sample (diluted volume).From the concentration of OH− ions obtained by Eq. 2, it is possible to find the pH of the solution by using Eq. 3.
where [OH−] = concentration of OH− ions in mole/liter.
3 Results
Tables 5 and 6 summarizes the results of the pH, OH− concentration, resistivity and polarization resistances of the mixtures obtained at several different ages.
It is possible to observe small, but consistent, alterations in the pH values (naturally accompanied by considerable changes in OH− concentrations) when comparing the age of the solution as well as to the substitution percentage. It should be noted that the pH values vary according to a logarithmic scale. Therefore, a unit variation in the pH—varying, for example, from 13.6 to 12.6 as it occurs at the age of 28 days for the 10% SF mixing ratio in comparison to the reference specimen—corresponds to a ten times greater reduction in the concentration of OH− ions in the solution.
As expected, an increase in the pH is noted at the first ages analyzed, between 4 h and 3 days, of all mixtures. Such increase is due to the fact that at the age of 4 h, the cement is still being dissolved and only hydration reactions of the aluminates are happening, whereas, at the age of 3 days the hydration of silicates is already initiated, and therefore the solution shows a higher alkalinity.
At the age of 3 days, the pH in all the mixtures with partial substitution of cement shows an inferior value compared to the reference. Such behavior is consistent with the beginning of the pozzolanic reactions, where there is a consumption of hydroxides originated from the cement hydration process. In addition, there is also a lower percentage of cement in the mixtures containing admixtures. Another fact that reinforces the assumption that the pozzolanic reactions depend on the occurrence of hydration of silicates is that, on the third day, there is a maximum peak in the values of pH in all of the mixtures containing mineral admixtures, and, after that, there is a consistent reduction in the pH value.
The mixture used as reference, without substitution of cement for pozzolans, showed a considerable increase in pH between 4 h and 3 days. After 3 days, the pH remained practically constant until the age of 91 days.
Only after the first 3 days will the difference in the percentage of substitution influence the pH value, having the mixture with the highest percentage of substitution the lowest pH value at all ages. Likewise, the mixture with the lowest percentage obtained the highest pH. Such behavior was expected because there is a reduction in the quantity of cement, and an increase in the consumption of calcium hydroxide by the pozzolanic reactions.
It is also noticeable that the solution 10% SF shows a lower value of pH at the end of the period of analysis (91 days) in relation to the reference sample and in relation to the mixtures with the same substitution material (lower percentages). From this, it is possible to verify that as the percentage of substitution of cement for silica fume increases; there is a decrease in the concentration of OH− ions as well as a decrease in the total percentage of alkaline cations in the solution from the third day on for all solutions analyzed.
Figure 3 shows the evolution of the polarization resistance and the pH development.
At first ages, 3 and 7 days, there is a low risk of corrosion and, as the cement hydration reactions occur, the probability of corrosion declines to passivation levels and remains there until the end of the period analyzed. Considering that the passive film formation process occurs mainly at these early ages (up to 7 days), as shown by Poursaee and Hansoon [27], and noting that at 3 and 7 days the concrete resistivity values remained nearly constant among all mixtures tested, the considerable increase observed in the polarization resistance values as silica fume is added must be attributed to a better passive film being formed, even though OH− concentration and pH are reduced. It should be noted that this observation is consistent with the argument presented in recent research about passive film formation, showing that such reductions in OH− concentrations reduce the solubility of film-forming Fe species and enhance corrosion resistance, as long as the ionic strength of the pore solution is also reduced [22–26].
At 28 days the samples are showed a behavior distinct of all other ages (likely due an experimental error), however, in readings at 7, 42 days, and the following ages until the end of the study, the concrete expressed similar behavior with few variations.
The reference sample shows a slight decline in the protection quality, moving from the passive zone into the zone of low risk of corrosion at the age of 42 days and remaining there until the age of 70 days, finishing the study in the passive zone. Therefore, this sample has a greater susceptibility of corrosion.
Furthermore, throughout the period of study, the samples with partial substitution of cement for silica fume show a tendency of having a better performance than those without substitutions.
A propensity of pozzolans to positively influence the performance of corrosion protection is noticeable, independently of the type of pozzolan and the percentage used. These results complement the study by Oliveira [20], in which it was noted that the admixtures are capable of improving the performance of concrete against corrosion, with a superior polarization resistance compared to the concrete without any substitution [20].
4 Conclusion
A greater influence of silica fume is noticeable until the seventh day, when the passivation film is being formed and stabilizing. At this point, it was observed a considerable increase in the polarization resistance when the pH is reduced due to the partial substitution of cement for the mineral admixture. The results presented in this study agree with recent research on passive film formation, showing that the reduction in pH values caused by the use of supplementary cementitious materials does not seem to have a negative effect regarding the protection against corrosion, but rather on the contrary: when mineral admixtures are used, the corresponding reductions in OH− concentrations (and ionic strength) lead to an enhanced passivation of the steel reinforcement, and increased resistivity (at later ages) further contributes to an improved corrosion resistance.
References
Araujo VRBS et al (2013) Estudo de prospecção do concreto verde. Congresso Brasileiro de Prospecção Tecnológica 6:106–114
Lima JAR (2010) Avaliação das consequências da produção de concreto no Brasil para as mudanças climáticas. Tese de Doutorado, Universidade de São Paulo, São Paulo
DA Costa EB et al (2013) Clínquer Portland com reduzido impacto ambiental. Ambiente Construído 13(2):75–86
Andrade MC (1992) Manual para diagnóstico de obras deterioradas por corrosão de armaduras. Pini, São Paulo
GENTIL, V. Corrosão. 3. ed. (1996) Rio de Janeiro: [s.n.]
Helene P (1993) Contribuição ao estudo da corrosão em armaduras de concreto armado. Tese de Doutorado, Universidade de São Paulo, São Paulo
Mehta PK, Monteiro PJM (2014) Concreto: microestrutura, propriedades e materiais, 20th edn. IBRACON, São Paulo
Baroghel-Bouny V, Capra B, Laurens S (2014) A durabilidade das armaduras e do concreto de cobrimento. In: Ollivier e Angélique Vichot JP, Cascudo e Helena Carasek O (eds) Durabilidade do Concreto: Bases científicas para a formulação de concretos duráveis de acordo com o ambiente. IBRACON, São Paulo
da Silva MG (2007) Cimentos Portland com adições minerais. In: Materiais de construção civil e princípios de ciência e engenharia de materiais, vol 1. IBRACON, São Paulo, pp 761–793
Dal Molin DCC (2011) Adições Minerais. Concreto: Ciência e Tecnologia, vol 1, 1a edn. IBRACON, São Paulo, pp 261–311
AMERICAN CONCRETE INSTITUTE. ACI 222R. (2001) Protection of metals in concrete against corrosion. ACI Committee Reports
Pereira VC, De O, Monteiro ECB (2011) Avaliação da capacidade de proteção de adições minerais em relação à corrosão de armaduras à corrosão de armaduras devido à carbonatação. Construindo 3:12
Cascudo O (1997) O controle da corrosão de armaduras em concreto: inspeção e técnicas eletroquímicas. Editora UFMG, São Paulo
Rilem TC (2004) 154-EMC. Recommendations: test methods for on-site corrosion rate measurement of polarization resistance method. Mater Struct 37:623–643
American Society Testing and Materials. ASTM G57 (2012) Test Method for Field Measurement of Soil Resistivity Using the Wenner Four-Electrode Method. [S.l.]: ASTM International
Rilem TC (2000) 154-EMC. Recommendations: test methods for on site measurement of resistivity of concrete. Mater Struct 33:603–611
Writing DA, Mohamad NA (2003) Electrical resistivity of concrete-a literature review. R&D Serial, Skokie, Illinois, USA, p 2457
Kulakowski MP (2002) Contribuição ao estudo da carbonatação em concretos e argamassas compostos com adição de sílica ativa. Universidade Federal do Rio Grande do Sul, Porto Alegre, Tese de Doutorado
Barneyback RS, Diamond S (1981) Expression and analysis of pore fluids from hardened cement pastes and mortars. Cem Concr Res 11(2):279–285
Oliveira AM (2007) Avaliação do desempenho de concreto com adições minerais quanto à corrosão das armaduras induzida por cloretos. Universidade Federal da Goiás, Goiânia
Shalon R, Raphael M (1959) Influence of sea water on corrosion of reinforcement. J Am Concr Inst 30(12):1251–1268
Ortolan VK (2015) Avaliação do pH e da força iônica da solução dos poros do concreto na resistência à corrosão da armadura. Dissertação—Universidade do Vale do Rio dos Sinos
Itty PA (2012) Microscale investigation of the corrosion performances of low-carbon and stainless steels in highly alkaline concretes. University of California, Berkeley (PhD Dissertation)
Mancio M (2008) Electrochemical and in situ surface-enhanced raman spectroscopic (SERS) study of passive films formed on low-carbon steel in highly alkaline environments. University of California, Berkeley (PhD Dissertation)
Mancio M, Kusinski G, Monteiro PJM, Devine TM (2009) Electrochemical and in situ SERS study of passive film characteristics and corrosion performance of 9% Cr microcomposite steel in highly alkaline environments. J ASTM Int 6:101903
Mancio M, Kusinski G, Devine TM, Monteiro PJM (2008) Electrochemical and in situ SERS study of passive film characteristics and corrosion performance of microcomposite steel in simulated concrete pore solutions. MMFX Technologies Corporation; University of California, Berkeley. p 104. http://mmfx.com/doc2/Electrochemical_Mancio_Kusinski.pdf. Accessed July 2015
Poursaee A, Hansson CM (2007) Reinforcing steel passivation in mortar and pore solution. Cem Concr Res 37(7):1127–1133
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ortolan, V.K., Mancio, M. & Tutikian, B.F. Evaluation of the influence of the pH of concrete pore solution on the corrosion resistance of steel reinforcement. J Build Rehabil 1, 10 (2016). https://doi.org/10.1007/s41024-016-0011-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41024-016-0011-8